Abstract
Targeting novel pathways associated with tumor angiogenesis, invasion and immunity, may lead to improvement in patient outcomes for renal cell carcinoma. Chemokines potentiate tumor growth, metastasis, angiogenesis and immune evasion through interactions with stromal cells and neoplastic cells. Further understanding of the mechanisms involved in chemokine-mediated angiogenesis and metastasis may lead to improved therapeutic strategies in this disease. Interactions between chemokine expression and signaling, and the VEGF and hypoxia-inducible factor pathways offer important opportunities to intervene in the process of renal cell carcinoma proliferation, angiogenesis and invasion. Modulation of the CXCR3/CXCR3-ligand or the CXCR4/CXCL12 biologic axis may be potential therapeutic targets for the treatment of renal cell carcinoma. Furthermore, combination treatment with agents targeting chemokine signaling with therapies directed at angiogenesis and tumor immunity may lead to improved outcomes in this disease.
Keywords: angiogenesis, chemokine, metastatic potential, renal cell carcinoma, treatment
Increased understanding of the molecular pathogenesis of renal cell carcinoma (RCC) has led to improved therapies that target important pathways involved in RCC angiogenesis, growth and invasion. Agents that target the VEGF and mTOR pathways, including sunitinib malate, sorafenib tosylate, bevacizumab ± IFNα, and temsirolimus, have improved clinical outcomes in randomized Phase III trials [1–5]. Tumor-associated angiogenesis, growth and invasion potential and the immunogenic nature of RCC have led to further investigation of these factors in the pathobiology of this cancer [6], and chemokines have demonstrated an important role. Chemokines are important for enhancing innate and adaptive immunity, regulating angiogenesis, and mediating tumor-cell metastases, and have been shown to have a direct impact on the biology of RCC. Therapeutically targeting these features may lead to novel interventions and improved response to therapy in patients with RCC.
Chemokines are produced by tumor cells, leukocytes, epithelial cells, stromal cells and endothelial cells [7,8]. In addition, functional chemokine receptors have been found on endothelial cells, leukocytes and transformed epithelial cells [9–11]. Through interactions with stromal cells and neoplastic cells, chemokines can potentiate tumor growth, metastasis, angiogenesis and immune evasion [12]. Chemokine ligand and receptor interactions then stimulate signaling cascades that result in the transcription of target genes responsible for tumor cell invasion, motility and survival [13]. An essential element of chemokine signaling depends on the chemotactic gradient achieved. Chemokine gradients within the tumor microenvironment and target organs can determine the metastatic potential of a tumor [7]. Immunotherapy and antibody treatments have been implicated in the upregulation of chemokines in RCC and other human cancers [14,15], which may be important in the reduction of tumor burden.
Although the importance of chemokines in cancer biology has been demonstrated, the development of inhibitors of chemokine pathways is still in an early phase. Attempts to block CXCR2 and inhibit tumor-cell angiogenesis have been successful in multiple tumor models [16–18]. This has been accomplished by utilizing CXCR2 small-molecule antagonists, substance P analogs, or CXCR2-knockout models. Strategies to attenuate the CXCL12/CXCR4 biological axis may potentially be employed as a new antimetastatic strategy to inhibit the metastatic potential of RCC. In fact, small-molecule antagonists to CXCR4 and RNA interference strategies have already been developed to inhibit the binding of lymphotropic strains of HIV to CXCR4 on T cells [19–21]. Multiple CXCL12/CXCR4 antagonists are under evaluation in Phase I testing for their safety and efficacy in various tumors [22]. Strategies to attenuate the CXCR2 or CXCL12/CXCR4 biological axis may potentially be employed as methods to enhance treatment of RCC.
Role of chemokines in angiogenesis
RCC growth and metastases have been associated with primary tumor-associated angiogenesis resulting from the mutation or hypermethylation of the von Hippel-Lindau (VHL) gene with subsequent hypoxia-inducible factor (HIF) activation accompanied by the downstream effects of VEGF induction [23,24]. Angiogenesis in the tumor environment is important for cancer growth, invasion and metastasis. The balance between proangiogenic and antiangiogenic factors determines the overall angiogenesis in the tumor microenvironment. Although VEGF and its receptors have been the most extensively studied, other factors that regulate angiogenesis in both a positive and negative manner are clearly involved in the process of angiogenesis in RCC. Targeting tumor immunity and angiogenesis through the inhibition of CXCR2 and its ligands, or the upregulation of CXCR3 and its ligands, may augment responses to therapy in RCC (Box 1). Chemokine members that contain the ‘ELR’ motif (ELR+) are equipotent angiogenic factors with bFGF and VEGF [25], and ELR+ CXC chemokines promote angiogenesis through CXCR2 [26]. By contrast, members that lack the ELR motif (ELR-) and are interferon-inducible, inhibit angiogenesis [25], and include CXCL4, CXCL9, CXCL10, and CXCL11 [25,27,28]. These interferon-inducible CXC chemokines act through CXCR3 to promote cell-mediated immunity and inhibit angiogenesis. CXCR2 and CXCR3 and their ligands have been shown to be expressed in RCC tumors [16,29,30]. Furthermore, CXCR3 expression has been associated with a favorable prognosis in RCC [31,32]. IL-12-mediated regression of murine RCC has been shown to be dependent on either CXCL9 or CXCL10 [33]. Therefore, on both structural and functional levels, the CXC chemokine family and their receptors play an integral role in the promotion or inhibition of angiogenesis relevant to RCC [16]. Proangiogenic CXCR2 ligands have been found in the plasma of patients with metastatic RCC, and CXCR2 was found to be expressed on the endothelial cells of RCC tumors [16]. Reduced tumor growth has been demonstrated in a CXCR2-knockout murine model, which correlated with a decrease in angiogenesis and an increase in tumor necrosis. This provides further evidence that modulating the CXCR3 and CXCR2 pathways may be a potential therapeutic modality for RCC.
