Abstract
Context
Pain and depression are the most common physical and psychological symptoms in primary care, respectively. Moreover, they co-occur 30-50% of the time and have adverse reciprocal effects on quality of life, disability, and health care costs.
Objectives
To determine if a combined pharmacological and behavioral intervention improves both depression and pain in primary care patients with musculoskeletal pain and comorbid depression.
Design, Setting, and Patients
Randomized controlled trial conducted at 6 community-based clinics of 1 university primary care system and 5 general medicine clinics of 1 Department of Veterans Affairs Medical Center. Recruitment occurred from January 2005 to June 2007 and follow-up concluded in June 2008. The 250 patients had low back, hip or knee pain for ≥ 3 months and at least moderate depression severity (Patient Health Questionnaire-9 score ≥ 10).
Intervention
Patients were randomly assigned to the intervention (n=123) or to usual care (n=127). The intervention consisted of 12 weeks of optimized antidepressant therapy (step 1) followed by 6 sessions of a pain self-management (PSM) program over 12 weeks (step 2) and, lastly, a continuation phase of therapy for 6 months (step 3).
Main Outcome Measures
Assessments at baseline, 1, 3, 6, and 12 months for depression (20-item Hopkins Symptom Checklist [HSCL-20]), pain severity and interference (Brief Pain Inventory [BPI]) and global improvement in pain.
Results
At 12 months, 46 (37.4%) of the 123 intervention patients had a 50% or greater reduction in depression severity from baseline compared with 21 (16.5%) of 127 usual care patients (relative risk [RR], 2.3; 95% CI, 1.5 to 3.2), corresponding to a much lower number with major depression (50 [40.7%] vs. 87 [68.5%]; RR, 0.6; 95% CI, 0.4-0.6). Also, a clinically significant (≥ 30%) reduction in pain was much more likely in intervention patients (51 [41.5%] vs. 22 [17.3%]; RR, 2.4; 95% CI, 1.6-3.2), as was global improvement in pain (58 [47.2%] vs. 16 [12.6%]; RR 3.7, 95% CI, 2.3-6.1). More intervention patients also experienced benefits in terms of our primary outcome, which was a combined improvement in both depression and pain (32 [26.0%] vs. 10 [7.9%]; RR = 3.3; 95% CI, 1.8 to 5.4).
Conclusion
Optimized antidepressant therapy followed by a pain self-management program resulted in substantial improvement in depression as well as moderate reductions in pain severity and disability.
Pain is the most common presenting somatic symptom in medical outpatients,1 and depression is the most common mental disorder.2 Pain complaints account for more than 40% of all symptom-related outpatient visits3, and depression is present in 10-15% of all patients attending primary care. Two-thirds of pain-related outpatient visits are due to musculoskeletal pain, accounting for nearly 70 million outpatient visits in the US each year.3 Back and joint pain result in an estimated 200 million lost work days per year.4 Moreover, pain and depression frequently coexist (30-50% co-occurrence) and have an additive effect on adverse health outcomes and treatment responsiveness of one another.5
Two types of treatment – one pharmacological and the other behavioral – could prove synergistic in the treatment of comorbid musculoskeletal pain and depression. Antidepressants are a well-established therapy for depression, and there is also evidence for at least moderate efficacy in pain which may vary by type of painful disorder and antidepressant class.6,7 Pain self-management (PSM) programs have proven efficacious for both low back pain and osteoarthritis (most commonly located in the hip and/or knee),8,9 with possible secondary benefits in reducing psychological distress.10-12 While literature syntheses have suggested that self-management programs may have a smaller effect on outcomes in musculoskeletal conditions than in other diseases such as diabetes, hypertension and asthma13,14, others argue that outcomes such as pain and function are more complex as are the components or PSM targeting painful conditions.15,16
The Stepped Care for Affective disorders and Musculoskeletal Pain (SCAMP) study was a randomized clinical trial consisting of 12 weeks of optimized antidepressant therapy (step 1) followed by 6 sessions of a pain self-management program delivered over 12 weeks (step 2) and finally a 6-month continuation phase in which symptoms were monitored and treatments reinforced. The study population comprised primary care patients with chronic musculoskeletal pain and comorbid depression. The co-primary outcomes were depression and pain severity at 12 months.
Methods
Study Sample
Details of the SCAMP trial design, study population, and measures have been previously described.17 Patients were recruited from two primary care clinical systems in Indianapolis: the Indiana University Medical Group Primary Care system (6 community-based clinical sites were used) and the Richard L Roudebush Veterans Administration Medical Center 5 general medicine clinics. The local institutional review board approved the study and all enrolled patients gave written informed consent.
Briefly, potential participants were primary care patients with comorbid musculoskeletal pain and depression The pain had to be: (a) located in the low back, hip or knee; (b) persistent for 3 months or longer despite conventional analgesic treatment, defined as prior use of at least two different analgesics; (c) at least moderate in severity, defined as a Brief Pain Inventory score of 5 or greater.18,19 The depression had to be of at least moderate severity, i.e., a PHQ-9 score ≥10 and endorsement of depressed mood and/or anhedonia. More than 90% of patients fulfilling this PHQ-9 criterion have major depression and/or dysthymia, and the remaining patients have clinically significant depression with substantial functional impairment.20,21 Patients on antidepressants who still met the entry criterion for clinical depression were eligible if they had been on an adequate dose of the antidepressant for at least 12 weeks.22,23. Excluded were individuals with severe cognitive impairment, bipolar disorder, substance use disorder, schizophrenia, a pain-related disability claim currently under adjudication, plans to become pregnant in the next year, a life expectancy less than 12 months, or inability to speak English. This trial was approved by the Indiana University Institutional Review Board and was monitored by a local independent Data Safety Monitoring Committee.
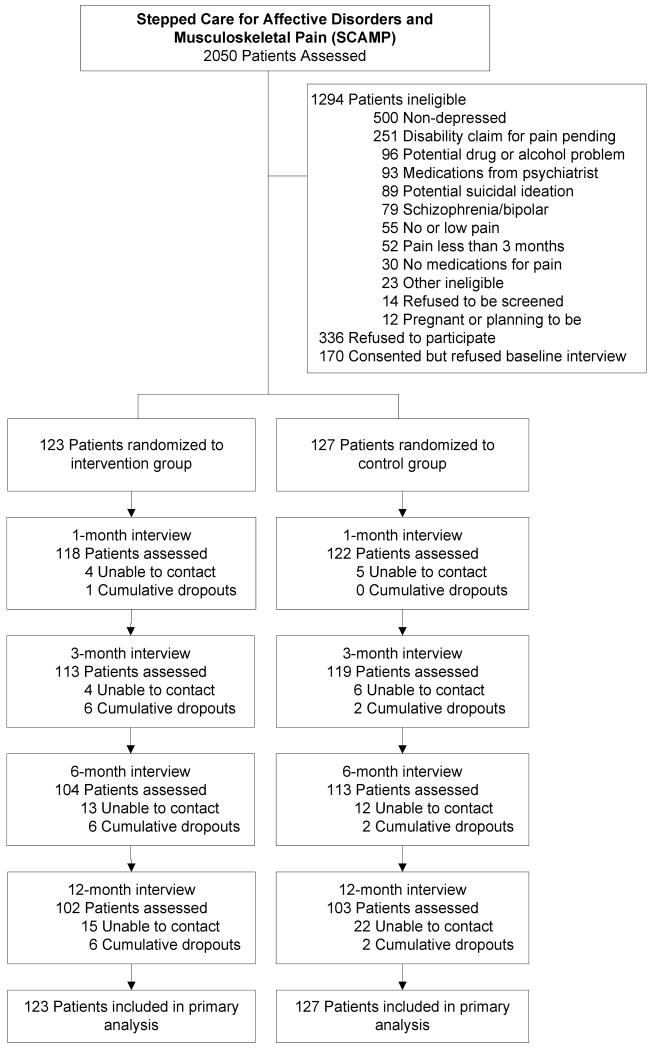
Figure 1 outlines the participant enrollment and follow-up in SCAMP. Of 2050 patients screened, 1294 were not eligible, most often because they were either not depressed (n = 500) or had pain that was minimal, of short duration, or did not require analgesics (n = 137). Of the 756 eligible patients, 250 (33%) enrolled in the trial by providing informed consent and completing a baseline interview after which they were randomized to one of the two study groups.
Figure 1.
Flowchart of participants in the SCAMP trial
Randomization and Blinding
Participants were randomized to intervention vs. usual care groups with randomization stratified by pain location (back vs. leg) and clinic site (university vs. VA). Randomization lists were computer-generated and treatment assignments were supplied in sealed opaque envelopes. The nurse care manager consented individuals and revealed the randomization assignment for each participant by opening the next envelope in the sequence after completion of baseline assessment. All baseline and follow-up outcome assessments were conducted by a research assistant blinded to treatment allocation and uninvolved in care management of participants.
