Abstract
Background
Pain intensity is commonly reported using a 0–10 numeric rating scale in breakthrough pain clinical trials. Analysis of the change on the Pain Intensity Numerical Rating Scale as a proportion as most consistently correlated with clinically important differences reported on the Patient Global Impression of Change. The analysis of data using a different global outcome measures and the pain relief scale will extend our understanding of these measures. Use of the pain relief scale is also explored in this study
Methods
Data came from the open titration phase of a multiple crossover, randomized, double-blind clinical trial comparing oral transmucosal fentanyl citrate to immediate-release oral morphine sulfate for treatment of cancer-related breakthrough pain. Raw and percent changes in the pain intensity scores on 1,307 from 134 oral transmucosal fentanyl citrate-naive patients were compared to the clinically relevant secondary outcomes of the pain relief verbal response scale and the global medication performance. The changes in raw and percent change were assessed over time and compared to the ordinal pain relief verbal response scale and global medication performance scales.
Results
The p-value of the interaction between the raw pain intensity difference was significant but not for the percent pain intensity difference score over 4 15 minute time periods (p = 0.034 and p = 0.26 respectively), in comparison with the ordinal pain relief verbal response scale (p = 0.0048 and p = 0.36 respectively), and global medication performance categories (p = 0.048 and p = 0.45 respectively).
Conclusion
The change in pain intensity in breakthrough pain was more consistent over time and when compared to both the pain relief verbal response scale and global medication performance scale when the percent change is used rather than raw pain intensity difference.
Introduction
Given the inherently subjective nature of the symptom, measurements of pain rely primarily on the verbal reports of patients 1–4. The multiple dimensions of pain such as intensity, characteristics, pain relief, and the global impressions of change are considered important additional endpoints for pain clinical trials 5–10. However, for studies of pain-specific therapies change in pain intensity over time is almost always the primary outcome. The pain intensity 0–100 millimeter visual analogue scale and the 0–10 numeric rating scale (PI-NRS) are commonly used metrics. The PI-NRS has become the more common choice because of its ease of use, a broader range of methods of administration, and evidence of consistent results across a wide range of languages and cultures 11,12.
For chronic pain studies a greater consistency between the change in the PI-NRS score and a clinically relevant outcome has been demonstrated using the percent change compared to the raw change in the analysis of pain intensity data 13. The calculation of the percent change converts the change PI-NRS to a proportional measure. The substantial improvement in the association with patient’s report of their global improvement supports the concept that patient’s use the PI-NRS proportional scale to report their change in pain intensity. To our knowledge this has not been investigated in studies of rapid onset breakthrough pain and how the measurements of pain change over time, given the relatively rapid resolution of this type of pain episode. In addition, no connection has been made to more specific global measures such as the patient’s assessment of the overall performance of their analgesic medication and the achievement of specific levels of pain relief. Demonstrating the consistency of these chronic pain findings in additional pain syndromes and using different global anchors will provide important information about the relationship of these measures and allow important comparisons across a wider array of pain studies.
MATERIALS AND METHODS
This study was approved by the Institutional Review Board of the University of Pennsylvania, Philadelphia, Pennsylvania. Each patient in the original clinical trial signed an informed consent before being enrolled.
Data Source
The data used in this analysis was obtained from a multicenter randomized, blinded, placebo-controlled clinical trial of oral transmucosal fentanyl citrate (OTFC - trade name ACTIQ® Cephalon, Inc, Frazer, PA) compared to oral immediate-release morphine sulfate for the treatment of cancer-related breakthrough pain. The study design and methods have been described in detail previously 14. In brief, all 134 outpatients enrolled had entered the trial with chronic cancer-related pain controlled with long-acting opioid drugs, and had recurrent episodes of breakthrough pain (BTP) adequately treated with immediate release morphine sulphate. Since all patients were OTFC naive, an initial titration phase was used to find the appropriate dose of OTFC for each patient starting at the lowest available dosage strength of 200 mcg per OTFC unit. Subjects were titrated up to a maximum of 1,600 mcg per OTFC unit or until a single lower dose was found that controlled more than two episodes of the patient’s target BTP in a row. All 1,307 treated episodes that had adequate data for analysis were included in this study. Given the rapid onset of action of OTFC, acceptable pain relief was expected within 30 minutes after initiation of the first dose 15 and at 45 min for the immediate release morphine sulphate. If not achieved, a second dose of the OTFC, or a dose of the patient’s original immediate release morphine sulphate, could be taken as an “additional dose of rescue medication” for that episode of BTP.