BOX 1. Chemokines in the regulation of angiogenesis in renal cell carcinoma.
Proangiogenic (ELR+ CXC chemokines)
CXCL1/GRO-α, CXCL2/GRO-β, CXCL3/GRO-γ, CXCL5/ENA-78, CXCL6/GCP-2, CXCL7/NAP-2, CXCL8/IL-8, PBP, CTAP-III, -TG
Receptor: CXCR2
Antiangiogenic (non-ELR+CXC Chemokines)
CXCL4/PF-4, CXCL4L1/PF-4, CXCL14, CXCL9/MIG, CXCL10/IP-10, CXCL11//ITAC
Receptor: CXCR3 (except CXCL14)
B-TG: β thromboglobulin; CTAP: Connective tissue-activating peptide; ENA: Epithelial neutrophil-activating peptide; GCP: Granulocyte chemotactic protein; GRO: Growth-related oncogene; IP: IFN-inducible protein; ITAC: IFN-inducible T cell chemoattractant; MIG: Monokine induced by IFN-; NAP: Neutrophil-activating peptide; PBP: Platelet basic protein; PF: Platelet factor.
Role of chemokines in tumor immunity
Previous treatments for metastatic RCC have focused on immune-modulating therapies [34–38], and chemokines play an important role at the interface of angiogenesis and tumor immunity in RCC. The combined effect of systemic induction of CXCR3 expression on circulating mononuclear cells by systemic IL-2 therapy in conjunction with concomitant intratumor spatial expression of CXCR3 ligands has been shown to be essential to create an optimal CXCR3/CXCR3 ligand-dependent chemotactic gradient in renal cell tumors [16]. The combined therapeutic intervention of systemic IL-2 and concomitant intratumor CXCR3 ligand (i.e., CXCL9) administration resulted in marked attenuation of tumor growth and tumor-associated angiogenesis [16]. Systemic priming with IL-2 could lead to the kinetic expression of chemokine receptor, CXCR3, from peripheral blood mononuclear cells (PBMCs) of patients with metastatic RCC receiving standard therapy with high-dose IL-2 [39]. IL-2 is the major agonist for the expression of CXCR3 on Th1 cells, which promote antitumor immune responses. Patients with metastatic clear-cell RCC and normal controls had similar PBMC CXCR3 levels and peripheral-blood angiostatic chemokines at baseline, but patients with metastatic RCC had elevated levels of CXCR2 angiogenic ligands [16,39]. CXCR3 ligands increased in response to high-dose IL-2 therapy and CXCR3 expression on circulating mononuclear cells of patients with metastatic RCC also increased. CXCR3 expression on CD4, CD8 and natural killer cells rose in response to high-dose IL-2 treatment. The induction of CXCR3 expression of PBMCs was most pronounced in a patient with a complete response to therapy [39]. Chemokines have been found to display pleiotropic activity, such as the recruitment of specific subsets of leukocytes and the regulation of angiogenesis [40]. Overexpression of a CXCR3 ligand alone at the local tumor level will not be sufficient to recruit PBMCs to the tumor unless they are primed and activated with upregulated expression of CXCR3. A study by Pan et al. described this phenomenon in a murine RCC model [41]. Combined systemic IL-2 with intratumor CXCL9 (a CXCR3 ligand) led to a significant reduction in tumor growth and angiogenesis, compared with either therapy alone [41]. Therefore, both components of the CXCR3/CXCR3 ligand biological axis must be optimized for recruitment of mononuclear cells. On the basis of the ability of interferon-inducible CXC chemokines to promote Th1 immunity and inhibit angiogenesis, combining immunotherapy with local induction of these chemokines may have a role in promoting tumor regression in RCC.
Role of chemokines in tumor metastasis
Chemokines have also been demonstrated to play a major role in mediating tumor metastasis [10,42–45]. Multiple cancers are found to express chemokine receptors, and their corresponding ligands are expressed at sites of tumor metastases [10,44,46,47]. However, CXCR4 appears to be the major chemokine receptor expressed on cancer cells [42,43,45], and CXCL12 (stromal-derived factor-1, [SDF-1]) is its lone ligand [48]. CXCR4 expression is required for tumor metastasis to other organs, and CXCR4 activation by CXCL12 induces migration of neoplastic cells [49]. CXCR4 expression has been correlated with the metastatic potential of multiple tumors, including RCC [10,44,50–54]. Müller and colleagues provided initial evidence linking the CXCL12/CXCR4 biological axis to breast cancer metastasis to specific organs [10], which was confirmed in non-small-cell lung cancer [44]. More recent studies have suggested that CXCR4 is expressed on various other cancer cells and its expression stimulated migration of cancer cells towards a CXCL12 gradient established in target organs for metastases [42,43,45]. Furthermore, elevated CXCR4 expression was detected in several human RCC cell lines and tumor samples, while only minimal CXCR4 expression was detected in normal kidney tissues [55]. Therefore, further understanding of the molecular mechanisms involved in the regulation of CXCR4 expression on tumor cells could lead to potential targets to modify the expression of CXCR4 and impact on metastases.
