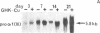Abstract
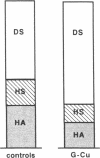
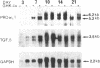
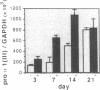
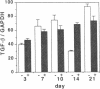
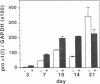
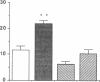
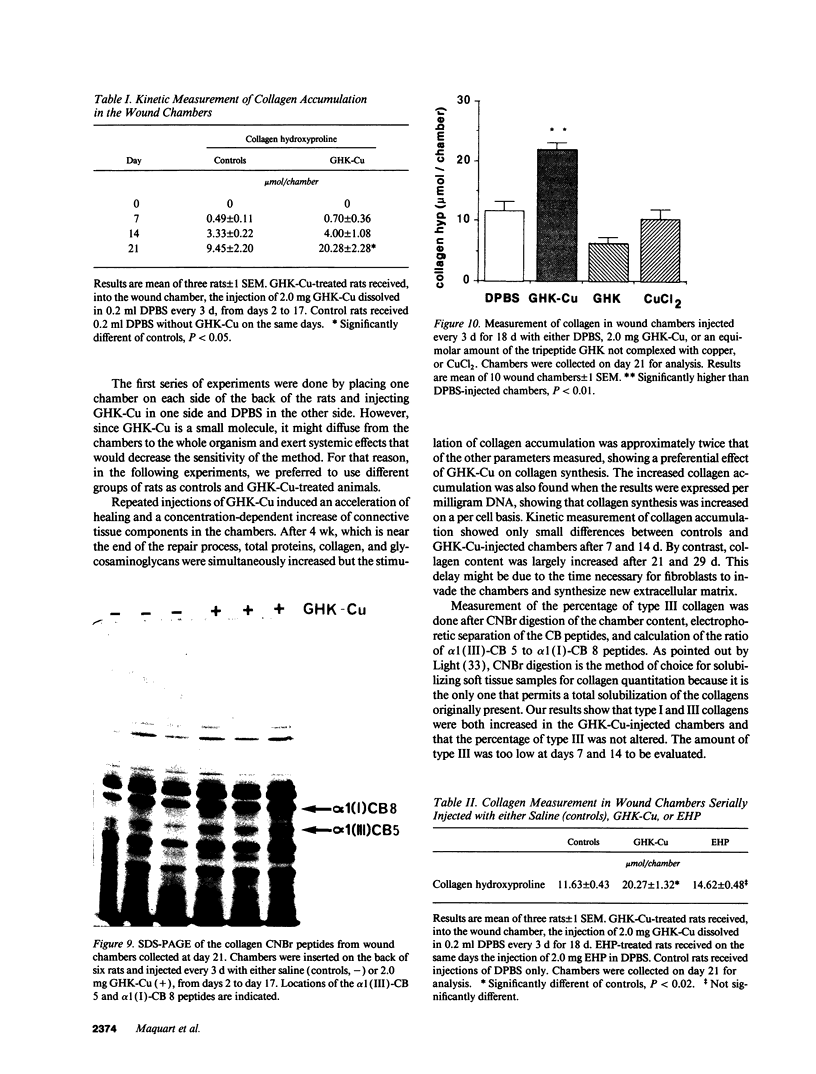
The tripeptide-copper complex glycyl-L-histidyl-L-lysine-Cu2+ (GHK-Cu) was first described as a growth factor for differentiated cells. Recent in vitro data showed that it possesses several properties of a potential activator of wound repair. We investigated the effects of GHK-Cu in vivo, using the wound chamber model described previously (Schilling, J.A., W. Joel, and M.T. Shurley, 1959. Surgery [St. Louis]. 46:702-710). Stainless steel wire mesh cylinders were implanted subcutaneously on the back of rats. The animals were divided into groups that received sequential injections into the wound chamber of either saline (control group) or various concentrations of GHK-Cu. At the end of the experiments, rats were killed, wound chambers were collected, and their content was analyzed for dry weight, total proteins, collagen, DNA, elastin, glycosaminoglycans, and specific mRNAs for collagens and TGF beta. In the GHK-Cu-injected wound chambers, a concentration-dependent increase of dry weight, DNA, total protein, collagen, and glycosaminoglycan contents was found. The stimulation of collagen synthesis was twice that of noncollagen proteins. Type I and type III collagen mRNAs were increased but not TGF beta mRNAs. An increase of the relative amount of dermatan sulfate was also found. A control tripeptide, L-glutamyl-L-histidyl-L-proline, had no significant effect. These results demonstrate that GHK-Cu is able to increase extracellular matrix accumulation in wounds in vivo.