The endpoints were collected at 15, 30, 45, and 60 min following the start of the administration of the OTFC dose, or until the time the patient decided to take an “additional dose of rescue medication.” The primary end point was the change in the PI-NRS, and the secondary endpoint was pain relief on a 5-point verbal rating scale [PR-VRS]. A global medication performance [GMP] rating was obtained at the end of treatment for each episode. The PR-VRS scale [0 (none), 1 (slight), 2 (moderate), 3 (lots), 4 (complete)], and the GMP scale [0 (poor), 1 (fair), 2 (good), 3 (very good), 4 (excellent)] were used as reported by patients as these were assumed to be zero at time zero. Data was collected using a paper patient diary. The data for each day was collected on a separate page so that the previous day’s information was not immediately available, but patients were not specifically blinded to their previous answer. The change in pain intensity was calculated as both: 1) the raw pain intensity difference [PID = PI-NRS value − PI-NRS baseline], and 2) the percentage pain intensity difference [%PID = (PID/PI-NRS baseline)*100].
Analysis
The first analysis evaluated the effect of baseline factors on the consistency of the patients’ pain reports by examining changes over the full 60-min time period. The values for each treatment episode were stratified into groups by a number of the patient characteristics. The mean value of the change in pain intensity was calculated for each patient group at 15, 30, 45, and 60 min. To provide the best evaluation over time, only data from the 1,105 episodes where the patient recorded outcomes for the full 60 min could be used (i.e., those that did not drop out to take an additional rescue dose). By definition, very little change was expected for episodes where the OTFC did not produce some degree of relief (i.e., episodes that required additional medication), and most of these records were truncated by 45 min. The data imputation methods necessary for the inclusion of these episodes added additional variability without useful data. However, as a sensitivity analysis we repeated the analysis with all episode data using a last observation carry forward. The outcome variable for these models was the change in pain at the given time point (or percent change) from baseline. Statistical interactions between patient characteristic groups and study time were tested using a linear regression analyses clustered by subject.
The second analysis compared changes in the pain intensity to the PR-VRS and to the GMP categorical scale, using the values measured at the end of each treated episode. For these analyses, the change in pain intensity was calculated as the difference between baseline and 45 minutes, because this was the last time point recorded for 95% of those patients who went on to take an “additional dose of rescue medication”, allowing us to use all treatment, regardless of whether or not they required extra doses of rescue medication. We tested different patient characteristic groups for an interaction between the average change in pain intensity for each level of the PR-VRS and the GMP, both of which were used as the dependent variable in a linear regression model. The p-values were adjusted for the lack of independence through clustering by patient.
