Abstract
Much research is focused on developing novel drugs to improve memory. In particular, psychostimulants have been shown to enhance memory and have a long history of safe use in humans. In prior work, we have shown that very low doses of amphetamine administered before training on a Pavlovian fear-conditioning task can dramatically facilitate the acquisition of cued fear. The current experiment sought to expand these findings to the extinction of cued fear, a well-known paradigm with therapeutic implications for learned phobias and post-traumatic stress disorder. If extinction reflects new learning, one might expect drugs that enhance the acquisition of cued fear to also enhance the extinction of cued fear. This experiment examined whether 0.005 or 0.05 mg/kg of d-amphetamine (therapeutic doses shown to enhance acquisition) also enhance the extinction of cued fear. Contrary to our hypothesis, amphetamine did not accelerate extinction. Thus, at doses that enhance acquisition of conditioned fear, amphetamine does not appear to enhance extinction.
Keywords: Amygdala, Freezing, Stimulants, Classical Conditioning, Mice
A large body of evidence suggests that psychostimulants can enhance learning and memory in both humans and rodents [6, 21, 30, 31, 33]. One such psychostimulant is amphetamine, a drug currently used to treat attention deficit hyperactivity disorder (ADHD; e.g. Adderall®) [1]. Our laboratory has previously found [37] that ultra low doses (0.005 and 0.05 mg/kg) of amphetamine, similar to the therapeutic doses for ADHD, administered to mice during training, dramatically enhance cued-fear memory when subjects are tested off-drug. It is clear that amphetamine can enhance the acquisition of aversive memories, but it is unclear whether amphetamine can also enhance the extinction of conditioned fear.
In Pavlovian fear conditioning, an initially neutral stimulus (the conditioned stimulus, CS, e.g. a tone) is paired with an aversive stimulus (the unconditioned stimulus, US, e.g. a footshock). Following repeated CS-US pairings, the CS alone can elicit fear in a subject. In rodents, freezing, or the absence of all movement with the exception of respiration, is often the measure of conditioned fear [2, 12]. The neurobiology underlying conditioned freezing is well understood; acquisition of cued fear depends critically on the convergence of CS and US information in the basolateral amygdala [20, 28]. This CS-US association is not necessarily permanent, however. Repeated presentations of the CS in the absence of the US lead to extinction of conditioned fear, evidenced by decreased freezing in response to the CS alone.
Extinction is thought to reflect new, inhibitory learning [24], whereby extinction training encodes a new memory of the CS that then competes with the original memory of the CS. Unlike acquisition of cued fear, the neural mechanisms underlying extinction are still poorly understood. For example, extinction seems to depend on the medial prefrontal cortex (which is not essential for fear acquisition) [22, 25], as well as the amygdala [5, 11].
Pavlovian fear conditioning can serve as a model for both the etiology and treatment of phobia because phobias, or maladaptive fear responses to conditioned stimuli [36], are frequently treated using extinction therapy [13, 14]. Extinction, however, is a relatively weak and unstable form of learning, so considerable research has focused on identifying pharmacological agents, which, if given during extinction therapy would strengthen and stabilize the reduction of fear [27, 35]. Therefore, if extinction reflects new, inhibitory learning, it is possible that drugs that enhance fear acquisition will also facilitate the extinction of fear memory. This study examined whether extinction could be facilitated using d-amphetamine, a psychostimulant drug previously shown to enhance acquisition of cued fear [37].
The effects of amphetamine on the extinction of conditioned freezing have only been examined in one other study. Mueller and colleagues [23] administered 1.0 mg/kg of amphetamine during extinction training. They found that amphetamine decreased freezing relative to saline controls during extinction training, but this effect was not seen when tested off-drug. Thus, they attributed the reduction in freezing to amphetamine-induced locomotor hyperactivity rather than enhanced extinction retention. Mueller’s results are not surprising in light of our recent findings, which found evidence for hyperactivity and no evidence of memory enhancement in animals administered 1 mg/kg d-amphetamine [37]. Only ultra-low doses of amphetamine (0.005-0.05 mg/kg) administered pre-training enhanced cued fear acquisition. Thus, these ultra-low doses of amphetamine are more likely than the moderate dose to enhance the extinction of Pavlovian fear conditioning. Therefore, we administered 0.005 and 0.05 mg/kg amphetamine during extinction training and found that neither dose altered the extinction of Pavlovian fear.
