Abstract
Background
Asymptomatic neurosyphilis is more difficult to diagnose in HIV-infected patients because HIV itself can cause cerebrospinal fluid (CSF) pleocytosis. The proportion of CSF lymphocytes that are B cells is elevated in neurosyphilis, suggesting that the CSF concentration of the B cell chemoattractant, chemokine (C-X-C motif) ligand 13 (CXCL13) concentration may also be elevated.
Methods
CSF and blood were collected from 199 HIV-infected patients with syphilis and neurosyphilis. Serum and CSF CXCL13 concentrations were determined.
Results
Patients with neurosyphilis had higher CSF and serum CXCL13 concentrations compared to patients with syphilis but not neurosyphilis. The odds of having symptomatic neurosyphilis were increased by 2.23 fold for every log increase in CSF CXCL13 concentration and were independent of CSF WBC and plasma HIV RNA concentrations, peripheral blood CD4+ T cell count and use of antiretroviral medications. A cut-off of 10 pg/mL CSF CXCL13 had high sensitivity and a cut-off of 250 pg/mL or evidence of intrathecal synthesis of CXCL13 had high specificity for diagnosis of both symptomatic and asymptomatic neurosyphilis. CSF concentrations of CXCL13 declined after treatment for neurosyphilis.
Conclusions
CSF CXCL13 concentration may be particularly useful for diagnosis of neurosyphilis in HIV-infected patients because it is independent of CSF pleocytosis and markers of HIV disease.
Keywords: syphilis, neurosyphilis, diagnosis, cerebrospinal fluid, CXCL13
Introduction
In 2000, the rate of primary and secondary syphilis was the lowest ever reported. However, the number of reported cases increased 92% between 2000 and 2007. In men, mainly men who have sex with men, the number of cases nearly tripled (1). As the rates of infectious syphilis have risen, the incidence and prevalence of neurosyphilis has increased, particularly among patients who also are infected with HIV. Cases of “neurorelapse,” meaning development of symptomatic neurosyphilis after standard treatment for early syphilis, continue to be reported in HIV-infected but not in HIV-uninfected patients (2-6).
There is no perfect test to establish or exclude the diagnosis of neurosyphilis. When the cerebrospinal fluid (CSF)-Venereal Disease Research Laboratory (VDRL) test is reactive, the diagnosis of neurosyphilis is established. When the CSF-VDRL is nonreactive, the laboratory diagnosis of neurosyphilis may rely on CSF pleocytosis alone. In patients with both HIV and syphilis, CSF pleocytosis can be due to syphilis, HIV or both infections. Because the CSF-VDRL is an imperfect “gold standard” for diagnosis of neurosyphilis, other markers of disease are needed. Studies whose goal is to identify a test that is “better” than the CSF-VDRL must use a gold standard other than CSF-VDRL for their analyses. Possibilities include combinations of CSF abnormalities or clinical abnormalities.
CSF from patients with syphilitic meningitis is unusual because it contains a high concentration of B lymphocytes (7). A small study suggested that the CSF concentration of the B lymphocyte chemoattractant chemokine (C-X-C motif) ligand 13 (CXCL13) is elevated in HIV-uninfected patients with neurosyphilis, but not uncomplicated syphilis (8). In this report, we explore the utility of CSF and serum CXCL13 concentrations as diagnostic tests for neurosyphilis using a clinical definition. This study was conducted on a large cohort of patients with concurrent syphilis and HIV, a group in which the diagnosis of neurosyphilis is particularly challenging.
Materials and Methods
Study Participants
One hundred ninety-nine patients with syphilis who were enrolled in a study of CSF abnormalities conducted in Seattle, WA (9) are included in this report. Individuals were eligible for enrollment if they had clinical or serological evidence of syphilis, and were deemed by the referring provider as possibly having neurosyphilis. In our community, reasons for referral to the study include neurological findings, hearing loss or visual loss, and HIV-infection, particularly if the peripheral blood CD4+ T cell count is ≤ 350 or the serum RPR titer is ≥ 1:32. The latter criteria are based on published data (9-11). All participants underwent a structured history and neurological examination that included assessment of cranial nerves, motor strength, sensation, coordination, reflexes and gait; lumbar puncture; and venipuncture. Participants who were treated for neurosyphilis returned for follow-up visits at 3, 6 and 12 months after therapy. At each follow-up visit, participants underwent the same structured history and neurological examination that was administered at study entry and venipuncture. Lumbar puncture was performed on all patients at the first follow-up visit, but was performed at subsequent visits only if the CSF profile from the previous lumbar puncture was abnormal.