Lastly, a linear regression model was also used to test for the interaction between the average values of the PR-VRS 1) compared over study time and 2) separately compared to the GMP categorical scale. The same patient characteristics used for the pain intensity comparison were used to define groups, including the initial level of reported pain.
In all analyses, the baseline characteristics considered were age categories (defined as 18–49, 50–59, 60–69, 70+ yr), sex, tumor types, final effective therapeutic dose, and the baseline pain intensity scores. The interaction with time and for each stratification factor was tested for both raw changes in pain intensity and percent change to assess if they differed according to the grouping patient characteristic factor.
Analyses were performed using STATA v8.0 (StataCorp LP, College Station, TX) and the graphs were produced using Excel 2007 (Microsoft, Redmond, WA).
RESULTS
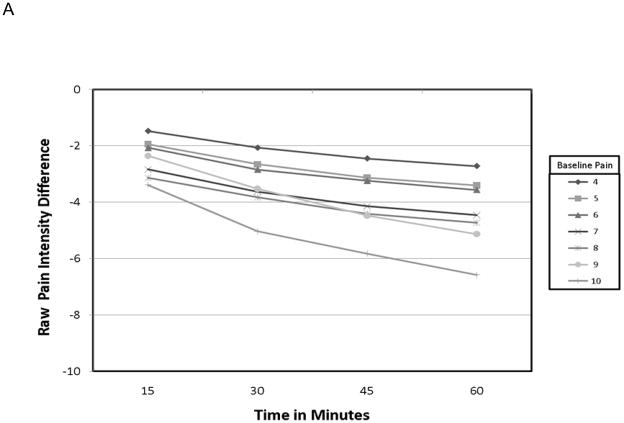
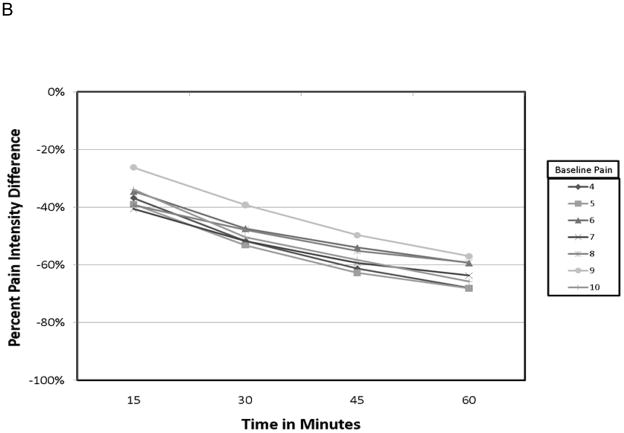
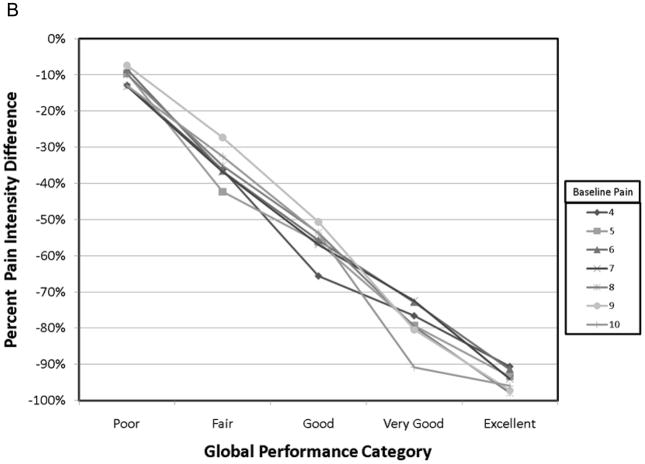
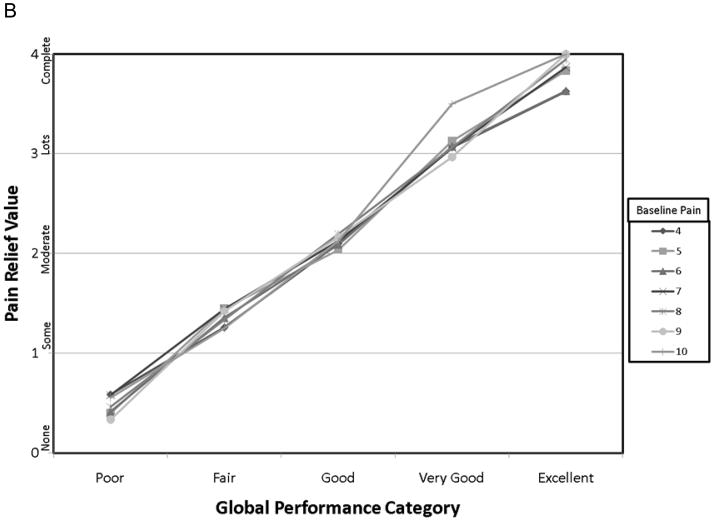
The demographic data from this cohort have previously been published 14. Pertinent data is summarized here for convenience. Of the 134 patients starting the study, 93 achieved adequate analgesia using a single dose of OTFC, and 89 agreed to enter the randomization phase. The reasons that patients dropped out have been carefully described elsewhere but were primarily due to standard opioid side-effects or cancer related events. The mean age of the 134 patients was 55 yr, 92% Caucasian, 47% women, and the primary cancers were colon, breast and lung. Of all the baseline patient characteristics used to group the various pain outcomes in this study, only the raw PID grouped by baseline pain intensity resulted in a statistically significant interaction with study time (fig. 1) and in the average value association with both PR-VRS (fig. 2) and GMP (fig. 3) categorical scales. In particular, patients who reported a higher numeric value for baseline pain intensity (say baseline =9 vs. baseline =4) demonstrated a larger change in raw pain intensity consistently over time (See fig. 1A: p=0.034), reported a greater level of relief (fig. 2A: p = 0.048), and displayed a higher performance level on the GMP scale (fig. 3A: p = 0.013). For a baseline = 9 versus baseline = 4 a change value of 8.75 versus 3.65 respectively was the average change see in patients who reported the GMP condition of excellent. Our sensitivity analysis of the pain level over time, using the whole data set demonstrated the same separation for raw pain intensity values but with increased variance in the analysis of the 45 and 60-min time points (not shown).
Figure 1.
Figure 2.
Figure 3.
Calculating the percent PID resulted in more consistent patterns across the patient characteristic groups over time (fig. 1B:), over the levels of PR-VRS (fig. 2b: p = 0.36), and compared to the ordinal GMP categories (fig. 3B, p = 0.45). These comparisons demonstrate that the response seen in the raw PID result was highly dependent on the baseline pain intensity over a full range of values and conditions. In contrast, the profile of the percent PID was less dependent on baseline pain intensity level over time or when compared to the ordinal categories of the GMP or the PR-VRS.