Pan et al. demonstrated that CXCR4 expression was markedly increased on circulating pan-cytokeratin+ cells of patients with metastatic RCC, as compared with normal control subjects, suggesting that these cells were comparable with circulating malignant cells [50]. When the cells from patients with metastatic RCC were examined for expression of CXCR4, over 90% of them were found to be CXCR4+ [50]. These findings suggest that CXCR4 is a predominant biomarker on pan-cytokeratin+ cells in the circulation of patients with metastatic RCC and the presence of CXCR4 expression on these cells may correlate with the metastatic potential of RCC. Strategies to block the activity of CXCR4 may be utilized as a new antimetastatic strategy to inhibit the metastatic potential of RCC.
Regulation of CXCR4 & HIF pathway
The VHL tumor suppressor gene is the most common mutated gene in RCC, and results in overexpression of HIF-1 and -2. Despite some conflicting evidence that VHL status can predict patient outcome in RCC, recent studies have shown that RCC tumor stage and prognosis were independent of VHL loss or methylation when compared with wild type [56–58]. Recent findings have linked HIF-1 and the expression of both CXCR3 and CXCR4 in RCC [59,60]. Hypoxia, and more specifically HIF-1, has been found to be a critical transcription factor for CXCR4 gene expression [60–62]. Moreover, VHL can negatively regulate the expression of CXCR4, owing to its capacity to target HIF-1 for degradation under normoxic conditions [60,61]. This process may be suppressed under hypoxic conditions in cells, allowing HIF-1-dependent induction of CXCR4 expression [60,61]. These findings suggest that HIF-1/VHL may play a significant role in regulating the expression of CXCR4 on tumor cells, and further understanding this molecular mechanistic link between HIF-1 and CXCR4 expression may enable the discovery of a novel means to intervene and impact on reducing metastatic RCC.
Ceradini et al. demonstrated that SDF-1/CXCL12 was regulated by HIF-1 in endothelial cells, increasing migration of circulating CXCR4+ cells to areas of ischemic tissue. Blocking CXCL12 or CXCR4 inhibited the recruitment of these cells to sites of regenerating tissue [63]. Hypoxia, particularly HIF-1α, has been shown to regulate the expression of CXCR4 in RCC [61,64,65]. Recent studies suggest that the loss or functional inactivation of the protein product of VHL resulted in persistent activation of HIF-1α and a dramatic increase in CXCR4 expression owing to the loss of its ability to target HIF-1 for degradation by 26S proteasome [61,64,65]. In addition, a recent study demonstrated that either knocking down VHL expression in human RCC cells or exposing these cells to hypoxic conditions can lead to markedly increased expression of CXCR4 mRNA and protein [50]. Metastases of human RCC cells was increased to adrenal glands, buffy coat, bone marrow, brain, kidney, spleen, liver and lung of mice bearing human RCC VHL-knockdown tumors, as compared with those bearing human RCC wild-type tumors [50]. Most of these organs have been found to express elevated levels of CXCL12 in severe combined immunodeficient mice in previous studies [10,44]. Furthermore, this study showed that the upregulation of CXCR4 expression in RCC induced by these conditions was mediated by the direct physical interaction between the HIF-1 transcriptional factor and its specific binding sites on the CXCR4 promoter region [50], which is probably the hypoxia response element as suggested by previous studies [64,66,67]. Finally, neutralization of CXCL12 inhibited metastasis of RCC to target organs expressing high levels of CXCL12. These studies establish a link between the VHL/HIF-1α axis to CXCL12/CXCR4-mediated metastases of human RCC.
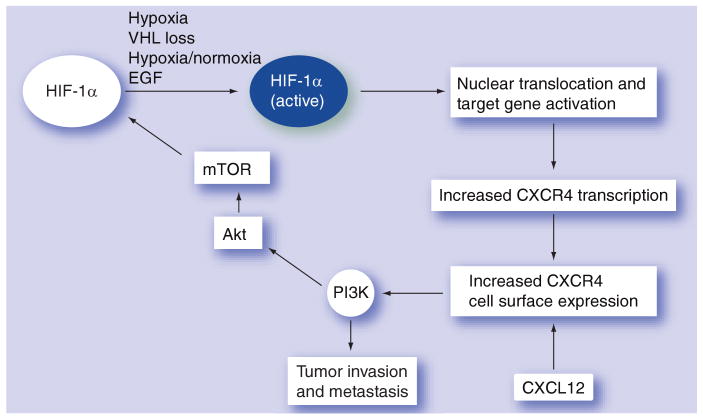
Regulation of CXCR4 expression and the metastatic potential of tumors involve the interaction of multiple signaling pathways. The upregulation of CXCR4 expression has been described to be mediated through the MEK/Erk signaling pathway and nuclear factor-B activation in prostate cancer cells [68]. Findings in lung cancer demonstrate that the combination of hypoxia and activation of the EGF receptor (EGFR) tyrosine kinase domain, led to additive to synergistic upregulation of CXCR4 expression via HIF-1α [67]. Moreover, EGFR activation in the presence of hypoxia further augments CXCR4 expression. EGFR is a receptor tyrosine kinase that activates PI3K and subsequent downstream targets including Akt and mTOR. In the presence of either PI3K inhibitors or mTOR inhibitors, HIF-1 activation and increases in CXCR4 expression was blocked. HIF-1 directly transactivated CXCR4 gene expression. Inhibitors of PI3K also prevented chemotaxis of tumor cells in response to CXCL12. These studies suggest that the PI3K pathway abrogates increased expression of CXCR4 induced by hypoxia and EGFR, and CXCL12-mediated chemotaxis [67]. EGFR activation/signaling and exposure to hypoxia, unlike the VHL mutation, are known to be shared by many cancers. These findings suggest that tyrosine-kinase receptor activation and a response to hypoxia, leading to increased HIF-1α, is critical for the regulation of the expression of CXCR4, and may represent a general scheme in the process of tumor metastasis (Figure 1). Combinations of both mTOR and EGFR inhibition may be a mechanism to block CXCR4-directed tumor metastasis in RCC.