Full text
PDF


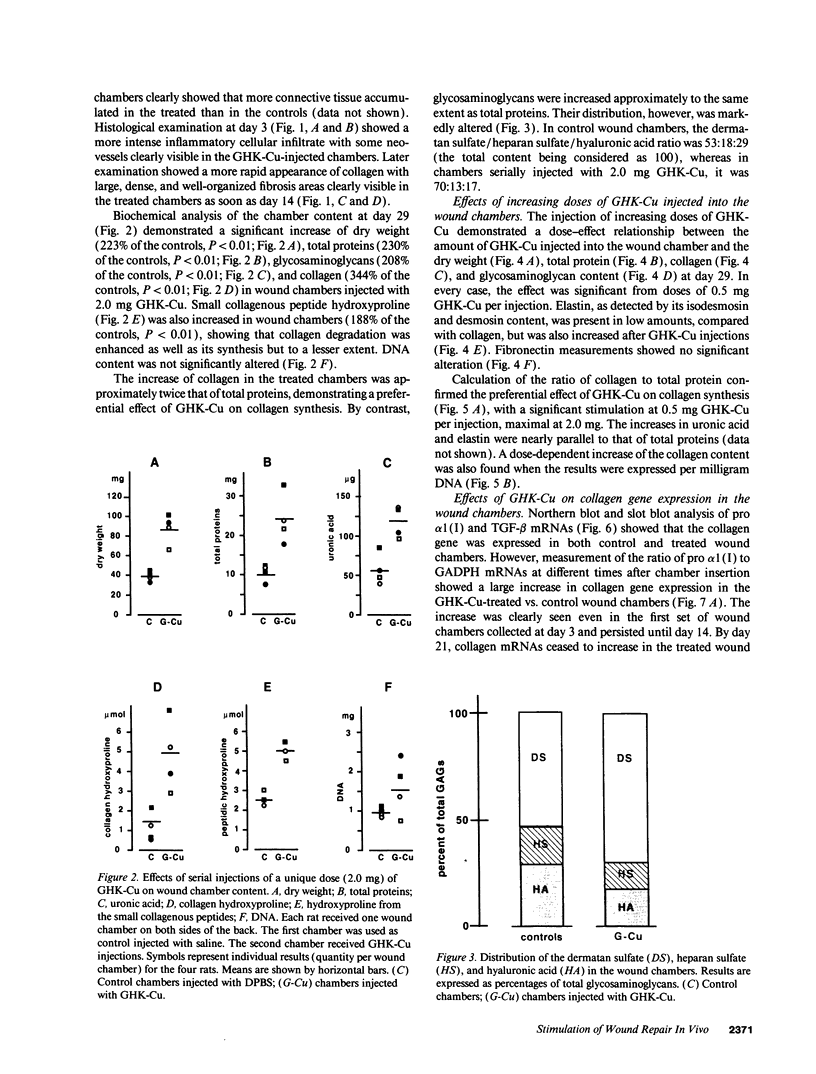


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
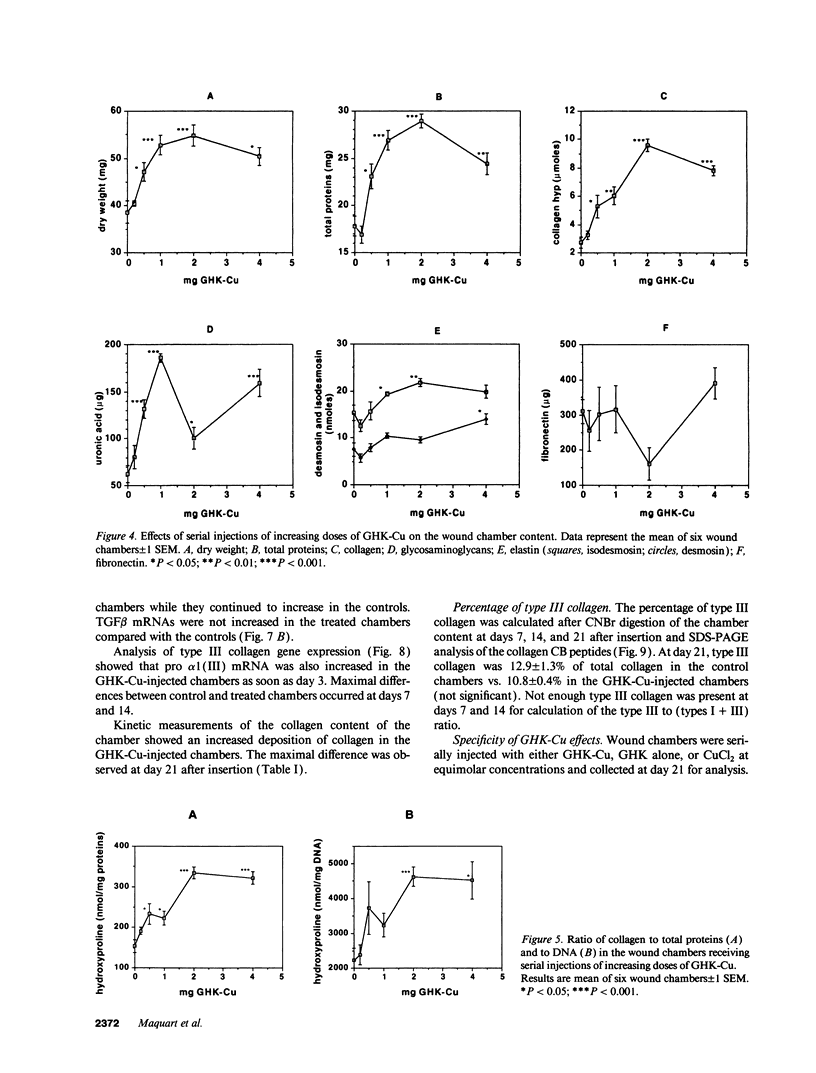
- BITTER T., MUIR H. M. A modified uronic acid carbazole reaction. Anal Biochem. 1962 Oct;4:330–334. doi: 10.1016/0003-2697(62)90095-7. [DOI] [PubMed] [Google Scholar]
- Bartold P. M., Wiebkin O. W., Thonard J. C. Molecular weight estimation of sulfated glycosaminoglycans in human gingivae. Connect Tissue Res. 1982;9(3):165–172. doi: 10.3109/03008208209160257. [DOI] [PubMed] [Google Scholar]
- Chan D., Cole W. G. Quantitation of type I and III collagens using electrophoresis of alpha chains and cyanogen bromide peptides. Anal Biochem. 1984 Jun;139(2):322–328. doi: 10.1016/0003-2697(84)90012-5. [DOI] [PubMed] [Google Scholar]
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Epstein E. H., Jr (Alpha1(3))3 human skin collagen. Release by pepsin digestion and preponderance in fetal life. J Biol Chem. 1974 May 25;249(10):3225–3231. [PubMed] [Google Scholar]
- Fiszer-Szafarz B., Szafarz D., Guevara de Murillo A. A general, fast, and sensitive micromethod for DNA determination application to rat and mouse liver, rat hepatoma, human leukocytes, chicken fibroblasts, and yeast cells. Anal Biochem. 1981 Jan 1;110(1):165–170. doi: 10.1016/0003-2697(81)90130-5. [DOI] [PubMed] [Google Scholar]
- Fleischmajer R., Fisher L. W., MacDonald E. D., Jacobs L., Jr, Perlish J. S., Termine J. D. Decorin interacts with fibrillar collagen of embryonic and adult human skin. J Struct Biol. 1991 Feb;106(1):82–90. doi: 10.1016/1047-8477(91)90065-5. [DOI] [PubMed] [Google Scholar]
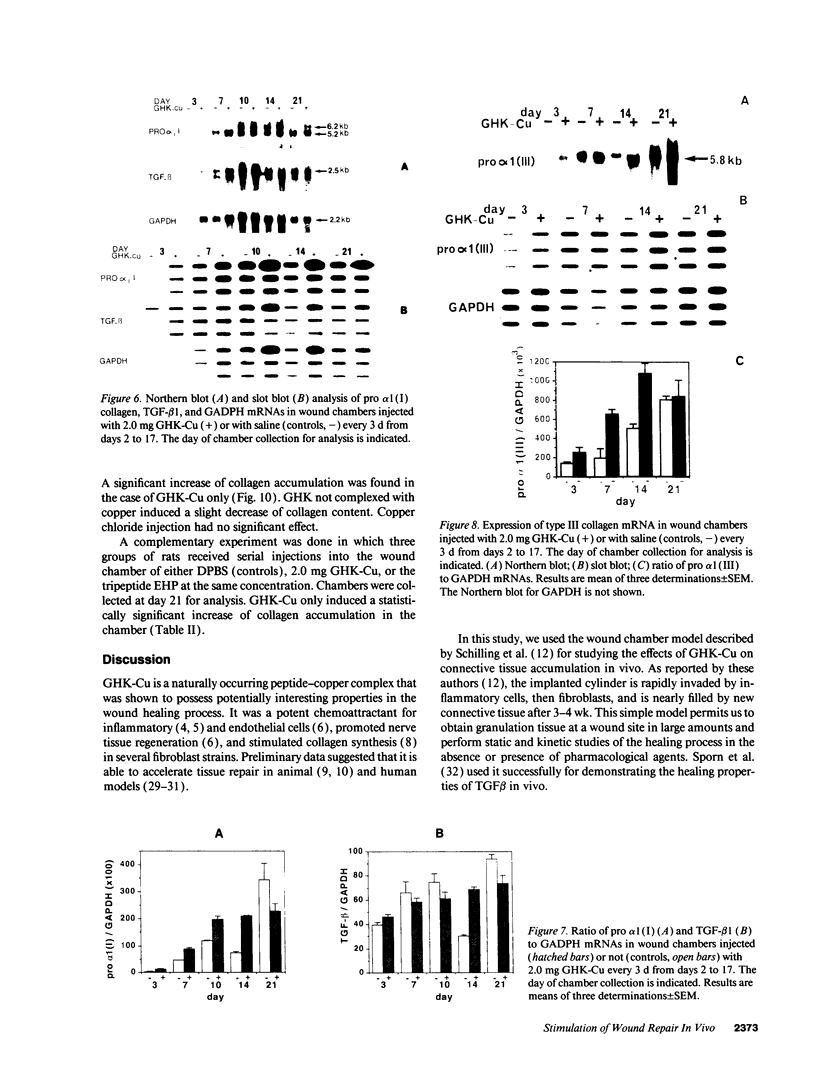