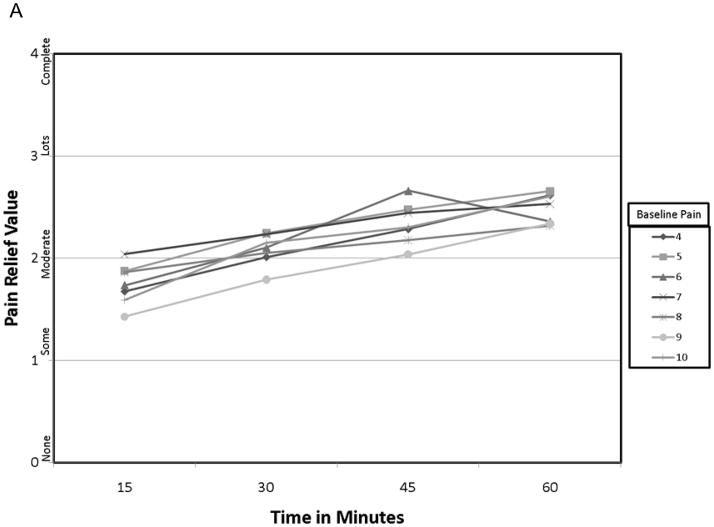
In considering the PR-VRS as a potential outcome, patient characteristic grouping of the PR-VRS score by the baseline factors showed no statistically significant interaction over time or with the GMP level including when grouped by the baseline pain intensity. In particular, the comparison over time (fig. 4A: p = 0.39), and comparison to the GMP (fig. 4B: p = 0.52) were similar between patient characteristic groups.
Figure 4.
DISCUSSION
Percentage Change versus Raw Change in Pain Intensity
Our findings support the improved consistency of a relationship between the percent change in the analysis of the PI-NRS and the clinically important changes measured on the global measures, in the context of a clinical trial for a rapid acting analgesic used to treat a rapid onset BTP in cancer patients. The additional demonstration of this finding in the setting of the use of different global outcome measures supports the consistency of this result across a broader array of study designs. The increased consistency of the percent change in PI-NRS compared to the global outcome and as measured across study time supports the calculation of percent as a way to adjust for the baseline pain, and potentially as an appropriate primary outcome for such clinical trials. This expands on the previous demonstration of the same relationship in patients from 10 chronic pain studies 13 and raises the possibility that the analysis of the PID raw scores can lead to inconsistent results, especially if the starting pain scores vary between groups or across studies. Calculating the % PID generates a result that is more consistent with clinically relevant global measures of the outcome, and has the potential to increase comparability across studies.
To put these findings into perspective, we should consider other studies and analysis techniques. For example, our results are consistent with a published study of 700 acute pain patients treated with multiple doses of medications. In that study, the difference in the average pain intensity results between baseline pain groups was also substantially reduced by the calculation of the percent change 16. In this study the authors reported a 10% difference in the level of change over the range of baseline pain scores even when analyzed using a percentage change in pain intensity related to differences in study design.
The most likely explanation for our findings is that the calculation of the PID as a percentage change brings this measure of in pain intensity in line with the patient’s global report of improvement, as represented here by the PR-VRS and GMP scores. This supports the concept that patients use the PI-NRS as a percentage scale. As a result, in situations where baseline pain is variable cross patients, failing to calculate treatment effects as a percent change consistently across the range of baseline values may obscure true differences in the group treatment effects within a study and complicate comparisons across studies. For example, in the published study of chronic pain data (see page 11), the average baseline pain intensity in patients ranged from 6.2 in one study to 7.0 in another 13. This difference in baseline value (7.0−6.2=0.8) at the start of the two studies could potentially result in a 13% variation (0.8/6.2) in the size of the efficacy outcome reported by these two studies based only on the difference in patient reported baseline pain. Since the range of the baseline pain intensity levels cannot be known prior to conducting a study, the percent PID appears to be a more appropriate a priori analysis decision as it provides a more consistent value across all initial pain values.
In considering the use of a percent change as the primary outcome for the analysis of group differences, there are additional statistical issues to consider. Vickers, et al have shown that in the analysis of a truly normal data set the calculation of a percentage change may not preserve the normality of the data and may have less statistical power17. However, this assumes that a constant absolute change is of equal importance across all levels of the scale, and the reduction of statistical power depends on the correlation between the baseline and final pain values. The primary finding of our study is that change in pain has a better association with clinically relevant levels of global measures, when it is considered as a proportion. In his article, Vickers states that the difference in statistical power will decrease if the treatment effect is proportional, as is supported by our data. Therefore, the cost in statistical efficiency in a parametric analysis is not as large for proportional data. In addition, since nonparametric approaches to the analysis of pain data are preferred by several other authors 18,19, additional work will be necessary to establish if the cost in statistical efficiency of the use of percent change rather than absolute change is of concern. Even if a small cost in efficiency remains, our data support the clinical relevance of the use of a proportion in the analysis of change in pain intensity, which is an important consideration in making an appropriate choice for the primary analysis of clinical trial data.
