Abstract
Objective
Having previously demonstrated that the complement system modulates mobilization of hematopoietic stem/progenitor cells (HSPC) in mice, we investigated the involvement of C5 cleavage fragments (C5a/desArgC5a) in human HSPC mobilization.
Methods
C5 cleavage fragments in the plasma were evaluated by ELISA using human anti-desArgC5a antibody, and the expression of the C5a/desArgC5a receptor (CD88) in hematopoietic cells by flow cytometry. We also examined the chemotactic responses of hematopoietic cells to C5 cleavage fragments and the expression of SDF-1-degrading proteases that perturb retention of HSPC in bone marrow (BM), namely matrix metalloproteinase (MMP)-9, membrane type (MT)1-MMP and carboxypeptidase M (CPM).
Results
We found that plasma levels of desArgC5a are significantly higher in patients who are good mobilizers and correlate with CD34+ cell and WBC counts in mobilized peripheral blood (mPB). C5 cleavage fragments did not chemoattract myeloid progenitors (CFU-GM) but desArgC5a did strongly chemoattract mature nucleated cells. Consistently, CD88 was not detected on CD34+ cells, but appeared on more mature myeloid precursors, monocytes and granulocytes. Moreover, G-CSF-mobilized PB MNC and PMN had a significantly higher percentage of cells expressing CD88 than non-mobilized PB. Furthermore, C5a stimulation of granulocytes and monocytes (i) decreased CXCR4 expression and chemotaxis towards an SDF-1 gradient, and ii) increased secretion of MMP-9 and expression of MT1-MMP and CPM.
Conclusion
C5 cleavage fragments not only induce a highly proteolytic microenvironment in the human BM which perturbs retention through the CXCR4/SDF-1 axis but also strongly chemoattract granulocytes, promoting their egress into mPB, which is crucial for subsequent mobilization of HSPC.
Keywords: Complement cleavage fragments, C5a, SDF-1/CXCR4 axis, HSPC mobilization, neutrophils
INTRODUCTION
It is known that interactions between the chemokine stromal cell-derived factor (SDF)-1 and its receptor CXCR4 generate signals that regulate the trafficking of hematopoietic stem/progenitor cells (HSPC) [reviewed in 1,2]. Bone marrow (BM) stromal cells and osteoblasts constitutively produce SDF-1, which is a potent chemoattractant for HSPC [3]. During HSPC mobilization, a perturbation of the SDF-1 chemotactic gradient in the BM occurs with a concomitant decrease in the responsiveness of HSPC towards it. Various molecules have been implicated in the process of HSPC mobilization, with emerging evidence that innate immunity components, including the complement cascade (CC), neutrophils and Toll-like receptors, play a key role in it [4,5].
The activation of complement triggers a cascade of reactions generating various bioactive peptides including the C5 cleavage fragments (C5a and desArgC5a) which are responsible for the recruitment of inflammatory cells to sites of infection [6]. The biological activities of C5a are specifically mediated by its interaction with its seven-transmembrane domain receptors, C5aR (C5R1, CD88) and C5L2 (GPR77), although fewer biologically relevant interactions have been found with the latter receptor [7,8]. Serum and cell-surface carboxypeptidases readily convert short-lived C5a to long-lived desArgC5a by removing the C-terminal arginine. Patients with inflammatory disorders have elevated levels of C5a and desArgC5a in their sera [9].
It has been reported that granulocyte-colony-stimulating factor (G-CSF)-induced mobilization is impaired in patients suffering from severe combined immunodeficiency disease (SCID), i.e., those who lack functional B and T lymphocytes [10], supporting the idea that the egress of HSPC from the BM occurs as part of the immune response. Interestingly, we have demonstrated that C3a enhances/primes the chemotactic responses of HSPC to SDF-1 resulting in faster engraftment of HSPC after their transplantation into lethally irradiated animals [11,12]. Subsequently, studies using C3-deficient (C3−/−) and C3a receptor-deficient (C3aR−/−) mice revealed that the C3a-C3aR axis protects HSPC from uncontrolled egress from the BM and that blockade of this axis increases mobilization [13]. On the other hand, C5- and Ig-deficient mice are poor mobilizers indicating that C5 cleavage fragments may be pivotal in HSPC mobilization [14]. Hence, we postulated that mobilization of HSPC is regulated differently at different levels of the complement activation cascade. In this study, we examined whether C5 cleavage fragments play a role in mobilization of human HSPC and how they affect the SDF-1/CXCR4 axis. We investigated whether C5a upregulates secreted and membrane-bound matrix metalloproteinases (MMPs) as well as carboxypeptidase M (CPM), known to inactivate SDF-1α [15,16]. We report for the first time, that C5 cleavage fragments may induce a highly proteolytic microenvironment in human BM that perturbs the homing effects of the CXCR4/SDF-1 axis and, as strong chemoattractants for granulocytes, they promote their egress into mobilized peripheral blood (mPB), facilitating subsequent mobilization of HSPC.
Materials and methods
Patients, cells and cultures
BM was obtained from unrelated donors and PB from normal donors and patients diagnosed with non-Hodgkin’s lymphoma, who had been mobilized with chemotherapy and G-CSF (Filgrastim μg/kg BID, Amgen, Thousand Oaks, CA, USA). All samples were obtained with donors’ and patients’ informed consent in accordance with the guidelines approved by the University of Alberta Health Research Ethics Board. During mobilization CD34+ cell and white blood cell (WBC) counts were monitored and leukapheresis carried out. Light-density mononuclear (MNC) cells from BM and from leukapheresis products were obtained by centrifugation (using a 60% Percoll density gradient, 1.077 g/mL; Amersham, Uppsala, Sweden). CD34+ cell separation was carried out using the Miltenyi MACS system (Miltenyi Biotec, Auburn, CA, USA) according to the manufacturer’s instructions. Polymorphonuclear (PMN) cells were isolated by centrifugation using Lympholyte-poly (Cedarlane, Burlington, ON, Canada). BM leukocytes were prepared by lysing red blood cells with lysis buffer (150 mM NH4Cl, 1 mM EDTA, 10 mM NaHCO3) for 10 min at room temperature. The cells were stimulated or not (controls) with either recombinant human (rh) G-CSF (100 ng/mL, R&D Systems, Minneapolis, MN, USA) or rh C5a (10 ng/mL or 100 ng/mL, Cedarlane) in RMPI 1640 with 5% bovine growth serum (BGS, Hyclone, ThermoFisher Scientific, Nepean, ON, Canada) for 15 h at 37°C.
