Abstract
Rationale and Objectives
With prescriptions of stimulants on the rise, it is important to examine the cognitive effects of low and moderate doses of stimulants, rather than only those typical of addicts.
Methods
The present study examined the effects a range of doses (0.005 – 8 mg/kg) of d-amphetamine sulfate on cued and contextual Pavlovian fear conditioning in mice.
Results
In agreement with previous research, subjects administered a moderately high dose of amphetamine (8 mg/kg) pre-training, typical of what addicts might take, displayed impaired memory when tested, off-drug. Alternately, subjects injected with a very low dose of amphetamine (0.005, 0.025, or 0.05 mg/kg), pre-training, similar to the therapeutic doses for attention deficit hyperactivity disorder, displayed enhanced memory when tested, off-drug. A control study showed these effects were not due to state-dependent learning.
Conclusions
Thus, dose is a critical determinant of the cognitive effects of psychostimulants.
Keywords: Amphetamine, Fear conditioning, Learning and memory, Mouse
Introduction
Amphetamine can be highly addictive, leading individuals to exhibit behaviors ranging from relatively minor cognitive impairments to severe psychotic symptoms. Methamphetamine abusers display worse performance on tests of word recall, perceptual speed, vocabulary, and abstract reasoning, compared with controls (Simon et al. 2002). After prolonged amphetamine abuse, addicts may also exhibit symptoms similar to schizophrenia, commonly referred to as amphetamine or stimulant psychosis (Harris and Batki 2000).
Conversely, amphetamine is also used to treat ailments such as narcolepsy and attention deficit hyperactivity disorder (ADHD). Amphetamine (e.g., Adderall®) improves ADHD symptoms for most affected children (Ahmann et al. 2001) and several related stimulants are similarly effective (e.g., methamphetamine, methylphenidate, atomoxetine, modafinil; Leonard et al. 2004).
While prescription stimulants containing amphetamine have proven beneficial to many people suffering from ADHD, excessive daytime sleepiness, and narcolepsy, off-label use of these drugs is a growing problem. The life-time prevalence of non-medical use of prescription stimulants in college students has been recently reported from 6.9% (McCabe et al. 2005) to 8.3% (Teter et al. 2006). These drugs tend to be regarded by the public as cognitive enhancers, presumably by promoting mental arousal or wakefulness. “Academic doping” is an emerging phenomenon in many educational settings (Butcher 2003). Stimulants also have documented benefits (and problems) in military use (Caldwell et al. 1995; Cornum 1994; Cornum et al. 1995). Indeed, a growing body of evidence demonstrates that healthy volunteers, not just those with ADHD, display cognitive benefits from low doses of stimulants (e.g., Barch and Carter 2005; Turner et al. 2003). These trends illuminate the importance of studying the effects of a variety of acute doses of amphetamine, rather than focusing on only the higher, chronic doses, typical of addicts. The current study examines the effects of a range of acute doses of d-amphetamine on a standard rodent learning and memory task, Pavlovian fear conditioning.
In Pavlovian fear conditioning, a discrete neutral stimulus (e.g., a tone) is paired with an aversive stimulus (e.g., a footshock). After training, when presented with the discrete neutral stimulus, alone, the subject exhibits fear. In addition, upon returning to the environmental chamber in which it had been trained, the subject also exhibits fear, a phenomenon known as context conditioning. A common measure of conditioned fear in rodents is freezing, or the absence of all movement, excluding respiration (Fanselow 1980).
The neurobiology of Pavlovian fear conditioning has been studied extensively. The dorsal hippocampus is critically involved in encoding memory of the context, in a time-graded manner (Anagnostaras et al. 2001; Anagnostaras et al. 1999a). Because of the efficiency of contextual fear conditioning, it has become a leading model of declarative memory in rats and mice (Anagnostaras et al. 2001; Anagnostaras et al. 2000). The amygdala, and specifically the basolateral/lateral complex of the amygdala, is involved in encoding an aversive association with both the context and tone (Fanselow and Gale 2003; Fanselow and Poulos 2005). Amphetamine has been shown to alter amygdalar activity in a number of ways, including potentiating the synaptic transmission between the amygdala and nucleus accumbens (Kessal et al. 2005). The hippocampus has also been implicated in amphetamine-induced locomotor activity, as lesions of the ventral hippocampus (VH) disrupt amphetamine-induced locomotion, and stimulation enhances it (White et al. 2006).
We previously found (Wood et al. 2007) that an acute dose of cocaine enhances learning on Pavlovian fear conditioning when given at a very low dose (0.1 mg/kg). Interestingly, we also found that a moderate dose of cocaine (15 mg/kg) produced a significant anterograde amnesia of fear conditioning. These effects were general to both contextual and cued fear. These data suggest that it is dose rather than abuse potential that is the critical determinant of whether a particular stimulant enhances or impairs memory. Moreover, one potential confound of the previous study is that cocaine produces local anesthesia, which could directly reduce fear conditioning by reducing the painfulness of the footshock. Therefore, in the present study, we examined the effects of amphetamine on conditioned fear in order to extend the findings to a widely prescribed stimulant as well as to control for local anesthesia. We also conducted a control study to address the issue of state-dependent effects on learning. We predicted that amphetamine would show the same pattern as cocaine, enhancing Pavlovian fear conditioning at low doses and interfering with fear conditioning at higher doses. The results of the current study are consistent with this hypothesis, showing enhanced learning during acquisition and recall of cued fear at low doses of amphetamine, and deficits in fear conditioning at higher doses. In addition, there was no evidence found to support the idea that state-dependent learning was a factor in the present results.
Materials and methods
Subjects
One-hundred-thirty-six (68 male, 68 female) C57BL6/NCrl (B6; Charles River Laboratories, San Diego, CA) inbred mice were used for Experiment 1 and 40 (19 male, 21 female) B6129SF1/J (H; Jackson Laboratory, West Sacramento, CA) hybrid mice were used for Experiment 2. Mice were weaned at 3 weeks of age and were group housed (2–5 mice per same sex cage) with continuous access to food and water. The animal colony was maintained on a 14:10 light/dark schedule, and all testing was performed during the light phase of the cycle. Mice were at least 8 weeks old before testing. All animal care and testing procedures were approved by the UCSD IACUC and were in accordance with the NIH “Principles of laboratory animal care.”