Outcome Assessment
Depression diagnoses were established with the PRIME-MD, which categorizes individuals into 3 DSM-IV diagnostic subgroups: major depression, dysthymia, and other depression.24 Depression severity was assessed, as a primary outcome, with the 20-item Hopkins Symptom Checklist (HSCL-20) which has established sensitivity to change25 and is widely used in effectiveness trials of depression in primary care.21-23,26,27 Pain outcomes included core domains recommended by IMMPACT guidelines for chronic pain clinical trials.28 The Brief Pain Inventory (BPI) was the primary pain outcome.29 The BPI includes a 4-item severity scale (current pain and worst, least, and average pain in past week) and a 7-item interference scale (the degree to which pain interferes with general activity, mood, walking, work, sleep, relations with other people, and enjoyment of life. Global change in pain was assessed with a 7-point scale with the options being worse, the same, or a little, somewhat, moderately, a lot, or completely better. Secondary measures of pain included the Graded Chronic Pain Scale (GCPS) and Roland Disability Scale. In addition to its pain severity and pain disability scales, the GCPS also asks how many days in the past 3 months usual activities have been limited by pain.30 The Roland Disability Scale is a 24-item pain-specific measure of physical disability validated in patients with back pain31 and chronic noncancer pain.32
Several scales or items from the SF-36 33,34 assessed social functioning, vitality, bodily pain, and a single general health perceptions item that has shown to predict long-term health outcomes.35 Anxiety was assessed by the GAD-7, a screening and severity measure validated for the four most common anxiety disorders: generalized anxiety, panic, social anxiety, and posttraumatic stress disorder.36,37 Because pain treatment and pain outcomes may vary by race or ethnicity,38 race/ethnicity (identified by the patient from preselected options) was also included as a demographic characteristic.
Medication refill (i.e., antidepressants and analgesics) and health care use data was extracted from electronic medical records for all participants for the 12-month study period following their enrollment interview. The one exception was that antidepressant use in the intervention group was extracted from care manager logs since intervention participants were provided antidepressants by the study rather than through a prescription. Most patients receive all or most of their medications from the pharmacies inputting prescription information into these EMRs. To assess additional co-interventions, a treatment survey asked participants about any treatments they received for pain and depression during the preceding 3 months at baseline and at the 3, 6, and 12-month follow-up interviews.
Intervention
The intervention model in SCAMP is based upon a depression care management team consisting of a nurse care manager (NCM) supervised by a physician depression specialist and proven effective in multiple primary care depression trials.39 The first 3 months consisted of optimized antidepressant therapy actively managed by the NCM (step1), followed by six sessions of a pain self-management program delivered every other week during the second 3 months (step 2). The final 6 months of the study was a continuation phase focused on relapse prevention. The protocol called for 5 in-person contacts (baseline, 6, 12, 16, and 20 weeks) and 8 telephone contacts (1, 3, 9, 14, 18 and 22 weeks; 8 and 10 months). Extra contacts could occur depending upon treatment changes or patient's clinical needs.
Step 1: Optimized Antidepressant Therapy (Weeks 1-12)
For intervention patients already on an antidepressant at baseline, dose adjustments or medication changes were considered since, despite antidepressant therapy, the patients remain clinically depressed. For patients not on an antidepressant or requiring antidepressant changes, the Appendix outlines the antidepressant algorithm for SCAMP, the rationale for which has been previously described.17 Since there is no evidence that one antidepressant is superior to another in terms of efficacy.40,41, SCAMP was not designed to test any particular antidepressant but, instead, optimal medication management that is both effective and tolerated in an individual patient. An SNRI was offered first in the algorithm because of evidence suggesting that norepinephrine reuptake inhibition (which occurs to a greater degree with tricyclic or SNRI than with SSRI antidepressants) may be particularly important in descending inhibitory pathways related to pain.7 Venlafaxine was selected for SCAMP because it was the most commonly used SNRI at the beginning of the trial and was also scheduled to become generic by the time of study completion.
Appendix. Antidepressant Selection and Dosing Details in SCAMP.
| Priority | Indications and Precautions | Class* | Drug | Dose (in mg) | |
|---|---|---|---|---|---|
| Initial | Dose Increases | ||||
| 1 | Avoid if cardiovascular disease, abnormal ECG, or hypertension not well controlled | SNRI | Venlafaxine | 75 | 150, 225 |
| 2 | SSRI of choice | SSRI | Fluoxetine | 20 | 30, 40 |
| 2 | SSRI of choice if cardiovascular disease | Sertraline | 50 | 100, 150 | |
| 3 | If failed first SSRI (fluoxetine or sertraline) | Citalopram | 20 | 30, 40 | |
| 4 | If obese or weight gain/sexual side effects | Other | Bupropion | 200 | 300, 400 |
| 4 | If insomnia a problem. Avoid if obese. | Mirtazapine | 15 | 30, 45 | |
| 5 | Avoid if cardiovascular disease, abnormal ECG, or hypertension not well controlled | TCA | Nortriptyline | 25 | 50, 75 |
SNRI = serotonin-norepinephrine reuptake inhibitor. SSRI = selective serotonin reuptake inhibitor. TCA = tricyclic antidepressant.
Clinical response was assessed at 3 weeks and, if there was not a clinically meaningful response – defined as a 5 point drop in the PHQ-925 -- a dose increase occurred. Participants who failed to achieve a PHQ-9 <10 and a 50% reduction in PHQ-9 score at 6 weeks were switched to a different antidepressant. Of note, depression rather than pain response dictated antidepressant adjustments. The target PHQ-9 was a score < 5 which approximates depression remission.42
Patients randomized to usual care were informed they had depressive symptoms and that they should seek advice about treatment. There were no other attempts by study personnel to influence depression or pain management unless a psychiatric emergency (e.g., suicidal ideation) arose.
Step 2: Pain Self-Management (PSM) Program (Weeks 13-26)
The PSM sessions were modeled after the successful Stanford self-management program8,9,43 and based on social-cognitive theory44 focusing on increasing self-efficacy and social support to self-manage low back pain or arthritis symptoms. Participants learn to modify their behavior through use and discussion of behavioral plans and problem-solving techniques to sustain behavioral change. The nurse care manager conducted the PSM sessions using a standardized, written protocol adapted from our previous work with musculoskeletal pain.45,46 Details of the PSM program including care manager training and procedures to assure fidelity are detailed elsewhere.17 Briefly, patients learn about chronic pain including triggers and flare-ups; coping with fear and other negative emotions; and strategies for physical activity, muscle relaxation, deep breathing, distraction, sleep hygiene, and working with providers and employers. Each session, the nurse care manager introduces new strategies for patient self-management, assists the patient in choosing strategies, and supervises the patient as he/she practices the chosen strategy. To promote perceived self-efficacy, patients receive individualized feedback about their progress.
Step 3: Continuation Phase (Weeks 27-52)
Intervention patients received scheduled nurse care manager calls at 8 and 10 months to assess symptoms and to evaluate antidepressant and PSM adherence. The care manager assessed current self-management strategies and assisted patients with new behavioral plans. Subjects with a PHQ-9 score ≥ 5 could have their antidepressant dose increased or be switched to a different antidepressant if they had not yet had trials of 2 different antidepressants. Those failing 2 different antidepressant trials were offered a referral to psychiatry. Only those refusing referral to psychiatry could have trials of several more antidepressants during the 12-month study. If antidepressant changes did occur, additional nurse care manager calls were scheduled as in Step 1.
Analysis
Analyses were based on intention-to-treat in all randomized participants. Our primary outcome was a composite depression-pain response at 12 months defined as both a ≥ 50% improvement in depression and ≥ 30% improvement in pain, the standard thresholds for moderate improvement in depression and pain clinical trials.47,48 This composite outcome used the HSCL-20 and BPI total scores; the latter was the average of the 4 BPI severity and the 7 BPI interference items, since both dimensions are considered essential in assessing outcomes of chronic pain therapy.28 The internal reliabilities of the BPI total, severity, and interference scores were similar in our sample (Cronbach's alpha = 0.89, 0.88, and 0.83, respectively). With 97 subjects per group, we projected 80% power to detect a 20% absolute difference in response rates with two-tailed α < .05. Enrolling 250 subjects allowed up to a 25% attrition rate. The resulting sample size also provided 80% power to detect a moderate treatment effect size of 0.4 SD on depression or pain as individual outcomes. While our pre-specified analysis was to compare groups primarily at 12 months and secondarily at intermediate time points, we also conducted repeated measures analyses on the primary depression (HSCL-20) and pain (BPI) outcomes using mixed-effects regression models. Analyses were not adjusted for multiple comparisons. This does not affect interpretation of our primary outcomes, but findings for secondary outcomes should be interpreted cautiously unless they are highly significant (P < .001). Analyses were performed using SAS Version 9.1 (SAS Institute, Cary, North Carolina).