Other precedents also exist for examining the percentage changes as a preferred method for defining clinically important differences for symptomatic conditions such as pain 20–23. For example, in the comprehensive structure for arthritis clinical trials developed by the Outcome Measures in Rheumatology Clinical Trials (OMERACT) group, experts derived levels of clinically important improvement from treatment, all of which are expressed as percent change 24. The percentage change is now endorsed by the American Rheumatology Association as the standard criterion, and is identified as an acceptable methodology in Food and Drug Administration guidelines for the development of new arthritis products 25. From the work of Moore and McQuay using meta-analysis to combine outcomes from smaller clinical trials, a 50% cut-off point for the percent maximum total pain relief was established as the point representing a clinically important change, reasoning that it “is a simple clinical endpoint… easily understood by professionals and patients” 26. Other investigators have employed percent changes in outcomes as the means for defining important and clinically meaningful treatment differences in analgesic trials 27–29.
Relationship of GMP and PR-VRS
The second important finding of our study is that a direct verbal measure of pain relief in short term pain treatment trials is inherently consistent with the a global outcome. The remarkably stability of the PR-VRS measurement scale over time and when compared to the GMP score supports the concept that the GMP and the PR-VRS appear to be measuring a similar patient construct in the cancer breakthrough pain setting. In studies where these endpoints may have been used as a primary outcome for pain treatment efficacy, the consistency of these findings with the percent PID should allow a better comparison of results for meta-analyses and systematic reviews of acute and breakthrough pain 5,30–35. However, caution should be taken in extending these findings to chronic pain studies where there is evidence that pain relief measures are associated more consistently with change in mood than changes in pain intensity 36.
Limitations
Although our findings are consistent with other studies, the potential limitations of this study must also be considered. First, is the potential generalizability issue to other types of rapid onset or breakthrough pain clinical studies. Cancer-related BTP is generally a self-limiting form of acute pain. It is possible that the response to breakthrough pain in non-cancer patients may be slightly different. In addition this population was mostly Caucasian, and testing in other populations would be appropriate. Third, the global measures used here are different than other studies. While a strength, it also is possible that other global measures may provide slightly different results. The limitations on the availability of other clinically relevant factors that can affect the perception and report of pain, such as affect, mood, expectations, and learning frequently, which are known to complicate the interpretation of the results of clinical trials of pain therapies 37,38, prevent our examining their effect on the relationship of pain intensity and global outcome. However, comparisons in our study were among different scales used by the same patients, and thus should not be affected by patient factors unless we presume that different measures are differentially affected in the same patient.
It is also important to acknowledge that the calculation of the percent change requires some practical consideration. Clearly, any formula requiring a division will not have a value when dividing by zero. Although true, this is not a major issue in most pain clinical trials, since patients have to have some pain in order to participate. In addition, the number of values a percent change can take gets smaller as the baseline is smaller. Again, this is usually not a major issue since entry criteria in a pain study is usually having enough pain (often ≥ 4/10) to warrant treatment. Lastly, it should be apparent that percentage up and percentage down are different. For example going from six to four is a decrease of 33%, while going from four to six is an increase of 50%. While this can be handled statistically, by dividing by the maximum of the baseline and final pain value, the appropriateness of this approach has not been adequately tested. Since most studies are conducted to evaluate therapies that improve pain, the majority of patients ending up at lower pain levels than baseline. For the comparison of two treatment groups, we would not expect the effect of such calculation issues to be different between treatment groups.
Ideally, the analytical techniques presented here will need to be tested using data sets from other populations of patients using different analgesics to verify that percent changes in pain intensity remain most consistent in other situations as well. However, since our findings are consistent with our previous analysis of a sample of patients with five different chronic pain syndromes 13, we have more confidence that replicating our procedures in data from other studies will demonstrate similar results.
Conclusion
We have reanalyzed data from the multiepisode titration phase of a clinical trial of breakthrough pain in cancer patients observing that a percent change pain intensity is better associated with PR-VRS and GMP, and may provide a more consistent representation of the patient’s response to treatment over time than the raw pain intensity difference. Although the choice of outcome measures and analyses for future clinical trials will depend on the study question and study design, the use of a percent change may provide as a more standardized approach to the evaluation and interpretation of clinical trials for pain therapies. A more standard approach may improve our ability to compare results across trials and in the evaluation of differences in the pain experiences between different populations such as the report of pain by males and females, and in different cultures 39–42. The consistency of the reported changes in the percent pain intensity with the reported level of clinical benefit that was demonstrated across all demographic factors suggests a way to handle the interperson differences in numeric pain measures to provide more consistent results across clinical studies.
Acknowledgments
Support Statement: This study was supported in part by grants from Cephalon, Inc., West Chester, Pennsylvania and The National Institutes of Health - R01 CA73797, Bethesda, Maryland.