Apparatus
Conditioning context
Testing was performed in a windowless room. Background noise (65-dB) was provided by a HEPA air cleaner and white light was provided by two 100W bulbs. The mice were continuously observed by a wall-mounted color video camera which was connected to a computer and video equipment in an adjacent room. Three to four mice were tested concurrently in individual conditioning chambers. Each chamber (32 cm wide, 25 cm high, 25 cm deep) was equipped with a stainless steel grid floor (36 rods, each rod 2-mm diameter, 8-mm center to center) and a speaker in the side wall. The sidewalls were white acrylic and the front wall was clear to allow for viewing (Med-Associates Inc., St. Albans, VT). A stainless steel drop-pan, scented with 7% isopropyl alcohol to provide a background odor, was located beneath each chamber. Between tests, the conditioning contexts were cleaned with 7% isopropyl alcohol solution. Each chamber was connected to a solid-state scrambler, providing AC constant current shock, and an audio stimulus generator located in an adjacent room, controlled via an interface connected to a Windows computer running Med-PC (Med-Associates Inc., St Albans, VT). Automated assessment of freezing and activity was provided by custom designed software adapted from NIH Image running on an Apple Macintosh G4 (automated algorithm validated elsewhere; Anagnostaras et al., 2000).
Alternate context
Multiple (3–4) mice were tested concurrently for tone fear in a separate room, in individual boxes measuring 30 cm wide, 25 cm high, 24 cm deep, and equipped with a speaker in the side walls. A clear Plexiglas front wall allowed the mice to be continually observed, while the ceiling, floor, and three interior walls of the chamber were solid white. To create a space distinct from the training context, a white plastic, triangular tent was placed inside each box; each side of the triangle measured 23 cm. Between tests, the chambers were cleaned and scented with a 5% white vinegar solution. The room was lit with dim red light and an infrared video camera, connected to the Macintosh G4 described above, was used to score freezing.
Drugs
Drugs were administered intraperitoneally (i.p.) in a volume of 5 or 10 ml/kg. D-amphetamine sulfate (Sigma-Aldrich Co., St. Louis, MO) was dissolved in 0.9% physiological saline. Amphetamine injections (salt weight; 0.005, 0.025, 0.05, 0.5, 1, 2, 4, or 8 mg/kg) were given 15 min before introduction to the testing equipment.
Experiment 1 Behavior Measurement
In order to measure the effects of amphetamine on exploratory locomotor activity, baseline activity in the 2 min before the first tone-shock pairing on the training day was assessed by counting the number of cross-overs each subject performed (Maren et al. 1998). A single cross-over was defined as the movement of a subject’s entire body from one half of the box to the other. Videotapes of the conditioning sessions were observed using a standard VCR and monitor. In addition to number of cross-overs, exploratory activity was also measured using an automated, video-based activity measure (Anagnostaras et al. 2000).
To assess whether amphetamine disrupted shock reactivity, mouse activity burst displayed during the 2 s of shock exposure (Unconditioned Response to shock), as well as activity during the 2 s leading up to the shock were measured as velocity (cm/s) (Anagnostaras et al. 2000). Full-screen video of this time period was digitized at 10 Hz using NIH Image. X-Y coordinates were obtained from each frame for every mouse using the wand auto-measure tool; these coordinates were imported into Microsoft Excel. Distance traveled (measured in pixels) between successive frames was computed using the distance formula [√((xn – xn+1)2 + (yn – yn+1)2)]; these values were converted into real distance in centimeters using known landmark distances in the video frame. Distance was then converted into velocity (cm/s) by dividing by time.
Experimental Procedures
Experiment 1
Conditioning
Mice were injected with saline or amphetamine 15 min prior to training. Training consisted of a 2 min baseline period, followed by 3 tone-shock pairings, each separated by 30 s. A tone-shock pairing consisted of a 30 s tone (2.8 kHz, 85 dB, A Scale), with a scrambled, constant current AC footshock (0.75 mA) administered during the last 2 s of the tone. Immediate post-shock freezing was measured for another 5 min. Thus, mice were inside the fear conditioning chambers for a total of 10 min before being returned to their home cages.
Testing
Mice were returned to the conditioning context without drug 24 h after training and freezing was scored for 5 min. Mice were placed in the alternate context 48 h after training, also off-drug. Tone testing consisted of a 2 min baseline, followed by a continuous 3 min tone identical to the training tone. Freezing was scored for the entire 5 min period.
Experiment 2
Procedures for Experiment 2 were identical to Experiment 1, with the exception that amphetamine or saline injections were administered 15 min before testing, in addition to those administered before training.
Results
Experiment 1
Generalized Activity
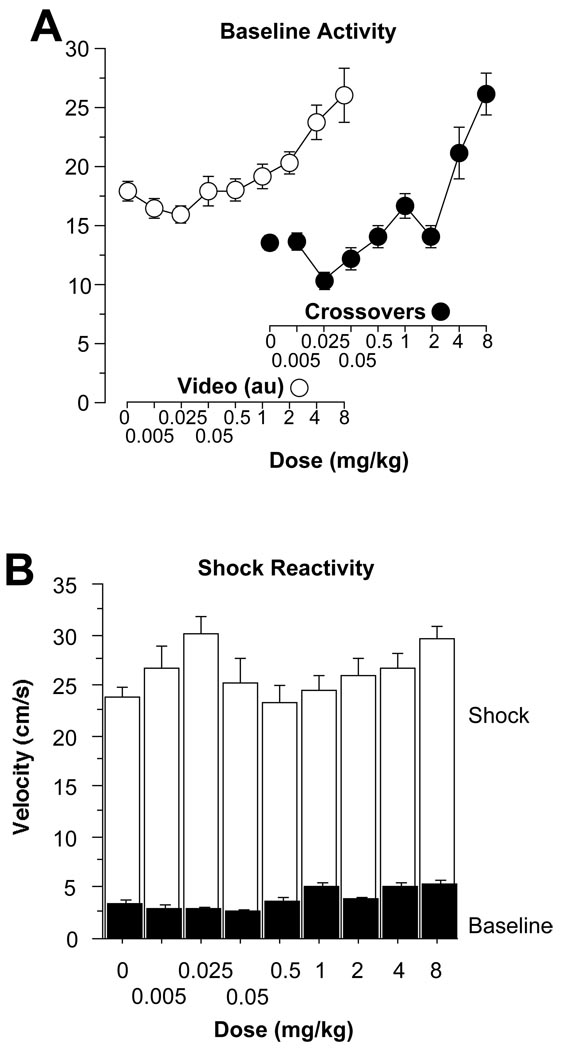
Amphetamine produced a dose-dependent increase in activity during the 2 min baseline period of training, measured both by an automated computer scoring system and by hand-counted cage cross-overs (Figure 1A). Group differences in cage cross-overs were found on an analysis of variance (ANOVA) [F(8,127) = 16.5, p < .0001]. Subjects administered 4–8 mg/kg amphetamine, pre-training, displayed significantly more cross-overs during baseline than the saline control group (Fisher’s protected least significant difference, PLSD, multiple post-hoc comparisons, p values < .01). No other doses differed significantly from saline controls (p values > .05). ANOVA demonstrated differences in the automated activity measure as well [F(8,127) = 7.5, p < .0001]. As with the cross-over measures, only mice given 4–8 mg/kg of amphetamine showed a significant difference in activity from saline controls (Fisher’s PLSD, p values < .01).
Figure 1.