Erythroid, myeloid and megakaryocytic progenitors were expanded from mobilized (m)PB CD34+ cells as described previously [17]. The cells were stained for CD88 on days 3, 6 and 11 of expansion, and on day 11 for CD34, glycophorin A (erythroid), CD33 (myeloid) and CD41 (megakaryocytic) lineage markers.
Enzyme-Linked Immunosorbent Assay (ELISA)
The levels of C5a in plasma samples from normal donors and mobilized patients were determined using the OptEIA Human C5a ELISA kit II (BD Biosciences, San Jose, CA, USA). Standards and samples (20-fold dilution) were added in duplicate to wells pre-coated with monoclonal antibody specific for human desArgC5a. After incubation for 2 h at room temperature, the wells were washed and a mixture of biotinylated anti-human C5a antibody and streptavidin-horseradish peroxidase was added, followed by incubation for 1 h at room temperature. The wells were washed and then 3,3′5,5′-tetramethylbenzidine substrate solution was added, which produced a blue color in direct proportion to the amount of desArgC5a present in the sample. Stop solution (1 M phosphoric acid) was added and optical density (OD) read at 450 nm.
FACS analysis
Expression of the CD88 antigen was examined with anti-CD88 MoAb (BD Biosciences, Mississauga, ON, Canada) followed by staining with goat anti-mouse AlexaFluor-488 secondary antibody (Invitrogen, Burlington, ON, Canada). Briefly, the cells were washed three times in buffer (PBS with 0.1% BSA) and incubated with isotype IgG, anti-CD88 MoAb for 45 min on ice followed by a further washing (three times) and staining with the secondary antibody for 30 min on ice. After the final wash, cells were fixed in 1% paraformaldehyde and analyzed by FACS (FACscan, Becton Dickinson, San Jose, CA, USA). Lymphocytes, monocytes and granulocytes were defined based on their forward scatter (FSC) and side scatter (SSC) using FCS Express (De Novo Software, Los Angeles, CA, USA). For CXCR4 expression, cells were stained with CD45-FITC (Beckman Coulter, Mississauga, ON, Canada) and CXCR4-PECy5 (BD Biosciences) for 45 min on ice, washed, fixed and analyzed by FACS using CD45 gating.
Gel-based and quantitative real-time RT-PCR
Total RNA from MNC and PMN cells was extracted using TRIzol Reagent (Invitrogen) according to the manufacturer’s instructions. Gel-based RT-PCR reactions were carried out as described previously using human GAPDH as internal control to evaluate transcripts for CD88, CXCR4, MT1-MMP and CPM [16,18]. The primer sequences used for CD88 (NM_001736.3) are as follows: sense, 5′-ATACCACCCCTGATTATGG and anti-sense, 5′-CCATCAATGCCATCTGGTTC. Gels were visualized under UV light and photographed using the FluorChem Imaging System (Alpha Innotech, San Leandro, CA, USA). Semi-quantitative evaluation by densitometric analysis of the bands in each sample was carried out using NIH Image J software (NIH, Bethesda, MD, USA). The relative level of target mRNA was regarded as the ratio between the intensities of the target primer and the GAPDH bands.
For quantitative real-time RT-PCR, the Superscript II reverse transcriptase kit (Invitrogen) was used to synthesize first-strand complementary DNA (cDNA). PCR amplification was carried out in triplicate in a 384-well plate on a 7900HT Fast Real 7500 Sequence Detector using the TaqMan Gene Expression Assay (Applied Biosystems, Foster City, CA, USA). Results were analyzed with the ABI Prism 7900HT Sequence Detection System software version 2.3 (Applied Biosystems). Each reaction was normalized by the cycle threshold (Ct) of human GAPDH cDNA expression (primer 4326317E, Applied Biosystems). Relative expression of human MT1-MMP (HS00237119_m1, Applied Biosystems) was calculated with the ΔΔCt method. The fold-change ratio was calculated and expressed as mean ± standard deviation.
Chemotaxis assay
Chemotaxis towards C5 cleavage fragments was performed using normal RBC-lysed PB or BM PMN cells resuspended in assay media (RPMI/0.5% BSA) and equilibrated for 30 min at 37°C as described [19]. Assay media (650 μL) containing rh C5a (70 ng/mL and 18 ng/mL; Calbiochem, La Jolla, CA, USA), purified human desArgC5a (70 ng/mL and 18 ng/mL; Calbiochem) or rh SDF-1α (50 ng/mL; PeproTech) was added to the lower chambers of a Costar Transwell 24-well plate (Costar Corning, Cambridge, MA, USA). Aliquots of cell suspension (1×106 cells/100 μL) were loaded onto the upper chambers with 5 μm-pore filters, and were incubated for 3 h (37°C, 95% humidity, 5% CO2) and cells from the lower chambers were scored using FACS analysis. In some experiments, migrated cells harvested from the lower chambers were plated in semisolid methylcellulose base media (R & D Systems), supplemented with rh GM-CSF (7.5 ng/mL) and rh IL-3 (15 ng/mL) and cultures were incubated for 7 days at which time they were scored for CFU-GM colonies. In another set of experiments, responses towards various concentrations of SDF-1 were evaluated as described previously [20] using media without (control) or with SDF-1α (50, 100 and 200 ng/mL; Biomedical Research Centre, University of British Columbia, Vancouver, BC, Canada) placed in the lower chambers. Aliquots of PMN cells (2 × 105 cells) without (control) or with rh C5a (100 ng/mL), and in the presence of epigallocatechin gallate (EGCG, 50 μM), o-phenanthroline (0.5 mM) or neutrophil serine protease inhibitor phenylmethylsulphonyl fluoride (PMSF, 1 mM), were loaded onto the upper chambers and incubated for 3 h (37°C, 95% humidity, 5% CO2). Cells that had migrated to the bottom chamber were harvested and counted using a hemocytometer.