Figure 1. EGF, hypoxia and CXCR4 in renal cell carcinoma tumor metastasis.
In RCC, the hypoxia-induced pathway is linked to VHL loss. Accumulation of HIF results in upregulation of genes and proteins involved in RCC tumorigenesis and metastasis, including CXCR4. EGF is also capable of upregulating CXCR4 expression through the PI3K, Akt, mTOR and HIF-1α pathway.
HIF: Hypoxia-inducible factor; RCC: Renal cell carcinoma; VHL: von Hippel-Lindau.
Expert commentary
The integral role of chemokines in the pathobiology of RCC has become increasingly apparent in the last decade. Chemokines are a family of cytokines involved in tumor blood vessel formation, invasion and cellular immunity. The CXCR2/CXCR2-ligand and CXCR3/CXCR3-ligand biologic axis require balance to maintain normal angiogenesis, and are dysregulated in RCC. The data presented indicated that systemic induction of CXCR3 expression by IL-2 combined with upregulation of intratumor CXCR3 ligands may effectively enhance immunotherapy for RCC. CXCR4 expression is found in RCC tumors and at sites of metastases, and mechanisms to block signaling through this chemokine receptor may inhibit micrometastases in RCC, and result in improved outcomes. CXCR4 expression and signaling may be attenuated by inhibition of the PI3K/mTOR pathway, blocking EGF signaling or direct antagonism with CXCR4 inhibitors. Studies are underway to assess the ability of mTOR and EGF inhibition to decrease the metastatic potential of tumors, and to understand the role of CXCR4 in predicting distant metastases. Overall, exploiting the multifaceted role of chemokines in RCC will be important for future treatments.
Five-year view
Chemokines and their receptors have been implicated in regulating RCC growth, angiogenesis and metastases. There is increasing evidence for the involvement of multiple chemokine pathways in the diverse process of renal cell tumorigenesis and invasion. The inhibition of angiogenesis, either directly through VEGF and its receptors, or indirectly through signaling pathways, has shown efficacy in advanced RCC, primarily of the clear cell histology. The importance of these agents and combining them with chemokine inhibition in the adjuvant setting or advanced disease, may lead to improvements in the treatment of RCC. Clinical trials studying the potential role of chemokine inhibition will help to determine mechanisms of chemokine-induced angiogenesis, growth and metastasis in RCC. In addition, delineation of how genes and proteins regulate chemokine activity and modulate the malignant phenotype will provide novel insights into kidney cancer pathogenesis and prognosis, which may lead to improved therapies.
Key issues.
Chemokines are important for tumor angiogenesis, metastasis and immunity in renal cell carcinoma (RCC).
Both angiogenic and antiangiogenic chemokines play a role in maintaining the balance of tumor angiogenesis in RCC. Inducing CXCR3 and its ligands, or inhibiting CXCR2 and its ligands, can prevent tumor growth and angiogenesis.
Combining immunotherapy with inhibition of angiogenesis may lead to more effective tumor regression in RCC.
CXCR4 is a mediator of tumor metastasis in a variety of cancers including RCC. It is overexpressed on primary tumor and metastatic lesions.
CXCR4 is regulated through the hypoxia-inducible factor-1 and the mTOR pathway; its expression is also upregulated by EGF.
Inhibition of CXCR4 by direct antagonists or by blocking mTOR and EGF receptor signaling pathways may be a means to decrease metastatic potential in RCC.
Improved understanding of the role of chemokines in RCC biology may lead to more effective therapy at all stages of disease.
Footnotes
For reprint orders, please contact: reprints@expert-reviews.com
Financial & competing interests disclosure
This review was supported by NCI K12 CA 01727 and Phase One Foundation (Karen L Reckamp); NIH HL66027 and CA87879 (Robert M Strieter). The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
Contributor Information
Karen L Reckamp, Assistant Professor of Medicine, Divisions of Medical Oncology and Therapeutics Research & Hematology and Hematopoeitic Transplantaion, City of Hope and Beckman Research Institute, 1500 E Duarte Road, MOB 1001, Duarte, CA 91010, USA, Tel.: +1 626 256 4673; +1 626 256 3155, Fax: +1 626 930 5461, kreckamp@coh.org.
Robert M Strieter, Henry B Mulholland Professor of Internal Medicine, Chairman, Department of Internal Medicine, University of Virginia School of Medicine, PO Box 800466, Charlottesville, VA 22908–0466, USA, Tel.: +1 434 982 6999, Fax: +1 434 243 0399, rms4w@virginia.edu.
Robert A Figlin, Arthur and Rosalie Kaplan Professor of Medical Oncology, Chair, Division of Medical Oncology & Therapeutics Research, City of Hope and Beckman Research Institute, Associate Director for Clinical Research, City of Hope Comprehensive Cancer Center, 1500 E Duarte Road, Duarte, CA 91010–3000, Tel.: +1 626 471 9290, Fax: +1 626 930 5461, rfiglin@coh.org.