(A) Baseline Activity. A dose-dependent increase in activity was observed during the 2 min baseline period, prior to the first tone-shock pairing (left, an automated video activity measure and right, full cage cross-overs; mean±SEM). (B) Shock Reactivity. All groups showed a significant increase in velocity (cm/s, mean±SEM) during the 2 s shock, compared with the 2 s baseline period leading up to the shock.
Activity Burst Velocity
A large difference in velocity was elicited by the first 2 s shock presentation (UR, unconditioned response), compared with the preceding 2 s, during which no shock was present (Figure 1B). A multivariate ANOVA revealed a significant effect of dose on velocity [F(8,127) = 2.3, p < .05], a significant effect of the shock [UR versus baseline; F(1,127) = 1687.5, p < .0001], along with a significant time period by dose interaction [F(8,127) = 2.5, p < .05], so the baseline and UR were considered separately. There were significant differences in the baseline velocity [F(8,127) = 5.8, p < .0001]. Post-hoc comparisons revealed similar differences in velocity as those seen in measurements of baseline activity, with subjects administered 1, 4, or 8 mg/kg amphetamine displaying increased velocity compared with subjects administered saline (Fisher’s PLSD, p values < .05). No other doses differed significantly in terms of baseline activity from saline. There were also significant differences during the UR [F(8,127) = 2.2, p < 0.05]. Only subjects given 0.025 or 8 mg/kg exhibited a larger UR to the shock compared to controls (p values < 0.05), but these differences were small and unrelated to the memory effects. Thus, amphetamine did not disrupt shock reactivity, and seemed to enhance the reactivity in certain cases.
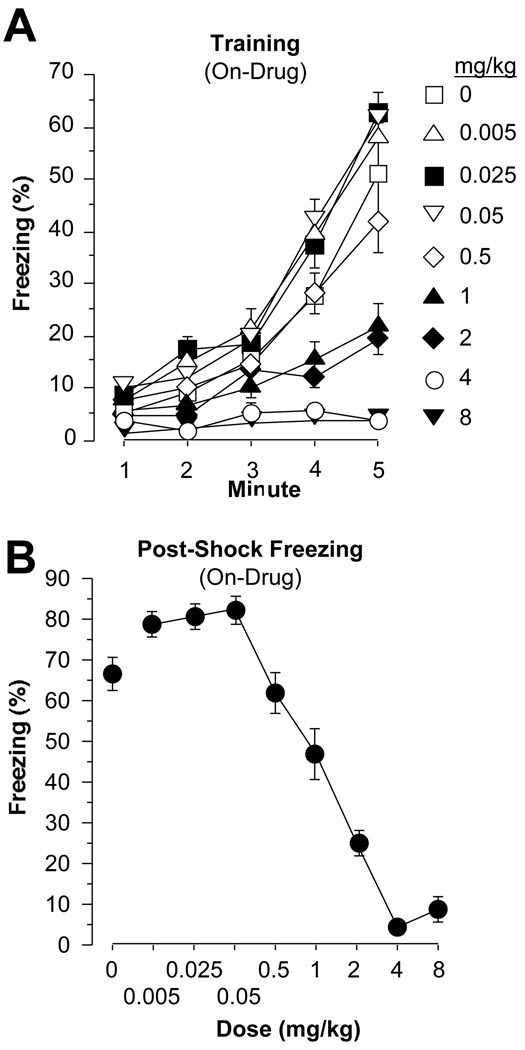
Training and Post-Shock Freezing
Subjects were inside the conditioning contexts on training day for a total of 10 min. Figure 2A depicts the first 5 min of training, consisting of a 2 min baseline period, followed by 3 tone-shock pairings during 3 min. A main effect for dose was present within the 5 min period [F(8,127) = 36.5, p < .0001], as was a main effect for minute [F(4,127) = 248.1, p < .0001], along with a significant dose by minute interaction [F(32,127) = 15.4, p < .0001]. Post-hoc comparisons revealed a dose-dependent effect of amphetamine on freezing, with subjects that had received 1, 2, 4, or 8 mg/kg freezing less than saline controls (Fisher’s PLSDs, p values < .0001). Interestingly, subjects that had been administered 0.005, 0.025, or 0.05 mg/kg amphetamine froze more than the saline control subjects (p values < .01). Subjects that received 0.5 mg/kg were not significantly different from saline controls (p > .05).
Figure 2.
(A) Training. Freezing (mean±SEM) was measured during all 5 min of training, including the 2 min baseline period and the 3 min period during which 3 tone-shock pairings were presented. Subjects on 1, 2, 4, or 8 mg/kg amphetamine froze less than saline control subjects, while subjects on 0.005, 0.025, or 0.05 mg/kg amphetamine froze more than controls. (B) Immediate Memory. Dose-dependent, post-training freezing was observed, with subjects on 0.005, 0.025, or 0.05 mg/kg amphetamine freezing more than controls, while those on 1, 2, 4, or 8 mg/kg amphetamine displayed less freezing than controls.
Immediate Memory
In minutes 6 through 10 of training, freezing was measured with no further presentation of tone or shock, as an index of immediate memory (Figure 2B). Dose-dependent differences in freezing were apparent [F(8,127) = 68.7, p < .0001]. Post-hoc tests revealed that subjects that had received 1, 2, 4, or 8 mg/kg amphetamine displayed less freezing than saline controls (Fisher’s PLSD, p values < .001). While these subjects were still on-drug, it is important to note that amphetamine’s locomotor effects could be influencing these results. Interestingly, however, subjects administered 0.005, 0.025 or 0.05, pre-training, froze more than saline controls (p values < .05). Subjects that received 0.5 mg/kg were not significantly different from saline controls (p > 0.05).
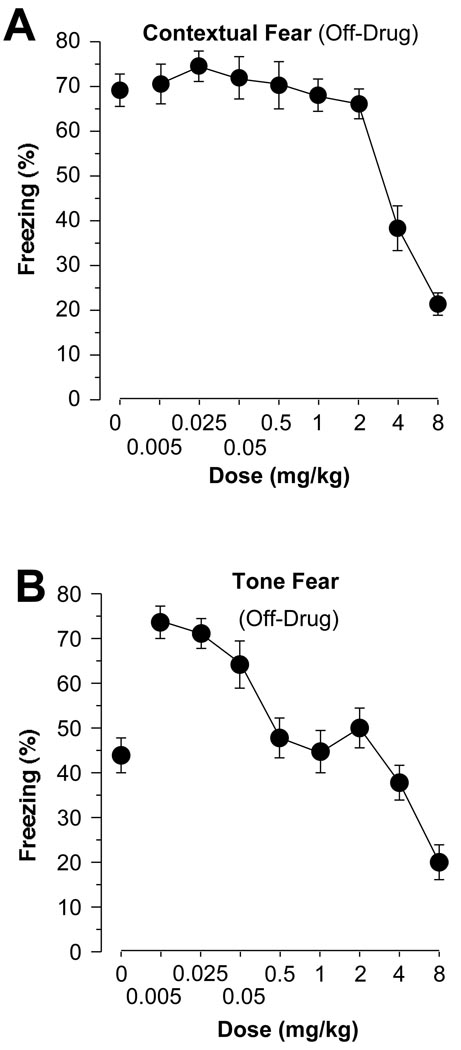
Context Fear
Subjects were returned to the conditioning chambers 24 h after training. Freezing was measured for 5 min, with all subjects off-drug (Figure 3A). Dose-dependent differences in freezing were apparent [F(8,127) = 20.7, p < .0001]. Subjects that had previously received 4 or 8 mg/kg amphetamine before training displayed less freezing, off-drug, when reintroduced to the training context compared with saline controls (Fisher’s PLSD, p values < .01). No other groups differed from saline controls (p values > 0.05).