Baseline characteristics were reported and comparisons were made between the two treatment groups. Categorical data were reported as frequencies (%); and differences between groups were compared with chi-square tests. Continuous data were reported as the mean and standard deviation (SD), and the difference between groups were tested using two-sample t-tests.
There was no difference in the magnitude of missing data between the treatment groups. Further, logistic regression models showed that intervention and control participants for which 12-month data was missing did not differ in terms of age, gender, pain location, clinic site, depression or pain severity. Missing outcomes during the follow-up period were imputed using the last-observation-carried-forward (LOCF) method. To assess the robustness of the analytical results under alternative imputation methods, we repeated analyses on all outcomes using multiple regression imputation as well as available data only (no imputation). Results did not differ between these 2 methods and LOCF; thus, we report the results from LOCF because it is the most conservative imputation strategy and also allows straightforward imputation of categorical as well as continuous variables.
Using the 1, 3, 6, and 12-month follow-up data, between-group differences were reported as mean (95% CI) differences for continuous variables and relative risks (95% CI) for categorical variables.49 For key pain and depression continuous outcomes, standardized effect sizes were calculated as the mean group difference divided by the pooled standard deviation for the measure at baseline. For key binary outcomes, the number needed to treat (NNT) was calculated as the reciprocal of the difference in the response rates.50
Results
Baseline Characteristics of Study Sample
As shown in Table 1, there were no significant baseline differences between the intervention and usual care groups. Overall, the mean age of the 250 participants was 55.5 years; 52.8% were women; 60.4% were white, 36.4% black, and 3.2% other. Work status was 25.6% employed, 31.6% unemployed or unable to work, and 42.8% retired. The site of pain was the back in 60.4% of subjects and the hip or knee in 39.6%.
Table 2. Primary Depression and Pain Outcomes (Last-Observation Carried Forward).
| Clinical Outcome | Intervention (123) | Usual Care (127) | Between-Group Difference or Relative Risk (95% CI) | P value |
|---|---|---|---|---|
| Depression outcomes | ||||
| SCL-20 depression (range, 0-4) | ||||
| Baseline | 1.83 (0.66) | 1.94 (0.65) | -0.11 (-0.27 to 0.06) | .20 |
| 6-Month follow-up | 1.16 (0.77) | 1.64 (0.70) | -0.47 (-0.66 to -0.29) | <.0001 |
| 12-Month follow-up | 1.14 (0.69) | 1.69 (0.74) | -0.55 (-0.73 to -0.37) | <.0001 |
| Major depressive disorder, n (%) | ||||
| Baseline | 90 (73.2) | 97 (76.4) | 0.9 (0.8 to 1.1) | .56 |
| 12-Month follow-up | 50 (40.7) | 87 (68.5) | 0.6 (0.4 to 0.8) | <.0001 |
| Depression responder (≥ 50% decrease in SCL-20 from baseline), n (%) | ||||
| 6-Month follow-up | 47 (38.2) | 18 (14.2) | 2.7 (1.8 to 3.8) | <.0001 |
| 12-Month follow-up | 46 (37.4) | 21 (16.5) | 2.3 (1.5 to 3.2) | .0002 |
| Pain outcomes | ||||
| Brief Pain Inventory severity (range, 0-10) | ||||
| Baseline | 6.16 (1.76) | 6.14 (1.78) | 0.02 (-0.42 to 0.46) | .92 |
| 6-Month follow-up | 5.24 (2.51) | 5.86 (2.20) | -0.63 (-1.22 to -0.04) | .0361 |
| 12-Month follow-up | 5.08 (2.54) | 6.03 (2.08) | -0.95 (-1.53 to -0.38) | .0014 |
| Brief Pain Inventory interference (range, 0-10) | ||||
| Baseline | 6.84 (2.15) | 7.09 (1.97) | -0.25 (-0.76 to 0.26) | .34 |
| 6-Month follow-up | 5.05 (2.84) | 6.30 (2.53) | -1.25 (-1.92 to -0.58) | .0003 |
| 12-Month follow-up | 4.96 (2.75) | 6.48 (2.43) | -1.52 (-2.16 to -0.87) | <.0001 |
| Brief Pain Inventory total (range, 0-10) * | ||||
| Baseline | 6.62 (1.85) | 6.77 (1.74) | -0.15 (-0.60 to 0.30) | .51 |
| 6-Month follow-up | 5.04 (2.57) | 6.14 (2.31) | -1.11 (-1.72 to -0.50) | .0004 |
| 12-Month follow-up | 4.94 (2.54) | 6.33 (2.18) | -1.39 (-1.98 to -0.80) | <.0001 |
| Pain responder (≥ 30% decrease in BPI total from baseline), n (%) | ||||
| 6-Month follow-up | 47 (38.2) | 22 (17.3) | 2.2 (1.4 to 3.0) | .0002 |
| 12-Month follow-up | 51 (41.5) | 22 (17.3) | 2.4 (1.6 to 3.2) | <.0001 |
| Composite outcome | ||||
| Composite responder (≥ 50% decrease in SCL-20 and ≥ 30% decrease in BPI total from baseline), n (%) | ||||
| 6-Month follow-up | 29 (23.6) | 10 (7.9) | 3.0 (1.6 to 5.1) | .0006 |
| 12-Month follow-up | 32 (26.0) | 10 (7.9) | 3.3 (1.8 to 5.4) | .0001 |
BPI total is the average of the 11 items of the BPI severity (4 items) and BPI interference (7 items) scales.
As shown in Table 2, intervention and usual care patients were also similar in terms of baseline depression and pain measures. In terms of depression diagnoses, 74.8% of the sample met DSM-IV criteria for major depression, 20.8% for dysthymia only, and 4.4% for minor depression. The mean HSCL-20 score of 1.89 (on a 0-4 scale) represents moderately severe depressive symptoms. Likewise, the mean BPI severity and interference scores of 6.15 and 6.97 (on a 0-10 scale) respectively represent moderately severe pain. This level of disability is confirmed by a Roland disability score of 17.4 (on a 0-24 scale, where a higher score indicates greater pain-related disability) and an SF-36 bodily pain score of 26.8 (on a 0 to 100 scale, where 0 represents the worst pain-related impairment).
Table 3. Secondary Pain and Quality of Life Outcomes (Last-Observation Carried Forward).