- Fort P., Marty L., Piechaczyk M., el Sabrouty S., Dani C., Jeanteur P., Blanchard J. M. Various rat adult tissues express only one major mRNA species from the glyceraldehyde-3-phosphate-dehydrogenase multigenic family. Nucleic Acids Res. 1985 Mar 11;13(5):1431–1442. doi: 10.1093/nar/13.5.1431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grosse G., Lindner G. Experimental influence of pharmacological agents on the regeneration of nervous tissue in vitro. Folia Morphol (Praha) 1980;28(4):345–347. [PubMed] [Google Scholar]
- LAGUNOFF D., WARREN G. Determination of 2-deoxy-2-sulfoaminohexose content of mucopolysaccharides. Arch Biochem Biophys. 1962 Dec;99:396–400. doi: 10.1016/0003-9861(62)90285-0. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lau S. J., Sarkar B. The interaction of copper(II) and glycyl-L-histidyl-L-lysine, a growth-modulating tripeptide from plasma. Biochem J. 1981 Dec 1;199(3):649–656. doi: 10.1042/bj1990649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Light N. D. Estimation of types I and III collagens in whole tissue by quantitation of CNBr peptides on SDS-polyacrylamide gels. Biochim Biophys Acta. 1982 Mar 18;702(1):30–36. doi: 10.1016/0167-4838(82)90024-3. [DOI] [PubMed] [Google Scholar]
- Maquart F. X., Pickart L., Laurent M., Gillery P., Monboisse J. C., Borel J. P. Stimulation of collagen synthesis in fibroblast cultures by the tripeptide-copper complex glycyl-L-histidyl-L-lysine-Cu2+. FEBS Lett. 1988 Oct 10;238(2):343–346. doi: 10.1016/0014-5793(88)80509-x. [DOI] [PubMed] [Google Scholar]
- Miller D. M., DeSilva D., Pickart L., Aust S. D. Effects of glycyl-histidyl-lysyl chelated Cu(II) on ferritin dependent lipid peroxidation. Adv Exp Med Biol. 1990;264:79–84. doi: 10.1007/978-1-4684-5730-8_11. [DOI] [PubMed] [Google Scholar]