References
Papers of special note have been highlighted as:
• of interest
•• of considerable interest
- 1.Motzer RJ, Hutson TE, Tomczak P, et al. Sunitinib versus interferon α in metastatic renal-cell carcinoma. N Engl J Med. 2007;356:115–124. doi: 10.1056/NEJMoa065044. [DOI] [PubMed] [Google Scholar]
- 2.Escudier B, Eisen T, Stadler WM, et al. Sorafenib in advanced clear-cell renal-cell carcinoma. N Engl J Med. 2007;356:125–134. doi: 10.1056/NEJMoa060655. [DOI] [PubMed] [Google Scholar]
- 3.Escudier B, Pluzanska A, Koralewski P, et al. Bevacizumab plus interferon α-2a for treatment of metastatic renal cell carcinoma: a randomised, double-blind Phase III trial. Lancet. 2007;370:2103–2111. doi: 10.1016/S0140-6736(07)61904-7. [DOI] [PubMed] [Google Scholar]
- 4.Hudes G, Carducci M, Tomczak P, et al. Temsirolimus, interferon α, or both for advanced renal-cell carcinoma. N Engl J Med. 2007;356:2271–2281. doi: 10.1056/NEJMoa066838. [DOI] [PubMed] [Google Scholar]
- 5.Rini BI, Halabi S, Rosenberg JE, et al. CALGB 90206: a Phase III trial of bevacizumab plus interferon-α versus interferon-α monotherapy in metastatic renal cell carcinoma. 2008 Genitourniary Cancers Symp; 2008. Abstract 350. [Google Scholar]
- 6.Atkins MB, Ernstoff MS, Figlin RA, et al. Innovations and challenges in renal cell carcinoma: summary statement from the Second Cambridge Conference. Clin Cancer Res. 2007;13:S667–S670. doi: 10.1158/1078-0432.CCR-06-2231. [DOI] [PubMed] [Google Scholar]
- 7.Balkwill F. Cancer and the chemokine network. Nat Rev Cancer. 2004;4:540–550. doi: 10.1038/nrc1388. [DOI] [PubMed] [Google Scholar]
- 8.Spring H, Schuler T, Arnold B, Hammerling GJ, Ganss R. Chemokines direct endothelial progenitors into tumor neovessels. Proc Natl Acad Sci USA. 2005;102:18111–18116. doi: 10.1073/pnas.0507158102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gupta SK, Lysko PG, Pillarisetti K, Ohlstein E, Stadel JM. Chemokine receptors in human endothelial cells. Functional expression of CXCR4 and its transcriptional regulation by inflammatory cytokines. J Biol Chem. 1998;273:4282–4287. doi: 10.1074/jbc.273.7.4282. [DOI] [PubMed] [Google Scholar]
- 10.Muller A, Homey B, Soto H, et al. Involvement of chemokine receptors in breast cancer metastasis. Nature. 2001;410:50–56. doi: 10.1038/35065016. [DOI] [PubMed] [Google Scholar]; •• First report of CXCR4 expression in breast tumors and metastases, and demonstrates that blocking CXCL12/CXCR4 interactions inhibits metastasis to regional lymph nodes and lung.
- 11.Scotton CJ, Wilson JL, Scott K, et al. Multiple actions of the chemokine CXCL12 on epithelial tumor cells in human ovarian cancer. Cancer Res. 2002;62:5930–5938. [PubMed] [Google Scholar]
- 12.Coussens LM, Werb Z. Inflammation and cancer. Nature. 2002;420:860–867. doi: 10.1038/nature01322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Locati M, Deuschle U, Massardi ML, et al. Analysis of the gene expression profile activated by the CC chemokine ligand 5/RANTES and by lipopolysaccharide in human monocytes. J Immunol. 2002;168:3557–3562. doi: 10.4049/jimmunol.168.7.3557. [DOI] [PubMed] [Google Scholar]
- 14.Roda JM, Parihar R, Magro C, Nuovo GJ, Tridandapani S, Carson WE., III Natural killer cells produce T cell-recruiting chemokines in response to antibody-coated tumor cells. Cancer Res. 2006;66:517–526. doi: 10.1158/0008-5472.CAN-05-2429. [DOI] [PubMed] [Google Scholar]
- 15.Bukowski RM, Rayman P, Molto L, et al. Interferon-gamma and CXC chemokine induction by interleukin 12 in renal cell carcinoma. Clin Cancer Res. 1999;5:2780–2789. [PubMed] [Google Scholar]
- 16.Mestas J, Burdick MD, Reckamp K, Pantuck A, Figlin RA, Strieter RM. The role of CXCR2/CXCR2 ligand biological axis in renal cell carcinoma. J Immunol. 2005;175:5351–5357. doi: 10.4049/jimmunol.175.8.5351. [DOI] [PubMed] [Google Scholar]
- 17.Guha S, Eibl G, Kisfalvi K, et al. Broad-spectrum G protein-coupled receptor antagonist, [D-Arg1,D-Trp5,7,9,Leu11]SP: a dual inhibitor of growth and angiogenesis in pancreatic cancer. Cancer Res. 2005;65:2738–2745. doi: 10.1158/0008-5472.CAN-04-3197. [DOI] [PubMed] [Google Scholar]
- 18.Wang B, Hendricks DT, Wamunyokoli F, Parker MI. A growth-related oncogene/CXC chemokine receptor 2 autocrine loop contributes to cellular proliferation in esophageal cancer. Cancer Res. 2006;66:3071–3077. doi: 10.1158/0008-5472.CAN-05-2871. [DOI] [PubMed] [Google Scholar]
- 19.Donzella GA, Schols D, Lin SW, et al. AMD3100, a small molecule inhibitor of HIV-1 entry via the CXCR4 co-receptor. Nat Med. 1998;4:72–77. doi: 10.1038/nm0198-072. [DOI] [PubMed] [Google Scholar]
- 20.Martinez MA, Gutierrez A, Armand-Ugon M, et al. Suppression of chemokine receptor expression by RNA interference allows for inhibition of HIV-1 replication. Aids. 2002;16:2385–2390. doi: 10.1097/00002030-200212060-00002. [DOI] [PubMed] [Google Scholar]
- 21.Zhang WB, Navenot JM, Haribabu B, et al. A point mutation that confers constitutive activity to CXCR4 reveals that T140 is an inverse agonist and that AMD3100 and ALX40–4C are weak partial agonists. J Biol Chem. 2002;277:24515–24521. doi: 10.1074/jbc.M200889200. [DOI] [PubMed] [Google Scholar]