| Clinical Outcome | Intervention (123) | Usual Care (127) | Between-Group Difference (95% CI) | P value |
|---|---|---|---|---|
| Roland pain disability (range, 0-24) | ||||
| Baseline | 17.3 (4.5) | 17.6 (4.1) | -0.3 (-1.4 to 0.8) | .57 |
| 12-Month follow-up | 14.0 (6.5) | 17.2 (5.3) | -3.2 (-4.7 to -1.8) | <.0001 |
| Graded chronic pain scale (GCPS) | ||||
| GCPS severity score (range, 0 to 100) | ||||
| Baseline | 72.7 (17.7) | 72.8 (15.4) | -0.1 (-4.2 to 4.1) | .97 |
| 12-Month follow-up | 67.8 (22.8) | 74.7 (17.2) | -6.9 (-12.0 to -1.9) | .007 |
| GCPS disability score (range, 0 to 100) | ||||
| Baseline | 67.8 (25.0) | 70.2 (24.8) | -2.4 (-8.6 to 3.8) | .45 |
| 12-Month follow-up | 52.5 (31.6) | 66.1 (27.3) | -13.6 (-20.9 to -6.2) | .0003 |
| GCPS disability days from pain in past 3 months (range, 0 to 90) | ||||
| Baseline | 34.9 (33.4) | 38.0 (33.1) | -3.1 (-11.4 to 5.2) | .46 |
| 3-Month follow-up | 33.2 (32.3) | 41.5 (33.2) | -8.3 (-16.5 to -0.1) | .046 |
| 6-Month follow-up | 28.2 (31.5) | 31.1 (30.9) | -2.8 (-10.6 to 5.0) | .47 |
| 12-Month follow-up | 31.4 (33.2) | 38.1 (31.8) | -6.8 (-14.9 to 1.3) | .10 |
| GAD-7 anxiety score (range, 0-21) | ||||
| Baseline | 8.7 (4.5) | 9.1 (4.4) | -0.4 (-1.5 to 0.7) | .48 |
| 12-Month follow-up | 5.8 (5.0) | 8.0 (5.1) | -2.2 (-3.5 to -0.9) | .0007 |
| SF functioning/quality of life (range, 0-100) | ||||
| General health perceptions | ||||
| Baseline | 33.1 (27.9) | 28.4 (26.6) | 4.7 (-2.1 to 11.5) | .18 |
| 12-Month follow-up | 35.2 (29.7) | 24.2 (25.5) | 11.1 (4.2 to 18.0) | .002 |
| Social functioning | ||||
| Baseline | 38.0 (25.2) | 40.5 (26.2) | -2.5 (-8.9 to 4.0) | .45 |
| 12-Month follow-up | 53.1 (30.9) | 47.0 (28.5) | 6.1 (-1.3 to 13.5) | .11 |
| Bodily pain | ||||
| Baseline | 26.5 (16.0) | 27.2 (14.1) | -0.7 (-4.5 to 3.0) | .70 |
| 12-Month follow-up | 37.3 (21.1) | 28.8 (16.9) | 8.5 (3.8 to 19.1) | .0005 |
| Vitality | ||||
| Baseline | 25.8 (16.6) | 24.6 (17.3) | 1.2 (-3.0 to 5.4) | .57 |
| 12-Month follow-up | 36.6 (22.7) | 27.8 (18.9) | 8.8 (3.6 to 14.0) | .001 |
Clinical Outcomes
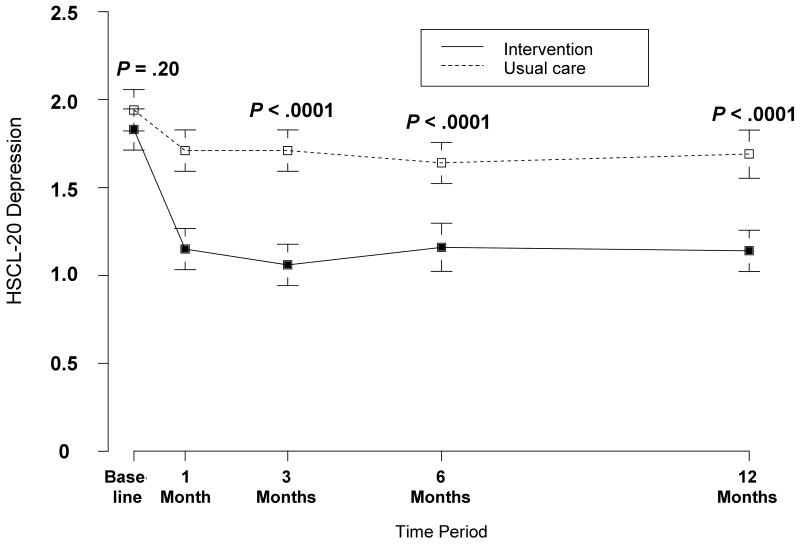
As shown in Table 2, the intervention group had significantly better HSCL-20 depression outcomes; the difference between groups at baseline and 12 months was -0.11 and -0.55, respectively. Accounting for baseline differences, this resulted in a net between-group difference of - 0.44 (95% CI, -0.62 to -0.26). This equates to a standardized treatment effect size of 0.67 (0.44 divided by the pooled SD for the HSCL-20 at baseline of 0.653). Figure 2 illustrates the substantial intervention effect that occurred by 1 month and was sustained over the 12-month trial. The intervention group also was much more likely to experience depression response (46 [37.4%] of the 123 intervention patients vs. 21 [16.5%] of the 127 patients; RR = 2.3, 95% CI, 1.5-3.2) and complete remission (22 [17.9%] vs. 6 [4.7%]; RR = 3.8, 95% CI, 1.6-7.6) at 12 months, corresponding to a much lower number with major depression (50 [40.7%] vs. 87 [68.5%]; RR = 0.6, 95% CI, 0.4-0.8). The number needed to treat (NNT) for depression response was 1/(.374 - .165), or 4.8 (95% CI, 3.4-8.3)
Figure 2.
Mean HSCL-20 depression scores in the intervention (solid line) and usual care (dashed line) groups. HSCL-20 scores can range from 0 to 4. Error bars indicate SEs. All study participants are included in each time period since last-observation carried forward imputation is used.
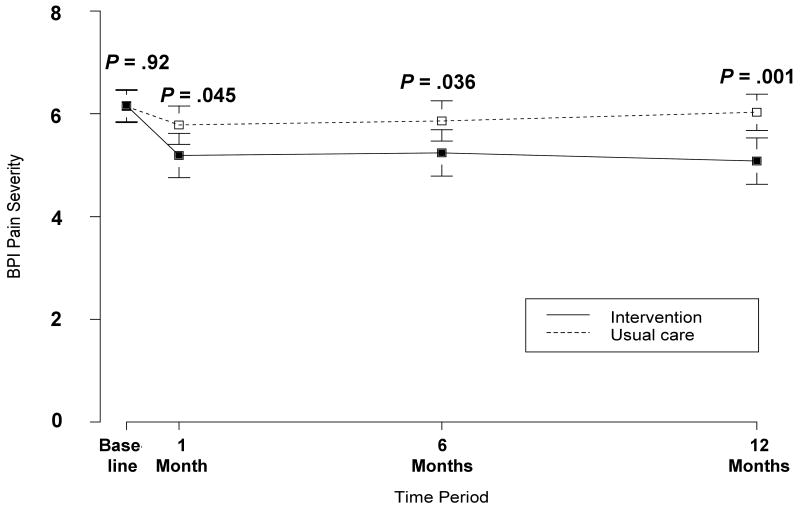
Table 2 also shows the effectiveness of the intervention on pain outcomes. The intervention group had significantly better BPI severity (net between-group difference of -0.98; 95% CI, -1.48 to -0.47) and BPI interference (net between-group difference of -1.27; 95% CI, -1.88 to -0.66) scores at 12 months. This represented standardized effect sizes of 0.54 for BPI severity and 0.62 for BPI interference. Figure 3 illustrates the significant intervention effect on BPI interference (Figure 3A) and BPI severity (Figure 3B) occurring by 1 month and sustained over the 12-month trial. Though both dimensions of pain improved significantly, the reductions in pain interference were even greater than in pain severity The intervention group was also much more likely to experience a ≥ 30% reduction in pain at 12 months (51 [41.5%] vs. 22 [17.3%]; RR = 2.4, 95% CI, 1.6-3.2). This corresponds to a NNT of 4.1 (95% CI, 3.0-6.5)
Figure 3.
Mean BPI pain interference (3A) and pain severity (3B) scores in the intervention (solid line) and usual care (dashed line) groups. BPI scores can range from 0 to 10. Error bars indicate SEs. All study participants are included in each time period since last-observation carried forward imputation is used.
In terms of our trial's primary and most conservative outcome, the intervention group was significantly more likely to experience a composite response, defined a priori as a ≥ 50% reduction in depression and a ≥ 30% reduction in pain. This difference in composite response rates was significant at both 6 months (23.6% vs. 7.9%; RR = 3.0, 95% CI, 1.6-5.1) and 12 months (32 [26.0%] vs. 10 [7.9%]; RR = 3.3, 95% CI, 1.8-5.4). This corresponds to a NNT of 5.5 (95% CI, 3.7-10.9). Finally, the statistical significance of the intervention effect was similar when fitting mixed effects regression models for our repeatedly measured primary outcomes, i.e., HSCL-20 depression score, BPI severity, and BPI interference.
Intervention patients were much more likely than those in usual care to report overall improvement in their pain at 6 months (61 [49.6%] vs. 19 [15.0%]; RR, 3.3; 95% CI, 2.2-5.2) which was sustained at 12 months (58 [47.2%] vs. 16 [12.6%; RR, 3.7; 95% CI, 2.3-6.1]. Correspondingly, there were fewer patients in the intervention group who were worse (15 vs. 44) and unchanged (50 vs. 67) at 12 months. Of the 58 intervention participants whose pain was better at 12 months, 8 were a little better, 21 were somewhat or moderately better, and 29 were a lot or completely better. In contrast, only 16 usual care participants reported improved pain at 12 months, of whom 3 were a little better, 6 were somewhat or moderately better, and 7 were a lot or completely better.
Table 3 compares the groups in terms of other pain and quality of life outcomes. The intervention group had better outcomes in terms of secondary pain measures (i.e., Roland pain disability, GCPS pain scores, and SF-36 bodily pain), less severe anxiety (GAD-7), and better health-related quality life (SF-36 vitality and general health perception scores).