Footnotes
Conflict of Interest: The laboratory has also received research funds from Pfizer, Inc., New York, New York; Endo Pharmaceuticals, Chadds Ford, Pennsylvania; Pharmacia, Kalamazoo, Michigan; Eli Lilly, Indianapolis, Indiana, and Glaxo Smith Kline, Research Triangle Park, North Carolina.
References
- 1.Bromm B. The measurement of pain in man. In: Bromm B, editor. Pain Measurement in Man. Amsterdam: Elsevier Science Publishers; 1984. pp. 3–13. [Google Scholar]
- 2.Katz J, Melzack R. Measurement of pain. Surg Clin North Am. 1999;79:231–52. doi: 10.1016/s0039-6109(05)70381-9. [DOI] [PubMed] [Google Scholar]
- 3.Farrar JT, Dworkin RH, Max MB. Use of the Cumulative Proportion of Responders Analysis (CPRA) graph to present pain data over a range of cut-off points: Making clinical trial data more understandable. J Pain Symptom Manage. 2006;30:369–77. doi: 10.1016/j.jpainsymman.2005.08.018. [DOI] [PubMed] [Google Scholar]
- 4.Donaldson G. Patient-reported outcomes and the mandate of measurement. Qual Life Res. 2008;17:1303–13. doi: 10.1007/s11136-008-9408-4. [DOI] [PubMed] [Google Scholar]
- 5.Chapman CR, Dunbar PJ. Measurement in pain therapy: Is pain relief really the endpoint? Curr Opin Anaesthesiol. 1998;11:533–7. doi: 10.1097/00001503-199810000-00015. [DOI] [PubMed] [Google Scholar]
- 6.Jensen MP. Using pain quality assessment measures for selecting analgesic agents. Clin J Pain. 2006;22:S9–13. doi: 10.1097/01.ajp.0000193829.45571.4f. [DOI] [PubMed] [Google Scholar]
- 7.Jensen MP, Martin SA, Cheung R. The meaning of pain relief in a clinical trial. J Pain. 2005;6:400–6. doi: 10.1016/j.jpain.2005.01.360. [DOI] [PubMed] [Google Scholar]
- 8.Tolle T, Freynhagen R, Versavel M, Trostmann U, Young JP., Jr Pregabalin for relief of neuropathic pain associated with diabetic neuropathy: A randomized, double-blind study. Eur J Pain. 2008;12:203–13. doi: 10.1016/j.ejpain.2007.05.003. [DOI] [PubMed] [Google Scholar]
- 9.Dworkin RH, Turk DC, Wyrwich KW, Beaton D, Cleeland CS, Farrar JT, Haythornthwaite JA, Jensen MP, Kerns RD, Ader DN, Brandenburg N, Burke LB, Cella D, Chandler J, Cowan P, Dimitrova R, Dionne R, Hertz S, Jadad AR, Katz NP, Kehlet H, Kramer LD, Manning DC, McCormick C, McDermott MP, McQuay HJ, Patel S, Porter L, Quessy S, Rappaport BA, Rauschkolb C, Revicki DA, Rothman M, Schmader KE, Stacey BR, Stauffer JW, von Stein T, White RE, Witter J, Zavisic S, Dworkin RH, Turk DC, Wyrwich KW, Beaton D, Cleeland CS, Farrar JT, Haythornthwaite JA, Jensen MP, Kerns RD, Ader DN, Brandenburg N, Burke LB, Cella D, Chandler J, Cowan P, Dimitrova R, Dionne R, Hertz S, Jadad AR, Katz NP, Kehlet H, Kramer LD, Manning DC, McCormick C, McDermott MP, McQuay HJ, Patel S, Porter L, Quessy S, Rappaport BA, Rauschkolb C, Revicki DA, Rothman M, Schmader KE, Stacey BR, Stauffer JW, von Stein T, White RE, Witter J, Zavisic S. Interpreting the clinical importance of treatment outcomes in chronic pain clinical trials: IMMPACT recommendations. J Pain. 2008;9:105–21. doi: 10.1016/j.jpain.2007.09.005. [DOI] [PubMed] [Google Scholar]
- 10.Turk DC, Dworkin RH, Revicki D, Harding G, Burke LB, Cella D, Cleeland CS, Cowan P, Farrar JT, Hertz S, Max MB, Rappaport BA, Turk DC, Dworkin RH, Revicki D, Harding G, Burke LB, Cella D, Cleeland CS, Cowan P, Farrar JT, Hertz S, Max MB, Rappaport BA. Identifying important outcome domains for chronic pain clinical trials: An IMMPACT survey of people with pain. Pain. 2008;137:276–85. doi: 10.1016/j.pain.2007.09.002. [DOI] [PubMed] [Google Scholar]
- 11.Cleeland CS, Ryan KM. Pain assessment: Global use of the Brief Pain Inventory. Ann Acad Med Singapore. 1994;23:129–38. [PubMed] [Google Scholar]