Zymography and Western blot
BM leukocytes, PMN from normal donors and MNC from cord blood or PB (2 × 106/mL) were incubated at 37°C in serum-free IMDM in the absence (control) or presence of 100 ng/mL C5a. After 24 h, the cell-conditioned media were collected and analyzed by zymography to determine MMP-2 and MMP-9 activities as previously described [21]. Cell lysates were also collected and analyzed for expression of MT1-MMP by Western blot as we previously described [18]. Densitometric analysis was carried out using the AlphaEase FCr image analysis software (Alpha Innotech).
Statistical analysis
The correlations between desArgC5a plasma levels and CD34+ cell and WBC counts were analyzed using linear regression. Correlation coefficient values (r) > 0.5 were considered significant. Arithmetic means and standard deviations were calculated and statistical significance was defined as p ≤ 0.05 using Student’s t test.
Results
C5 cleavage fragment levels are higher in the plasma of good mobilizers
Since mobilization is impaired in C5-deficient mice [14,19], we first evaluated whether plasma levels of C5 cleavage fragments differ in patients who are good versus poor mobilizers. Because in human plasma or serum C5a is rapidly cleaved to the stable desArgC5a form, we employed an ELISA assay using monoclonal antibody specific for human desArgC5a. Initially, we evaluated three plasma samples obtained from patients having broadly different mobilization responses and which had been stored at −70°C for less than a month. Patient #1 was a very poor mobilizer (CD34+ cells, 4/μL; WBC, 0.9 × 106/mL); patient #2 was an intermediate mobilizer (CD34+ cells, 78/μL; WBC, 14.2 × 106/mL) and patient #3 a very good mobilizer (CD34+ cells, 321/μL; WBC, 47.9 × 106/mL). Figure 1A shows that patient #3 exhibited the highest plasma level of desArgC5a (37.9 ± 1.0 ng/mL), while patient #1 had the lowest (1.4 ± 1.9 ng/mL). We then screened plasma samples taken from more mobilized patients (n = 9) and stored for longer periods of time, and found significant positive correlations between the number of CD34+ cells/μL in the blood and plasma desArgC5a levels (r = 0.81, p = 0.04, Fig. 1B, left panel), and between WBC count and desArgC5a levels (r = 0.87, p = 0.005, Fig. 1B, right panel) on the day of leukapheresis. It is worth noting that the ELISA results of plasma samples that were stored for a longer period had relatively higher levels of desArgC5a, reflecting gradual in vitro complement activation during storage.
Figure 1.
desArgC5a levels correlate with mobilization responses. (A) desArgC5a in plasma was measured by ELISA, and CD34+ cell and WBC counts were evaluated in the G-CSF-mobilized patients, on the day of leukapheresis. Levels of desArgC5a in the plasma of three selected patients are shown: patient #1, a poor mobilizer, patient #2 intermediate, and patient #3 a very good mobilizer. (B) Positive correlations between desArgC5a levels and CD34+ cell counts and WBC counts and are shown for more plasma samples (n = 9 patients). Samples for ELISA were analyzed in duplicates.
desArgC5a but not C5a chemoattracts PMN cells at physiological concentrations
Previously we have shown that desArgC5a chemoattracts mouse BM MNC [19]. To evaluate the role of C5 cleavage fragments in the mobilization of human HSPC we examined the chemotactic responses of PB and BM nucleated cells to physiological concentrations of C5a and desArgC5a. We found that these cells were strongly chemoattacted to desArgC5a but not to C5a (Fig. 2A) and, interestingly, chemoattraction towards desArgC5a was several times stronger than that towards SDF-1 (50 ng/mL). On the other hand, neither desArgC5a nor C5a chemoattracted human CFU-GM progenitor cells (Fig. 2B).
Figure 2.
Chemotactic effect of C5a and desArgC5a on human PB and BM nucleated cells. (A) Migration of PB (left) and BM (right) nucleated cells (NC). (B) Migration of BM CFU-GM progenitors. In physiological concentrations, desArgC5a but not C5a, strongly chemoattracts both PB and BM nucleated cells. Chemotactic responses to desArgC5a are several times stronger than those to SDF-1. On the other hand, neither desArgC5a nor C5a chemoattracted BM CFU-GM progenitor cells. Values are the fold-increases of migrated cells compared to media alone (M). *p < 0.05 as compared with media alone (control). The data shown represents the combined results of three independent experiments carried out in triplicate per group (n = 9).
C5a/desArgC5a receptor CD88 is expressed on myeloid precursors and mature cells and G-CSF upregulates it
Next, we examined the expression of CD88 on human hematopoietic cells at different levels of maturation/differentiation. CD88 could not be detected in CD34+ cells isolated from BM, mPB and CB (Fig. 3A), which is consistent with their lack of response to C5a and desArgC5a as described above (Fig. 2B). However, CD88 appeared first on the surface of ex vivo-expanded myeloid and megakaryocytic precursors (day 6) (Fig. 3B). When we compared CD88 expression on normal (steady-state) PB lymphocytes, monocytes and PMN cells with their mPB counterpart cells, we found that while surface CD88 was undetectable on lymphocytes from either population, it was expressed on normal PB monocytes and PMN cells and even more highly on monocytes and PMN cells from mPB (Fig. 3C). When we stimulated normal steady-state BM leukocytes with G-CSF in vitro, we found that it upregulated CD88 surface expression on these cells (Fig. 3D). Taken together, these data indicate that during in vivo G-CSF-induced mobilization or in vitro pre-incubation with G-CSF, CD88 expression increases on more mature myeloid cells.
