Abstract
In the present study we made an attempt to understand the skin irritation cascade of selected aliphatic hydrocarbons using microdialysis technique. Microdialysis probes were inserted into dermis in the dorsal skin of hairless rats. After 2 h of probes insertion, occlusive dermal exposure (2 h) was carried out with 230 μl of nonane, dodecane and tetradecane, using Hill top chambers®. Inflammatory biomarkers such as substance P (SP), α-melanocyte stimulating hormone (α-MSH) Interleukin 6 (IL-6) and prostaglandin E2 (PGE2) were analyzed in the dialysis samples by enzyme immunoassay (EIA). SP, α-MSH and IL6 were released in significant amounts following the dermal exposure of nonane and dodecane, whereas tetradecane did not induce any of these markers in significant amounts compared to control. Nonane increased the PGE2 levels in significant amounts within 2 h of chemical exposure compared to dodecane and tetradecane. IL-6 response was found to be slow and 2–3-fold increase in IL-6 levels was observed after 5 h following nonane and dodecane application. The magnitude of skin irritation exerted by all three chemicals was in the order of nonane ≥ dodecane ≥ tetradecane. The results demonstrate that microdialysis can be used to measure the inflammatory biomarkers in the skin irritation studies and irritation response of chemicals was quantifiable by this method. In conclusion, microdialysis was found to be an excellent tool to measure several inflammatory biomarkers as a function of time after dermal exposures with irritant chemicals.
Keywords: Microdialysis, Skin irritation, Jet fuels, Alpha MSH, Interleukin 6, Aliphatic hydrocarbons
1. Introduction
For many years assessment of skin response to irritants or noxious stimuli had to be performed from outside the skin surface. Thus, the methods have been limited to observing changes in skin color (erythema), transepidermal water loss (TEWL) and measuring temperature changes to gain information about dermal blood flow. The application of molecular biology to the study of these functional responses in animals or humans in vivo, has been proven difficult because these techniques require direct access to the dermal tissues while causing minimal disruption or damage to the local environment (Clough and Church, 2002). Microdialysis is a widely used technique to determine the endogenous and exogenous solutes in the extra-cellular space of tissues under minimally invasive conditions. The microdialysis principle is based on the sampling of soluble molecules from the interstitial spaces of the tissues where dialysis probe is inserted into the tissue and perfused at an optimal flow rate with a physiological solution (Muller, 2002; Schmidt et al., 2008). Initially microdialysis technique was used for the recovery of brain neuropeptides (Ungerstedt and Pycock, 1974) and later on this technique was adopted to use in various tissues like skin (Kreilgaard, 2002; Fulzele et al., 2007), adipose tissue (Lindberger et al., 2001), muscle (Newman et al., 2001) and gastrointestinal tract (Iversen et al., 1997). Very few studies have been carried out on the dermal microdialysis to assess the skin irritation of irritant chemicals. During the inflammatory cascade, numerous soluble components such as cytokines and chemokines (IL-1α, IL-1β, IL-6, IL-8 and IL-10), and free radicals are released in to the surrounding tissue medium (Angst et al., 2008). Application of dermal microdialysis to the area of skin irritation/inflammation can be a very useful tool to quantify the release of neuropeptides, cytokines or chemokines as a function of time.
Microdialysis technique is minimally invasive and provides biomarkers in relatively pure form and no further purification process is required for their quantification. In an earlier report, we have shown that dermal microdialysis can be used for skin irritation assessment of jet fuel (JP8), by measuring substance P (SP) and prostaglandin E2 (PGE2) levels (Fulzele et al., 2007). The current study is focused on the evaluation of skin irritation potential of selected aliphatic hydrocarbons (nonane, dodecane and tetradecane) by measuring various inflammatory biomarkers such as SP, PGE2, alpha melanocyte stimulating hormone (α-MSH) and interleukin 6 (IL-6) using dermal microdialysis technique. We selected these biomarkers on the basis of their molecular weight, and easy recovery by microdialysis. Furthermore, these compounds are most commonly expressed in a wide variety of cutaneous irritation and inflammatory conditions in response to noxious chemicals after dermal exposures. Aliphatic hydrocarbons are the primary hydrocarbon components; C8–C14 hydrocarbons constitute about 74% of the jet fuel composition (Chou et al., 2002). Dermal exposures of individual hydrocarbons and quantification of biological markers as a function of time could provide an in depth understanding of events of skin irritation and inflammatory cascade by jet fuels. In the present study we selected nonane, dodecane and tetradecane as representative aliphatic hydrocarbons and measured inflammatory biomarkers by a microdialysis technique following dermal exposures.
Important aspect in the microdialysis studies is the selection of suitable probes for the recovery of various biomarkers; wide range of microdialysis probes of different molecular weight (MW) cutoffs and configurations (concentric and linear) are commercially available. The low MW compounds (2–5 kDa) can easily be recovered from linear probes with a MW cutoff below 30 kDa. However, recovery of large MW compounds (MW above 8 kDa) is challenging and the performance of microdialysis probe will depend on the several factors like flow rate, probe selection, perfusion fluid and nature of the substance to be recovered. With recent introduction of large MW cutoff probes up to 3000 kDa, it is possible to conduct microdialysis studies of large MW compounds including proteins and peptides. Angst et al. (2008) measured several cytokines and nerve growth factors using a larger MW cutoff microdialysis probe (3000 kDa) with an outside diameter of 400 μm. The 3000 kDa probe is not easily available for research purpose and most of the studies have used CMA 20 PES probe (100 kDa) for the recovery of cytokines (Ao and Stenken, 2006; Rosenbloom et al., 2005). In the present study we used both linear low molecular weight LM-10 (30 kDa) and CMA 20 (MW cut off 100 kDa) microdialysis probes for the recovery of four inflammatory markers, SP, PGE2, α-MSH and IL-6 (Table 1). We selected these biomarkers on the basis of their molecular weight, and easy recovery by microdialysis. Furthermore, these compounds are most commonly expressed in a wide variety of cutaneous irritation and inflammatory conditions in response to noxious chemicals after dermal exposures. The outcome of these studies will help in evaluating the usefulness of microdialysis technique in determining the structure activity relationship of aliphatic hydrocarbons in terms of skin irritation.