Fifty-two C57B6/J inbred mice from Jackson Laboratory (West Sacramento, CA) were used in approximately equal numbers of males and females, balanced across groups. Mice were weaned at 3 weeks of age and were group housed (2-5 mice per cage) with continuous access to food and water. Mice were at least 10 weeks old before testing and subjects were handled for 5 days prior to training. The vivarium was maintained on a 14:10 light:dark schedule, and all testing was performed during the light phase of the cycle. All animal care and testing procedures were approved by the UCSD IACUC and were in accordance with the National Research Council Guide for the Care and Use of Laboratory Animals.
Mice underwent acquisition training (tone-shock pairings) for 1 day, off-drug, followed by 6 days of extinction trials (tone-alone presentations) under saline or amphetamine conditions. One final day of extinction was conducted off-drug. Three to four mice were tested concurrently, in individual conditioning chambers housed in a windowless room. Conditioning chambers were setup as described previously [29, 37]. Each conditioning chamber (32 cm × 25 cm × 25 cm) was located within a sound-attenuating chamber (63.5 cm × 35.5 cm × 76 cm) (Med-Associates Inc., St. Albans, VT) and equipped with a speaker in the sidewall. During acquisition training, the context consisted of a stainless steel grid floor (36 rods, each rod 2 mm in diameter, 8 mm center to center; Med-Associates Inc., St. Albans, VT) and a stainless steel drop pan. The sidewalls were white acrylic, and the front wall was clear to allow for viewing. Between each trial, the chambers were cleaned and scented with 7% isopropyl alcohol to provide a background odor. Ventilation fans provided background noise (65 dB). Each sound-attenuating chamber was equipped with an overhead LED light source, providing white and near-infrared light. The mice were continuously observed by a wall-mounted IEEE 1394 progressive scan video camera with a visible light filter (VID-CAM-MONO-2A; Med-Associates Inc., St. Albans, VT) connected to a computer in an adjacent room. Each chamber was connected to a solid-state scrambler, providing AC constant current shock, and an audio stimulus generator, controlled via an interface connected to a Windows computer running Video Freeze (Med-Associates, Inc., St. Albans, VT), a program designed for the automated assessment of freezing and locomotor activity. In results that will be published more fully elsewhere, computer and human scored data had a correlation of 0.971 and a fit of computer = -.007 + .974 × human (for more detail on this calculation, see [3]).
The conditioning context was altered along several dimensions for the extinction trials. White acrylic sheets were placed over the grid floors and a black plastic, triangular tent (23 cm, each side), translucent to near infrared light, was placed inside each box. Only near-infrared light was used, creating a dark environment visible only to the video camera. Between extinction trials, the chambers were cleaned and scented with a 5% vinegar solution.
Acquisition training was conducted off-drug and consisted of a 2-min baseline activity period, followed by 9 tone-shock pairings, each separated by 20-s. During each tone-shock pairing, a 10-s tone (conditioned stimulus: 2.8 kHz, 90 dB, A scale) was presented and co-terminated with a scrambled footshock (unconditioned stimulus: 2-s, 0.75 mA, AC constant current) delivered through the floor of the cages. Freezing behavior, defined as the absence of all movement with the exception of respiration [12], was scored automatically using Video Freeze software (Med-Associates, Inc., St. Albans, VT). Mice were inside the fear-conditioning chambers for a total of 9-min before being returned to their home cages.