- 12.Keller S, Bann CM, Dodd SL, Schein J, Mendoza TR, Cleeland CS, Keller S, Bann CM, Dodd SL, Schein J, Mendoza TR, Cleeland CS. Validity of the brief pain inventory for use in documenting the outcomes of patients with noncancer pain. Clin J Pain. 2004;20:309–18. doi: 10.1097/00002508-200409000-00005. [DOI] [PubMed] [Google Scholar]
- 13.Farrar JT, Young JP, Jr, LaMoreaux L, Werth JL, Poole RM. Clinical importance of changes in chronic pain intensity measured on an 11-point numerical pain rating scale. Pain. 2001;94:149–58. doi: 10.1016/S0304-3959(01)00349-9. [DOI] [PubMed] [Google Scholar]
- 14.Coluzzi PH, Schwartzberg L, Conroy JD, Charapata S, Gay M, Busch MA, Chavez J, Ashley J, Lebo D, McCracken M, Portenoy RK. Breakthrough cancer pain: A randomized trial comparing oral transmucosal fentanyl citrate (OTFC) and morphine sulfate immediate release (MSIR) Pain. 2001;91:123–30. doi: 10.1016/s0304-3959(00)00427-9. [DOI] [PubMed] [Google Scholar]
- 15.Lichtor JL, Sevarino FB, Joshi GP, Busch MA, Nordbrock E, Ginsberg B. The relative potency of oral transmucosal fentanyl citrate compared with intravenous morphine in the treatment of moderate to severe postoperative pain. Anesth Analg. 1999;89:732–8. doi: 10.1097/00000539-199909000-00038. [DOI] [PubMed] [Google Scholar]
- 16.Cepeda MS, Africano JM, Polo R, Alcala R, Carr DB. What decline in pain intensity is meaningful to patients with acute pain? Pain. 2003;105:151–7. doi: 10.1016/s0304-3959(03)00176-3. [DOI] [PubMed] [Google Scholar]
- 17.Vickers AJ. The use of percentage change from baseline as an outcome in a controlled trial is statistically inefficient: A simulation study. BMC Med Res Methodol. 2001;1:6. doi: 10.1186/1471-2288-1-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Berger VW, Lunneborg CE, Ernst MD, Levine JG. Parametric analyses in randomized clinical trials. J Mod Appl Stat Methods. 2002;1:74–82. [Google Scholar]
- 19.Svensson E. Guidelines to statistical evaluation of data from rating scales and questionnaires. J Rehabil Med. 2001;33:47–8. doi: 10.1080/165019701300006542. [DOI] [PubMed] [Google Scholar]
- 20.Beecher HK. Measurement of subjective responses. New York: Oxford University Press; 1959. [Google Scholar]
- 21.Houde RW. Methods for measuring clinical pain in humans. Acta Anaesthesiol Scand Suppl. 1982;74:25–9. doi: 10.1111/j.1399-6576.1982.tb01840.x. [DOI] [PubMed] [Google Scholar]
- 22.Lasagna L. The clinical measurement of pain. Ann N Y Acad Sci. 1960;86:28–37. doi: 10.1111/j.1749-6632.1960.tb42788.x. [DOI] [PubMed] [Google Scholar]
- 23.Turk DC, Rudy TE, Sorkin BA. Neglected topics in chronic pain treatment outcome studies: Determination of success. Pain. 1993;53:3–16. doi: 10.1016/0304-3959(93)90049-U. [DOI] [PubMed] [Google Scholar]
- 24.Goldsmith CH, Boers M, Bombardier C, Tugwell P. Criteria for clinically important changes in outcomes: Development, scoring and evaluation of rheumatoid arthritis patient and trial profiles. OMERACT Committee. J Rheumatol. 1993;20:561–5. [PubMed] [Google Scholar]
- 25.FDA Guidance for Industry. Clinical Development Programs for Drugs, Devices, and Biological Products for the Treatment of Rheumatoid Arthritis (RA) Rockville, MD: Food and Drug Administration; 1999. [Google Scholar]
- 26.Moore A, McQuay H, Gavaghan D. Deriving dichotomous outcome measures from continuous data in randomised controlled trials of analgesics. Pain. 1996;66:229–37. doi: 10.1016/0304-3959(96)03032-1. [DOI] [PubMed] [Google Scholar]
- 27.Rowbotham MC, Reisner LA, Davies PS, Fields HL. Treatment response in antidepressant-naive postherpetic neuralgia patients: Double-blind, randomized trial. J Pain. 2005;6:741–6. doi: 10.1016/j.jpain.2005.07.001. [DOI] [PubMed] [Google Scholar]
- 28.Simpson DM, Brown S, Tobias J. Controlled trial of high-concentration capsaicin patch for treatment of painful HIV neuropathy. Neurology. 2008;70:2305–13. doi: 10.1212/01.wnl.0000314647.35825.9c. [DOI] [PubMed] [Google Scholar]