The study protocol was reviewed and approved by the University of Washington Institutional Review Board, and human experimentation guidelines were followed in the conduct of this research. Written informed consent was obtained from all participants.
Laboratory Methods
CXCL13 was measured in CSF and serum using the Human CXCL13/BLC/BCA-1 Quantikine ELISA Kit (R&D Systems, Minneapolis, MN) according to manufacturer’s instructions for serum. Reverse-transcriptase polymerase chain reaction (RT-PCR) was used to detect T. pallidum in CSF (9). Cerebrospinal fluid Venereal Disease Research Laboratory (VDRL) test, and measurement of CSF WBCs, blood CD4+ T cells and plasma HIV RNA were performed in CLIA-approved laboratories. Blood CD4+ T cell count and plasma HIV RNA values collected within 90 days before or after the diagnosis of neurosyphilis were used in the analyses. Serum rapid plasma reagin (RPR) test was performed in a single research laboratory according to standard methods (12).
Statistical Methods
The most common objective neurological abnormalities in our patients were hearing loss and visual loss (9). Participants were divided into three groups: 1) symptomatic neurosyphilis was defined as hearing or visual loss regardless of CSF abnormalities (n=29); 2) asymptomatic neurosyphilis was defined as a reactive CSF-VDRL or CSF WBCs > 20/uL in the absence of hearing or vision loss (n=54); and 3) uncomplicated syphilis was defined as a nonreactive CSF-VDRL, CSF WBCs ≤ 20/uL and normal hearing and vision (n=106). One hundred eighty-nine patients had data for all variables used to differentiate symptomatic from asymptomatic neurosyphilis and neurosyphilis from uncomplicated syphilis. Symptomatic neurosyphilis was considered to be the most rigorous neurosyphilis definition, and, because it does not include CSF measures, enabled us to take into account CSF WBC concentration and CSF-VDRL in our analyses.
CXCL13 concentrations at or below the level of detection were set at the level of detection (1.64 pg/mL in 8 CSF and in no serum) and plasma HIV RNA concentration at or below 50 copies/mL was set at 50 copies/mL (n=47 of 195). Elevated CSF:serum CXCL13 ratio was defined as > 1.0, suggesting intrathecal CXCL13 synthesis. Pairwise comparisons were made using the Mann Whitney U test and the chi-square test or Fisher’s Exact test. Relationships between clinical and laboratory variables were determined using logistic regression, and results were expressed as odds ratios (OR) with 95% confidence intervals (95% CI). For these analyses, CSF CXCL13 and serum CXCL13 (n=157) concentrations and peripheral blood CD4+ T cell counts (n=196) were log transformed. CSF WBC numbers were dichotomized as > 20 cells/uL vs. ≤ 20 cells/uL as previously described (9). Multivariate models were developed testing all variables that showed a trend towards significance in univariate analyses. Diagnostic sensitivity and specificity were calculated using standard formulae and expressed as percent (95% CI). Comparisons between sensitivities or specificities were performed using the two sample test of proportions. Two-tailed P-values < 0.05 were considered significant for all analyses. The Bonferroni correction was used to correct for multiple comparisons.
Results
Patient Characteristics
Characteristics of the 199 patients are shown in Table 1. Most study participants were men with early syphilis, median (IQR) peripheral blood CD4+ T cell count was 432 (238-572), and 42% were taking combination antiretroviral therapy (CART) at the time of enrollment. Fifteen percent of patients had symptomatic neurosyphilis, 29% had asymptomatic neurosyphilis and the remaining 56% had uncomplicated syphilis. CSF:serum CXCL13 ratio was > 1 in 37 (24%) of 157 patients.
Table 1.
Characteristics of 199 Study Participants
| Characteristic or Value | Percent or Median Value |
|---|---|
| Male | 198/199 (99%) |
| Age (median, IQR) | 37 (33-42) |
| Syphilis Stage1 Early Late |
112/158 (71%) 46/158 (29%) |
| Taking CART | 58/139 (42%) |
| 1/Serum RPR titer (median, IQR) | 64 (16-256) |
| CD4 (median, IQR) | 432 (238-572) |
| Log 10 plasma HIV RNA (median, IQR) | 3.68 (1.78-4.84) |
| T. pallidum detected in CSF by RT-PCR | 30/167 (18%) |
| Reactive CSF-VDRL test | 40/199 (20%) |
| Symptomatic neurosyphilis2 | 29/189 (15%) |
| Asymptomatic neurosyphilis3 | 54/189 (29%) |
CART, combination antiretroviral therapy; RPR, Rapid Plasma Regain test; CSF, cerebrospinal fluid; RT-PCR, reverse transcriptase polymerase chain reaction; VDRL, Venereal Research Laboratory.