Figure 3.
(A) Contextual Fear. When returned to the training context 24 h after training, off-drug, subjects that had received 4 or 8 mg/kg pre-training amphetamine displayed less freezing (mean±SEM) than all other groups. (B) Tone Fear. Subjects were introduced to a new context 48 h post-training, off-drug, and, after a 2 min baseline period, were presented with the training tone for 3 min. Freezing (mean±SEM) during the 3 min period when the tone was presented is depicted. Those that had been injected with 8 mg/kg, pre-training, displayed less freezing than all other groups, while those that had been administered 0.005, 0.025, or 0.05 mg/kg displayed more freezing than all other groups.
Tone Fear
Subjects were introduced to a new context 48 h after training, off-drug. Freezing was measured for 5 min, consisting of a 2 min baseline period, and a 3 min tone presentation (Figure 3B). The tone was the same frequency and volume as that which had been paired with the shock during training. Dose-dependent differences in freezing during the tone presentation were apparent [F(8,127) = 17.1, p < .0001]. Only subjects that had received 8 mg/kg amphetamine, pre-training, showed decreased freezing during the tone test compared with saline controls (Fisher’s PLSD, p < .0001). Interestingly, those that had been injected with 0.005, 0.025, or 0.05 mg/kg amphetamine, pre-training, showed increased freezing compared with saline controls (p values < .001). No other doses differed from saline controls (p values > 0.05).
Experiment 1 Summary
Overall, contextual fear memory tested off drug was sensitive to disruption by 4–8 mg/kg, whereas tone fear was sensitive only to 8 mg/kg. Immediate memory was more sensitive to disruption by amphetamine, with deficits appearing at 1–8 mg/kg, suggesting this represents a somewhat different action of the drug. Nonetheless, deficit of long-term memory was not specific to context or tone fear. These data indicate that amphetamine is disrupting fear conditioning by acting in an area of the brain that generalizes to both tests, potentially the amygdala, but likely not the hippocampus. Finally, an enhancement in immediate and cued, but not contextual, memory was observed at 0.005, 0.025, and 0.05 mg/kg. Indeed, the enhancement in tone fear was quite substantial, with the lowest dose of amphetamine nearly doubling tone fear. This suggests some potential selectivity in terms of the enhancement; this possibility is further discussed below.
Experiment 2
Training, Post-Shock, and Immediate Memory
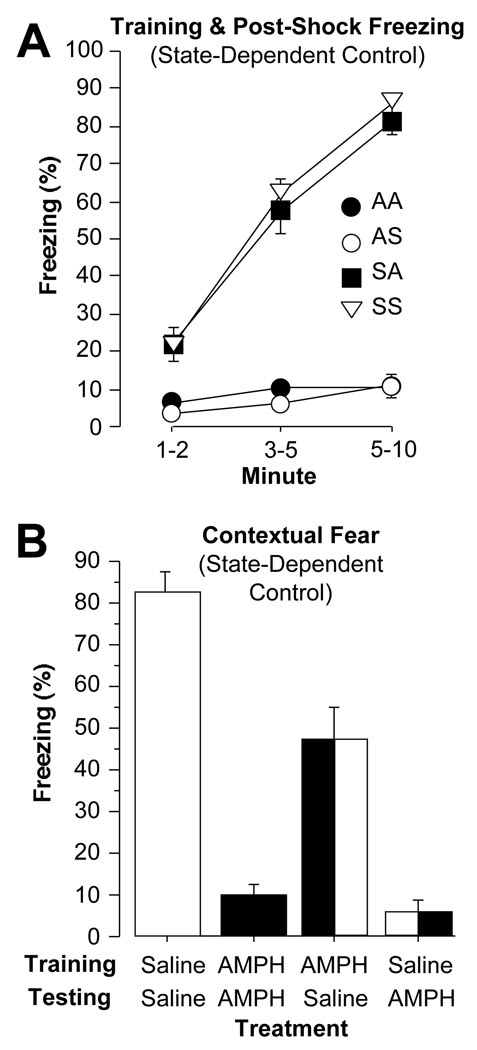
The training protocol for the state-dependent control study was the same as the dose-response study. Fifteen min after drug or saline injection, subjects were exposed to the conditioning chambers for a total of 10 min, composed of a 2 min baseline period, 3 min during which 3 tone-shock pairings were presented, and a 5 min immediate memory test (Figure 4A). Drug-dependent effects on freezing were evident [F(3,36) = 88.7, p < .0001], with mice injected with saline, pre-training, showing markedly more freezing throughout training than those injected with 8 mg/kg amphetamine (Fisher’s PLSD, p values < .0001). A main effect for training period was evident as well [F(2,36) = 259.9, p < .0001], with average freezing increasing throughout each period of training (p values < .0001). A significant drug by training period interaction [F(6,36) = 59.7, p < .0001], however, demonstrated that this effect was driven differentially by the two saline groups, as the two groups on amphetamine did not show an increase in freezing.
Figure 4.
(A) State-Dependent Control – Training and Immediate Memory. Freezing (mean±SEM) was measured during the baseline period (min 1–2), the period during which 3 tone-shock pairings were presented (min 3–5), and during the 5 min immediately following training (min 6–10). While subjects administered saline before training showed a steady increase in freezing, subjects administered 8 mg/kg amphetamine showed no increase in freezing. (B) State-Dependent Control - Contextual Fear. Regardless of treatment before training, subjects administered 8 mg/kg amphetamine before contextual testing displayed very little freezing. In contrast, mice injected with saline before contextual testing showed some freezing if injected with 8 mg/kg before training, and greater levels of freezing if injected with saline before training. There was no evidence of state-dependent memory.
Context Fear
Using the same protocol as Experiment 1, subjects were returned to the conditioning chambers 24 h after training to measure contextual fear memory (Figure 4B). Drug treatment had significant effects on contextual fear expression [F(3,36) = 66.5, p < .0001], with both groups injected with 8 mg/kg amphetamine, pre-testing, displaying less freezing than those injected with saline (Fisher’s PLSD, p values < .0001). Mice that received amphetamine during testing exhibited very little freezing and did not differ from one another (p > 0.05). Consistent with Experiment 1, mice that had been injected with 8 mg/kg amphetamine, pre-training, but then injected with saline, pre-testing, displayed less contextual fear than those that had been injected with saline both pre-training and pre-testing (p < .0001). Overall, no state-dependent learning effects were evident. Consistent with Experiment 1, amphetamine was able to disrupt performance of freezing when animals were trained on drug, as well as contextual memory when those animals were tested 24 h later, off-drug.