Table 4. 12-Month Medication and Health Care Use.
| Variable | Intervention (123) | Usual Care (127) | P value * | ||
|---|---|---|---|---|---|
| Mean (SD) | Median (Range) | Mean (SD) | Median (Range) | ||
| Medication use, months | |||||
| Antidepressants | 9.3 (4.2) | 12 (0-12) | 2.0 (3.2) | 0 (0-12) | < .0001 |
| Tricyclics † | 1.2 (2.7) | 0 (0-12) | 1.2 (2.6) | 0 (0-12) | .99 |
| Other psychotropics | 0.7 (2.2) | 0 (0-12) | 0.7 (2.3) | 0 (0-12) | .93 |
| Opioid analgesics | 3.5 (4.6) | 1 (0-12) | 3.0 (4.2) | 1 (0-12) | .40 |
| Other analgesics ‡ | 2.6 (3.1) | 1 (0-12) | 2.8 (3.4) | 1 (0-12) | .57 |
| Health care use, number | |||||
| Outpatient visits | |||||
| Primary care | 6.4 (5.8) | 5 (0-35) | 5.8 (5.4) | 4 (0-33) | .11 |
| Medical specialty | 1.3 (2.3) | 0 (0-16) | 1.6 (2.9) | 0 (0-19) | .02 |
| Surgical specialty | 2.7 (4.0) | 1 (0-24) | 2.4 (3.5) | 1 (0-26) | .11 |
| Mental health | 1.6 (7.9) | 0 (0-82) | 0.7 (2.9) | 0 (0-24) | < .0001 |
| Other | 1.3 (3.3) | 0 (0-23) | 1.3 (2.6) | 0 (0-18) | .89 |
| Emergency dept visits | 1.8 (3.4) | 1 (0-27) | 1.2 (2.1) | 0 (0-14) | < .0001 |
| Hospital days | 1.5 (5.8) | 0 (0-49) | 0.8 (2.5) | 0 (0-15) | < .0001 |
Group differences on medication use were tested using t-test and on health care use using Poisson modeling.
Typically low-dose (< 100 mg amitriptyline or equivalent) rather than antidepressant dose level
The use of “other analgesics” may be underestimated since simple analgesics are often obtained without a prescription and would not be captured by electronic prescribing data.
Care Manager Contacts and Antidepressants Used in the Intervention Group
The intervention protocol called for 5 in-person care manager contacts and 8 telephone contacts over 12 months, with extra contacts allowed depending upon treatment changes or patient's clinical needs. The mean (SD) number of in-person contacts that actually occurred was 2.5 (1.3), and the mean (SD) number of telephone contacts was 11.5 (5.1). Thus, the average intervention patient had 14 contacts over the 12-month study, 82% of which were by phone. The variability was due to early dropout by some intervention patients, extra contacts required for others, and substitution of telephone contacts when an in-person contact was not feasible for the patient.
The antidepressant initiated or continued at baseline was venlafaxine in 75 (61%) of the intervention patients, an SSRI in 39 (32%), or another antidepressant including combinations in 9 (7%). At 12 months, there were 26 (21%) intervention patients on venlafaxine, 36 (29%) on an SSRI, 22 (18%) on other antidepressants including combinations, 16 (13%) on no antidepressant, and 23 (19%) for which the information was not known. Of the 100 intervention subjects whose antidepressant status was known at 12 months, 41% had continued their initial antidepressant, 43% had switched to a different antidepressant (n = 38) or combination pharmacotherapy (n = 5), and 16% were on no antidepressant. Thus, we know that, at a minimum, 43 (35%) of the 123 patients in the intervention group had an antidepressant switched or added during the study. The mean dose for the 79 patients known to be on antidepressant monotherapy at 12 months was 147 mg for venlafaxine (n = 26), 140 mg for sertraline (n = 24), 295 mg for buproprion (n = 10), 36 mg for mirtazapine (n = 7), 27 mg for fluoxetine (n = 6), 40 mg for citalopram (n = 4), and 15 mg for paroxetine (n = 2).
12-Month Medication and Health Care Use
Table 4 summarizes the antidepressant and analgesic refill information as well as health care use for all intervention and usual care participants during the 12 month study period following their enrollment interview. All data was derived from the electronic medical records, except antidepressant use in the intervention participants since they were provided antidepressants free of charge as part of the study. Compared to the usual care, those in the intervention group were on antidepressants during the 12-month study for a much longer period of time (9.3 vs. 2.0 months, P < .0001). Notably, 83 of the participants in the intervention group were on antidepressants all 12 months of the study compared to only 5 in the usual care group (67.5% vs. 3.9%). Of the 52 usual care participants for whom there was EMR data showing any antidepressant use, only 9 (17%) had EMR evidence of an antidepressant being switched or added during their 12 months in the study. There were no significant group differences in terms of low-dose tricyclic, opioid, or non-opioid analgesic use.
Table 5. 12-Month Medication and Health Care Use.
| Variable | No (%) of Patients with Any Use Over 12 Months | Amount of Use in Entire Sample Over 12 Months Mean (SD); Median (Range) | P Value a | ||
|---|---|---|---|---|---|
| Intervention Group (n = 123) | Usual Care Group (n = 127) | Intervention Group (n = 123) | Usual Care Group (n = 127) | ||
| Medication use, mo | |||||
| Antidepressants | 121 (98) | 54 (43) | 9.2 (4.2); 12 (0-12) | 2.0 (3.3); 0 (0-12) | < .001 |
| Tricyclics b | 32 (26) | 35 (28) | 1.2 (2.7); 0 (0-12) | 1.2 (2.6); 0 (0-12) | .98 |
| Other psychotropics | 20 (16) | 16 (13) | 0.7 (2.2); 0 (0-12) | 0.7 (2.2); 0 (0-12) | .89 |
| Opioid analgesics | 67 (54) | 67 (53) | 3.5 (4.6); 1 (0-12) | 3.0 (4.2); 1 (0-12) | .35 |
| Other analgesics c | 74 (60) | 82 (66) | 2.5 (3.1); 1 (0-12) | 2.8 (3.4); 1 (0-12) | .44 |
| Health care use, No. | |||||
| Outpatient visits | |||||
| Primary care | 115 (94) | 119 (94) | 6.3 (5.8) 5 (0-35) | 5.9 (5.3); 4 (0-33) | .16 |
| Medical specialty | 52 (42) | 53 (42) | 1.3 (2.3); 0 (0-16) | 1.6 (2.8); 0 (0-19) | .03 |
| Surgical specialty | 77 (63) | 86 (68) | 2.7(4.0); 1 (0-24) | 2.4 (3.5); 1 (0-26) | .10 |
| Mental health | 31 (25) | 21 (17) | 1.6 (7.9); (0-82) | 0.7 (2.9); 0 (0-24) | < .001 |
| Other | 45 (37) | 53 (42) | 1.4 (3.4); 0 (0-23) | 1.2 (2.4); 0 (0-18) | .16 |
| Emergency dept visits | 61 (50) | 59 (46) | 1.8 (3.5); 0 (0-27) | 1.2 (2.1); 0 (0-14) | < .001 |
| Time in hospital, d | 25 (20) | 18 (14) | 1.5 (5.9); 0 (0-49) | 0.8 (2.5); 0 (0-15) | < .001 |
Differences between group means were compared using t-test for amount of medication use and Poisson modeling for amount of health care use
Typically low-dose (< 100 mg amitriptyline or equivalent) rather than antidepressant dose level
The use of “other analgesics” may be underestimated since simple analgesics are often obtained without a prescription and would not be captured by electronic prescribing data.
Intervention participants had slightly more mental health visits, emergency department visits and hospital days and slightly fewer medical specialty visits. Removing extreme outliers from the analyses did not change the results. Although statistically significant, the absolute magnitude of these differences was small.
Patient-Reported Co-Interventions
Participants were asked to report treatments received for pain or depression since their last interview at 3 timepoints: 3, 6, and 12 months. Patients were classified as having each type of treatment either not at all or at least once during the 12-month period. Intervention patients were slightly less likely than usual care patients to report changes in their pain medicine (52% vs. 63%, P =.016) but did not differ significantly in their likelihood of visiting a pain clinic (34% vs. 28%) or having pain-related emergency department visits (22% vs. 30%), hospitalizations (10% vs. 8%), X-rays (41% vs. 49%), or laboratory tests (20% vs. 31%, P = .06). There were also no differences in visits to a psychiatrist, psychologist or counselor (11% vs. 13%) or to specialists for pain such as physical therapists, orthopedists, rheumatologists, neurologists, or complementary medicine providers. Intervention and usual care participants were also equally likely (50% vs. 49%) to report a change in their medication for “mood, nerves or depression”.
Discussion
The SCAMP trial has several important findings. First, optimized antidepressant therapy coupled with a pain self-management program produced substantial reductions in depression severity as well as enhanced response and remission rates. Second, the intervention also resulted in moderate reductions in both pain severity and pain-related disability. Third, the benefits on both depression and pain outcomes were sustained over the 12 months of the trial, including the 6-month continuation phase.