Twenty-four hours after training, mice began the first of 6 days of extinction trials in the alternate context described above, on-drug. Extinction consisted of a 1-min baseline, followed by 15 presentations of the training tone (10-s tone, 20-s interval between tones). Mice were removed from the chambers 30-s later and returned to their home cages. Freezing and activity were scored for the entire 9-min period during each extinction day. Drugs were administered intraperitoneally (i.p.) in a volume of 10 ml/kg. d-amphetamine hemisulfate (Sigma-Aldrich Co., St. Louis, MO, USA) was dissolved in 0.9% sodium chloride. Amphetamine injections (salt weight: 0.005 or 0.05 mg/kg) were given i.p. 15-min prior to extinction trials. Mice were randomly assigned to one of three groups indicating the amount of amphetamine administered: 0 mg/kg (saline control, n = 20), 0.005 mg/kg (n = 16), and 0.05 mg/kg amphetamine (n = 16). Doses were chosen based on a previous study of cued fear acquisition [37]. A single, additional day of extinction (Day 7) was conducted off-drug, to serve as a state-dependent control.
Figure 1 depicts each min of acquisition training, consisting of a 2-min baseline period, followed by 9 tone-shock pairings, and a 2.5-min post-shock period. There was a main effect for minute [F(8,392) = 66.1, p < 0.0001], with freezing increasing after the onset of the toneshock pairings. The animals were off-drug and no group differences [F(2, 49) = 0.819, p = 0.447] or group by minute interactions [F(2,49) = 0.388, p = 0.681] were observed. On the first day of extinction, baseline locomotor activity (measured in arbitrary units by an automated computer scoring system) did not differ between groups [F(2,49) = 0.156, p = 0.856], suggesting that the low doses of amphetamine did not influence locomotor activity (data not depicted; see also [37]).
Figure 1.
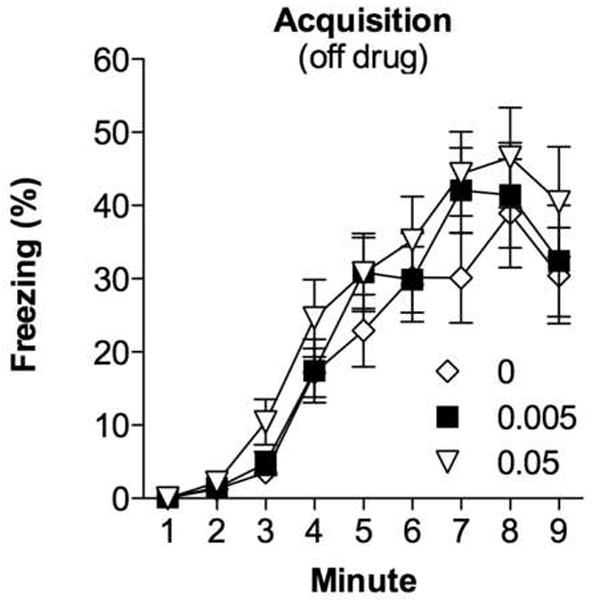
Percentage of time spent freezing during training. The shocks were presented starting at 2-min. All subjects were off-drug and all groups showed the same freezing behavior. Each group represents the dose (mg/kg) of amphetamine given prior to each extinction trial (not given during acquisition). Each point represents the M ± SEM.