Figure 3.
C5a/desArgC5a receptor (CD88) expression on myeloid precursors, monocytes and PMN. (A) Isolated BM, mPB and CB CD34+ cells were stained with either mouse IgG or anti-CD88 MoAb followed by incubation with goat anti-mouse AlexaFluor-488 secondary antibody. Filled and open histograms correspond to isotype IgG and CD88, respectively. (B) CD88 expression on ex vivo-expanded erythroid, myeloid and megakaryocytic precursors. Cells were expanded as described in Materials and methods and FACS analysis was carried out on days 3, 6 and 11 of expansion. Lineage marker expression was evaluated on day 11 of expansion. (C) FACS analysis of CD88 expression on lymphocytes, monocytes and PMN cells derived from normal (n) and mobilized (m) PB. (D) FACS analysis of CD88 expression on BM leukocytes stimulated or not (control) with G-CSF (100 ng/mL, 48 h). Representative data from three independent experiments are shown.
C5a decreases surface CXCR4 expression on PMN cells
As the SDF-1/CXCR4 axis plays a critical role in HSPC mobilization, we evaluated the effect of C5a on CXCR4 expression in various leukocyte subpopulations derived from normal (n)PB and G-CSF-mobilized PB. CXCR4 expression on each subpopulation was evaluated using CD45 gating (Fig. 4A). We found that CXCR4 expression is significantly lower in PMN cells obtained from mPB than from nPB but does not differ between the monocyte and lymphocyte populations from either source (Fig. 4B). We also found that C5a reduces CXCR4 surface expression (in a dose-dependent manner) on monocytes and PMN, but not on lymphocytes (Fig. 4C).
Figure 4.
C5a downregulates surface CXCR4 expression in monocytes and PMN. (A) Definition of subsets of leukocytes (lymphocytes, monocytes and PMN cells) using FSC and SSC analysis. Leukocyte subpopulations were gated using CD45-FITC staining. (B) PB leukocytes were obtained from either normal donors (n = 4) or G-CSF-mobilized patients (n = 6) and co-stained with anti-CXCR4-PECy5; mean percentage of CXCR4 expression is presented. * p ≤ 0.05 (C) Various populations of PB leukocytes were stimulated or not (control) with C5a or G-CSF (100 ng/mL) for 15 h and CXCR4 expression was evaluated by FACS analysis. Cells were stained and gated as described in A. (D) CXCR4 expression in PMN cells after treatment with C5a and anti-C5a antibody. The colors of the histograms (left panel) and bars (right panel) correspond to the percentage of CXCR4 expression in cells labelled with isotype control IgG (black), untreated cells (red), after treatment with C5a (green), and after treatment with C5a and anti-C5a antibody (blue). Representative data from four independent experiments are shown.
To confirm that C5a suppressed CXCR4 surface expression, we stained PMN cells for CXCR4 after incubating them with C5a and anti-C5a antibody. We found that anti-C5a antibody reversed the reduction in CXCR4 expression brought about by C5a (Fig. 4D). We also observed that G-CSF reduces the expression of CXCR4 (Fig. 4C), which is consistent with the findings by others that G-CSF down-regulates CXCR4 on myeloid cells [22].
C5a stimulates expression of proteolytic enzymes in PMN cells and MNC
As proteolytic enzymes play a critical role in the mobilization of HSPC, we examined whether C5a treatment induces MMPs and CPM expression. MMP secretion was quantified by densitometric analysis of zymograms in the absence or presence of exogenous C5a, corresponding to basal and induced levels of MMP activity, respectively. We found that C5a treatment of PMN cells and MNC derived from normal PB enhances MMP-9 activity up to 3-fold (Fig. 5A). As measured by gel-based RT-PCR, C5a also slightly upregulated or had no effect on the expression of MT1-MMP in MNC and PMN cells, but when real-time RT-PCR was used a significant upregulation could be observed in MNC (Fig. 5B). Using Western blotting, we confirmed an upregulation of MT1-MMP by C5a in leukocytes and MNC. Furthermore, after incubation of PMN cells and MNC, expression of the CPM gene increased about 2-fold with C5a (Fig. 5C).
Figure 5.
C5a enhances production of proteases. (A) MMP-9 secretion by PMN cells (n = 4) and MNC (n = 3) after stimulation with C5a (100 ng/mL) for 24 h at 37°C as analyzed by zymography. Medium conditioned by fibrosarcoma HT-1080 cells was used as a standard (Std) to indicate the position of soluble MMPs. (B) MT1-MMP gene expression (by semi-quantitative and real-time RT-PCR) and protein expression (by Western blotting) in PMN (n = 4) and MNC (n = 3) in the absence (control) or presence of C5a (100 ng/mL for 24 h at 37°C). * p ≤ 0.05. (C) CPM gene expression in PMN cells (n = 3) and MNC (n = 3) after incubation or not (control) with C5a (100 ng/mL for 24 h at 37°C). GAPDH was used as internal loading control for the RT-PCR. The numbers at the bottom of the gels indicate fold-increase in expression relative to control as determined by densitometric analysis.