- 29.Wu CL, Agarwal S, Tella PK, Klick B, Clark MR, Haythornthwaite JA, Max MB, Raja SN. Morphine versus mexiletine for treatment of postamputation pain: A randomized, placebo-controlled, crossover trial. Anesthesiology. 2008;109:289–96. doi: 10.1097/ALN.0b013e31817f4523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lin PL, Fan SZ, Huang CH, Huang HH, Tsai MC, Lin CJ, Sun WZ. Analgesic effect of lidocaine patch 5% in the treatment of acute herpes zoster: A double-blind and vehicle-controlled study. Reg Anesth Pain Med. 2008;33:320–5. doi: 10.1016/j.rapm.2007.02.015. [DOI] [PubMed] [Google Scholar]
- 31.Moore RA, Moore OA, Derry S, McQuay HJ. Numbers needed to treat calculated from responder rates give a better indication of efficacy in osteoarthritis trials than mean pain scores. Arthritis Res Ther. 2008;10:R39. doi: 10.1186/ar2394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Stacey BR, Swift JN. Pregabalin for neuropathic pain based on recent clinical trials. Curr Pain Headache Rep. 2006;10:179–84. doi: 10.1007/s11916-006-0043-x. [DOI] [PubMed] [Google Scholar]
- 33.Stegmann JU, Weber H, Steup A, Okamoto A, Upmalis D, Daniels S. The efficacy and tolerability of multiple-dose tapentadol immediate release for the relief of acute pain following orthopedic (bunionectomy) surgery. Curr Med Res Opin. 2008;10:10. doi: 10.1185/03007990802448056. [DOI] [PubMed] [Google Scholar]
- 34.Sultan A, Gaskell H, Derry S, Moore RA. Duloxetine for painful diabetic neuropathy and fibromyalgia pain: Systematic review of randomised trials. BMC Neurol. 2008;8:29. doi: 10.1186/1471-2377-8-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Viscusi ER, Martin G, Hartrick CT, Singla N, Manvelian G. Forty-eight hours of postoperative pain relief after total hip arthroplasty with a novel, extended-release epidural morphine formulation. Anesthesiology. 2005;102:1014–22. doi: 10.1097/00000542-200505000-00022. [DOI] [PubMed] [Google Scholar]
- 36.Fishman B, Pasternak S, Wallenstein SL, Houde RW, Holland JC, Foley KM. The Memorial Pain Assessment Card. A valid instrument for the evaluation of cancer pain. Cancer. 1987;60:1151–8. doi: 10.1002/1097-0142(19870901)60:5<1151::aid-cncr2820600538>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- 37.Breivik H, Borchgrevink PC, Allen SM, Rosseland LA, Romundstad L, Hals EK, Kvarstein G, Stubhaug A. Assessment of pain. Br J Anaesth. 2008;101:17–24. doi: 10.1093/bja/aen103. [DOI] [PubMed] [Google Scholar]
- 38.Fishbain D. Evidence-based data on pain relief with antidepressants. Ann Med. 2000;32:305–16. doi: 10.3109/07853890008995932. [DOI] [PubMed] [Google Scholar]
- 39.Aubrun F, Salvi N, Coriat P, Riou B, Aubrun F, Salvi N, Coriat P, Riou B. Sex- and age-related differences in morphine requirements for postoperative pain relief. Anesthesiology. 2005;103:156–60. doi: 10.1097/00000542-200507000-00023. [DOI] [PubMed] [Google Scholar]
- 40.Edrington JM, Paul S, Dodd M, West C, Facione N, Tripathy D, Koo P, Schumacher K, Miaskowski C, Edrington JM, Paul S, Dodd M, West C, Facione N, Tripathy D, Koo P, Schumacher K, Miaskowski C. No evidence for sex differences in the severity and treatment of cancer pain. J Pain Symptom Manage. 2004;28:225–32. doi: 10.1016/j.jpainsymman.2003.12.013. [DOI] [PubMed] [Google Scholar]
- 41.Hobara M, Hobara M. Beliefs about appropriate pain behavior: Cross-cultural and sex differences between Japanese and Euro-Americans. Eur J Pain. 2005;9:389–93. doi: 10.1016/j.ejpain.2004.09.006. [DOI] [PubMed] [Google Scholar]
- 42.Wijnhoven HA, de Vet HC, Picavet HS, Wijnhoven HAH, de Vet HCW, Picavet HSJ. Explaining sex differences in chronic musculoskeletal pain in a general population. Pain. 2006;124:158–66. doi: 10.1016/j.pain.2006.04.012. [DOI] [PubMed] [Google Scholar]