As we were interested in examining between-trial extinction (extinction retention, [24]) and not within-trial extinction, we calculated the average freezing during the first 5 tones each day (Fig. 2a). Between-trial extinction seems more relevant to the treatment of learned fear because it is long lasting. We encountered moderately high baseline freezing during each extinction session (Fig. 2a, dashed lines), so we also measured tone freezing by subtracting baseline freezing from tone-elicited freezing (Fig. 2b). Subjects underwent 6 days of extinction trials (on-drug), and a main effect of day on average freezing over baseline during the first five tones was present [F(5, 245) = 15.890, p < 0.0001] (Fig. 2b). Cued fear decreased as the number of extinction trials increased. Thus, all of the groups showed cued fear extinction; freezing decreased by at least 50% between Day 1 and Day 6 of the extinction trials. No group differences in between-trial extinction [F(2, 49) = 0.223, p = 0.801] or group-by-day interaction [F(10, 245) = 0.498, p = 0.89] were observed. To purely measure extinction, we generated a difference score by subtracting average freezing during the first 5 tones of extinction Day 1 from average freezing during the first 5 tones of extinction Day 6 (Fig. 2c). Again, all of the groups showed extinction, as demonstrated by the negative difference scores (percent freezing was greater on Day 1 than on Day 6 for all groups). No group differences were observed [F(2, 49) = 0.280, p = 0.757]. Finally, although this experiment was not optimally designed to examine within-trial extinction because of the very close spacing of the tone presentations, no group differences were found in terms of short-term extinction during extinction Day 1 across the 15 tones [MANOVA, group by time interaction F(2,49) = 0.81, p = 0.738, or the difference between the average of tones 1-3 and 13-15, F(2,49) = 0.925, p = 0.404; data not depicted].
Figure 2.
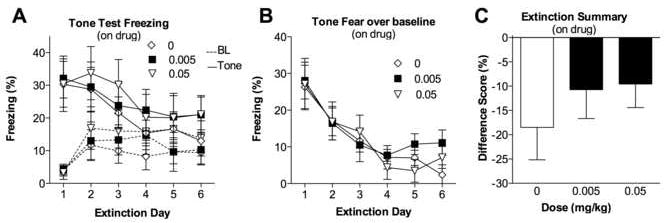
(A) Percentage of time spent freezing during baseline (BL) and the average of the first 5 tone presentations (Tone) for each of the six on-drug extinction trial days. Each group represents the dose (mg/kg) of amphetamine given prior to each extinction trial. (B) Percentage of time spent freezing during the first tone block (first 5 tone presentations averaged) over baseline for each extinction day. (C) Difference between the percentages time spent freezing over baseline during the first tone block (first 5 tone presentations) on extinction Day 6 and extinction Day 1. All groups show evidence of extinction. Amphetamine did not affect between-trial or overall extinction. Each point represents the M ± SEM.
We also examined locomotor activity during the extinction trials as an alternate index of fear [3]. As in our previous analyses, we examined activity across the first five tone presentations to compare between-trial, rather than within-trial, changes in activity. We generated a suppression ratio to control for baseline differences in subjects’ activity. The suppression ratio was defined as: (average activity during the first five tones)/(activity during the first five tones + activity during extinction trial baseline). Very low values indicate a high level of fear, 0.5 indicates no fear, and values greater than 0.5 can indicate conditioned safety [3, 4]. There was a significant effect of day on the activity suppression ratios [F(5, 245) = 27.102, p < 0.0001] (Fig. 3a), with suppression scores increasing (indicating decreased fear) as the number of extinction trials increased. By extinction Day 6, the suppression ratios were significantly larger (indicating less fear) than they had been on Day 1. No main effect of group [F(2, 49) = 0.337, p = 0.715], or day-by-group interaction [F(10, 245) = 1.09, p = 0.370] was observed.
Figure 3.
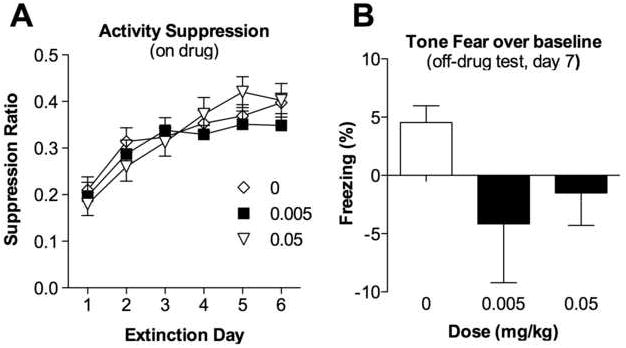
(A) Activity suppression for each of the six on-drug extinction trial days. Activity suppression was computed as suppression ratio = (average activity during the first five tones)/(activity during the first five tones + activity during baseline). Values close to 0.0 reflect high levels of fear; values close to 0.5 reflect no fear [4]. Amphetamine administered before each extinction trial did not affect activity suppression. Each point represents the M ± SEM. (B) Percentage time spent freezing during the state-dependent control test (extinction Day 7). All animals were off drug and there was no evidence of state-dependent memory. Each bar represents the M ± SEM.