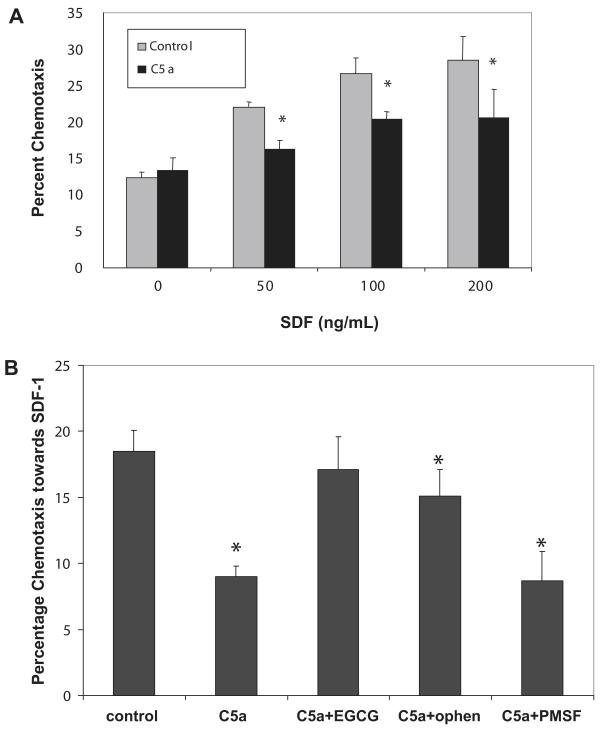
C5a decreases chemotactic responsiveness of PMN cells to an SDF-1 gradient, which is restored by MMP inhibitors
We then used a chemotaxis assay to determine whether C5a-induced reduction of CXCR4 expression on PMN cells results in abrogated responses to SDF-1 and found lower chemoattraction of C5a-treated cells towards various gradients of SDF-1 (Fig. 6A). Moreover, in the presence of the MMP inhibitors (EGCG and o-phenanthroline), but not with neutrophil serine protease inhibitor (PMSF), chemotaxis towards SDF-1 was restored, which further confirms that CXCR4 down-regulation is mediated by MMPs (Fig. 6B).
Figure 6.
Chemotaxis of C5a-treated PMN cells towards SDF-1 in the presence of protease inhibitors. (A) Chemotaxis towards media alone or various concentrations of SDF-1 of normal PMN cells (n = 2) after incubation or not (control) with 100 ng/mL C5a for 15 h. * p ≤ 0.05. (B) Chemotaxis towards SDF-1 (100 ng/mL) of normal PMN cells (n = 2) after incubation or not (control) with C5a (100 ng/mL) in the presence of the MMP inhibitors, epigallocatechin gallate (EGCG, 50 μM) and o-phenanthroline (ophen, 0.5 mM) or neutrophil serine protease inhibitor phenylmethylsulphonyl fluoride (PMSF, 1 mM). Data is representative of two independent experiments done in quadruplicates. * p ≤ 0.05 relative to control.
Discussion
The increased use of mPB as a source of HSPC has challenged clinical investigators to find new targets for agents that can improve the efficiency of stem cell mobilization and collection [23]. The initial report that mobilization in patients suffering from SCID was significantly lower than in normal donors [10] suggested that the immune system could be linked to the mobilization process. Indeed, our recent studies show that the CC is activated during mobilization and that complement cleavage fragments modulate HSPC trafficking [4]. Previously, we reported that C3a/C3aR-deficient mice are good mobilizers [13], and conversely cells from C3aR-deficient mice display defective homing after hematopoietic transplant [24]. On the other hand, C5- and Ig-deficient mice (which do not activate complement by the classic pathway) are poor mobilizers [14]. Hence, the aim of this study was to elucidate C5a/desArgC5a-mediated mechanisms that govern egress of HSPC from the BM into the circulation during G-CSF-induced mobilization. This could shed more light on why some patients mobilize HSPC better than others do.
We found that, like murine HSPC, immature human HSPC do not express the C5a/desArgC5a receptor (CD88) [19], although more mature myeloid cells do. This supports the idea that the pro-mobilization effects of C5a/desArgC5a are mediated indirectly through more mature cells rather than directly on HSPC [1]. In particular, we know that neutrophils are essential for HSPC mobilization as neutropenic mice do not mobilize, but this process can be restored by the infusion of purified mature neutrophils [25]. Moreover, we and others have shown that the release of granulocytes from the BM always precedes mobilization of HSPC in murine models as well as in mobilized patients [19,26]. Furthermore, we demonstrated here that in the PB of mobilized patients, monocytes and PMN cells express CD88 in significantly higher levels than in normal unmobilized PB, and that in vitro G-CSF stimulation upregulates CD88 surface expression in steady-state BM leukocytes. In fact, a variety of chemotactic factors, including C5a/desArgC5a, has been shown to induce a rapid neutrophilia in PB when injected intravenously into rabbits and mice, indicating that these factors can create gradients from the blood across the sinusoidal endothelium, thereby driving the egress of neutrophils from the BM [27]. Moreover, we recently showed that desArgC5a but not C5a is a strong chemoattractant for murine granulocytes [19]. Consistently, in this work we found that desArgC5a strongly chemoattracted human PB and BM nucleated cells and that high desArgC5a levels in plasma correlated with good HSPC mobilization response as well as WBC count in the PB.
The next important finding we report here is the decrease in surface expression of CXCR4 in PMN cells treated with C5a, an effect that was reversed by anti-C5a antibody. More interestingly, we demonstrated that the C5a-mediated attenuation in CXCR4 expression in PMN results in reduced chemotactic responsiveness to an SDF-1 gradient.
In addition to reduced CXCR4 expression, the decrease in retention of HSPC in BM could be due to a declining concentration of functional SDF-1 [28]. As reported, a drop in SDF-1 levels in the BM occurs during G-CSF-induced mobilization and coincides with a peak in proteolytic activity [29]. Once released into the BM microenvironment various proteases can cleave and inactivate a number of molecules essential to the retention of HSPC within the BM. In particular, SDF-1 is a substrate for neutrophil elastase, cathepsin G, MMP-2, MMP-9, CD26/DPPIV and CPM [15,16,30-32]. Here we demonstrated significant upregulation of MMP-9 in PMN and MNC by C5a, consistent with a previous study [33]. Increased MMP-9 in neutrophil cytoplasm has been detected by immunohistochemical staining of BM sections after mobilization with G-CSF [34]. For the most part, neutrophils are considered the predominant MMP-9-secreting cells involved in HSPC mobilization. We envision that SDF-1 in the BM is inactivated by MMP-9, thereby weakening HSPC tethering in the BM niche and subsequently causing their egress. Although in this work we did not employ enzymatic assays using fluorescent peptide substrates to establish enhanced MMP-9 activities induced by C5a, we were nevertheless able to indirectly demonstrate that the effects mediated by C5a (such as decreased cell surface CXCR4 expression and abrogated chemotactic response towards an SDF-1 gradient) may be mediated through MMPs as these effects were reversed in the presence of MMP inhibitors.