Discussion
We predicted that amphetamine would disrupt Pavlovian fear conditioning at moderate to high doses, like those that addicts take, and enhance fear conditioning at low doses similar to those used to treat ADHD. As expected, moderate doses of amphetamine administered pre-training inhibited contextual and cued fear memory, off-drug, while very low doses enhanced immediate and cued fear memory. These data are in agreement with the results of our previous work with cocaine, which induced a similar pattern of enhancements and deficits in memory (Wood et al. 2007). A low dose of cocaine (0.1 mg/kg) produced an enhancement in immediate, contextual and cued fear, whereas a high dose (15 mg/kg) produced an impairment in all three measures. The combined results of these studies lead us to theorize that it is not the addictive properties of the drugs or the clinical profile of the subjects taking the drugs but, rather, the dose of drugs that lead to the divergent cognitive effects associated with psychostimulants.
One difference in the results of the present study and our previous research with cocaine (Wood et al. 2007) is that a range of low doses of amphetamine studied did not enhance contextual fear 24 h after training. It is unclear if the memory enhancement provided by a low dose of amphetamine is specific to cued fear, because immediate fear memory is believed to be a form of contextual fear (Fanselow 1986). Indeed, while low doses of amphetamine induced small, but significantly higher levels of freezing during the immediate memory test compared to saline controls, levels of freezing off-drug during the tone test almost doubled, compared to controls. This evidence suggests that local actions of the drug (e.g., action on autoreceptors), alone, cannot account for the observed inhibition of activity during the immediate memory test. It may be the case that some other, untested low dose of amphetamine would reveal enhanced memory on both contextual and cued tests, off-drug, but it seems likely that a very low dose of amphetamine enhances cued memory, exclusively, since we tried a good range of low doses (0.005–0.5 mg/kg) and induced large enhancements of tone memory. Another possibility is that the enhancement was not observed in contextual fear because of a “ceiling effect;” about 70% freezing is potentially the maximum that B6 mice may freeze under these training parameters (Anagnostaras et al. 2000; Anagnostaras et al. 2003). Clearly, a moderate or high dose of amphetamine impairs both forms of fear memory; there were some differences in terms of the deficits, in that 4 and 8 mg/kg produced significant deficits in contextual fear, where only 8 mg/kg was effective in disrupting tone fear. However, overall, the deficits seem similar.
Research with a variety of human populations has produced mixed findings about the cognitive effects of stimulant use in humans. One study found cognitive benefits with amphetamine administration (0.25 mg/kg, orally) to people with schizophrenia, already treated with antipsychotics (Barch and Carter 2005), despite the common notion that amphetamine exacerbates schizophrenia. The control subjects of this study, as well, exhibited improved language production and reaction time, but had no benefit in working memory accuracy. These findings demonstrate the ability of stimulants to improve certain cognitive functions in a healthy population. Many studies have found cognitive deficits in amphetamine and cocaine addicts (e.g., Rogers and Robbins 2001). Currently using methamphetamine abusers were found to have worse performance on word recall, perceptual speed, an abbreviated IQ test, and the Wisconsin Card Sorting Task, compared with controls (Simon et al. 2002). In addition, methamphetamine addicts were found to perform more poorly on a decision-making task than their matched controls, while displaying less activation in a number of frontal cortical areas, as measured by fMRI (Paulus et al. 2002).
Previous research on amphetamine administration and associative learning in rodents has also yielded mixed findings. Rats administered d-amphetamine (5 mg/kg, i.p.) or cocaine (40 mg/kg, i.p.) during extinction of fear potentiated startle continued to show high startle amplitudes after 120 presentations of the nonreinforced CS (Borowski and Kokkinidis 1998). These results indicate that amphetamine and cocaine impaired the extinction of fear potentiated startle. Extinction is generally conceived as the acquisition of new, inhibitory learning (Barad 2006). These doses of amphetamine and cocaine would produce impairments if given during training on our conditioned fear task. Post-training injections of d-amphetamine at 1.0 mg/kg (i.p.), but not 0.25 or 4.0 mg/kg, have been shown to enhance inhibitory avoidance learning (Martinez et al. 1980). It is unclear if pre- versus post-training injections would share the same dose-response curve, so it is difficult to directly compare the results of the Martinez study with the current results.
As with cocaine, several possible reasons exist as to why conditioning was inhibited (Wood et al. 2007). While amphetamine does not have anesthetic properties, it could have induced a positive hedonic state in the mice. Those on higher doses may have perceived the shocks as less aversive than those on lower doses because of a drug-induced feeling of well being. While this experiment is not designed to fully explore this possibility, there is little evidence that this explanation accounts for the results. We gathered data measuring the shock reactivity of subjects (Fig. 1B). All subjects showed a large increase in velocity during the first shock presentation, in comparison to baseline velocity. Mice on 8 mg/kg amphetamine seemed to perceive the shock at least as aversive as those on lower doses. In addition, this explanation of altered perception would imply that amphetamine produces a positive hedonic state disruptive to fear conditioning, whereas much research has shown that amphetamine is anxiogenic, enhancing most defensive behaviors in rodents (Markham et al. 2006).
The dose-response curves for context and tone tests revealed a pattern of results inconsistent with action in the hippocampus. Although context conditioning was slightly more sensitive to disruption by high doses (4–8 mg/kg) of amphetamine than tone conditioning, context conditioning did not exhibit any enhancement from low doses, whereas tone conditioning exhibited large enhancements. This suggests that amphetamine is probably acting somewhere other than the hippocampus, which plays a selective role in contextual fear (Anagnostaras et al. 1999a). For example, scopolamine produced a selective deficit in contextual fear consistent with anticholinergic action in the hippocampus (Anagnostaras et al. 1995; Anagnostaras et al. 1999b; Gale et al. 2001). Previous research on moderate or high doses of amphetamine supports an interpretation of attentional deficits resulting in poor performance. Rats showed impairment on a delayed matching to sample task at low doses of 0.6 and 1.0 mg/kg amphetamine, i.p. (Harper et al. 2005). At these doses, the responses rats gave for a given trial were greatly influenced by their responses from the preceding trial. These results were interpreted to stem from poor attention or confusion, as opposed to a pure associative memory deficit. Evidence for attentional deficits due to chronic amphetamine (5.0 mg/kg, i.p.) use was also found in extinction deficits of active and passive avoidance responses in mice (Kokkinidis 1983). Frontal lobe dysfunction, resulting in poor attention and decision-making, has been documented in a number of studies, including those utilizing fMRI (Paulus et al. 2002).