- 22.Kakinuma T, Hwang ST. Chemokines, chemokine receptors, and cancer metastasis. J Leukoc Biol. 2006;79:639–651. doi: 10.1189/jlb.1105633. [DOI] [PubMed] [Google Scholar]
- 23.Gnarra JR, Tory K, Weng Y, et al. Mutations of the VHL tumour suppressor gene in renal carcinoma. Nat Genet. 1994;7:85–90. doi: 10.1038/ng0594-85. [DOI] [PubMed] [Google Scholar]
- 24.Herman JG, Latif F, Weng Y, et al. Silencing of the VHL tumor-suppressor gene by DNA methylation in renal carcinoma. Proc Natl Acad Sci USA. 1994;91:9700–9704. doi: 10.1073/pnas.91.21.9700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Strieter RM, Polverini PJ, Kunkel SL, et al. The functional role of the ELR motif in CXC chemokine-mediated angiogenesis. J Biol Chem. 1995;270:27348–27357. doi: 10.1074/jbc.270.45.27348. [DOI] [PubMed] [Google Scholar]; • First description of a functional role of the ELR motif in determining the angiogenic or angiostatic potential of CXC chemokines, supporting their role in regulating angiogenesis.
- 26.Wente MN, Keane MP, Burdick MD, et al. Blockade of the chemokine receptor CXCR2 inhibits pancreatic cancer cell-induced angiogenesis. Cancer Lett. 2006;241:221–227. doi: 10.1016/j.canlet.2005.10.041. [DOI] [PubMed] [Google Scholar]
- 27.Strieter RM, Belperio JA, Arenberg DA, Smith MI, Burdick MD, Keane MP. CXC chemokines in angiogenesis. In: Ransohoff RM, Suzuki K, Proudfoot AEI, Hickey WF, editors. Universes in Delicate Balance: Chemokines and the Nervous System. Vol. 129. Elsevier Science BV; Amsterdam, The Netherlands: 2002. [Google Scholar]
- 28.Belperio JA, Burdick MD, Keane MP, et al. The role of the CC chemokine, RANTES, in acute lung allograft rejection. J Immunol. 2002;165:461–472. doi: 10.4049/jimmunol.165.1.461. [DOI] [PubMed] [Google Scholar]
- 29.Johrer K, Zelle-Rieser C, Perathoner A, et al. Up-regulation of functional chemokine receptor CCR3 in human renal cell carcinoma. Clin Cancer Res. 2005;11:2459–2465. doi: 10.1158/1078-0432.CCR-04-0405. [DOI] [PubMed] [Google Scholar]
- 30.Suyama T, Furuya M, Nishiyama M, et al. Up-regulation of the interferon γ (IFN-γ)-inducible chemokines IFN-inducible T-cell α chemoattractant and monokine induced by IFN-γ and of their receptor CXC receptor 3 in human renal cell carcinoma. Cancer. 2005;103:258–267. doi: 10.1002/cncr.20747. [DOI] [PubMed] [Google Scholar]
- 31.Kondo T, Nakazawa H, Ito F, et al. Favorable prognosis of renal cell carcinoma with increased expression of chemokines associated with a Th1-type immune response. Cancer Sci. 2006;97:780–786. doi: 10.1111/j.1349-7006.2006.00231.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Klatte T, Seligson DB, Leppert JT, et al. The chemokine receptor CXCR3 is an independent prognostic factor in patients with localized clear cell renal cell carcinoma. J Urol. 2008;179:61–66. doi: 10.1016/j.juro.2007.08.148. [DOI] [PubMed] [Google Scholar]
- 33.Tannenbaum CS, Tubbs R, Armstrong D, Finke JH, Bukowski RM, Hamilton TA. The CXC chemokines IP-10 and Mig are necessary for IL-12-mediated regression of the mouse RENCA tumor. J Immunol. 1998;161:927–932. [PubMed] [Google Scholar]
- 34.Ravaud A, Trufflandier N, Ferriere JM, et al. Subcutaneous interleukin-2, interferon α-2b and 5-fluorouracil in metastatic renal cell carcinoma as second-line treatment after failure of previous immunotherapy: a Phase II trial. Br J Cancer. 2003;89:2213–2218. doi: 10.1038/sj.bjc.6601419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Negrier S, Escudier B, Lasset C, et al. Recombinant human interleukin-2, recombinant human interferon α-2a, or both in metastatic renal-cell carcinoma. Groupe Francais d'Immunotherapie. N Engl J Med. 1998;338:1272–1278. doi: 10.1056/NEJM199804303381805. [DOI] [PubMed] [Google Scholar]
- 36.McDermott DF, Regan MM, Clark JI, et al. Randomized Phase III trial of high-dose interleukin-2 versus subcutaneous interleukin-2 and interferon in patients with metastatic renal cell carcinoma. J Clin Oncol. 2005;23:133–141. doi: 10.1200/JCO.2005.03.206. [DOI] [PubMed] [Google Scholar]
- 37.Fyfe G, Fisher RI, Rosenberg SA, Sznol M, Parkinson DR, Louie AC. Results of treatment of 255 patients with metastatic renal cell carcinoma who received high-dose recombinant interleukin-2 therapy. J Clin Oncol. 1995;13:688–696. doi: 10.1200/JCO.1995.13.3.688. [DOI] [PubMed] [Google Scholar]
- 38.Yang JC, Sherry RM, Steinberg SM, et al. Randomized study of high-dose and low-dose interleukin-2 in patients with metastatic renal cancer. J Clin Oncol. 2003;21:3127–3132. doi: 10.1200/JCO.2003.02.122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Reckamp KL, Figlin RA, Moldawer N, et al. Expression of CXCR3 on mononuclear cells and CXCR3 ligands in patients with metastatic renal cell carcinoma in response to systemic IL-2 therapy. J Immunother. 2007;30(4):417–24. doi: 10.1097/CJI.0b013e31802e089a. [DOI] [PubMed] [Google Scholar]
- 40.Strieter RM. Chemokines: not just leukocyte chemoattractants in the promotion of cancer. Nat Immunol. 2001;2:285–286. doi: 10.1038/86286. [DOI] [PubMed] [Google Scholar]; • Review of chemokines in the pathogenesis of cancer.