Aside from soluble MMP-9, C5a also enhanced the expression in PMN cells and MNC of two membrane-bound enzymes known to inactivate SDF-1, namely MT1-MMP and CPM. MT1-MMP degrades extracellular matrix macromolecules, cytokines and chemokines, including SDF-1, and stimulates cell mobility and migration [35]. We previously showed that mesenchymal stem cells, BM CD34+ cells and hematopoietic progenitors such as CFU-Meg express MT1-MMP, which mediates their migration [18,20,36]. MT1-MMP also mediates migration of endothelial cells and monocytes [37,38]. Moreover, MT1-MMP activates proMMP-2, which contributes to the elevation of the proteolytic activity in the BM microenvironment and subsequently mobilization of HSPC [39]. Recently, we reported that CPM is ubiquitously expressed by cells in the BM microenvironment and is upregulated by treatment with G-CSF [16]. Unlike the other proteases (e.g., elastases, cathepsin G and MMP-9, released in large amounts in the BM by neutrophils during G-CSF-induced mobilization), which inactivate SDF-1α by cleaving amino acids in the N-terminal region, CPM rapidly cleaves the C-terminal lysine of SDF-1α resulting in attenuated chemotactic responses of HSPC in vitro [16]. Moreover, as it has been suggested that the C-terminal lysine of SDF-1α contributes to its binding on the cell surface and preserving the activity of SDF-1α [40], we proposed that CPM cleavage of lysine could facilitate the release of SDF-1α from the cell surface, rendering it more susceptible to degradation by other proteases.
Our results underscore the contribution of C5a in creating a highly proteolytic microenvironment in the BM that is conducive to the egress of HSPC, as well as the indispensable role of neutrophils in creating a path through the endothelial barrier for the HSPC to pass through [19,24,41,42]. Treatment with Flt3 ligand and SCF is also associated with a decrease in SDF-1 in the BM and it has been shown that suppression of SDF-1-producing osteoblast lineage cells appears to be a shared feature of cytokine-induced HSPC mobilization [43]. Here we show that C5a cleavage fragments affect SDF-1 level indirectly by promoting proteolytic degradation of this chemokine.
Taken together, our findings support a model in which signals induced by G-CSF that contribute to mobilization are generated as part of the innate immunity response and converge at the SDF-1/CXCR4 axis. The experiments performed here on human cells as well as our recently published data on an animal model [19] support the idea that C5 cleavage fragments modulate egress of HSPC into mPB at different stages. First, by increasing the secretion of proteolytic enzymes from granulocytes, they attenuate the function of the SDF-1/CXCR4 axis that plays a crucial role in retention of HSPC in BM. Next, by chemoattracting granulocytes, they promote their egress through the endothelial barrier, thus facilitating (or “paving the way”) for subsequent egress of HSPC. Finally, as we recently demonstrated, some cationic peptides secreted by granulocytes activated in PB by C5a may increase the responsiveness of HSPC to the serum SDF-1 level [19]. On this basis, the role of C5a in HSPC mobilization that we show here lends credence to the notion that HSPC trafficking and innate immunity are dynamically linked, andare regulated in a cooperative and reciprocal manner. Our results provide additional insights into an understanding of the directional cues governing the migration of HSPC from the BM, and thus may help promote the development of more efficient mobilization strategies.
Acknowledgments
We would like to thank Jencet Montaño, April Xu and Barbara Pedrycz for excellent technical support. This work was supported by grants from Canadian Blood Services (CBS) R & D/Canadian Institutes of Health Research, Blood Utilization and Conservation Initiative to AJ-W and a CBS Postdoctoral Fellowship award to AJ.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest disclosure
No financial interest/relationships with financial interest relating to the topic of this article have been declared.
References
- 1.Levesque JP, Winkler IG. Mobilization of hematopoietic stem cells: state of the art. Curr Opin Organ Transplant. 2008;13:53–58. doi: 10.1097/MOT.0b013e3282f42473. [DOI] [PubMed] [Google Scholar]
- 2.Pelus LM. Peripheral blood stem cell mobilization: new regimens, new cells, where do we stand. Curr Opin Hematol. 2008;15:285–292. doi: 10.1097/MOH.0b013e328302f43a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Aiuti A, Webb IJ, Bleul C, Springer T, Gutierrez-Ramos JC. The chemokine SDF-1 is a chemoattractant for human CD34(+) hematopoietic progenitor cells and provides a new mechanism to explain the mobilization of CD34(+) progenitors to peripheral blood. J Exp Med. 1997;185:111–120. doi: 10.1084/jem.185.1.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lee H, Ratajczak MZ. Innate immunity: a key player in the mobilization of hematopoietic stem/progenitor cells. Arch Immunol Ther Exp. 2009;57:1–9. doi: 10.1007/s00005-009-0037-6. [DOI] [PubMed] [Google Scholar]
- 5.Spiegel A, Kalinkovich A, Shivtiel S, Kollet O, Lapidot T. Stem cell regulation via dynamic interactions of the nervous and immune systems with the microenvironment. Cell Stem Cell. 2008;3:484–492. doi: 10.1016/j.stem.2008.10.006. [DOI] [PubMed] [Google Scholar]