The effect size of the SCAMP intervention on depression outcomes was similar to that seen in patient populations without chronic pain. In a systematic review of 28 randomized controlled trials of multi-component interventions for primary care patients receiving acute-phase treatment for depression, Williams et al found an 18.4% median absolute increase in patients with a 50% improvement in symptoms (range, 8.3–46%) and a median absolute increase of 16.7% (range, 10.6–40%) in remission from depression.39 The median absolute increases in SCAMP were 20.9% for a 50% response (i.e., 37.4% response in the intervention group vs. 16.5% in the usual care group) and 13.2% for remission (17.9% vs. 4.7%). Also, the number needed to treat (NNT) of 4.8 to achieve a treatment response for depression is similar to the NNT of 4 in a Cochrane review of antidepressants compared with placebo or no treatment in medically ill adults.51
Some of the reasons for improved depression outcomes in SCAMP may be that intervention patients were on antidepressants more months than usual care patients (9.3 vs. 2.0 months), were more likely to be on an antidepressant all 12 months of the study (67.5% vs. 3.9%), and more likely to have an antidepressant switched or added during the study (35% vs. 17%). Continuing an antidepressant at least 6-12 months is known to enhance depression outcomes, and STAR*D and other trials have taught us that an inadequate response to the initial antidepressant is not uncommon and may require a change in medication.39,52 The assessment of antidepressant use may have been more accurate in intervention patients whose medication was provided by the nurse care manager and documented in the study logs, while antidepressant use in usual care patients depended entirely on refill information in the electronic medical record. However, it is unlikely that group differences as large as we found for antidepressant usage are entirely due to ascertainment bias.
The effect size of the SCAMP intervention on pain outcomes − 0.54 for pain severity and 0.62 for pain interference was notable. Chronic pain is difficult to treat and a 30% reduction is typically judged a clinical response – as determined by patient-rated quality of life and perceptions of analgesic efficacy53 – in contrast to the 50% reduction required for depression. Using this threshold, a clinical response in pain was much more likely in the intervention compared to the control group (41.5% vs. 17.3%, or a NNT of 4.1). Impressively, when rating overall change in pain, 47.2% of intervention patients reported improvement at 12 months, compared to only 12.6% of control patients. Thus, the SCAMP intervention showed benefits in terms of 3 pain outcomes considered relevant in clinical trials: pain severity, pain interference, and global pain improvement. It is possible that pain improvement in our trial reflected a main effect of improved mood (i.e., an antidepressant effect on mood rather than an analgesic effect), and that as depression lifts, patients may experience pain as being less intense and less disabling. Conversely, it is also possible that the improvement in depression was mediated by an improvement in pain (i.e., as pain improves, patients feel less depressed) or that both depression and pain lessened as a result of treatment effects on a common pathway.
The largest reductions in depression and pain were seen early (i.e., during the first month of optimized antidepressant therapy) and were sustained during the remainder of the trial. Thus, the added value of the pain self-management program cannot be ascertained in SCAMP. We had postulated that improvements in pain that might occur with optimized antidepressant therapy would be further enhanced with a behavioral intervention designed to improve pain coping and other self-management skills. It is possible that, without the pain self-management program, patients whose pain initially improved might have suffered a relapse. However, it is also possible that antidepressant continuation, as occurred in SCAMP, is sufficient. To test whether pain self-management provides any benefits beyond optimized antidepressant therapy would require a parallel group or factorial trial design rather than the sequential approach used in the current study.
While the between-group differences (i.e., treatment effect) were similar to previous depression trials, the absolute response and remission rates in both intervention and control groups were low compared to other depression care management studies.39,54 and closer to that seen in populations with more medical comorbidity.22,51 Indeed, secondary analyses of two collaborative care interventions found that high baseline pain reduces depression improvement rates.55,56 A secondary analysis of patients with comorbid pain in a geriatric depression trial57 found a much more modest ES for pain outcomes than for depression. Thus, additional interventions to co-manage pain (e.g., optimized analgesic management) may be necessary to further improve response and remission rates. For example, Dobscha and colleagues recently found that a collaborative care intervention for chronic pain that included clinician education, patient education and activation, symptom monitoring, feedback and recommendations to clinicians, and facilitation of specialty care produced modest improvement in both pain and depression outcomes.58
In addition to improving depression and pain outcomes, the SCAMP intervention also demonstrated benefits in terms of secondary measures such as anxiety, functional impairment, and quality of life. There was also a nonsignificant trend towards fewer pain-related disability days. Since pain and depression are among the two leading causes of decreased work productivity59,60, interventions that improve both of these symptoms as well as their adverse impact on functional status might be particularly desirable from not only the patient but also the employer and societal perspectives.
SCAMP used an antidepressant algorithm rather than a single antidepressant, and more than half of the patients discontinued or switched from their initial antidepressant by 12 months. Therefore, the superiority of one antidepressant over another in comorbid depression and pain cannot be determined. Studies to date have failed to establish differential efficacy among antidepressants in terms of depression outcomes.21,52 For pain, tricyclic antidepressants (TCAs) may be somewhat more effective than SSRI antidepressants.6,61,62 However, head-to-head comparisons are few, previous trials are short in duration, and many trials have used lower doses of TCAs than are normally required for an optimal effect on depression. Several SNRI antidepressants now have FDA indications for treating neuropathic pain and fibromyalgia7 but their efficacy in the painful conditions studied in our SCAMP trial (low back pain and osteoarthritis of the hip and knee) requires further research. Also their relative superiority compared to other antidepressants in terms of improving pain is less certain63 and would require active comparator trials. Notably, all antidepressants used in our SCAMP trial are now available in generic formulations.
Neither data from electronic medical records nor patient self-report suggested that group differences were significantly confounded by co-interventions. The average intervention patient averaged slightly more than one care manager contact per month over the 12-month study, with most of these (82%) occurring by telephone. Health care use was slightly higher for intervention patients in a few categories of visits, but these differences did not appear clinically significant. Though we did not design our single-site study to conduct a formal cost-effectiveness analysis, the care manager contacts together with greater antidepressant use and no decrease in health care use certainly indicates there was some added cost to achieve the improved depression and pain outcomes in the intervention group. This is consistent with many other primary care trials comparing enhanced depression care to usual care,64,65. However, a recent multi-center trial with sophisticated cost analyses found that the cost per quality-adjusted life year for enhanced depression care compared favorably with many other medical interventions66 and it is possible that increased costs incurred during the first year may be recouped with cost savings in subsequent years.67
Our study has several limitations. First, since patients were enrolled from urban underserved and veteran clinics, the generalizability of our results to other primary care populations needs to be demonstrated. However, adverse socioeconomic factors more prevalent in our sample tend to make treatment of depression and pain more difficult, so the substantial effects we observed are noteworthy. Second, since only one-third of eligible patients agreed to enroll, the extent to which the benefits we found are generalizable to patients not desiring participation in a trial is uncertain. Third, SCAMP is a multi-component effectiveness trial. Therefore, to what degree benefits can be specifically attributed to the antidepressant-behavioral intervention vs. the nonspecific effects of care manager contacts cannot be precisely determined. Also, the lack of blinding in an effectiveness design may inflate somewhat the benefits specifically attributable to the intervention. However, a recent literature synthesis of depression care management trials showed that, like our SCAMP study, an effectiveness trial with a usual care control group has been the most common study design.39 Also, a number of patients in the usual care group received antidepressants which might tend to reduce the effect size of the intervention. Importantly, the two treatment groups did not differ in terms of self-reported pain or depression co-interventions over the 12-month trial.
In summary, SCAMP showed that optimized antidepressant therapy coupled with pain self-management in patients with comorbid pain and depression can produce substantial improvements in both depression and pain. At the same time, additional interventions may be needed to produce larger improvements in pain and higher depression response and remission rates. Strategies might include optimized analgesic management, cognitive-behavioral therapy6,68, or augmentation strategies.41 While numerous trials have shown that enhanced care for depression is equally or more cost-effective than the treatment of chronic medical diseases, the lack of parity for mental disorders leads some payers to insist that depression care must be cost-neutral or even cost-savings.64,65 A recent trial demonstrated that depression care management improved workplace as well as clinical outcomes.69 Since pain and depression are among the leading causes of decreased work productivity, an intervention that is effective for both conditions may further strengthen the “business case”. Also, an intervention that allows a care manager to cover several conditions rather than a single disorder may enhance its implementation and cost-effectiveness. Given the prevalence, morbidity, disability, and costs of the pain-depression dyad, the SCAMP results have important implications.