Late stage includes syphilis of unknown duration.
Hearing loss, vision loss or both, regardless of CSF findings. Data on one or more components of the neurosyphilis diagnosis were missing for 10 patients.
CSF white blood cells >20/uL or CSF-VDRL reactive or both in absence of hearing or vision loss.
CSF and Serum CXCL13 Concentration by Patient Group
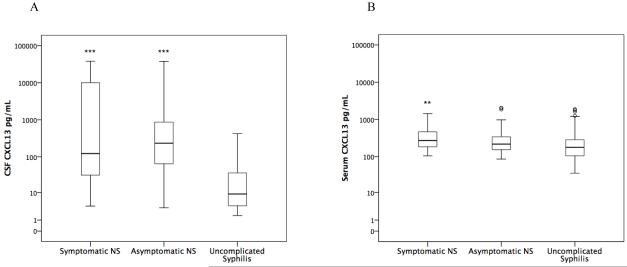
Compared to patients with uncomplicated syphilis, CSF CXCL13 concentration was significantly higher in both neurosyphilis groups (p≤0.001; Figure 1A), and serum CXCL13 was significantly higher in patients with symptomatic neurosyphilis (p=0.003; Figure 1B). These relationships remained statistically significant after taking into account multiple comparisons. There was no significant difference in CSF or serum CXCL13 concentration between those with symptomatic vs. asymptomatic neurosyphilis.
Figure 1.
Box plots of CSF CXCL13 concentration (Figure 1A) and serum CXCL13 concentration (Figure 1B) in three patient groups. Patients with symptomatic neurosyphilis (Symptomatic NS) had hearing or vision loss with or without cerebrospinal fluid (CSF) abnormalities. Patients with asymptomatic neurosyphilis (Asymptomatic NS) had CSF white blood cells > 20/uL or a reactive CSF-VDRL or both, but no hearing or vision loss. Patients with uncomplicated syphilis had no hearing or vision loss, CSF white blood cells < 20/uL and nonreactive CSF-VDRL.
** P<0.01 compared to uncomplicated syphilis
***P<0.001 compared to uncomplicated syphilis
CSF CXCL13 Concentration and Neurosyphilis
In these analyses, we used symptomatic neurosyphilis as our outcome variable because it allowed us to evaluate associations with CSF WBC concentration. In univariate models, the odds of symptomatic neurosyphilis increased significantly with increasing CSF CXCL13 concentration, CSF WBCs > 20/uL, detection of T. pallidum in CSF by RT-PCR and increasing plasma HIV RNA (Table 2). The odds of symptomatic neurosyphilis decreased with increasing peripheral blood CD4+ T cell count (Table 2). The odds of symptomatic neurosyphilis were not significantly different in patients who were or were not taking CART. In a multivariate model, the odds of symptomatic neurosyphilis remained significantly higher with higher CSF CXCL13 and plasma HIV RNA concentration, and lower with increasing peripheral blood CD4+ T cell count. In this multivariate model, CSF WBC > 20/uL and detection of T. pallidum in CSF were not significantly associated with symptomatic neurosyphilis (Table 2).
Table 2.
Odds of Symptomatic Neurosyphilis
| Odds Ratio (95% Confidence Interval) | ||
|---|---|---|
| Univariate | Multivariate | |
| CSF CXCL13 per log10 | 2.07 (1.45-2.97), p<0.001 | 2.23 (1.46-3.39), p<0.001 |
| CSF WBCs > 20/uL | 2.66 (1.19-5.94), p=0.02 | NS |
| Detection of T. pallidum in CSF | 3.20 (1.14-9.01), p=0.03 | NS |
| Plasma HIV RNA per log10 | 1.77 (1.27-2.47), p=0.001 | 1.54 (1.09-2.18), p=0.01 |
| Blood CD4+ T cells per log 10 | 0.33 (0.13-0.86), p=0.02 | 0.31 (0.10-0.96), p=0.04 |
Diagnostic Sensitivity and Specificity of CSF CXCL13 Concentration and CSF:Serum CXCL13 Ratio > 1
We categorized CSF CXCL13 concentration into a low value (10 pg/mL) and a high value (250 pg/mL) based roughly on the 25th percentile and 75th percentile of all values. We first calculated sensitivity and specificity of low and high CSF CXCL13 concentrations and CSF:serum CXCL13 ratio > 1 using symptomatic neurosyphilis as the gold standard. We took this approach because the clinical diagnosis was independent of CSF abnormalities and thus allowed us to compare sensitivity and specificity to that of the CSF-VDRL. As shown in Table 3, the 10 pg/mL CSF CXCL13 cut-off had significantly higher sensitivity than the CSF-VDRL (p=0.003) for diagnosis of symptomatic neurosyphilis. The diagnostic specificity of the 250 pg/mL CSF CXCL13 cut-off and CSF:serum CXCL13 ratio > 1 was comparable to the CSF-VDRL.