- 6.Guo R-F, Ward PA. Role of C5a in inflammatory responses. Annu Rev Immunol. 2005;23:821–852. doi: 10.1146/annurev.immunol.23.021704.115835. [DOI] [PubMed] [Google Scholar]
- 7.Lee H, Whitfield PL, Mackay CR. Receptors for complement C5a. The importance of C5aR and the enigmatic role of C5L2. Immunol Cell Biol. 2008;86:153–160. doi: 10.1038/sj.icb.7100166. [DOI] [PubMed] [Google Scholar]
- 8.Monk PN, Scola A-M, Madala P, Fairlie DP. Function, structure and therapeutic potential of complement C5a receptors. Br J Pharmacol. 2007;152:429–448. doi: 10.1038/sj.bjp.0707332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Burger R, Zilow G. Complement-derived anaphylatoxins in natural immunity. In: Sim E, editor. The Natural Immune System: Humoral Factors. Oxford; New York, NY: 1993. p. 209. [Google Scholar]
- 10.Sekhsaria S, Fleisher TA, Vowells S, et al. Granulocyte colony-stimulating factor recruitment of CD34+ progenitors to peripheral blood: Impaired mobilization in chronic granulomatous disease and adenosine deaminase-deficient severe combined immunodeficiency disease patients. Blood. 1996;88:1104–1112. [PubMed] [Google Scholar]
- 11.Reca R, Mastellos D, Majka M, et al. Functional receptor for C3a anaphylatoxin is expressed by normal hematopoietic stem/progenitor cells, and C3a enhances their homing-related responses to SDF-1. Blood. 2003;101:3784–3793. doi: 10.1182/blood-2002-10-3233. [DOI] [PubMed] [Google Scholar]
- 12.Ratajczak MZ, Reca R, Wysoczynski M, Yan J, Ratajczak J. Modulation of the SDF-1–CXCR4 axis by third complement component (C3) – Implications for trafficking of CXCR4+ stem cells. Exp Hematol. 2006;34:986–995. doi: 10.1016/j.exphem.2006.03.015. [DOI] [PubMed] [Google Scholar]
- 13.Ratajczak J, Reca R, Kucia M, et al. Mobilization studies in mice deficient in either C3 or C3a receptor (C3aR) reveal a novel role for complement in retention of hematopoietic stem/progenitor cells in bone marrow. Blood. 2004;103:2071–2078. doi: 10.1182/blood-2003-06-2099. [DOI] [PubMed] [Google Scholar]
- 14.Reca R, Cramer D, Yan J, et al. A novel role of complement in mobilization: immunodeficient mice are poor granulocyte-colony stimulating factor mobilizers because they lack complement-activating immunoglobulins. Stem Cells. 2007;25:3093–3100. doi: 10.1634/stemcells.2007-0525. [DOI] [PubMed] [Google Scholar]
- 15.McQuibban GA, Butler GS, Gong JH, et al. Matrix metalloproteinase activity inactivates the CXC chemokine stromal cell-derived factor-1. J Biol Chem. 2001;276:43503–43508. doi: 10.1074/jbc.M107736200. [DOI] [PubMed] [Google Scholar]
- 16.Marquez-Curtis L, Jalili A, Deiteren K, Shirvaikar N, Lambeir A-M, Janowska-Wieczorek A. Carboxypeptidase M expressed by human bone marrow cells cleaves the C-terminal lysine of stromal cell-derived factor 1 alpha: another player in hematopoietic stem/progenitor cell mobilization? Stem Cells. 2008;26:1211–1220. doi: 10.1634/stemcells.2007-0725. [DOI] [PubMed] [Google Scholar]
- 17.Majka M, Janowska-Wieczorek A, Ratajczak J, et al. Numerous growth factors, cytokines, and chemokines are secreted by human CD34+ cells, myeloblasts, erythroblasts, and megakaryoblasts and regulate normal hematopoiesis in an autocrine/paracrine manner. Blood. 2001;97:3075–3085. doi: 10.1182/blood.v97.10.3075. [DOI] [PubMed] [Google Scholar]
- 18.Son BR, Marquez-Curtis LA, Kucia M, et al. Migration of bone marrow and cord blood mesenchymal stem cells in vitro is regulated by stromal-derived factor-1-CXCR4 and hepatocyte growth factor-c-met axes and involves matrix metalloproteinases. Stem Cells. 2006;24:1254–1264. doi: 10.1634/stemcells.2005-0271. [DOI] [PubMed] [Google Scholar]
- 19.Lee HM, Wu W, Wysoczynski M, et al. Impaired mobilization of hematopoietic stem/progenitor cells in C5-deficient mice supports the pivotal involvement of innate immunity in this process and reveals novel promobilization effects of granulocytes. Leukemia. 2009;23:2052–2062. doi: 10.1038/leu.2009.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Janowska-Wieczorek A, Marquez LA, Dobrowsky A, Ratajczak MZ, Cabuhat ML. Differential MMP and TIMP production by human marrow and peripheral blood CD34+ cells in response to chemokines. Exp Hematol. 2000;28:1274–1285. doi: 10.1016/s0301-472x(00)00532-4. [DOI] [PubMed] [Google Scholar]
- 21.Janowska-Wieczorek A, Marquez LA, Nabholtz JM, et al. Growth factors and cytokines upregulate gelatinase expression in bone marrow CD34+ cells and their transmigration through reconstituted basement membrane. Blood. 1999;93:3379–3390. [PubMed] [Google Scholar]
- 22.Kim HK, De La Luz Sierra M, Williams CK, Gulino AV, Tosato G. G-CSF down-regulation of CXCR4 expression identified as a mechanism for mobilization of myeloid cells. Blood. 2006;108:812–820. doi: 10.1182/blood-2005-10-4162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bensinger W, DiPersio JF, McCarty JM. Improving stem cell mobilization strategies: future directions. Bone Marrow Transplant. 2009;43:181–195. doi: 10.1038/bmt.2008.410. [DOI] [PubMed] [Google Scholar]