Table 1.
In vitro recovery of SP, PGE2, α-MSH and IL6 at two different flow rates.
| Chemical | Molecular weight (kDa) | In vitro relative recovery (%) | |
|---|---|---|---|
| 2 μl/min | 4 μl/min | ||
| SP | 1.35 | 13 ± 0.49 | 2.76 ± 0.07 |
| PGE2 | 0.35 | 33.85 ± 0.72 | 34.73 ± 0.44 |
| α-MSH | 1.67 | 27.36 ± 6.7 | 21 ± 3.8 |
| IL6* | 21.7 | 15 ± 0.3a | 11 ± 0.9b |
IL6 recovery was carried out with 0.1%, w/v BSA in Krebs ringer solution at a flow rate of (a) 0.5 μl/min and (b) 1 μl/min. Values represent mean ± S.D. (n = 3).
2. Materials and methods
2.1. Materials
The nonane, dodecane, tetradecane, urethane, and halothane were obtained from Sigma–Aldrich (St. Louis, MO). α-MSH (Assay > 98%) was obtained from Biopeptide Co. LLC (San Diego CA). Bovine serum albumin (BSA) was obtained from Cell Signaling Technology (Denver, MA). Enzyme immunoassay (EIA) kits for SP and rat IL-6 were purchased from Cayman Chemicals (Ann Arbor, MC) and Pierce Biotechnology Inc (Thermo scientific, Rockford, IL), respectively. EIA kits for α-MSH and PGE2 were procured from Phoenix Pharmaceuticals (Belmont, CA) and R&D Systems (Minneapolis, MN), respectively. Linear microdialysis probe, 30 kDa MW cut off and 10 mm dialysis membrane (LM-10) was procured from Bio-analytical Systems (West Lafayette, IN) and non-linear CMA20 microdialysis polyethersulfone (PES) probe with a 100-kDa MW cutoff and 10 mm dialysis membrane was obtained from CMA Microdialysis (North Chelmsford, MA). All other chemicals used in this research were of analytical or US pharmacopeial grade.
2.2. Animals
CD®(SD) hrBi hairless rats (250–300 g; Charles River Laboratories) were utilized for the studies. The protocol for in vivo experiments was approved by the Animal Care and Use Committee, Florida A & M University. The animals were acclimatized to laboratory conditions for 1 week prior to experiments and were on standard animal chow and water ad libitum. The temperature of the room was maintained at 22 ± 1 °C and the relative humidity of the experimentation room was found in the range of 35–50%. For microdialysis experiments the animals were anesthetized by intraperitoneal (i.p.) injection of Urethane (1.5 g/kg; 300 mg/ml; i.p.) with the anesthesia lasting for the entire period of experiment and after completion of the study animals were sacrificed with an overdose of halothane
2.3. In vitro recovery
To characterize the transfer rate of the probes, in vitro recovery of α-MSH and IL-6 was assessed. In case of SP, PGE2 and α-MSH recovery studies, a LM-10 microdialysis probe was placed in a 5 ml vial containing 1000 pg/ml stock solution in Krebs–Ringer solution. The inlet end of the probe was connected to a CMA/102 microinjection pump (CMA microdialysis, North Chelmsford, MA) using a tubing connector while the outlet was connected to a CMA/142 micro-fraction collector (CMA microdialysis, North Chelmsford, MA). The probe was perfused with Krebs–Ringer solution at a flow rate of 2.0 and 4.0 μl/min for 60 min in two different experiments. Dialysate samples were collected every 30 min for 2 h. For the recovery of IL-6, CMA 20 microdialysis probe was placed in 2000 pg/ml IL-6 stock solution and perfused with 0.1%, w/v BSA dissolved in Krebs–Ringer solution at a flow rate of 0.5 and 1 μl/min, respectively. To obtain adequate amount of sample for IL-6 analysis, prior to the start of the experiment, 30 μl of 0.1%, w/v BSA solution was added to each vial and samples were collected every 60 min for 2 h. The inflammatory biomarkers concentration was measured in the dialysate (Cout) along with the concentration in the surrounding medium (Cm). The relative recovery was calculated by the equation: dialysate concentration (Cout) × 100/standard concentration (Cm).
2.4. In vivo studies
For in vivo recovery of SP, α-MSH and PGE2 the linear microdialysis LM-10 probe was used. The inlet end of the probe was connected to a CMA microinjection pump using a tubing connector. In case of LM-10 catheter, Krebs–Ringer solution (138 mM NaCl, 5 mM KCl, 1 mM MgCl2, 1 mM CaCl2, 11 mM NaHCO3, 1 mM NaH2 PO4) was pumped at a flow rate of 2 μl/min. For in vivo IL-6 recovery, 100 kDa microdialysis CMA-20 probe was used. The probe was perfused at a flow rate of 0.5 μl/min with 0.1%, w/v BSA dissolved in Krebs–Ringer solution. In order to ensure the proper fluid filling, prior to start of the experiment all the probes were perfused for 1 h with perfusion fluid. In case of IL-6 recovery studies the sample vials were filled with 30 μl perfusion fluid as described in in vitro recovery studies. Dermal implantation of the probes was carried out as per manufacturer's instructions. After implantation of microdialysis probes in the rat skin, the outlet end of the probe was connected to a refrigerated micro-fraction collector and throughout the study period samples were maintained at 4 °C. Two baseline dialysate samples were collected at 1 h intervals during initial equilibration period.