An additional, potential confound to be addressed is state-dependent learning. According to this theory, subjects on-drug during training would remember what they learned better if also tested on-drug. A discrepancy in drug state between training and testing has been shown to be detrimental to learning, on certain drugs. For example, one representative study examined the effects of benzodiazepine (chlordiazepoxide, CDP) administration during extinction of fear conditioning (Bouton et al. 1990). Rats given CDP 30 min before extinction trials showed no decrease in freezing during an off-drug test performed after extinction. The authors performed a control study to decipher whether this effect was due to interference in learning during extinction or state-dependent learning by running all extinction trials on-drug and running each subject on two tests, one on-drug and one off-drug, after extinction. ANOVA revealed a main effect of drug during testing, and a planned comparison showed a significant difference in freezing during testing on-drug versus off-drug in rats that had undergone extinction on CDP. A similar effect was not found during testing sessions in rats that had undergone extinction without drug, suggesting CDP’s effects were due to state-dependent learning. It is worthy to point out, however, that no difference was reported between the two groups of rats given CDP during testing, nor between the two groups that were off-drug during testing, regardless of whether or not extinction had been performed with or without CDP. That is to say, there was evidence that CDP administration during testing could have increased the freezing response. Interestingly, measures of lick suppression, a measure not overtly confounded by the sedative effects of CDP, showed the same pattern. Overall, past studies that have shown state-dependent learning with benzodiazepine have also had to address possible locomotor confounds.
In the present study, subjects that received 0.05, 0.025, or 0.05 mg/kg amphetamine, pre-training, displayed higher levels of freezing than controls when tested, off-drug. This finding contradicts the prediction of state-dependent learning. In addition, the immediate memory test, performed on-drug, elicited a greater deficit in freezing than when subjects were tested off-drug. While the immediate memory test for a study of this design is clearly confounded with the motor stimulant properties of amphetamine, it is worthy to note that animals administered a high dose of amphetamine during both testing and training showed no increase in freezing over those that had received drug only during testing in Experiment 2. Indeed, subjects given amphetamine during both training and testing exhibited almost no evidence of learning, suggesting there was no state-dependent learning. Rather, amphetamine disrupted performance of the freezing response when given during testing and not training, and disrupted memory formation when given during training and not testing. Overall, amphetamine and other stimulants have not been shown to produce a high level of state-dependent learning, compared with other addictive drugs (Overton 1972).
As the amygdala and hippocampus are critical for contextual and cued fear conditioning (Fanselow and Poulos 2005), it is possible that amphetamine acted on one or both structures. An acute, low dose of amphetamine (1.5 mg/kg) has been shown to enhance synaptic transmission between the basolateral amygdala and nucleus accumbens, 30 min after the injection (Kessal et al. 2005). Chronic, but not acute, doses of amphetamine (between 1 and 4 mg/kg) have been shown to induce an up-regulation of glial fibrillary acidic protein (GFAP), which is considered a marker of astrogliosis and neurotoxicity, in the rat hippocampus (Frey et al. 2006). Therefore, neurotoxicity in the hippocampus is unlikely to account for our study which used acute dosing. A fear conditioning experiment analyzing the effects of caffeine in rats found a selective disruption of context memory (Corodimas et al. 2000), suggesting hippocampal-specific deficits. However, cocaine produced enhancements (at low doses) and deficits (at high doses) that were not specific to context or tone fear memory (Wood et al. 2007).
The effects of amphetamine on neural plasticity have also been examined in human, as well as animal, populations. It has been reported that low-dose amphetamine (10 mg, p.o., d-amphetamine, approximately 0.12 mg/kg for an 80 kg person) given during speech therapy, leads to improved recovery from aphasia (Walker-Batson et al. 2001). In order to examine amphetamine’s effects on experience-dependent plasticity in rats, researchers examined fear conditioning in rats housed in a complex environment for 3 months (Briand et al. 2005). While rats showed enhanced learning after living in the complex environment, rats treated with d-amphetamine (4.0 mg/kg) for 21 days, then housed in the enriched environment for 3 months, did not show the same enhancement. The authors hypothesized that this moderate dose of amphetamine limited the experience-dependent structural plasticity normally engaged by a complex environment.
Studies on the effects of amphetamine on long-term potentiation (LTP), an experience-dependent increase in synaptic efficacy thought to be associated with learning, have produced conflicting results. Broadly, some research on methamphetamine users has shown a decreased density of dopamine transporter in the striatum (e.g., Lundqvist 2005). As activation of DA receptors can modulate the expression of glutamate receptors, DA receptor activity can, in turn, alter synaptic plasticity (Sun et al. 2005). LTP in the dentate gyrus was also found to be enhanced by amphetamine, in a dose-dependent manner (Gold et al. 1984). Increased potentiation of population spike amplitude was found in rats that had been administered 0.01, 0.1, 1.0, or 3.0 mg/kg amphetamine, i.p., while doses higher (10.0 mg/kg) and lower (0.001 mg/kg) produced no differences, compared to controls. In other research, however, acute amphetamine administration (5.0 mg/kg, i.p.) did not alter perforant path LTP of EPSP slope but caused a small reduction in LTP of the population spike amplitude (Morimoto et al. 1987). The authors interpreted these results as showing amphetamine reduces cellular excitability. LTP was blocked in nucleus accumbens neurons when slices were bathed in 2.5 µM amphetamine solution (Li and Kauer 2004). Interestingly, this effect was attenuated when rats were administered amphetamine (2.5 mg/kg, i.p.) in vivo for 6 d, and their nucleus accumbens slices were prepared 8–10 d after the last injection. Additionally, a higher AMPA-receptor/ NMDA-receptor ratio at the glutamatergic synapses in the ventral tegmental area (VTA) was associated with higher behavioral sensitization to a single dose of amphetamine in young rats (Faleiro et al. 2004). These results indicate that LTP occurred rapidly in the VTA, and with a single amphetamine exposure. Overall, studies of amphetamine and synaptic plasticity have yielded mixed results, but demonstrate that low doses of amphetamine appear to enhance cell excitability and synaptic plasticity.
In general, research in humans and rodents on memory and synaptic plasticity is in agreement with the current study on acute doses, suggesting that very low doses are beneficial to cognition and moderate or high doses are detrimental. We have previously found similar results with cocaine (Wood et al. 2007). The implications of this research is broad, ranging from addicts, to students using stimulants for academic doping, and those prescribed stimulants for learning disabilities. An improved understanding of when these drugs produce improvements in cognition as opposed to deficits in cognition and addiction is of critical importance. The present study suggests that very low dosing is a critical determinant of stimulant efficacy in enhancing cognition, more so than the addictive properties of the drug, or the clinical profile of the subject taking the drug.
Acknowledgements
We thank Jakyong Lee, Angela Smith and Jason Yeatman for excellent technical assistance, and Tristan Shuman for helpful comments on the manuscript. SCW was supported by an NSF Graduate Research Fellowship. These studies were supported by NIH grant (DA020041) and Hellman Fellowship to SGA. The above experiments comply with the current laws of the United States, the country in which they were performed.
