Abstract
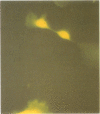
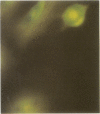
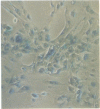
A system for targeting foreign DNA to epithelial cells in vitro has been developed by exploiting receptor-mediated endocytosis. The polymeric immunoglobulin receptor transports dimeric immunoglobulin A and immunoglobulin M through epithelial cells, including those of the respiratory tract, by binding the immunoglobulins at the basolateral surface and transporting them across the cell. Fab fragments of antibodies directed against the extracellular portion of the receptor, secretory component, are similarly transported. Anti-human secretory component Fab fragments were covalently linked to a polycation, and complexed to various expression plasmids. When bound to an expression plasmid containing the Escherichia coli lacZ gene ligated to the Rous sarcoma virus promoter, the complexes transfected HT29.74 human colon carcinoma cells induced to express polymeric immunoglobulin receptor, but not those lacking the receptor. Primary cultures of human tracheal epithelial cells grown on collagen gels, which induce the expression of polymeric immunoglobulin receptor, were also transfected with the complexes. From 5 to 66% of the respiratory epithelial cells had beta-galactosidase activity after treatment, comparable to the percentage of cultured human tracheal epithelial cells that express polymeric immunoglobulin receptor (8-35%). The addition of excess human secretory component (Fab ligand) to the culture medium at the time of transfection blocked the delivery of DNA. The expression plasmid, either alone, complexed to the polycation, or complexed to a carrier based on an irrelevant Fab fragment, was not effective in transfecting either cell type. This DNA carrier system introduces DNA specifically into epithelial cells that contain pIgR in vitro.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Chintalacharuvu K. R., Piskurich J. F., Lamm M. E., Kaetzel C. S. Cell polarity regulates the release of secretory component, the epithelial receptor for polymeric immunoglobulins, from the surface of HT-29 colon carcinoma cells. J Cell Physiol. 1991 Jul;148(1):35–47. doi: 10.1002/jcp.1041480105. [DOI] [PubMed] [Google Scholar]
- Chu C. S., Trapnell B. C., Curristin S. M., Cutting G. R., Crystal R. G. Extensive posttranscriptional deletion of the coding sequences for part of nucleotide-binding fold 1 in respiratory epithelial mRNA transcripts of the cystic fibrosis transmembrane conductance regulator gene is not associated with the clinical manifestations of cystic fibrosis. J Clin Invest. 1992 Sep;90(3):785–790. doi: 10.1172/JCI115952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins F. S. Cystic fibrosis: molecular biology and therapeutic implications. Science. 1992 May 8;256(5058):774–779. doi: 10.1126/science.1375392. [DOI] [PubMed] [Google Scholar]
- Cotten M., Längle-Rouault F., Kirlappos H., Wagner E., Mechtler K., Zenke M., Beug H., Birnstiel M. L. Transferrin-polycation-mediated introduction of DNA into human leukemic cells: stimulation by agents that affect the survival of transfected DNA or modulate transferrin receptor levels. Proc Natl Acad Sci U S A. 1990 Jun;87(11):4033–4037. doi: 10.1073/pnas.87.11.4033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drumm M. L., Pope H. A., Cliff W. H., Rommens J. M., Marvin S. A., Tsui L. C., Collins F. S., Frizzell R. A., Wilson J. M. Correction of the cystic fibrosis defect in vitro by retrovirus-mediated gene transfer. Cell. 1990 Sep 21;62(6):1227–1233. doi: 10.1016/0092-8674(90)90398-x. [DOI] [PubMed] [Google Scholar]
- Engelhardt J. F., Yankaskas J. R., Ernst S. A., Yang Y., Marino C. R., Boucher R. C., Cohn J. A., Wilson J. M. Submucosal glands are the predominant site of CFTR expression in the human bronchus. Nat Genet. 1992 Nov;2(3):240–248. doi: 10.1038/ng1192-240. [DOI] [PubMed] [Google Scholar]
- Fiedler M. A., Kaetzel C. S., Davis P. B. Sustained production of secretory component by human tracheal epithelial cells in primary culture. Am J Physiol. 1991 Oct;261(4 Pt 1):L255–L261. doi: 10.1152/ajplung.1991.261.4.L255. [DOI] [PubMed] [Google Scholar]
- Flotte T. R., Solow R., Owens R. A., Afione S., Zeitlin P. L., Carter B. J. Gene expression from adeno-associated virus vectors in airway epithelial cells. Am J Respir Cell Mol Biol. 1992 Sep;7(3):349–356. doi: 10.1165/ajrcmb/7.3.349. [DOI] [PubMed] [Google Scholar]
- Hazinski T. A., Ladd P. A., DeMatteo C. A. Localization and induced expression of fusion genes in the rat lung. Am J Respir Cell Mol Biol. 1991 Mar;4(3):206–209. doi: 10.1165/ajrcmb/4.3.206. [DOI] [PubMed] [Google Scholar]
- Infeld M. D., Brennan J. A., Davis P. B. Human tracheobronchial epithelial cells direct migration of lung fibroblasts in three-dimensional collagen gels. Am J Physiol. 1992 May;262(5 Pt 1):L535–L541. doi: 10.1152/ajplung.1992.262.5.L535. [DOI] [PubMed] [Google Scholar]