On the last extinction day (Day 7), subjects underwent the same extinction protocol as Days 1 through 6, but were tested off-drug. This trial served as a state-dependent control. Regardless of treatment on prior extinction trial days, subjects displayed low levels of freezing when tested off-drug; tone elicited freezing (average of the 3 tones) minus baseline freezing (average of the first 2-min) is depicted (Fig. 3b). The extinction memory was retained and there was no evidence of state-dependent memory. No group differences in tone-elicited freezing were found [F(2, 49) = 0.007, p = 0.993]. These results provide no evidence that amphetamine altered the extinction of cued fear.
We examined the effects of amphetamine on the extinction of cued fear. As has been reported with higher doses [23], we found that low (therapeutic) doses of amphetamine do not facilitate extinction of conditioned fear. We hypothesized that because cued fear extinction involves new learning, ultra-low doses of amphetamine, previously shown to dramatically enhance cued fear acquisition [37], would also enhance extinction. Mueller et al. [23] failed to observe a facilitatory effect of amphetamine on cued-fear extinction, perhaps because they used a dose (1mg/kg) that does not affect cued-fear acquisition [37]. Our results, however, are not consistent with this hypothesis.
Prior research has also found that amphetamine does not affect extinction on other behavioral paradigms. For example, a moderately high dose of amphetamine (5 mg/kg) given during extinction of fear-potentiated startle in rats failed to alter extinction [8]. Also, amphetamine (1mg/kg) had no effect on extinction of conditioned approach [7, 10]. Amphetamine (5 mg/kg) has even been found to impair extinction of passive avoidance [15, 16]. As with Mueller et al. [23], however, all of these studies used moderate to high doses of amphetamine that induce locomotor hyperactivity and impair the acquisition of fear conditioning [37]. Thus, to address this confound we used very low doses of amphetamine that do not influence activity, but can enhance memory [37]. As expected, baseline activity measurements during the first day of extinction did not differ between the amphetamine and saline groups. Thus, amphetamine’s lack of effect on extinction in the current experiment cannot be attributed to amphetamine-induced alterations in locomotor activity.
One explanation for our finding is that the acquisition of aversive memories and their extinction reflect different types of new memory formation. Early evidence that N-methyl-D-aspartate (NMDA) receptors are essential for both acquisition and extinction of fear fostered enthusiasm that the mechanisms of acquisition and extinction may be similar [19, 34, 35]. However, more recent evidence suggests that the neural circuitry and pharmacology of fear acquisition and extinction are dissociable [for a review see 24, 26]. Li et al. [18] provide a model demonstrating how the amygdala could encode fear acquisition and extinction memories independently using discrete neural pathways. At the synaptic level, extinction, but not acquisition, depends on cannabinoid receptors [32]. At the systems level, extinction, but not acquisition, may depend on the medial prefrontal cortex [22, 25]. If the neural mechanisms were different, then a drug would not necessarily be expected to enhance both acquisition and extinction. Additionally, acquisition and extinction may have different dose-response curves for pharmacological manipulation, though this seems unlikely as 1.0 mg/kg [23], and now 0.005 and 0.05 mg/kg, amphetamine have been shown to have no effect on cued fear extinction.