Occlusive dermal exposure (2 h) was carried out with 230 μl each of nonane, dodecane and tetradecane using Hill top chambers® (Babu et al., 2004a). Following dermal exposures, dialysate samples were collected for 5 h at 1 h intervals and stored at −80 °C until analyzed.
2.5. Evaluation of probe depth
To measure the probe depth after implantation and to determine the exact location of the probe in the dermis, histological evaluation was performed (Mathy et al., 2005). After biopsy, the tissue was fixed in 4% formalin solution and embedded in paraffin wax. Sections were cut perpendicular to the surface of the skin. Tissues were processed and stained with hematoxylin/eosin following standard procedure. Location of the probe in dermis was performed using optical microscope with graduated lens.
2.6. Analysis
The concentration of SP, PGE2, α-MSH and IL-6 in the dialysis samples was analyzed by using EIA kits as per manufacturer's instructions.
2.7. Data analysis
The amount of SP, PGE2, α-MSH and IL-6 in the dialysate were expressed a pg/ml and the differences in the means and variances between various groups were examined using one-way analysis of variance (ANOVA) and Tukey multiple comparison test at a 95% confidence interval (P < 0.05). The statistical analysis was performed using GraphPad PRISM version 2.0 software (San Diego, CA).
3. Results
3.1. In vitro recovery
The SP, PGE2, α-MSH and IL-6 recovery from LM-10 and CMA-20 probes are given in Table 1. The perfusion fluid flow rate influenced the relative recovery of SP, where at lower flow rate (2 μl/min) the relative recovery was 13% versus 2.8% at a flow rate of 4 μl/min. There was no appreciable difference in the recovery of PGE2 between flow rates of 2 and 4 μl/min. The relative in vitro recovery of α-MSH with LM-10 probe at a flow rate of 4 and 2 μl/min with Krebs–Ringer buffer as perfusion medium was found to be 21% and 27%, respectively. At a flow rate of 1 and 0.5 μl/min, the relative recovery of IL-6 with CMA-20 microdialysis probe was found to be 11% and 15%, respectively.
3.2. In vivo recovery after occlusive exposure of aliphatic chemicals
In the present study, four inflammatory markers (SP, PGE2, α-MSH and IL-6) were recovered following occlusive dermal exposure (2 h) with irritant chemicals. Insertion of needle in to the dermis resulted the release of inflammatory markers and in all samples, baseline values were achieved within 2 h after retrieval of the needle.
3.3. Substance P release
Dermal exposure with selected aliphatic hydrocarbons resulted in variable levels of SP release. A significant increase in SP concentration was found between baseline and 1 and 2 h of occlusive exposure of dodecane and nonane (P < 0.001) (Fig. 1A and B). Tetradecane did not increase SP levels (Fig. 1C), while nonane and dodecane increased the SP release by 3- and 2-fold compared to control, immediately after their application, which was maintained above baseline levels till the end of the study. After 1 h of nonane occlusive exposure, the SP release increased from 20 to 70 pg/ml and the same levels were maintained until 2 h after removal of occlusion. Whereas with dodecane, SP release reached maximal levels (55 pg/ml) after 2 h of chemical application and maintained statistically significant levels (P < 0.05) up to 4 h after removal of occlusion.
Fig. 1.
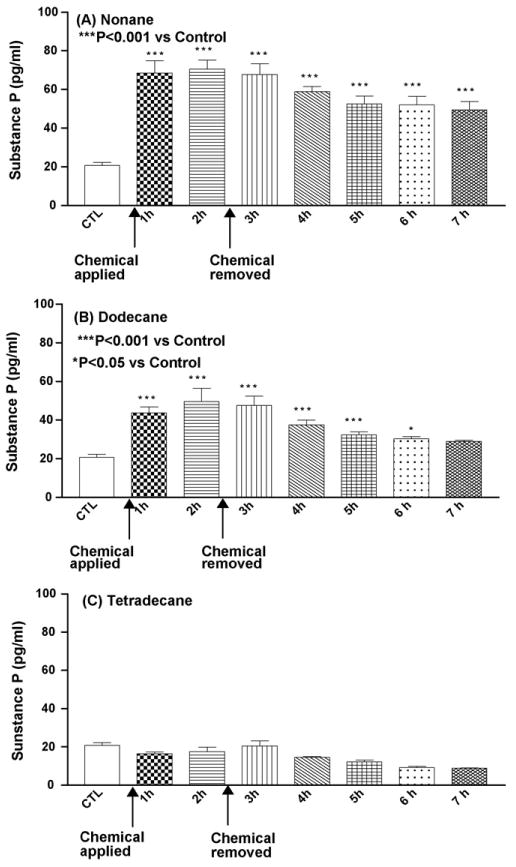
Mean concentration of substance P (pg/ml) following insertion of the probe, application of test chemical and 5 h after removal of the chemical. CTL: control baseline and n = 6 animals (A–C).
3.4. Prostaglandin E2 (PGE2) release
Nonane exposure resulted in significant increase (P < 0.001) in PGE2 levels at the end of 1 h and 2 h of exposure (Fig. 2A). Following removal of occlusion, there was a drop in PGE2 levels and reached normal baseline after 4 h of chemical exposure. There was no significant increase in the PGE2 levels over the baseline values after treatment with dodecane and tetradecane (Fig. 2B and C).
Fig. 2.