References
- Ahmann PA, Theye FW, Berg R, Linquist AJ, Van Erem AJ, Campbell LR. Placebo-controlled evaluation of amphetamine mixture-dextroamphetamine salts and amphetamine salts (Adderall): efficacy rate and side effects. Pediatrics. 2001;107:E10. doi: 10.1542/peds.107.1.e10. [DOI] [PubMed] [Google Scholar]
- Anagnostaras SG, Gale GD, Fanselow MS. Hippocampus and contextual fear conditioning: recent controversies and advances. Hippocampus. 2001;11:8–17. doi: 10.1002/1098-1063(2001)11:1<8::AID-HIPO1015>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- Anagnostaras SG, Josselyn SA, Frankland PW, Silva AJ. Computer-assisted behavioral assessment of Pavlovian fear conditioning in mice. Learn Mem. 2000;7:58–72. doi: 10.1101/lm.7.1.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anagnostaras SG, Maren S, Fanselow MS. Scopolamine selectively disrupts the acquisition of contextual fear conditioning in rats. Neurobiol Learn Mem. 1995;64:191–194. doi: 10.1006/nlme.1995.0001. [DOI] [PubMed] [Google Scholar]
- Anagnostaras SG, Maren S, Fanselow MS. Temporally graded retrograde amnesia of contextual fear after hippocampal damage in rats: within-subjects examination. J Neurosci. 1999a;19:1106–1114. doi: 10.1523/JNEUROSCI.19-03-01106.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anagnostaras SG, Maren S, Sage JR, Goodrich S, Fanselow MS. Scopolamine and Pavlovian fear conditioning in rats: dose-effect analysis. Neuropsychopharmacology. 1999b;21:731–744. doi: 10.1016/S0893-133X(99)00083-4. [DOI] [PubMed] [Google Scholar]
- Anagnostaras SG, Murphy GG, Hamilton SE, Mitchell SL, Rahnama NP, Nathanson NM, Silva AJ. Selective cognitive dysfunction in acetylcholine M1 muscarinic receptor mutant mice. Nat Neurosci. 2003;6:51–58. doi: 10.1038/nn992. [DOI] [PubMed] [Google Scholar]
- Barad M. Is extinction of fear erasure or inhibition? Why both, of course. Learn Mem. 2006;13:108–109. doi: 10.1101/lm.211306. [DOI] [PubMed] [Google Scholar]
- Barch DM, Carter CS. Amphetamine improves cognitive function in medicated individuals with schizophrenia and in healthy volunteers. Schizophr Res. 2005;77:43–58. doi: 10.1016/j.schres.2004.12.019. [DOI] [PubMed] [Google Scholar]
- Borowski TB, Kokkinidis L. The effects of cocaine, amphetamine, and the dopamine D1 receptor agonist SKF 38393 on fear extinction as measured with potentiated startle: implications for psychomotor stimulant psychosis. Behav Neurosci. 1998;112:952–965. doi: 10.1037//0735-7044.112.4.952. [DOI] [PubMed] [Google Scholar]
- Bouton ME, Kenney FA, Rosengard C. State-dependent fear extinction with two benzodiazepine tranquilizers. Behav Neurosci. 1990;104:44–55. doi: 10.1037//0735-7044.104.1.44. [DOI] [PubMed] [Google Scholar]
- Briand LA, Robinson TE, Maren S. Enhancement of auditory fear conditioning after housing in a complex environment is attenuated by prior treatment with amphetamine. Learn Mem. 2005;12:553–556. doi: 10.1101/lm.95905. [DOI] [PubMed] [Google Scholar]
- Butcher J. Cognitive enhancement raises ethical concerns. Academics urge pre-emptive debate on neurotechnologies. Lancet. 2003;362:132–133. doi: 10.1016/s0140-6736(03)13897-4. [DOI] [PubMed] [Google Scholar]
- Caldwell JA, Caldwell JL, Crowley JS, Jones HD. Sustaining helicopter pilot performance with Dexedrine during periods of sleep deprivation. Aviat Space Environ Med. 1995;66:930–937. [PubMed] [Google Scholar]
- Cornum K. Extended Air Combat Operations: F-15s over Iraq. Aviation, Space and Environmental Medicine. 1994 [Google Scholar]
- Cornum K, Cornum R, Storm W. Use of psychostimulants in extended flight operations: a Desert Shield experience Aerospace Medical Panel Symposium (Advisory Group for Aerospace Research and Development Conference Proceedings 579, Neurological Limitations of Aircraft Operations: Human Performance Implications); Koln, Germany. 1995. pp. 371–374. [Google Scholar]
- Corodimas KP, Pruitt JC, Stieg JM. Acute exposure to caffeine selectively disrupts context conditioning in rats. Psychopharmacology (Berl) 2000;152:376–382. doi: 10.1007/s002130000557. [DOI] [PubMed] [Google Scholar]
- Faleiro LJ, Jones S, Kauer JA. Rapid synaptic plasticity of glutamatergic synapses on dopamine neurons in the ventral tegmental area in response to acute amphetamine injection. Neuropsychopharmacology. 2004;29:2115–2125. doi: 10.1038/sj.npp.1300495. [DOI] [PubMed] [Google Scholar]
- Fanselow MS. Conditioned and unconditional components of post-shock freezing. Pavlov J Biol Sci. 1980;15:177–182. doi: 10.1007/BF03001163. [DOI] [PubMed] [Google Scholar]
- Fanselow MS. Associative vs. topographical accounts of the immediate shock freezing deficit in rats: Implications for the response selection rules governing species specific defensive reactions. Learning & Motivation. 1986;17:16–39. [Google Scholar]
- Fanselow MS, Gale GD. The amygdala, fear, and memory. Ann N Y Acad Sci. 2003;985:125–134. doi: 10.1111/j.1749-6632.2003.tb07077.x. [DOI] [PubMed] [Google Scholar]
- Fanselow MS, Poulos AM. The neuroscience of mammalian associative learning. Annu Rev Psychol. 2005;56:207–234. doi: 10.1146/annurev.psych.56.091103.070213. [DOI] [PubMed] [Google Scholar]
- Frey BN, Andreazza AC, Cereser KM, Martins MR, Petronilho FC, de Souza DF, Tramontina F, Goncalves CA, Quevedo J, Kapczinski F. Evidence of astrogliosis in rat hippocampus after d-amphetamine exposure. Prog Neuropsychopharmacol Biol Psychiatry. 2006;30:1231–1234. doi: 10.1016/j.pnpbp.2006.03.016. [DOI] [PubMed] [Google Scholar]
- Gale GD, Anagnostaras SG, Fanselow MS. Cholinergic modulation of pavlovian fear conditioning: effects of intrahippocampal scopolamine infusion. Hippocampus. 2001;11:371–376. doi: 10.1002/hipo.1051. [DOI] [PubMed] [Google Scholar]