Several limitations in this study need to be addressed. The mice showed somewhat low levels of freezing to the tone on the first day of extinction (about 30%, after correcting for baseline, for all groups). As a result, there may have been insufficient ability to detect subtle differences in extinction. The mice were trained in a context with a bright light and underwent extinction trials in the dark. As mice are nocturnal, their activity increases in the dark and freezing behavior to the tone may have been confounded by increased activity simply due to the darker environment. Despite this, mice showed robust between-trial extinction and there was ample opportunity to observe differences between saline and amphetamine-treated mice. To address these concerns, future studies will look at the effect of different conditioning parameters (e.g. increased shock intensity and/or a different number of tone-shock pairings), and extinction training in a bright context.
To conclude, amphetamine does not appear to be a suitable candidate for facilitating fear extinction. As neural mechanisms underlying extinction learning are identified, so are potential targets for pharmacological manipulation. Exposure therapy can successfully be augmented pharmacologically [27], and it would be of significant clinical value to continue searching for those drugs that may enhance extinction. Additionally, to further investigate the dissociation between fear acquisition and extinction learning, it would be useful to concurrently examine acquisition and extinction of fear with a variety of memory-enhancing drugs [9, 17, 29, 35, 37].
Acknowledgments
We thank Jennifer Sage and Tristan Shuman for helpful comments on the manuscript. We also thank Denise Cai for excellent data analysis assistance. These studies were supported by NSF Graduate Research Fellowship (SCW), NIH NRSA DA026259 (SCW), NIH grant DA020041 (SGA) and Hellman Fellowship (SGA).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Ahmann PA, Theye FW, Berg R, Linquist AJ, Van Erem AJ, Campbell LR. Placebo-controlled evaluation of amphetamine mixture-dextroamphetamine salts and amphetamine salts (Adderall): efficacy rate and side effects. Pediatrics. 2001;107:E10. doi: 10.1542/peds.107.1.e10. [DOI] [PubMed] [Google Scholar]
- 2.Anagnostaras SG, Gale GD, Fanselow MS. Hippocampus and contextual fear conditioning: recent controversies and advances. Hippocampus. 2001;11:8–17. doi: 10.1002/1098-1063(2001)11:1<8::AID-HIPO1015>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- 3.Anagnostaras SG, Josselyn SA, Frankland PW, Silva AJ. Computer-assisted behavioral assessment of Pavlovian fear conditioning in mice. Learn Mem. 2000;7:58–72. doi: 10.1101/lm.7.1.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Annau Z, Kamin LJ. The conditioned emotional response as a function of intensity of the US. J Comp Physiol Psychol. 1961;54:428–432. doi: 10.1037/h0042199. [DOI] [PubMed] [Google Scholar]
- 5.Barad M, Gean PW, Lutz B. The role of the amygdala in the extinction of conditioned fear. Biol Psychiatry. 2006;60:322–328. doi: 10.1016/j.biopsych.2006.05.029. [DOI] [PubMed] [Google Scholar]
- 6.Barch DM, Carter CS, Perlstein W, Baird J, Cohen JD, Schooler N. Increased stroop facilitation effects in schizophrenia are not due to increased automatic spreading activation. Schizophr Res. 1999;39:51–64. doi: 10.1016/s0920-9964(99)00025-0. [DOI] [PubMed] [Google Scholar]