Table 1. Baseline Characteristics of the 250 Participants in the SCAMP Trial.
| Baseline Patient Characteristic | Intervention Group (N=123) | Usual Care Group (N=127) | P Value |
|---|---|---|---|
| Mean (SD) age, yr | 55.2 (12.6) | 55.8 (11.1) | .70 |
| Women, n (%) | 69 (56) | 63 (50) | .30 |
| Race, n (%) | .29 | ||
| White | 75 (61) | 76 (60) | |
| Black | 42 (34) | 49 (39) | |
| Other | 6 (5) | 2 (2) | |
| Education, n (%) | .65 | ||
| Less than High school | 28 (23) | 32 (25) | |
| High school | 54 (44) | 48 (38) | |
| At least some college or trade school | 41 (33) | 46 (37) | |
| Married, n (%) | 48 (39) | 44 (35) | .47 |
| Employment status, n (%) | .35 | ||
| Employed | 36 (29) | 28 (22) | |
| Unemployed or unable to work | 39 (32) | 40 (32) | |
| Retired | 48 (39) | 59 (47) | |
| Pain location, n (%) | .66 | ||
| Back | 76 (62) | 75 (59) | |
| Hip or knee | 47 (38) | 52 (41) | |
| Clinical site, n (%) | .96 | ||
| Veteran administration (VA) | 50 (41) | 52 (41) | |
| University clinics | 73 (59) | 75 (59) | |
| Median duration of pain, yr (interquartile range) * | 8 (3-21) | 10 (4-20) | .81 |
| Mean (SD) no. of medical diseases | 2.7 (1.6) | 2.7 (1.4) | .62 |
| Baseline medications, n (%) † | |||
| Opioid analgesics | 63 (52) | 49 (42) | .12 |
| Non-opioid analgesics | 88 (72) | 85 (72) | .99 |
| Tricyclic (TCA) antidepressants | 29 (24) | 16 (14) | .04 |
| Non-TCA antidepressants | 28 (23) | 17 (14) | .09 |
| Nonpharmacological treatments for pain | 31 (25) | 28 (22) | .56 |
| Have seen mental health specialist | 51 (41) | 59 (46) | .43 |
Middle 50% range of values, i.e., difference between 1st and 3rd quartiles (25th-75th percentiles)
Baseline medication data was available for 122 (99%) of stepped care and 118 (93%) of usual care subjects. These numbers were used as the denominators for calculating the proportion of patients on various medications.
Acknowledgments
The authors gratefully acknowledgement the care management provided by Carol Kempf and Gloria Nicholas and the research assistance provided by Monica Huffman.
Funding/Support: This work was supported by grant R01 MH-071268 from the National Institute of Mental Health, National Institutes of Health. Dr. Kroenke was the principal investigator.
Role of the Sponsor: The sponsor provided financial support for the study only and had no role in the design and conduct of the study; the collection, management, analysis, and interpretation of the study; or in the preparation, review, or approval of the manuscript.
Funding: This study was supported by a grant from the National Institute of Mental Health to Dr. Kroenke (MH-071268)
Footnotes
Trial Registration clinicaltrials.gov Identifier: NCT00118430
Author Contributions: Drs. Kroenke had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Kroenke, Bair, Damush, Sutherland
Acquisition of data: Kroenke, Bair, Damush, Hoke
Analysis and interpretation of data: Kroenke, Bair, Damush, Wu, Sutherland, Tu
Drafting of the manuscript: Kroenke, Wu, Hoke
Critical revision of the manuscript for important intellectual content: Kroenke, Bair, Damush, Wu, Sutherland, Tu
Statistical analysis: Kroenke, Wu, Sutherland, Tu.
Obtained funding: Kroenke.
Administrative, technical, or material support: Kroenke, Bair, Damush, Hoke.
Study supervision: Kroenke, Bair, Damush, Hoke
Financial Disclosures. Dr. Kroenke has received research funding from Eli Lilly, Pfizer, and Wyeth, and honoraria as a speaker, consultant, or advisory board member from Eli Lilly, Pfizer, Wyeth, Astra-Zeneca and Forest Laboratories. Dr. Bair has received one-time consultant fees from Wyeth, Abbott, and Cephalon. No other authors reported disclosures.
References
- 1.Kroenke K. Patients presenting with somatic complaints: epidemiology, psychiatric comorbidity and management. Int J Methods Psychiatr Res. 2003;12:34–43. doi: 10.1002/mpr.140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Spitzer RL, Kroenke K, Linzer M, et al. Health-related quality of life in primary care patients with mental disorders: results from the PRIME-MD 1000 study. JAMA. 1995;274:1511–1517. [PubMed] [Google Scholar]
- 3.Schappert SM. National Ambulatory Medical Care Survey: 1989 summary National Center for Health Statistics. Vital Health Stat. 1992;13(110) [PubMed] [Google Scholar]
- 4.Sternbach RA. Survey of Pain in the United States: The Nuprin Pain Report. Clin J Pain. 1986;2:49–53. doi: 10.1016/0304-3959(86)90224-1. [DOI] [PubMed] [Google Scholar]
- 5.Bair MJ, Robinson RL, Katon W, Kroenke K. Depression and pain comorbidity: a literature review. Arch Intern Med. 2003;163:2433–2445. doi: 10.1001/archinte.163.20.2433. [DOI] [PubMed] [Google Scholar]
- 6.Jackson JL, O'Malley PG, Kroenke K. Antidepressants and cognitive-behavioral therapy for symptom syndromes. CNS Spectr. 2006;11:212–222. doi: 10.1017/s1092852900014383. [DOI] [PubMed] [Google Scholar]
- 7.Kroenke K, Krebs EE, Bair MJ. Pharmacotherapy of chronic pain: a synthesis of recommendations from systematic reviews. Gen Hosp Psychiatry. doi: 10.1016/j.genhosppsych.2008.12.006. In press. [DOI] [PubMed] [Google Scholar]
- 8.Lorig K. Self-management education: more than a nice extra. Med Care. 2003;41:699–701. doi: 10.1097/01.MLR.0000072811.54551.38. [DOI] [PubMed] [Google Scholar]
- 9.Von Korff M, Moore JC. Stepped care for back pain: activating approaches for primary care. Ann Intern Med. 2001;134:911–917. doi: 10.7326/0003-4819-134-9_part_2-200105011-00016. [DOI] [PubMed] [Google Scholar]
- 10.Lorig KR, Holman H. Self-management education: history, definition, outcomes, and mechanisms. Ann Behav Med. 2003;26:1–7. doi: 10.1207/S15324796ABM2601_01. [DOI] [PubMed] [Google Scholar]
- 11.Damush TM, Weinberger M, Perkins SM, et al. Randomized trial of a self-management program for primary care patients with acute low back pain: short-term effects. Arthritis Rheum. 2003;49:179–186. doi: 10.1002/art.10995. [DOI] [PubMed] [Google Scholar]
- 12.Pariser D, O'Hanlon A, Espinoza L. Effects of telephone intervention on arthritis self-efficacy, depression, pain, and fatigue in older adults with arthritis. J Geriatr Phys Ther. 2006;28:67–73. [PubMed] [Google Scholar]
- 13.Warsi A, LaValley MP, Wang PS, Avorn J, Solomon DH. Arthritis self-management education programs. Arthritis Rheum. 2003;48:2207–2213. doi: 10.1002/art.11210. [DOI] [PubMed] [Google Scholar]
- 14.Chodosh J, Morton SC, Mojica W, et al. Meta-analysis: chronic disease self-management programs for older adults. Ann Intern Med. 2005;143:427–438. doi: 10.7326/0003-4819-143-6-200509200-00007. [DOI] [PubMed] [Google Scholar]
- 15.Newman S, Steed L, Mulligan K. Self-management interventions for chronic illness. Lancet. 2004;364:1523–1537. doi: 10.1016/S0140-6736(04)17277-2. [DOI] [PubMed] [Google Scholar]
- 16.Holman HR, Lorig K. Self-management education for osteoarthritis. Ann Intern Med. 2006;144:618. doi: 10.7326/0003-4819-144-8-200604180-00014. [DOI] [PubMed] [Google Scholar]
- 17.Kroenke K, Bair M, Damush T, et al. Stepped Care for Affective Disorders and Musculoskeletal Pain (SCAMP) study Design and practical implications of an intervention for comorbid pain and depression. Gen Hosp Psychiatry. 2007;29:506–517. doi: 10.1016/j.genhosppsych.2007.08.005. [DOI] [PubMed] [Google Scholar]
- 18.Cleeland CS. Measurement of pain by subjective report. In: Foley KM, editor. Advances in Pain Research and Therapy. 1989. pp. 391–403. [Google Scholar]