- 41.Pan J, Burdick MD, Belperio JA, et al. CXCR3/CXCR3 ligand biological axis impairs RENCA tumor growth by a mechanism of immunoangiostasis. J Immunol. 2006;176:1456–1464. doi: 10.4049/jimmunol.176.3.1456. [DOI] [PubMed] [Google Scholar]
- 42.Balkwill F. The significance of cancer cell expression of the chemokine receptor CXCR4. Semin Cancer Biol. 2004;4:171–179. doi: 10.1016/j.semcancer.2003.10.003. [DOI] [PubMed] [Google Scholar]
- 43.Kucia M, Jankowski K, Reca R, et al. CXCR4-SDF-1 signalling, locomotion, chemotaxis and adhesion. J Mol Histol. 2004;35:233–245. doi: 10.1023/b:hijo.0000032355.66152.b8. [DOI] [PubMed] [Google Scholar]
- 44.Phillips RJ, Burdick MD, Lutz M, Belperio JA, Keane MP, Strieter RM. The stromal derived factor-1/CXCL12-CXC chemokine receptor 4 biological axis in non-small cell lung cancer metastases. Am J Respir Crit Care Med. 2003;167:1676–1686. doi: 10.1164/rccm.200301-071OC. [DOI] [PubMed] [Google Scholar]
- 45.Zlotnik A. Chemokines in neoplastic progression. Semin Cancer Biol. 2004;14:181–185. doi: 10.1016/j.semcancer.2003.10.004. [DOI] [PubMed] [Google Scholar]
- 46.Balkwill F. Chemokine biology in cancer. Semin Immunol. 2003;15:49–55. doi: 10.1016/s1044-5323(02)00127-6. [DOI] [PubMed] [Google Scholar]
- 47.Murphy PM. Chemokines and the molecular basis of cancer metastasis. N Engl J Med. 2001;345:833–835. doi: 10.1056/NEJM200109133451113. [DOI] [PubMed] [Google Scholar]
- 48.Horuk R. Chemokine receptors. Cytokine Growth Factor Rev. 2001;12:313–335. doi: 10.1016/s1359-6101(01)00014-4. [DOI] [PubMed] [Google Scholar]
- 49.Burger JA, Kipps TJ. CXCR4: a key receptor in the crosstalk between tumor cells and their microenvironment. Blood. 2006;107:1761–1767. doi: 10.1182/blood-2005-08-3182. [DOI] [PubMed] [Google Scholar]; • Extensive review of the importance of CXCR4 in tumor progression for a number of malignancies. The development and use of CXCR4 antagonists in cancer are also described.