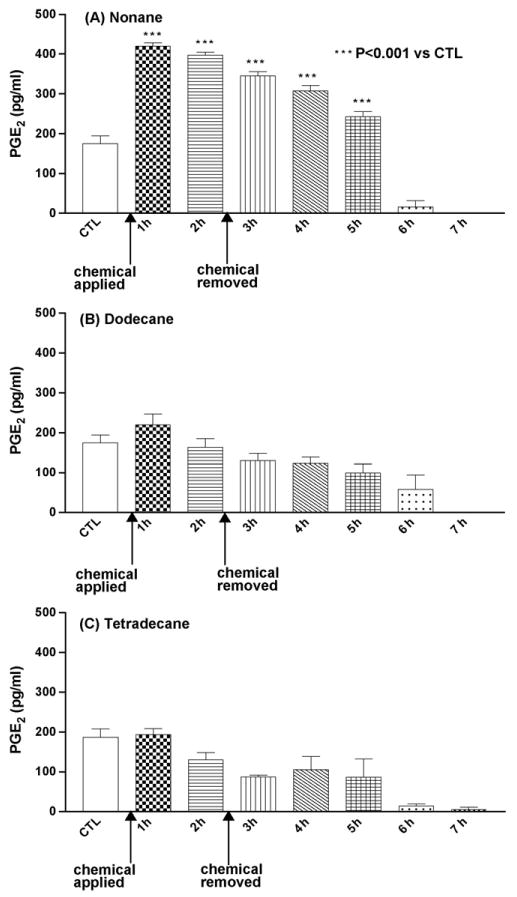
Mean concentration of PGE2 (pg/ml) following insertion of the probe, application of test chemical and 5 h after removal of the chemical. CTL: control baseline and n = 6 animals (A–C).
3.5. Alpha melanocyte stimulating hormone (α-MSH) release
α-MSH was detected in all samples after 1 h of dermal exposure and significantly higher α-MSH levels were observed after 3 h of occlusive exposure in the nonane treatment group (P < 0.001) (Fig. 3A). Dodecane showed a significant increase in α-MSH release immediately after chemical application (P < 0.001) and after 3 h following the chemical removal, α-MSH levels were gradually decreased (Fig. 3B). Tetradecane did not have any significant effect on α-MSH release, as compared to control (Fig. 3C).
Fig. 3.
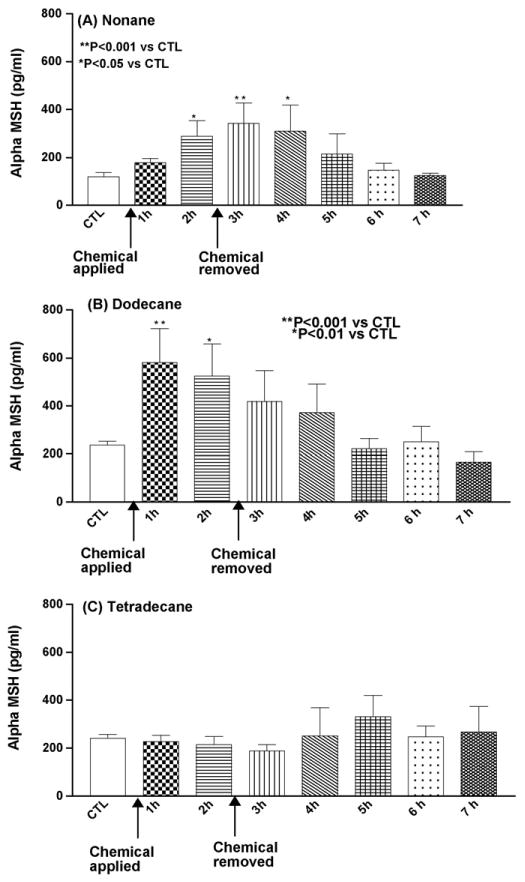
Mean concentration of α-MSH (pg/ml) following insertion of the probe, application of test chemical and 5 h after removal of the chemical. CTL: control baseline and n = 6 animals (A–C).
3.6. Interleukin 6 (IL-6) release
Fig. 4 shows the effect of dermal occlusive exposures of different chemicals on the release of IL-6. Following application of chemicals IL-6 release was increased gradually and reached maximal levels after 3 h of occlusion removal. Dodecane and nonane maintained very high IL-6 levels even after 5 h postapplication (P < 0.001), where as tetradecane did not elicit IL-6 release in significant amounts compared to control.
Fig. 4.
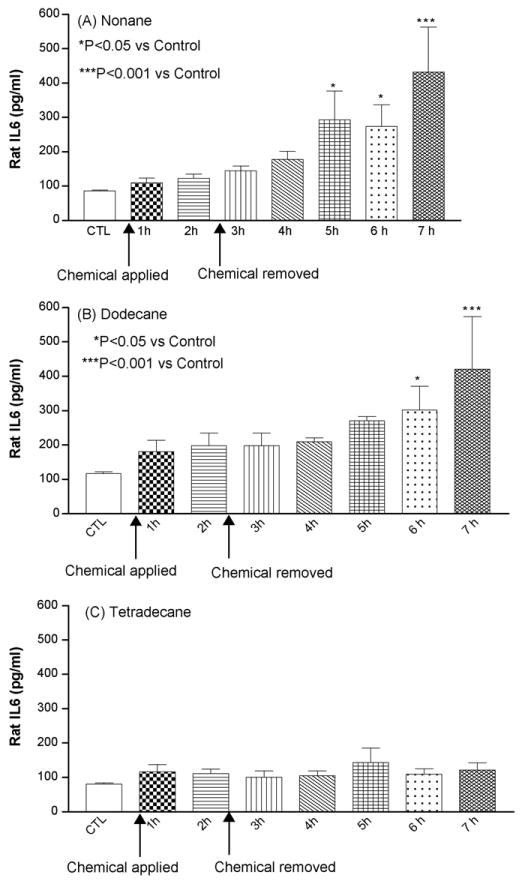
Mean concentration of IL-6 (pg/ml) following insertion of the probe, application of test chemical and 5 h after removal of the chemical. CMA-20 microdialysis probe perfused with 0.1%, w/v BSA in Krebs ringer solution at flow rate of 0.5 μg/ml and samples vials were diluted with 30 μl of 0.1%, w/v BSA solution. CTL: Control baseline and n = 6 animals (A–C).