- Gold PE, Delanoy RL, Merrin J. Modulation of long-term potentiation by peripherally administered amphetamine and epinephrine. Brain Res. 1984;305:103–107. doi: 10.1016/0006-8993(84)91124-7. [DOI] [PubMed] [Google Scholar]
- Harper DN, Wisnewski R, Hunt M, Schenk S. (+/−)3,4-methylenedioxymethamphetamine, d-amphetamine, and cocaine impair delayed matching-to-sample performance by an increase in susceptibility to proactive interference. Behav Neurosci. 2005;119:455–463. doi: 10.1037/0735-7044.119.2.455. [DOI] [PubMed] [Google Scholar]
- Harris D, Batki SL. Stimulant psychosis: symptom profile and acute clinical course. Am J Addict. 2000;9:28–37. doi: 10.1080/10550490050172209. [DOI] [PubMed] [Google Scholar]
- Kessal K, Chessel A, Spennato G, Garcia R. Ketamine and amphetamine both enhance synaptic transmission in the amygdala-nucleus accumbens pathway but with different time-courses. Synapse. 2005;57:61–65. doi: 10.1002/syn.20154. [DOI] [PubMed] [Google Scholar]
- Kokkinidis L. The effects of chronic amphetamine administration on the acquisition and extinction of an active and passive avoidance response in mice. Pharmacol Biochem Behav. 1983;19:593–598. doi: 10.1016/0091-3057(83)90333-7. [DOI] [PubMed] [Google Scholar]
- Leonard BE, McCartan D, White J, King DJ. Methylphenidate: a review of its neuropharmacological, neuropsychological and adverse clinical effects. Hum Psychopharmacol. 2004;19:151–180. doi: 10.1002/hup.579. [DOI] [PubMed] [Google Scholar]
- Li Y, Kauer JA. Repeated exposure to amphetamine disrupts dopaminergic modulation of excitatory synaptic plasticity and neurotransmission in nucleus accumbens. Synapse. 2004;51:1–10. doi: 10.1002/syn.10270. [DOI] [PubMed] [Google Scholar]
- Lundqvist T. Cognitive consequences of cannabis use: comparison with abuse of stimulants and heroin with regard to attention, memory and executive functions. Pharmacol Biochem Behav. 2005;81:319–330. doi: 10.1016/j.pbb.2005.02.017. [DOI] [PubMed] [Google Scholar]
- Maren S, Anagnostaras S, Fanselow M. The startled seahorse: is the hippocampus necessary for contextual fear conditioning? Trends Cogn Sci. 1998;2:39–42. doi: 10.1016/s1364-6613(98)01123-1. [DOI] [PubMed] [Google Scholar]
- Markham CM, Yang M, Blanchard RJ, Blanchard DC. Effects of D-amphetamine on defensive behaviors related to fear and anxiety. Pharmacol Biochem Behav. 2006;83:490–499. doi: 10.1016/j.pbb.2006.03.009. [DOI] [PubMed] [Google Scholar]
- Martinez JL, Jr, Jensen RA, Messing RB, Vasquez BJ, Soumireu-Mourat B, Geddes D, Liang KC, McGaugh JL. Central and peripheral actions of amphetamine on memory storage. Brain Res. 1980;182:157–166. doi: 10.1016/0006-8993(80)90838-0. [DOI] [PubMed] [Google Scholar]
- McCabe SE, Knight JR, Teter CJ, Wechsler H. Non-medical use of prescription stimulants among US college students: prevalence and correlates from a national survey. Addiction. 2005;100:96–106. doi: 10.1111/j.1360-0443.2005.00944.x. [DOI] [PubMed] [Google Scholar]
- Morimoto K, Otani S, Goddard GV. Effects of acute and long-term treatment with amphetamine on evoked responses and long-term potentiation in the dentate gyrus of anesthetized rats. Brain Res. 1987;407:137–143. doi: 10.1016/0006-8993(87)91227-3. [DOI] [PubMed] [Google Scholar]
- Overton DA. State-dependent learning produced by addicting drugs. In: Fisher C, Freedman AM, editors. Opiate addiction: origins and treatment. New York: Halsted Press Division of Wiley; 1972. pp. 61–75. [Google Scholar]
- Paulus MP, Hozack NE, Zauscher BE, Frank L, Brown GG, Braff DL, Schuckit MA. Behavioral and functional neuroimaging evidence for prefrontal dysfunction in methamphetamine-dependent subjects. Neuropsychopharmacology. 2002;26:53–63. doi: 10.1016/S0893-133X(01)00334-7. [DOI] [PubMed] [Google Scholar]
- Rogers RD, Robbins TW. Investigating the neurocognitive deficits associated with chronic drug misuse. Curr Opin Neurobiol. 2001;11:250–257. doi: 10.1016/s0959-4388(00)00204-x. [DOI] [PubMed] [Google Scholar]
- Simon SL, Domier CP, Sim T, Richardson K, Rawson RA, Ling W. Cognitive performance of current methamphetamine and cocaine abusers. J Addict Dis. 2002;21:61–74. doi: 10.1300/j069v21n01_06. [DOI] [PubMed] [Google Scholar]
- Sun X, Zhao Y, Wolf ME. Dopamine receptor stimulation modulates AMPA receptor synaptic insertion in prefrontal cortex neurons. J Neurosci. 2005;25:7342–7351. doi: 10.1523/JNEUROSCI.4603-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teter CJ, McCabe SE, LaGrange K, Cranford JA, Boyd CJ. Illicit use of specific prescription stimulants among college students: prevalence, motives, and routes of administration. Pharmacotherapy. 2006;26:1501–1510. doi: 10.1592/phco.26.10.1501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner DC, Robbins TW, Clark L, Aron AR, Dowson J, Sahakian BJ. Cognitive enhancing effects of modafinil in healthy volunteers. Psychopharmacology (Berl) 2003;165:260–269. doi: 10.1007/s00213-002-1250-8. [DOI] [PubMed] [Google Scholar]
- Walker-Batson D, Curtis S, Natarajan R, Ford J, Dronkers N, Salmeron E, Lai J, Unwin DH. A double-blind, placebo-controlled study of the use of amphetamine in the treatment of aphasia. Stroke. 2001;32:2093–2098. doi: 10.1161/hs0901.095720. [DOI] [PubMed] [Google Scholar]
- White IM, Whitaker C, White W. Amphetamine-induced hyperlocomotion in rats: Hippocampal modulation of the nucleus accumbens. Hippocampus. 2006;16:596–603. doi: 10.1002/hipo.20189. [DOI] [PubMed] [Google Scholar]
- Wood SC, Fay J, Sage JR, Anagnostaras SG. Cocaine and Pavlovian fear conditioning: dose-effect analysis. Behav Brain Res. 2007;176:244–250. doi: 10.1016/j.bbr.2006.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]