Table 3.
Sensitivity and Specificity for Neurosyphilis Diagnosis
| Diagnostic Criterion | ||
|---|---|---|
| Symptomatic Neurosyphilis | ||
| Sensitivity (95% CI) | Specificity (95% CI) | |
| CSF CXCL13 ≥ 10 pg/mL | 90% (73-98%) | 37% (29-45%) |
| CSF CXCL13 ≥ 250 pg/mL | 41% (24-61%) | 79% (72-85%) |
| CSF:Serum CXCL13 > 1 | 58% (37-78%) | 84% (76-90%) |
| CSF-VDRL | 55% (36-74%) | 86% (80-91%) |
We then repeated the above analysis addressing the situation with the greatest clinical uncertainty: neurologically asymptomatic individuals with a nonreactive CSF-VDRL but with CSF pleocytosis. As shown in Table 4, using WBCs > 20/uL cut-off as the gold standard, the above sensitivity and specificities were greater than those calculated using symptomatic neurosyphilis as the gold standard. For CSF CXCL13 concentration ≥ 250 pg/mL and for CSF:serum CXCL13 ratio > 1 the specificities were significantly higher than when symptomatic neurosyphilis was used as the gold standard (p=0.002 and p=0.001, respectively).
Table 4.
Sensitivity and Specificity for Neurosyphilis Diagnosis in Neurologically Asymptomatic Patients with a Nonreactive CSF-VDRL
| Diagnostic Criterion | ||
|---|---|---|
| CSF WBCs > 20/ul | ||
| Sensitivity (95% CI) | Specificity (95% CI) | |
| CSF CXCL13 ≥ 10 pg/mL | 97% (84%-100%) | 53% (43%-63%) |
| CSF CXCL13 ≥ 250 pg/mL | 41% (24%-59%) | 93% (87%-97%) |
| CSF:Serum CXCL13 ≥ 1 | 46% (26%-67%) | 98% (92%-100%) |
Decline in CSF CXCL13 Concentration After Neurosyphilis Therapy
CSF CXCL13 concentrations were available at baseline and at one or more follow-up visits for 34 patients, and in 31, the baseline value was >10. Before treatment, all 31 had a reactive CSF-VDRL or CSF WBCs > 20/uL. Of the 31 participants, 26 received an effective treatment regimen with penicillin or ceftriaxone based on current standards (13). The most common regimen was IM procaine penicillin G (APPG) 2.4 MU per day with probenecid 500 mg po per day. Twenty-one individuals were treated with this regimen for at least 10 days; 2 additional individuals received shorter courses (6 and 8 days). The remaining 8 individuals received IV penicillin G 20-24 MU per day divided into 6 doses for 10 days (n=4) or 14 days (n=1), IV ceftriaxone 2000 mg IV per day for 10 days (n=1) or oral doxycycline 200 mg by mouth twice a day for 21 days (n=2). Figure 2 shows that CSF CXCL13 concentrations decreased after treatment. This was apparent for the two participants who received 6 and 8 days of APPG treatment as well as for the two patients treated with doxycycline (data not shown).
Figure 2.