4. Discussion
Jet fuels are complex aromatic and aliphatic hydrocarbon mixtures known to cause skin irritation and sensitization. Each hydrocarbon component in the jet fuels behaves differently and the toxicity effects will depend on the chemical nature of individual hydrocarbon. Therefore, it is very important to understand the toxicity profile of individual aliphatic or aromatic hydrocarbons. Very few studies have been carried out on the dermal toxicity of aliphatic hydrocarbons, which occupy more than 80% of jet fuel composition. In vitro skin permeation studies carried out with aromatic and aliphatic hydrocarbons (C9–C14) indicate that aliphatic hydrocarbons retain in the skin in higher amounts than aromatic hydrocarbons and therefore can produce higher skin toxicity (McDougal et al., 2000). Generally, non-invasive techniques like TEWL and Draize scoring are widely used for skin irritation assessment of jet fuels. However, the main drawback of non-invasive or biophysical techniques is that these methods are highly subjective and TEWL in particular is influenced by environmental humidity and temperature, if these conditions are not controlled, this method can lead to variable results (Heylings et al., 2003). Dermal exposure of chemicals may induce invisible but functional changes in the skin and biophysical methods such as TEWL, Draize testing, etc. will give false implication. Furthermore, occlusive chemical exposure gives false increase or reduction in TEWL measurements because the applied dose will influence the TEWL values. Therefore, measurement of inflammatory changes within the skin will give the exact skin irritation profile of the chemicals. Microdialysis is one of the viable techniques, which can be used for the assessment and understanding of the skin irritation cascade. This is a well-established technique for the continuous sampling of biomarkers of disease within the extracellular fluid space in vivo. It has an advantage over other sampling techniques in that it can be used to follow temporal variations in the generation and release of a substance at a discrete location within the tissue space. Furthermore this technique can be directly adapted to humans to measure molecular responses in the skin, without having to collect biopsy samples.
The effect of nonane, dodecane and tetradecane on skin irritation was studied by measuring the expression of four inflammatory biomarkers, SP, PGE2, α-MSH and IL-6 in rat skin using microdialysis technique. In order to minimize the interactions during the analysis of low MW biomarkers, SP, PGE2 and α-MSH, in vitro and in vivo recovery studies were carried out with low MW cutoff LM-10 (MW cutoff 30 kDa) linear microdialysis probe. In vitro recovery results showed excellent recovery of SP, PGE2 and α-MSH with LM-10 probe (Table 1). For the recovery of higher MW biomarker, IL-6, CMA20 (MW cut off 100 kDa) microdialysis probe was used with a 0.1%, w/v BSA solution as a perfusion fluid. Serum proteins and detergents are commonly added to microdialysis perfusion fluids to improve recovery of higher MW peptides (Trickler and Miller, 2003; Ao et al., 2006). Our results demonstrated that addition of 0.1%, w/v BSA to the perfusion fluid enhanced the recovery of IL-6 by several fold and with the Ringer solution alone IL-6 recovery was below detectable levels. Generally, recovery of biomarkers is inversely proportional to the perfusion fluid flow rate. To determine the performance of CMA20 probe over a period of time, in vitro IL-6 recovery studies were carried out for 5 h and it was observed that IL-6 recovery at a flow rate of 0.5 μl/min with CMA20 probe was consistent even after 5 h (data not shown). Following probe insertion, hairless rat skin was subjected to histological examination, which demonstrated that the probe was implanted just below the dermal–epidermal junction.
Substance P, a neuropeptide, is released during the induction of neurogenic inflammation and is mainly active in inducing protein extravagation by activation of NK-1 receptors (Kramer et al., 2005). There is a relation between skin irritation and SP release; it is known that exposure to certain organic solvents (cyclohexane, toluene and m-xylene) will induce the expression of SP and several cytokines (Iyadomi et al., 1998). Our previous studies with JP8 showed that occlusive dermal exposure of hairless rats can induce the release of SP in significant amounts compared to control group (Fulzele et al., 2007). In the current study, following nonane and dodecane occlusive exposure, SP was released and reached maximal levels after 1 h of chemical exposure and decreased gradually thereafter, whereas tetradecane did not show any increase in SP levels even after 1 h of exposure (Fig. 1). These results support our previous findings with these chemicals, where nonane and dodecane showed significant increases in TEWL and erythema values following 1 h occlusive exposure while tetradecane did not show any increase in TEWL compared to control (Babu et al., 2004a). However, tetradecane showed significant irritation under long-term unocclusive exposures (Babu et al., 2004b), indicating that skin irritation of aliphatic hydrocarbons dependent on the exposure conditions. This was further demonstrated by Muhammad et al. (2005), 1-day occlusive exposure to tetradecane did not yield any significant changes, whereas 4-day exposure resulted changes in skin morphology and erythema.
Prostaglandins (PGs) are diverse group of hormone like substances that mediate many cellular and physiological processes. Mize et al. (1997) studied the correlation between the visual erythema scores and PGE2 efflux and observed that there is a direct relationship between the PGE2 efflux and intensity of skin irritation. Our results show an increase in PGE2 levels following exposure to nonane, whereas dodecane and tetradecane did not release PGE2 in significant amounts (Fig. 2). Fang et al. (2003) characterized the irritation potential of various skin permeation enhancers by measuring the TEWL and PGE2 levels and identified that skin PGE2 expression levels are well correlated with the TEWL values. PGE2 is a potent mediator generated in immune tissues by cyclooxygenation of arachidonic acid in response to irritation. During skin irritation process, the time required to release PGE2 is short, following nonane application. PGE2 was released rapidly; however, the levels were decreased steadily after 1 h of chemical application. This may be due to the depletion of arachidonic acid depots in skin tissue. This may be one of the reasons for observed decline in PGE2 levels below the equilibration value as a course of time (Fig. 3). Tetradecane did not show any irritation upon occlusive dermal exposure and it appeared to have no influence on the PGE2 release.