- 24.Wysoczynski M, Reca R, Lee H, Wu W, Ratajczak J, Ratajczak MZ. Defective engraftment of C3aR−/− hematopoietic stem cells reveals a novel role of the C3a-C3aR axis in bone marrow homing. Leukemia. 2009;23:1455–1461. doi: 10.1038/leu.2009.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Prujit JF, Verzaal P, van Os R, et al. Neutrophils are indispensable for hematopoietic stem cell mobilization induced by interleukin-8 in mice. Proc Natl Acad Sci USA. 2002;99:6228–6233. doi: 10.1073/pnas.092112999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Molineux G, McCrea C, Yan XQ, Kerzic P, McNiece I. Flt-3 ligand synergizes with granulocyte colony-stimulating factor to increase neutrophil numbers and to mobilize peripheral blood stem cells with long-term repopulating potential. Blood. 1997;89:3998–4004. [PubMed] [Google Scholar]
- 27.Furze RC, Rankin SM. Neutrophil mobilization and clearance in the bone marrow. Immunol. 2008;125:281–288. doi: 10.1111/j.1365-2567.2008.02950.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Petit I, Szyper-Kravitz M, Nagler A, et al. G-CSF induces stem cell mobilization by decreasing bone marrow SDF-1 and up-regulating CXCR4. Nat Immunol. 2002;3:687–694. doi: 10.1038/ni813. [DOI] [PubMed] [Google Scholar]
- 29.Winkler IG, Levesque J-P. Mechanisms of hematopoietic stem cell mobilization: when innate immunity assails the cells that make blood and bone. Exp Hematol. 2006;34:996–1009. doi: 10.1016/j.exphem.2006.04.005. [DOI] [PubMed] [Google Scholar]
- 30.Valenzuela-Fernandez A, Planchenault T, Baleux F, et al. Leukocyte elastase negatively regulates stromal cell-derived factor-1 (SDF-1)/CXCR4 binding and functions by amino-terminal processing of SDF-1 and CXCR4. J Biol Chem. 2002;277:15677–15689. doi: 10.1074/jbc.M111388200. [DOI] [PubMed] [Google Scholar]
- 31.Delgado MB, Clark-Lewis I, Loetscher P, et al. Rapid inactivation of stromal cell-derived factor-1 by cathepsin G associated with lymphocytes. Eur J Immunol. 2001;31:699–707. doi: 10.1002/1521-4141(200103)31:3<699::aid-immu699>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 32.Christopherson KW, 2nd, Cooper S, Broxmeyer HE. Cell surface peptidase CD26/DPPIV mediates G-CSF mobilization of mouse progenitor cells. Blood. 2003;101:4680–4686. doi: 10.1182/blood-2002-12-3893. [DOI] [PubMed] [Google Scholar]
- 33.DiScipio RG, Schraufstatter IU, Sikora L, Zuraw BL, Srimarao P. C5a mediates secretion and activation of matrix metalloproteinase 9 from human eosinophils and neutrophils. Int Immunopharmacol. 2006;6:1109–1118. doi: 10.1016/j.intimp.2006.02.006. [DOI] [PubMed] [Google Scholar]
- 34.Jin F, Zhai Q, Qiu L, et al. Degradation of BM SDF-1 by MMP-9: the role in G-CSF-induced hematopoietic stem/progenitor cell mobilization. Bone Marrow Transplant. 2008;42:581–588. doi: 10.1038/bmt.2008.222. [DOI] [PubMed] [Google Scholar]
- 35.Barbolina MV, Stack MS. Membrane type 1-matrix metalloproteinase: Substrate diversity in pericellular proteolysis. Semin Cell Dev Biol. 2008;19:24–33. doi: 10.1016/j.semcdb.2007.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shirvaikar N, Reca R, Jalili A, et al. CFU-Meg progenitors ex vivo-expanded from cord blood maintain their in vitro homing potential and express matrix metalloproteinases. Cytotherapy. 2008;10:182–192. doi: 10.1080/14653240801910897. [DOI] [PubMed] [Google Scholar]
- 37.Gálvez BG, Matías Román S, Albar JP, et al. Membrane type 1-matrix metalloproteinase is activated during migration of human endothelial cells and modulates endothelial motility and matrix remodelling. J Biol Chem. 2001;276:37491–37500. doi: 10.1074/jbc.M104094200. [DOI] [PubMed] [Google Scholar]
- 38.Matías Román S, Gálvez BG, Genis L, et al. Membrane type 1-matrix metalloproteinase is involved in migration of human monocytes and is regulated through their interaction with fibronectin or endothelium. Blood. 2005;105:3956–3964. doi: 10.1182/blood-2004-06-2382. [DOI] [PubMed] [Google Scholar]
- 39.Vagima Y, Avigdor A, Goichberg P, et al. MT1-MMP and RECK are involved in human CD34+ progenitor cell retention, egress and mobilization. J Clin Invest. 2009;119:492–503. doi: 10.1172/JCI36541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.De La Luz Sierra MD, Yang FQ, Narazaki M, et al. Differential processing of stromal-derived factor 1-alpha and stromal-derived factor-1 beta explains functional diversity. Blood. 2004;103:2452–2459. doi: 10.1182/blood-2003-08-2857. [DOI] [PubMed] [Google Scholar]
- 41.Levesque JP, Hendy J, Takamatsu Y, Williams B, Winkler IG, Simmons PJ. Mobilization by either cyclophosphamide or granulocyte colony-stimulating factor transforms the bone marrow into a highly proteolytic environment. Exp Hematol. 2002;5:440–449. doi: 10.1016/s0301-472x(02)00788-9. [DOI] [PubMed] [Google Scholar]
- 42.Velders GA, Fibbe WE. Involvement of proteases in cytokine-induced hematopoietic stem cell mobilization. Ann NY Acad Sci. 2005;1044:60–69. doi: 10.1196/annals.1349.008. [DOI] [PubMed] [Google Scholar]
- 43.Christopher MJ, Liu F, Hilton MJ, Long F, Link DC. Suppression of CXCL12 production by BM osteoblasts is a common and critical pathway for cytokine-induced mobilization. Blood. 2009;114:1331–1339. doi: 10.1182/blood-2008-10-184754. [DOI] [PMC free article] [PubMed] [Google Scholar]