- Odedra R., Weiss J. B. Low molecular weight angiogenesis factors. Pharmacol Ther. 1991;49(1-2):111–124. doi: 10.1016/0163-7258(91)90025-h. [DOI] [PubMed] [Google Scholar]
- Pickart L., Freedman J. H., Loker W. J., Peisach J., Perkins C. M., Stenkamp R. E., Weinstein B. Growth-modulating plasma tripeptide may function by facilitating copper uptake into cells. Nature. 1980 Dec 25;288(5792):715–717. doi: 10.1038/288715a0. [DOI] [PubMed] [Google Scholar]
- Pickart L., Thaler M. M. Tripeptide in human serum which prolongs survival of normal liver cells and stimulates growth in neoplastic liver. Nat New Biol. 1973 May 16;243(124):85–87. [PubMed] [Google Scholar]
- Pickart L. The use of glycylhistidyllysine in culture systems. In Vitro. 1981 Jun;17(6):459–466. doi: 10.1007/BF02633506. [DOI] [PubMed] [Google Scholar]
- Poole T. J., Zetter B. R. Stimulation of rat peritoneal mast cell migration by tumor-derived peptides. Cancer Res. 1983 Dec;43(12 Pt 1):5857–5861. [PubMed] [Google Scholar]
- Qian S. W., Kondaiah P., Casscells W., Roberts A. B., Sporn M. B. A second messenger RNA species of transforming growth factor beta 1 in infarcted rat heart. Cell Regul. 1991 Mar;2(3):241–249. doi: 10.1091/mbc.2.3.241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raju K. S., Alessandri G., Ziche M., Gullino P. M. Ceruloplasmin, copper ions, and angiogenesis. J Natl Cancer Inst. 1982 Nov;69(5):1183–1188. [PubMed] [Google Scholar]
- SCHILLING J. A., JOEL W., SHURLEY H. M. Wound healing: a comparative study of the histochemical changes in granulation tissue contained in stainless steel wire mesh and polyvinyl sponge cylinders. Surgery. 1959 Oct;46:702–710. [PubMed] [Google Scholar]