α-MSH plays an important role in the skin inflammation. Under normal conditions, α-MSH can be found in detectable levels in the skin, whereas, under inflammatory condition α-MSH expression levels increase several folds (Schiller et al., 2004). Several studies have demonstrated that α-MSH exerts potent immunoregulatory effects by interacting with melamocortin-1R expressing monocytes, macrophages or dendritic cells (Luger et al., 2000; Slominski et al., 2000). However, α-MSH release depends on the optimal generation of the proinflammatory cytokines such as IL-1, which induces the release of α-MSH (Schauer et al., 1994). In our case, we observed an increase in α-MSH release following nonane and dodecane treatment, initially α-MSH release was faster and after removal of occlusion there was a gradual decrease in α-MSH release (Fig. 3).
Interleukin 6 (IL-6) is a multifunctional cytokine produced in a variety of inflammatory conditions and plays central role in modulating immunity (Akira et al., 1990). Production of IL-6 is tightly regulated; upon chemical insult or irritation, IL-6 levels increase transiently and return to normal levels after resolution of insult (Hirano, 1992). During the skin irritation assessment of aliphatic hydrocarbons, the chemicals were applied after 2 h equilibration period, which was found to be optimal time period to minimize the needle-induced trauma. IL-6 is a secondary proinflammatory cytokine produced by stimulation of several other pathways, such as IL-1α, TNF and SP. Both nonane and dodecane showed increase in IL-6 release after 5 h of application of chemicals, where as tetradecane did not induce any IL-6 release. These findings are well in agreement with the recent reports, where 1 h occlusive exposure of undecane and tetradecane significantly altered the expression of several genes and only undecane altered the IL-6 and TNF gene (McDougal and Garrett, 2007). TNF is produced by keratinocytes and langherhans cells in response to a variety of noxious stimuli resulting in the production of IL-6 and several other inflammatory mediators (Luster et al., 1999). In the present study microdialysis samples were collected for 5 h after removal of occlusion where as the decrease in IL-6 release was not possible to determine for longer time points. This can be overcome using return awake animal containment system (Holovics et al., 2008) where the microdialysis sampling can be carried out in conscious rats for extended period of time. These experiments are presently being pursued in our laboratory.
The current study was focused on the molecular mechanisms involved in the skin irritation of aliphatic hydrocarbons. Evaluation of SP revealed that, initially SP was released in very high amounts upon occlusive chemical exposures and at later stages the release was gradually decreased. During the skin irritation process as a first response primary inflammatory mediators such as SP and IL-1α, are released and later stages primary inflammatory markers will trigger the release of secondary inflammatory markers like IL-6, IL-8, etc. (Fig. 5). In the present study SP levels were increased by about 4-fold within 1 h after nonane exposure (Fig. 1A) and the IL-6 peaked to a maximum level after 7 h of application of nonane (Fig. 4A). These results strongly suggest that there is a correlation between SP expression and its effect on inducing IL-6 release upon dermal exposures. Inflammation is a complex phenomenon and involves many events in up and down regulation of many inflammatory biomarkers, in the present study it cannot be ruled out the involvement of IL-1α in the induction of IL-6 release. Several reports indicate that there is a relation between increased SP levels and increased production of proinflammatory biomarkers like IL-6, IL-8 and TNF-alpha (Zhao et al., 2002; Yamaguchi et al., 2008). SP in association with IL-1α can induce mast cell degranulation leading to the expression of IL-6 in the skin (Theoharides et al., 2004).
Fig. 5.
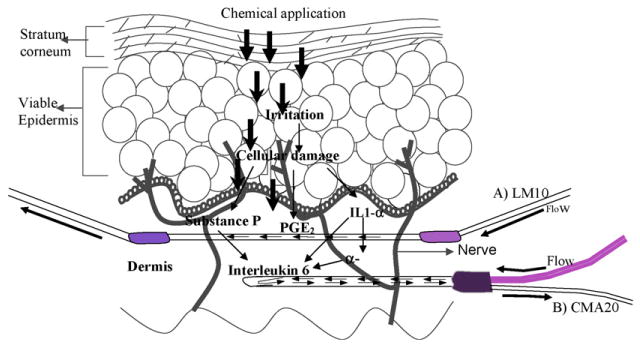
Hypothesized biomolecular interactions in the skin following application of aliphatic hydrocarbons. A) LM-10 linear microdialysis probe B) CMA 20 concentric micordialysis probe. The figure shows that IL-1α, PGE2 and SP are primary biomarkers in response to skin irritation, these in turn induce the expression of IL-6.
In conclusion, microdialysis was demonstrated to be excellent tool to measure several inflammatory biomarkers as a function of time after dermal exposures with irritant chemicals. Microdialysis technique also provided information to discriminate the dermal irritancy of different aliphatic hydrocarbons such as nonane, dodecane and tetradecane at cellular levels.
Acknowledgments
The authors gratefully acknowledge the financial assistance provided by the Department of Defense, AFOSR, AFMC and RCMI (NIH).
Footnotes
Conflicts of Interest: None.