- 7.Blaiss CA, Janak PH. Post-training, but not post-reactivation, administration of amphetamine and anisomycin modulates Pavlovian conditioned approach. Neurobiol Learn Mem. 2007;87:644–658. doi: 10.1016/j.nlm.2006.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Borowski TB, Kokkinidis L. The effects of cocaine, amphetamine, and the dopamine D1 receptor agonist SKF 38393 on fear extinction as measured with potentiated startle: implications for psychomotor stimulant psychosis. Behav Neurosci. 1998;112:952–965. doi: 10.1037//0735-7044.112.4.952. [DOI] [PubMed] [Google Scholar]
- 9.Chhatwal JP, Davis M, Maguschak KA, Ressler KJ. Enhancing cannabinoid neurotransmission augments the extinction of conditioned fear. Neuropsychopharmacology. 2005;30:516–524. doi: 10.1038/sj.npp.1300655. [DOI] [PubMed] [Google Scholar]
- 10.Dudderidge HJ, Gray JA. Joint effects of sodium amylobarbitone and amphetamine sulphate on resistance to extinction of a rewarded running response in the rat. Psychopharmacologia. 1974;35:365–370. doi: 10.1007/BF00429227. [DOI] [PubMed] [Google Scholar]
- 11.Falls WA, Miserendino MJ, Davis M. Extinction of fear-potentiated startle: blockade by infusion of an NMDA antagonist into the amygdala. J Neurosci. 1992;12:854–863. doi: 10.1523/JNEUROSCI.12-03-00854.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fanselow MS. Conditioned and unconditional components of post-shock freezing. Pavlov J Biol Sci. 1980;15:177–182. doi: 10.1007/BF03001163. [DOI] [PubMed] [Google Scholar]
- 13.Foa EB. Psychosocial treatment of posttraumatic stress disorder. J Clin Psychiatry. 2000;61(Suppl 5):43–48. discussion 49-51. [PubMed] [Google Scholar]
- 14.Garakani A, Mathew SJ, Charney DS. Neurobiology of anxiety disorders and implications for treatment. Mt Sinai J Med. 2006;73:941–949. [PubMed] [Google Scholar]
- 15.Kokkinidis L. The effects of chronic amphetamine administration on the acquisition and extinction of an active and passive avoidance response in mice. Pharmacol Biochem Behav. 1983;19:593–598. doi: 10.1016/0091-3057(83)90333-7. [DOI] [PubMed] [Google Scholar]
- 16.Kumar R. Extinction of fear. I. Effects of amylobarbitone and dexamphetamine given separately and in combination on fear and exploratory behaviour in rats. Psychopharmacologia. 1971;19:163–187. doi: 10.1007/BF00402640. [DOI] [PubMed] [Google Scholar]
- 17.Ledgerwood L, Richardson R, Cranney J. Effects of D-cycloserine on extinction of conditioned freezing. Behav Neurosci. 2003;117:341–349. doi: 10.1037/0735-7044.117.2.341. [DOI] [PubMed] [Google Scholar]
- 18.Li G, Nair SS, Quirk GJ. A biologically realistic network model of acquisition and extinction of conditioned fear associations in lateral amygdala neurons. J Neurophysiol. 2009;101:1629–1646. doi: 10.1152/jn.90765.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lu KT, Walker DL, Davis M. Mitogen-activated protein kinase cascade in the basolateral nucleus of amygdala is involved in extinction of fear-potentiated startle. J Neurosci. 2001;21:RC162. doi: 10.1523/JNEUROSCI.21-16-j0005.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Maren S, Aharonov G, Stote DL, Fanselow MS. N-methyl-D-aspartate receptors in the basolateral amygdala are required for both acquisition and expression of conditional fear in rats. Behav Neurosci. 1996;110:1365–1374. doi: 10.1037//0735-7044.110.6.1365. [DOI] [PubMed] [Google Scholar]
- 21.Martinez JL, Jr, Jensen RA, Messing RB, Vasquez BJ, Soumireu-Mourat B, Geddes D, Liang KC, McGaugh JL. Central and peripheral actions of amphetamine on memory storage. Brain Res. 1980;182:157–166. doi: 10.1016/0006-8993(80)90838-0. [DOI] [PubMed] [Google Scholar]