- Saito H., Yamagata T., Suzuki S. Enzymatic methods for the determination of small quantities of isomeric chondroitin sulfates. J Biol Chem. 1968 Apr 10;243(7):1536–1542. [PubMed] [Google Scholar]
- Scott J. E. Proteoglycan-fibrillar collagen interactions. Biochem J. 1988 Jun 1;252(2):313–323. doi: 10.1042/bj2520313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sporn M. B., Roberts A. B., Shull J. H., Smith J. M., Ward J. M., Sodek J. Polypeptide transforming growth factors isolated from bovine sources and used for wound healing in vivo. Science. 1983 Mar 18;219(4590):1329–1331. doi: 10.1126/science.6572416. [DOI] [PubMed] [Google Scholar]
- Vuento M., Salonen E., Pasanen M., Stenman U. H. Competitive enzyme immunoassay for human plasma fibronectin. J Immunol Methods. 1981;40(1):101–108. doi: 10.1016/0022-1759(81)90085-5. [DOI] [PubMed] [Google Scholar]
- Vuorio T., Mäkelä J. K., Kähäri V. M., Vuorio E. Coordinated regulation of type I and type III collagen production and mRNA levels of pro alpha 1(I) and pro alpha 2(I) collagen in cultured morphea fibroblasts. Arch Dermatol Res. 1987;279(3):154–160. doi: 10.1007/BF00413250. [DOI] [PubMed] [Google Scholar]
- Wegrowski Y., Maquart F. X., Borel J. P. Stimulation of sulfated glycosaminoglycan synthesis by the tripeptide-copper complex glycyl-L-histidyl-L-lysine-Cu2+. Life Sci. 1992;51(13):1049–1056. doi: 10.1016/0024-3205(92)90504-i. [DOI] [PubMed] [Google Scholar]
- Zetter B. R., Rasmussen N., Brown L. An in vivo assay for chemoattractant activity. Lab Invest. 1985 Sep;53(3):362–368. [PubMed] [Google Scholar]