- Johnson L. G., Olsen J. C., Sarkadi B., Moore K. L., Swanstrom R., Boucher R. C. Efficiency of gene transfer for restoration of normal airway epithelial function in cystic fibrosis. Nat Genet. 1992 Sep;2(1):21–25. doi: 10.1038/ng0992-21. [DOI] [PubMed] [Google Scholar]
- Kaetzel C. S., Robinson J. K., Chintalacharuvu K. R., Vaerman J. P., Lamm M. E. The polymeric immunoglobulin receptor (secretory component) mediates transport of immune complexes across epithelial cells: a local defense function for IgA. Proc Natl Acad Sci U S A. 1991 Oct 1;88(19):8796–8800. doi: 10.1073/pnas.88.19.8796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kvale D., Brandtzaeg P., Løvhaug D. Up-regulation of the expression of secretory component and HLA molecules in a human colonic cell line by tumour necrosis factor-alpha and gamma interferon. Scand J Immunol. 1988 Sep;28(3):351–357. doi: 10.1111/j.1365-3083.1988.tb01460.x. [DOI] [PubMed] [Google Scholar]
- Lim K., Chae C. B. A simple assay for DNA transfection by incubation of the cells in culture dishes with substrates for beta-galactosidase. Biotechniques. 1989 Jun;7(6):576–579. [PubMed] [Google Scholar]
- Martinez-Tello F. J., Braun D. G., Blanc W. A. Immunoglobulin production in bronchial mucosa and bronchial lymph nodes, particularly in cystic fibrosis of the pancreas. J Immunol. 1968 Nov;101(5):989–1003. [PubMed] [Google Scholar]
- Musil L. S., Baenziger J. U. Cleavage of membrane secretory component to soluble secretory component occurs on the cell surface of rat hepatocyte monolayers. J Cell Biol. 1987 Jun;104(6):1725–1733. doi: 10.1083/jcb.104.6.1725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips J. O., Everson M. P., Moldoveanu Z., Lue C., Mestecky J. Synergistic effect of IL-4 and IFN-gamma on the expression of polymeric Ig receptor (secretory component) and IgA binding by human epithelial cells. J Immunol. 1990 Sep 15;145(6):1740–1744. [PubMed] [Google Scholar]
- Piskurich J. F., France J. A., Tamer C. M., Willmer C. A., Kaetzel C. S., Kaetzel D. M. Interferon-gamma induces polymeric immunoglobulin receptor mRNA in human intestinal epithelial cells by a protein synthesis dependent mechanism. Mol Immunol. 1993 Mar;30(4):413–421. doi: 10.1016/0161-5890(93)90071-i. [DOI] [PubMed] [Google Scholar]
- Raphael G. D., Druce H. M., Baraniuk J. N., Kaliner M. A. Pathophysiology of rhinitis. 1. Assessment of the sources of protein in methacholine-induced nasal secretions. Am Rev Respir Dis. 1988 Aug;138(2):413–420. doi: 10.1164/ajrccm/138.2.413. [DOI] [PubMed] [Google Scholar]
- Rosenfeld M. A., Siegfried W., Yoshimura K., Yoneyama K., Fukayama M., Stier L. E., Päkkö P. K., Gilardi P., Stratford-Perricaudet L. D., Perricaudet M. Adenovirus-mediated transfer of a recombinant alpha 1-antitrypsin gene to the lung epithelium in vivo. Science. 1991 Apr 19;252(5004):431–434. doi: 10.1126/science.2017680. [DOI] [PubMed] [Google Scholar]
- Rosenfeld M. A., Yoshimura K., Trapnell B. C., Yoneyama K., Rosenthal E. R., Dalemans W., Fukayama M., Bargon J., Stier L. E., Stratford-Perricaudet L. In vivo transfer of the human cystic fibrosis transmembrane conductance regulator gene to the airway epithelium. Cell. 1992 Jan 10;68(1):143–155. doi: 10.1016/0092-8674(92)90213-v. [DOI] [PubMed] [Google Scholar]
- Sollid L. M., Kvale D., Brandtzaeg P., Markussen G., Thorsby E. Interferon-gamma enhances expression of secretory component, the epithelial receptor for polymeric immunoglobulins. J Immunol. 1987 Jun 15;138(12):4303–4306. [PubMed] [Google Scholar]
- Trapnell B. C., Chu C. S., Paakko P. K., Banks T. C., Yoshimura K., Ferrans V. J., Chernick M. S., Crystal R. G. Expression of the cystic fibrosis transmembrane conductance regulator gene in the respiratory tract of normal individuals and individuals with cystic fibrosis. Proc Natl Acad Sci U S A. 1991 Aug 1;88(15):6565–6569. doi: 10.1073/pnas.88.15.6565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner E., Cotten M., Foisner R., Birnstiel M. L. Transferrin-polycation-DNA complexes: the effect of polycations on the structure of the complex and DNA delivery to cells. Proc Natl Acad Sci U S A. 1991 May 15;88(10):4255–4259. doi: 10.1073/pnas.88.10.4255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watts C. L., Fanaroff A. A., Bruce M. C. Elevation of fibronectin levels in lung secretions of infants with respiratory distress syndrome and development of bronchopulmonary dysplasia. J Pediatr. 1992 Apr;120(4 Pt 1):614–620. doi: 10.1016/s0022-3476(05)82492-8. [DOI] [PubMed] [Google Scholar]
- Wu R., Smith D. Continuous multiplication of rabbit tracheal epithelial cells in a defined, hormone-supplemented medium. In Vitro. 1982 Sep;18(9):800–812. doi: 10.1007/BF02796504. [DOI] [PubMed] [Google Scholar]
- Zenke M., Steinlein P., Wagner E., Cotten M., Beug H., Birnstiel M. L. Receptor-mediated endocytosis of transferrin-polycation conjugates: an efficient way to introduce DNA into hematopoietic cells. Proc Natl Acad Sci U S A. 1990 May;87(10):3655–3659. doi: 10.1073/pnas.87.10.3655. [DOI] [PMC free article] [PubMed] [Google Scholar]