References
- Akira S, Hirano T, Taga T, Kishimoto T. Biology of multifunctional cytokines: IL 6 and related molecules (IL 1 and TNF) FASEB J. 1990;4:2860–2867. [PubMed] [Google Scholar]
- Angst MS, Clark JD, Carvalho B, Tingle M, Schmelz M, Yeomans DC. Cytokine profile in human skin in response to experimental inflammation, noxious stimulation, and administration of a COX-inhibitor: a microdialysis study. Pain. 2008;139:15–17. doi: 10.1016/j.pain.2008.02.028. [DOI] [PubMed] [Google Scholar]
- Ao X, Stenken JA. Microdialysis sampling of cytokines. Methods. 2006;38:331–341. doi: 10.1016/j.ymeth.2005.11.012. [DOI] [PubMed] [Google Scholar]
- Ao X, Wang X, Lennartz RM, Loegering JD, Stenken JA. Multiplexed cytokine detection in microliter microdialysis samples obtained from activated cultured macrophages. J Pharm Biomed Anal. 2006;40:915–921. doi: 10.1016/j.jpba.2005.07.044. [DOI] [PubMed] [Google Scholar]
- Babu RJ, Chatterjee A, Singh M. Assessment of skin irritation and molecular responses in rat skin exposed to nonane, dodecane and tetradecane. Toxicol Lett. 2004a;153:255–266. doi: 10.1016/j.toxlet.2004.04.036. [DOI] [PubMed] [Google Scholar]
- Babu RJ, Chatterjee A, Ahaghotu E, Singh M. Percutaneous absorption and skin irritation upon low-level prolonged dermal exposure to nonane, dodecane and tetradecane in hairless rats. Toxicol Ind Health. 2004b;20:109–118. doi: 10.1191/0748233704th197oa. [DOI] [PubMed] [Google Scholar]
- Chou CC, Riviere JE, Monteiro-Riviere NA. Differential relationship between the carbon chain length of jet fuel aliphatic hydrocarbons and their ability to induce cytotoxicity vs. interleukin-8 release in human epidermal keratinocytes. Toxicol Sci. 2002;69:226–233. doi: 10.1093/toxsci/69.1.226. [DOI] [PubMed] [Google Scholar]
- Clough GF, Church MK. Vascular responses in the skin: an accessible model of inflammation. News Physiol Sci. 2002;17:170–174. doi: 10.1152/nips.01378.2001. [DOI] [PubMed] [Google Scholar]
- Fang JY, Hwang TL, Fang CL, Chiu HC. In vitro and in vivo evaluations of the efficacy and safety of skin permeation enhancers using flurbiprofen as a model drug. Int J Pharm. 2003;255:153–166. doi: 10.1016/s0378-5173(03)00086-3. [DOI] [PubMed] [Google Scholar]
- Fulzele SV, Babu RJ, Ahaghotu E, Singh M. Estimation of proinflammatory biomarkers of skin irritation by dermal microdialysis following exposure with irritant chemicals. Toxicology. 2007;31:77–88. doi: 10.1016/j.tox.2007.05.005. [DOI] [PubMed] [Google Scholar]
- Heylings JR, Diot S, Esdaile DJ, Fasano WJ, Manning LA, Owen HM. A prevalidation study on the in vitro skin irritation function test (SIFT) for prediction of acute skin irritation in vivo: results and evaluation of ECVAM Phase III. Toxicol In Vitro. 2003;17:123–138. doi: 10.1016/s0887-2333(02)00130-3. [DOI] [PubMed] [Google Scholar]
- Hirano T. Interleukin-6 and its relation to inflammation and disease. Clin Immunol Immunopathol. 1992;62:S60–S65. doi: 10.1016/0090-1229(92)90042-m. [DOI] [PubMed] [Google Scholar]
- Holovics JH, Anderson CR, Levine BR, Hui WH, Lunte RC. Investigation of drug delivery by iontophoresis in a surgical wound utilizing microdialysis. Pharm Res. 2008;25:1762–1770. doi: 10.1007/s11095-007-9490-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iversen HH, Celsing F, Leone AM, Gustafsson LE, Wiklund NP. Nerve-induced release of nitric oxide in the rabbit gastrointestinal tract as measured by in vivo microdialysis. Br J Pharmacol. 1997;120:702–706. doi: 10.1038/sj.bjp.0700967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iyadomi M, Higaki Y, Ichiba M, Morimoto M, Tokokuni K. Evaluation of organic solvent-induced inflammation modulated by neuropeptides in abdominal skin of hairless rats. Ind Health. 1998;36:40–51. doi: 10.2486/indhealth.36.40. [DOI] [PubMed] [Google Scholar]
- Kramer HH, Schmidt K, Leis S, Schmelz M, Sommer C, Birklein F. Inhibition of neutral endopeptidase (NEP) facilitates neurogenic inflammation. Exp Neurol. 2005;195:179–184. doi: 10.1016/j.expneurol.2005.04.015. [DOI] [PubMed] [Google Scholar]
- Kreilgaard M. Assessment of cutaneous drug delivery using microdialysis. Adv Drug Deliv Rev. 2002;1(Suppl (54)):S99–S121. doi: 10.1016/s0169-409x(02)00117-5. [DOI] [PubMed] [Google Scholar]
- Lindberger M, Tomson T, Wallstedt L, Stahle L. Distribution of valproate to subdural cerebrospinal fluid, subcutaneous extracellular fluid, and plasma in humans: a microdialysis study. Epilepsia. 2001;42:256–261. doi: 10.1046/j.1528-1157.2001.26600.x. [DOI] [PubMed] [Google Scholar]