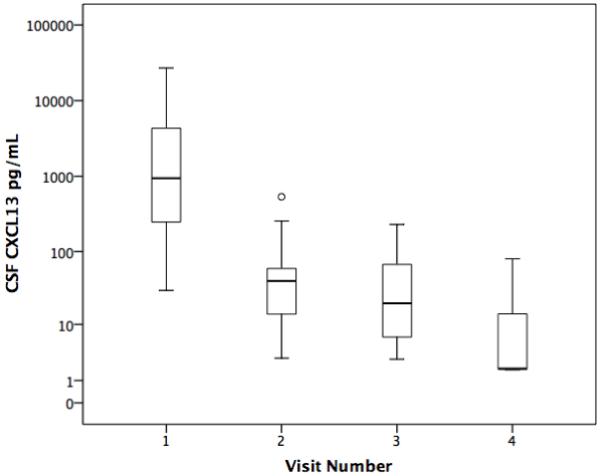
Box plots of CSF CXCL13 concentration before and after neurosyphilis treatment. Visit 1 is the study entry visit (n=31). Visit 2 is the visit closest to 3 months after treatment (n=29), Visit 3 is the visit closest to 6 months after treatment (n=14) and Visit 4 is the visit closest to 12 months after treatment (n=3).
Discussion
The diagnosis of neurosyphilis is not difficult when patients have typical symptoms and signs of the disease. However, the diagnosis of asymptomatic neurosyphilis is based solely on CSF abnormalities, including CSF-VDRL reactivity or CSF pleocytosis. In some instances of asymptomatic neurosyphilis, the CSF-VDRL may be nonreactive and the diagnosis must be based solely on CSF pleocytosis. Thus diagnosis of asymptomatic neurosyphilis can be especially difficult in patients who are also infected with HIV because HIV itself causes CSF pleocytosis. Moreover, several HIV-related factors influence the likelihood of HIV-related CSF pleocytosis. For example, in a separate study, we showed that the odds of CSF pleocytosis (defined as CSF WBCs > 5/uL) were 6-fold higher in patients who were not taking antiretrovirals compared to those who were taking them, 23-fold higher in those with a peripheral blood CD4+ T cell count above 200/uL compared to individuals with counts ≤ 200/uL, and 3-fold higher in patients with detectable plasma HIV RNA compared to those with undetectable plasma HIV RNA (14).
In this study, we examined the relationship between neurosyphilis and CSF concentration of CXCL13 in HIV-infected individuals with syphilis. Our ultimate goal was to determine whether this measure could be used to diagnose asymptomatic neurosyphilis in HIV-infected individuals with syphilis when the CSF-VDRL is nonreactive. We found that, compared to patients with uncomplicated syphilis, CSF CXCL13 concentration is significantly higher in patients with both asymptomatic and symptomatic neurosyphilis, and CSF CXCL13 concentration declines after neurosyphilis treatment.
We used symptomatic neurosyphilis as an irrefutable gold standard for neurosyphilis diagnosis. This approach allowed us to include CSF WBC concentration in the analysis, which we would not have been able to do had we used CSF abnormalities as our gold standard. We showed that the odds of neurosyphilis are significantly higher with increasing CSF CXCL13 concentration, even taking into account CSF WBC numbers and markers of HIV infection, including use of antiretroviral therapy. Using this same rigorous definition, which also allowed us to compare to the CSF-VDRL, we demonstrated the diagnostic utility of CSF CXCL13 concentration. Finally, turning to the most vexing clinical situation, an HIV infected patient with syphilis and a nonreactive CSF-VDRL, we showed that CSF CXCL13 concentration and the ratio of CSF:serum CXCL13 have high sensitivity and specificity for diagnosis of asymptomatic neurosyphilis.
Our study has some limitations. The patient group was a convenience sample taken from a larger sample of HIV-infected patients with syphilis and was enriched for individuals with symptomatic (15%) and asymptomatic (29%) neurosyphilis to increase the power of our analyses. We made the assumption that CXCL13 measures that are diagnostically specific and sensitive for diagnosis of symptomatic neurosyphilis will also be diagnostically specific and sensitive for asymptomatic neurosyphilis. Our results are uniformly consistent with this assumption. One could argue that measurement of CSF and serum CXCL13 concentration is costly (approximately $12 per tests for the reagents). However, the cost of inappropriate neurosyphilis treatment or of failure to treat neurosyphilis could be far greater.
Our results have important implications for neurosyphilis diagnosis in HIV-infected individuals because they suggest that there are diagnostic test(s) for neurosyphilis that are not confounded by HIV infection itself. Importantly, they suggest that CSF and serum CXCL13 concentrations can be used to establish or refute a diagnosis of neurosyphilis when the CSF-VDRL is nonreactive but the CSF WBC concentration is elevated. Ideally, our findings should be confirmed in a large longitudinal study in which neurosyphilis treatment decisions take into account CXCL13 concentrations in CSF and serum, and both treated and untreated patients are followed with serial CSF and clinical examinations. Such a study is unlikely to be undertaken for many reasons, including cost and logistical issues. Thus clinicians will need to weigh the applicability of our findings to their patients on an individual basis.