- 50.Pan J, Mestas J, Burdick MD, et al. Stromal derived factor-1 (SDF-1/CXCL12) and CXCR4 in renal cell carcinoma metastasis. Mol Cancer. 2006;5:56. doi: 10.1186/1476-4598-5-56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hu J, Deng X, Bian X, et al. The expression of functional chemokine receptor CXCR4 is associated with the metastatic potential of human nasopharyngeal carcinoma. Clin Cancer Res. 2005;11:4658–4665. doi: 10.1158/1078-0432.CCR-04-1798. [DOI] [PubMed] [Google Scholar]
- 52.Schimanski CC, Schwald S, Simiantonaki N, et al. Effect of chemokine receptors CXCR4 and CCR7 on the metastatic behavior of human colorectal cancer. Clin Cancer Res. 2005;11:1743–1750. doi: 10.1158/1078-0432.CCR-04-1195. [DOI] [PubMed] [Google Scholar]
- 53.Cabioglu N, Yazici MS, Arun B, et al. CCR7 and CXCR4 as novel biomarkers predicting axillary lymph node metastasis in T1 breast cancer. Clin Cancer Res. 2005;11:5686–5693. doi: 10.1158/1078-0432.CCR-05-0014. [DOI] [PubMed] [Google Scholar]
- 54.Yasumoto K, Koizumi K, Kawashima A, et al. Role of the CXCL12/CXCR4 axis in peritoneal carcinomatosis of gastric cancer. Cancer Res. 2006;66:2181–2187. doi: 10.1158/0008-5472.CAN-05-3393. [DOI] [PubMed] [Google Scholar]
- 55.Schrader AJ, Lechner O, Templin M, et al. CXCR4/CXCL12 expression and signalling in kidney cancer. Br J Cancer. 2002;86:1250–1256. doi: 10.1038/sj.bjc.6600221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Yao M, Yoshida M, Kishida T, et al. VHL tumor suppressor gene alterations associated with good prognosis in sporadic clear-cell renal carcinoma. J Natl Cancer Inst. 2002;94:1569–1575. doi: 10.1093/jnci/94.20.1569. [DOI] [PubMed] [Google Scholar]
- 57.Banks RE, Tirukonda P, Taylor C, et al. Genetic and epigenetic analysis of von Hippel-Lindau (VHL) gene alterations and relationship with clinical variables in sporadic renal cancer. Cancer Res. 2006;66:2000–2011. doi: 10.1158/0008-5472.CAN-05-3074. [DOI] [PubMed] [Google Scholar]
- 58.Smits KM, Schouten LJ, van Dijk BA, et al. Genetic and epigenetic alterations in the von Hippel-Lindau gene: the influence on renal cancer prognosis. Clin Cancer Res. 2008;14:782–787. doi: 10.1158/1078-0432.CCR-07-1753. [DOI] [PubMed] [Google Scholar]
- 59.Klatte T, Seligson DB, Riggs SB, et al. Hypoxia-inducible factor 1 α in clear cell renal cell carcinoma. Clin Cancer Res. 2007;13:7388–7393. doi: 10.1158/1078-0432.CCR-07-0411. [DOI] [PubMed] [Google Scholar]
- 60.Schioppa T, Uranchimeg B, Saccani A, et al. Regulation of the chemokine receptor CXCR4 by hypoxia chemokine receptor CXCR4 downregulated by von Hippel-Lindau tumour suppressor pVHL. J Exp Med. 2003;198:1391–1402. doi: 10.1084/jem.20030267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Staller P, Sulitkova J, Lisztwan J, Moch H, Oakeley EJ, Krek W. Chemokine receptor CXCR4 downregulated by von Hippel-Lindau tumour suppressor pVHL. Nature. 2003;425:307–311. doi: 10.1038/nature01874. [DOI] [PubMed] [Google Scholar]; •• Demonstration of CXCR4 regulation by the von Hippel-Lindau tumor suppressor protein von Hippel-Lindau, and hypoxia-inducible factor (HIF)-dependent CXCR4 activation during hypoxic conditions. An association of strong CXCR4 expression with poor tumor-specific survival was found in clear cell renal cell carcinoma.
- 62.Pantuck AJ, Zeng G, Belldegrun AS, Figlin RA. Pathobiology, prognosis, and targeted therapy for renal cell carcinoma: exploiting the hypoxia-induced pathway. Clin Cancer Res. 2003;9:4641–4652. [PubMed] [Google Scholar]
- 63.Ceradini DJ, Kulkarni AR, Callaghan MJ, et al. Progenitor cell trafficking is regulated by hypoxic gradients through HIF-1 induction of SDF-1. Nat Med. 2004;10:858–864. doi: 10.1038/nm1075. [DOI] [PubMed] [Google Scholar]
- 64.Schioppa T, Uranchimeg B, Saccani A, et al. Regulation of the chemokine receptor CXCR4 by hypoxia. J Exp Med. 2003;198:1391–1402. doi: 10.1084/jem.20030267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Zagzag D, Krishnamachary B, Yee H, et al. Stromal cell-derived factor-1α and CXCR4 expression in hemangioblastoma and clear cell-renal cell carcinoma: von Hippel-Lindau loss-of-function induces expression of a ligand and its receptor. Cancer Res. 2005;65:6178–6188. doi: 10.1158/0008-5472.CAN-04-4406. [DOI] [PubMed] [Google Scholar]
- 66.Caruz A, Samsom M, Alonso JM, et al. Genomic organization and promoter characterization of human CXCR4 gene. FEBS Lett. 1998;426:271–278. doi: 10.1016/s0014-5793(98)00359-7. [DOI] [PubMed] [Google Scholar]
- 67.Phillips RJ, Mestas J, Gharaee-Kermani M, et al. EGF and hypoxia-induced expression of CXCR4 on non-small cell lung cancer cells is regulated by the phosphatidylinositol 3-kinase/ PTEN/AKT/mammalian target of rapamycin signaling pathway and activation of hypoxia inducible factor-1α. J Biol Chem. 2005;280:22473–22481. doi: 10.1074/jbc.M500963200. [DOI] [PubMed] [Google Scholar]; •• CXCR4 expression was regulated by the phosphatidylinositol 3-kinase/PTEN/mTOR signal transduction pathway, activation of HIF-1 α, and, ultimately, HIF-1-dependent transcription of the CXCR4 gene.
- 68.Kukreja P, Abdel-Mageed AB, Mondal D, Liu K, Agrawal KC. Up-regulation of CXCR4 expression in PC-3 cells by stromal-derived factor-1α (CXCL12) increases endothelial adhesion and transendothelial migration: role of MEK/ERK signaling pathway-dependent NF-κB activation. Cancer Res. 2005;65:9891–9898. doi: 10.1158/0008-5472.CAN-05-1293. [DOI] [PubMed] [Google Scholar]