- 22.Milad MR, Quirk GJ. Neurons in medial prefrontal cortex signal memory for fear extinction. Nature. 2002;420:70–74. doi: 10.1038/nature01138. [DOI] [PubMed] [Google Scholar]
- 23.Mueller D, Olivera-Figueroa LA, Pine DS, Quirk GJ. The effects of yohimbine and amphetamine on fear expression and extinction in rats. Psychopharmacology (Berl) 2009 doi: 10.1007/s00213-009-1491-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Myers KM, Davis M. Mechanisms of fear extinction. Mol Psychiatry. 2007;12:120–150. doi: 10.1038/sj.mp.4001939. [DOI] [PubMed] [Google Scholar]
- 25.Quirk GJ, Garcia R, Gonzalez-Lima F. Prefrontal mechanisms in extinction of conditioned fear. Biol Psychiatry. 2006;60:337–343. doi: 10.1016/j.biopsych.2006.03.010. [DOI] [PubMed] [Google Scholar]
- 26.Quirk GJ, Mueller D. Neural mechanisms of extinction learning and retrieval. Neuropsychopharmacology. 2008;33:56–72. doi: 10.1038/sj.npp.1301555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ressler KJ, Rothbaum BO, Tannenbaum L, Anderson P, Graap K, Zimand E, Hodges L, Davis M. Cognitive enhancers as adjuncts to psychotherapy: use of D-cycloserine in phobic individuals to facilitate extinction of fear. Arch Gen Psychiatry. 2004;61:1136–1144. doi: 10.1001/archpsyc.61.11.1136. [DOI] [PubMed] [Google Scholar]
- 28.Rogan MT, Staubli UV, LeDoux JE. Fear conditioning induces associative long-term potentiation in the amygdala. Nature. 1997;390:604–607. doi: 10.1038/37601. [DOI] [PubMed] [Google Scholar]
- 29.Shuman T, Wood SC, Anagnostaras SG. Modafinil and memory: effects of modafinil on Morris water maze learning and Pavlovian fear conditioning. Behav Neurosci. 2009;123:257–266. doi: 10.1037/a0014366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Soetens E, Casaer S, D’Hooge R, Hueting JE. Effect of amphetamine on long-term retention of verbal material. Psychopharmacology (Berl) 1995;119:155–162. doi: 10.1007/BF02246156. [DOI] [PubMed] [Google Scholar]
- 31.Soetens E, D’Hooge R, Hueting JE. Amphetamine enhances human-memory consolidation. Neurosci Lett. 1993;161:9–12. doi: 10.1016/0304-3940(93)90127-7. [DOI] [PubMed] [Google Scholar]
- 32.Suzuki A, Josselyn SA, Frankland PW, Masushige S, Silva AJ, Kida S. Memory reconsolidation and extinction have distinct temporal and biochemical signatures. J Neurosci. 2004;24:4787–4795. doi: 10.1523/JNEUROSCI.5491-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Turner DC, Robbins TW, Clark L, Aron AR, Dowson J, Sahakian BJ. Cognitive enhancing effects of modafinil in healthy volunteers. Psychopharmacology (Berl) 2003;165:260–269. doi: 10.1007/s00213-002-1250-8. [DOI] [PubMed] [Google Scholar]
- 34.Walker DL, Davis M. The role of amygdala glutamate receptors in fear learning, fear-potentiated startle, and extinction. Pharmacol Biochem Behav. 2002;71:379–392. doi: 10.1016/s0091-3057(01)00698-0. [DOI] [PubMed] [Google Scholar]
- 35.Walker DL, Ressler KJ, Lu KT, Davis M. Facilitation of conditioned fear extinction by systemic administration or intra-amygdala infusions of D-cycloserine as assessed with fear-potentiated startle in rats. J Neurosci. 2002;22:2343–2351. doi: 10.1523/JNEUROSCI.22-06-02343.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Watson JB, Rayner R. Conditioned emotional reactions. 1920. Am Psychol. 2000;55:313–317. doi: 10.1037//0003-066x.55.3.313. [DOI] [PubMed] [Google Scholar]
- 37.Wood SC, Anagnostaras SG. Memory and psychostimulants: modulation of Pavlovian fear conditioning by amphetamine in C57BL/6 mice. Psychopharmacology (Berl) 2009;202:197–206. doi: 10.1007/s00213-008-1185-9. [DOI] [PMC free article] [PubMed] [Google Scholar]