- Luger TA, Brzoska T, Scholzen TE, Kalden DH, Sunderkötter C, Armstrong C, Ansel J. The role of alpha-MSH as a modulator of cutaneous inflammation. Ann NY Acad Sci. 2000;917:232–238. doi: 10.1111/j.1749-6632.2000.tb05388.x. [DOI] [PubMed] [Google Scholar]
- Luster MI, Simeonova PP, Gallucci R, Matheson J. Tumor necrosis factor alpha and toxicology. Crit Rev Toxicol. 1999;29:491–511. doi: 10.1080/10408449991349258. [DOI] [PubMed] [Google Scholar]
- Mathy FX, Ntivunwa D, Verbeek R, Preat V. Fluconazole distribution in rat dermis following intravenous and topical application: a microdialysis study. J Pharm Sci. 2005;94:770–780. doi: 10.1002/jps.20290. [DOI] [PubMed] [Google Scholar]
- McDougal JN, Garrett CM. Gene expression and target tissue dose in the rat epidermis after brief JP-8 and JP-8 aromatic and aliphatic component exposures. Toxicol Sci. 2007;97:569–581. doi: 10.1093/toxsci/kfm037. [DOI] [PubMed] [Google Scholar]
- McDougal JN, Pollard DL, Wiesman W, Garrett CM, Miller TE. Assessment of skin absorption and penetration of JP-8 jet fuel and its components. Toxicol Sci. 2000;55:247–255. doi: 10.1093/toxsci/55.2.247. [DOI] [PubMed] [Google Scholar]
- Mize NK, Buttery M, Daddona P, Morales C, Cormier M. Reverse iontophoresis: monitoring prostaglandin E2 associated with cutaneous inflammation in vivo. Exp Dermatol. 1997;6:298–302. doi: 10.1111/j.1600-0625.1997.tb00176.x. [DOI] [PubMed] [Google Scholar]
- Muhammad F, Monteiro-Riviere NA, Riviere JE. Comparative in vivo toxicity of topical JP-8 jet fuel and its individual hydrocarbon components: identification of tridecane and tetradecane as key constituents responsible for dermal irritation. Toxicol Pathol. 2005;33:258–266. doi: 10.1080/01926230590908222. [DOI] [PubMed] [Google Scholar]
- Muller M. Science, medicine, and the future: microdialysis. Brit Med J. 2002;324:588–591. doi: 10.1136/bmj.324.7337.588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newman JM, Di Maria CA, Rattigan S, Clark MG. Nutritive blood flow affects microdialysis O/I ratio for [(14)C]ethanol and (3)H(2)O in perfused rat hindlimb. Am J Physiol Heart Circ Physiol. 2001;281:H2731–H2737. doi: 10.1152/ajpheart.2001.281.6.H2731. [DOI] [PubMed] [Google Scholar]
- Rosenbloom AJ, Sipe DM, Weedn VW. Microdialysis of proteins: performance of the CMA/20 probe. J Neurosci Methods. 2005;30:147–153. doi: 10.1016/j.jneumeth.2005.04.018. [DOI] [PubMed] [Google Scholar]
- Schauer E, Trautinger F, Köck A, Schwarz A, Bhardwaj R, Simon M, Ansel JC, Schwarz T, Luger TA. Proopiomelanocortin-derived peptides are synthesized and released by human keratinocytes. J Clin Invest. 1994;93:2258–2262. doi: 10.1172/JCI117224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiller M, Brzoska T, Bohm M, Metz D, Scholzen ET, Rougier A. Solar-simulated ultraviolet radiation-induced upregulation of the melanocortin-1 receptor, proopiomelanocortin, and α-melanocyte-stimulating hormone in human epidermis in vivo. J Invest Dermatol. 2004;122:468–476. doi: 10.1046/j.0022-202X.2004.22239.x. [DOI] [PubMed] [Google Scholar]
- Schmidt S, Banks R, Kumar V, Rand HK, Derendorf H. Clinical microdialysis in skin and soft tissues. J Clin Pharmacol. 2008;48:351–364. doi: 10.1177/0091270007312152. [DOI] [PubMed] [Google Scholar]
- Slominski A, Wortsman J, Luger T, Paus R, Solomon S. Corticotropin releasing hormone and proopiomelanocortin involvement in the cutaneous response to stress. Physiol Rev. 2000;80:979–1020. doi: 10.1152/physrev.2000.80.3.979. [DOI] [PubMed] [Google Scholar]
- Theoharides TC, Donelan JM, Papadopoulou N, Cao J, Kempuraj D, Conti P. Mast cells as targets of corticotropin-releasing factor and related peptides. Trends Pharmacol Sci. 2004;25:563–568. doi: 10.1016/j.tips.2004.09.007. [DOI] [PubMed] [Google Scholar]
- Trickler WJ, Miller DW. Use of osmotic agents in microdialysis studies to improve the recovery of macromolecules. J Pharm Sci. 2003;92:1419–1427. doi: 10.1002/jps.10410. [DOI] [PubMed] [Google Scholar]
- Ungerstedt U, Pycock C. Functional correlates of dopamine neurotransmission. Bull Schweiz Akad Med Wiss. 1974;30:44–55. [PubMed] [Google Scholar]
- Yamaguchi M, Ozawa Y, Mishima H, Aihara N, Kojima T, Kasai K. Substance P increases production of proinflammatory cytokines and formation of osteoclasts in dental pulp fibroblasts in patients with severe orthodontic root resorption. Am J Orthod Dentofac Orthoped. 2008;133:690–698. doi: 10.1016/j.ajodo.2006.03.043. [DOI] [PubMed] [Google Scholar]
- Zhao D, Kuhnt-Moore S, Zeng H, Pan A, Wu JS, Simeonidis S, Moyer MP, Pothoulakis C. Substance P-stimulated interleukin-8 expression in human colonic epithelial cells involves Rho family small GTPases. Biochem J. 2002;368:665–672. doi: 10.1042/BJ20020950. [DOI] [PMC free article] [PubMed] [Google Scholar]