Article Summary.
In 199 patients with HIV and syphilis, CSF CXCL13 was sensitive and specific for the diagnosis of neurosyphilis and was independent of CSF pleocytosis and markers of HIV disease.
Acknowledgments
This study was supported by grant #NS34235 from the National Institutes of Health/National Institute of Neurological Disorders and Stroke.
Footnotes
This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Centers for Disease Control and Prevention . Sexually Transmitted Disease Surveillance 2007. U.S. Department of Health and Human Services; Atlanta, GA: Dec, 2008. [Google Scholar]
- 2.Berry CD, Hooton TM, Collier AC, Lukehart SA. Neurologic relapse after benzathine penicillin therapy for secondary syphilis in a patient with HIV infection. N Engl J Med. 1987;316:1587–9. doi: 10.1056/NEJM198706183162507. [DOI] [PubMed] [Google Scholar]
- 3.Walter T, Lebouche B, Miailhes P, Cotte L, Roure C, Schlienger I, et al. Symptomatic relapse of neurologic syphilis after benzathine penicillin G therapy for primary or secondary syphilis in HIV-infected patients. Clin Infect Dis. 2006;43:787–90. doi: 10.1086/507099. [DOI] [PubMed] [Google Scholar]
- 4.Richards BW, Hessburg TJ, Nussbaum JN. Recurrent syphilitic uveitis. N Engl J Med. 1989;320:62. [PubMed] [Google Scholar]
- 5.Mishra S, Walmsley SL, Loutfy MR, Kaul R, Logue KJ, Gold WL. Otosyphilis in HIV-coinfected individuals: a case series from Toronto, Canada. AIDS Patient Care STDS. 2008;22:213–9. doi: 10.1089/apc.2007.0019. [DOI] [PubMed] [Google Scholar]
- 6.Symptomatic early neurosyphilis among HIV-positive men who have sex with men--four cities, United States, January 2002-June 2004. MMWR Morb Mortal Wkly Rep. 2007;56:625–8. [PMC free article] [PubMed] [Google Scholar]
- 7.Marra CM, Tantalo LC, Maxwell CL, Dougherty K, Wood B. Alternative cerebrospinal fluid tests to diagnose neurosyphilis in HIV-infected individuals. Neurology. 2004;63:85–8. doi: 10.1212/01.wnl.0000131902.69113.34. [DOI] [PubMed] [Google Scholar]
- 8.Rupprecht TA, Kirschning CJ, Popp B, Kastenbauer S, Fingerle V, Pfister HW, et al. Borrelia garinii induces CXCL13 production in human monocytes through Toll-like receptor 2. Infect Immun. 2007;75:4351–6. doi: 10.1128/IAI.01642-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Marra CM, Maxwell CL, Smith SL, Lukehart SA, Rompalo AM, Eaton M, et al. Cerebrospinal fluid abnormalities in patients with syphilis: association with clinical and laboratory features. J Infect Dis. 2004;189:369–76. doi: 10.1086/381227. [DOI] [PubMed] [Google Scholar]
- 10.Ghanem KG, Moore RD, Rompalo AM, Erbelding EJ, Zenilman JM, Gebo KA. Neurosyphilis in a clinical cohort of HIV-1-infected patients. Aids. 2008;22:1145–51. doi: 10.1097/QAD.0b013e32830184df. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Libois A, De Wit S, Poll B, Garcia F, Florence E, Del Rio A, et al. HIV and syphilis: when to perform a lumbar puncture. Sex Transm Dis. 2007;34:141–4. doi: 10.1097/01.olq.0000230481.28936.e5. [DOI] [PubMed] [Google Scholar]
- 12.Larsen SA, Pope V, Johnson RE, Kennedy EJJ. A Manual of Tests for Syphilis. 9th ed. American Public Health Association; Washington, DC: 1998. [Google Scholar]
- 13.Workowski KA, Berman SM. Sexually transmitted diseases treatment guidelines, 2006. MMWR Recomm Rep. 2006;55:1–94. [PubMed] [Google Scholar]
- 14.Marra CM, Maxwell CL, Collier AC, Robertson KR, Imrie A. Interpreting cerebrospinal fluid pleocytosis in HIV in the era of potent antiretroviral therapy. BMC Infect Dis. 2007;7:37. doi: 10.1186/1471-2334-7-37. [DOI] [PMC free article] [PubMed] [Google Scholar]