- 19.Williams LS, Jones WJ, Shen J, Robinson RL, Weinberger M, Kroenke K. Prevalence and impact of pain and depression in neurology outpatients. J Neurol Neurosurg Psychiatry. 2003;74:1587–1589. doi: 10.1136/jnnp.74.11.1587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kroenke K, Spitzer RL, Williams JBW. The PHQ-9: Validity of a brief depression severity measure. J Gen Intern Med. 2001;16:606–613. doi: 10.1046/j.1525-1497.2001.016009606.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kroenke K, West SL, Swindle R, et al. Similar effectiveness of paroxetine, fluoxetine, and sertraline in primary care: a randomized trial. JAMA. 2001;286:2947–2955. doi: 10.1001/jama.286.23.2947. [DOI] [PubMed] [Google Scholar]
- 22.Unutzer J, Katon W, Callahan CM, et al. Collaborative care management of late-life depression in the primary care setting: a randomized controlled trial. JAMA. 2002;288:2836–2845. doi: 10.1001/jama.288.22.2836. [DOI] [PubMed] [Google Scholar]
- 23.Dietrich AJ, Oxman TE, Williams JW, Jr, et al. Re-engineering systems for the treatment of depression in primary care: cluster randomised controlled trial. BMJ. 2004;329:602–605. doi: 10.1136/bmj.38219.481250.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Spitzer RL, Williams JB, Kroenke K, et al. Utility of a new procedure for diagnosing mental disorders in primary care. The PRIME-MD 1000 study. JAMA. 1994;272:1749–1756. [PubMed] [Google Scholar]
- 25.Lowe B, Unutzer J, Callahan CM, Perkins AJ, Kroenke K. Monitoring depression treatment outcomes with the patient health questionnaire-9. Med Care. 2004;42:1194–1201. doi: 10.1097/00005650-200412000-00006. [DOI] [PubMed] [Google Scholar]
- 26.Katon W, Von Korff M, Lin E, et al. Stepped collaborative care for primary care patients with persistent symptoms of depression: a randomized trial. Arch Gen Psychiatry. 1999;56:1109–1115. doi: 10.1001/archpsyc.56.12.1109. [DOI] [PubMed] [Google Scholar]
- 27.Williams JW, Jr, Barrett J, Oxman T, et al. Treatment of dysthymia and minor depression in primary care: A randomized controlled trial in older adults. JAMA. 2000;284:1519–1526. doi: 10.1001/jama.284.12.1519. [DOI] [PubMed] [Google Scholar]
- 28.Dworkin RH, Turk DC, Farrar JT, et al. Core outcome measures for chronic pain clinical trials: IMMPACT recommendations. Pain. 2005;113:9–19. doi: 10.1016/j.pain.2004.09.012. [DOI] [PubMed] [Google Scholar]
- 29.Cleeland CS, Gonin R, Hatfield AK, et al. Pain and its treatment in outpatients with metastatic cancer. N Engl J Med. 1994;330:592–596. doi: 10.1056/NEJM199403033300902. [DOI] [PubMed] [Google Scholar]
- 30.Von Korff M, Ormel J, Keefe FJ, Dworkin SF. Grading the severity of chronic pain. Pain. 1992;50:133–149. doi: 10.1016/0304-3959(92)90154-4. [DOI] [PubMed] [Google Scholar]
- 31.Roland M, Morris R. A study of the natural history of back pain. Part I: development of a reliable and sensitive measure of disability in low-back pain. Spine. 1983;8:141–144. doi: 10.1097/00007632-198303000-00004. [DOI] [PubMed] [Google Scholar]
- 32.Jensen MP, Strom SE, Turner JA, Romano JM. Validity of the Sickness Impact Profile Roland scale as a measure of dysfunction in chronic pain patients. Pain. 1992;50:157–162. doi: 10.1016/0304-3959(92)90156-6. [DOI] [PubMed] [Google Scholar]
- 33.McHorney CA, Ware JE, Raczek AE. The MOS 36-Item Short-Form Health Survey (SF-36): II. Psychometric and clinical tests of validity in measuring physical and mental health constructs. Med Care. 1993;31:247–263. doi: 10.1097/00005650-199303000-00006. [DOI] [PubMed] [Google Scholar]
- 34.Ware JE, Gandek B. The SF-36 Health Survey: development and use in mental health research and the IQOLA Project. Int J Ment Health. 1994;23:49–73. [Google Scholar]
- 35.Idler EL, Benyamini Y. Self-rated health and mortality: a review of twenty-seven community studies. J Health Soc Behav. 1997;38:21–37. [PubMed] [Google Scholar]
- 36.Spitzer RL, Kroenke K, Williams JB, Lowe B. A brief measure for assessing generalized anxiety disorder: the GAD-7. Arch Intern Med. 2006;166:1092–1097. doi: 10.1001/archinte.166.10.1092. [DOI] [PubMed] [Google Scholar]
- 37.Kroenke K, Spitzer RL, Williams JBW, Monahan PO, Lowe B. Anxiety disorders in primary care: prevalence, impairment, comorbidity, and detection. Ann Intern Med. 2007;146:317–325. doi: 10.7326/0003-4819-146-5-200703060-00004. [DOI] [PubMed] [Google Scholar]
- 38.Cintron A, Morrison RS. Pain and ethnicity in the United States: A systematic review. J Palliat Med. 2006;9:1454–1473. doi: 10.1089/jpm.2006.9.1454. [DOI] [PubMed] [Google Scholar]
- 39.Williams JW, Jr, Gerrity M, Holsinger T, Dobscha S, Gaynes B, Dietrich A. Systematic review of multifaceted interventions to improve depression care. Gen Hosp Psychiatry. 2007;29:91–116. doi: 10.1016/j.genhosppsych.2006.12.003. [DOI] [PubMed] [Google Scholar]
- 40.Rush AJ, Trivedi MH, Wisniewski SR, et al. Bupropion-SR, sertraline, or venlafaxine-XR after failure of SSRIs for depression. N Engl J Med. 2006;354:1231–1242. doi: 10.1056/NEJMoa052963. [DOI] [PubMed] [Google Scholar]
- 41.Trivedi MH, Fava M, Wisniewski SR, et al. Medication augmentation after the failure of SSRIs for depression. N Engl J Med. 2006;354:1243–1252. doi: 10.1056/NEJMoa052964. [DOI] [PubMed] [Google Scholar]
- 42.Kroenke K, Spitzer RL. The PHQ-9: A new depression and diagnostic severity measure. Psychiatric Annals. 2002;32:509–521. [Google Scholar]
- 43.Lorig K, Holman H. Arthritis self-management studies: a twelve-year review. Health Educ Q. 1993;20:17–28. doi: 10.1177/109019819302000104. [DOI] [PubMed] [Google Scholar]
- 44.Bandura A. Social Foundations of Thought and Action: A Social Cognitive Theory. Prentice Hall; 1986. [Google Scholar]
- 45.Damush TM, Weinberger M, Perkins SM, et al. The long-term effects of a self-management program for inner-city, primary care patients with acute low back pain. Arch Intern Med. 2003;163:2632–2638. doi: 10.1001/archinte.163.21.2632. [DOI] [PubMed] [Google Scholar]
- 46.Damush TM, Weinberger M, Clark DO, et al. Acute low back pain self-management intervention for urban primary care patients: rationale, design, and predictors of participation. Arthritis Rheum. 2002;47:372–379. doi: 10.1002/art.10382. [DOI] [PubMed] [Google Scholar]
- 47.Keller MB. Past, present, and future directions for defining optimal treatment outcome in depression: remission and beyond. JAMA. 2003;289:3152–3160. doi: 10.1001/jama.289.23.3152. [DOI] [PubMed] [Google Scholar]
- 48.Dworkin RH, Turk DC, Wyrwich KW, et al. Interpreting the clinical importance of treatment outcomes in chronic pain clinical trials: IMMPACT recommendations. J Pain. 2008;9:105–121. doi: 10.1016/j.jpain.2007.09.005. [DOI] [PubMed] [Google Scholar]
- 49.Zhang J, Yu KF. What's the relative risk? A method of correcting the odds ratio in cohort studies of common outcomes. JAMA. 1998;280:1890–1891. doi: 10.1001/jama.280.19.1690. [DOI] [PubMed] [Google Scholar]
- 50.Herbert RD. How to estimate treatment effects from reports of clinical trials. II: Dichotomous outcomes. Austral J Physiother. 2000;46:309–313. doi: 10.1016/s0004-9514(14)60292-0. [DOI] [PubMed] [Google Scholar]
- 51.Gill D, Hatcher S. Antidepressants for depression in medical illness. Cochrane Database Syst Rev. 2000:CD001312. doi: 10.1002/14651858.CD001312. [DOI] [PubMed] [Google Scholar]
- 52.Rush AJ, Trivedi MH, Wisniewski SR, et al. Acute and longer-term outcomes in depressed outpatients requiring one or several treatment steps: a STAR*D report. Am J Psychiatry. 2006;163:1905–1917. doi: 10.1176/ajp.2006.163.11.1905. [DOI] [PubMed] [Google Scholar]
- 53.Farrar JT, Portenoy RK, Berlin JA, Kinman JL, Strom BL. Defining the clinically important difference in pain outcome measures. Pain. 2000;88:287–294. doi: 10.1016/S0304-3959(00)00339-0. [DOI] [PubMed] [Google Scholar]
- 54.Gilbody S, Bower P, Fletcher J, Richards D, Sutton AJ. Collaborative care for depression: a cumulative meta-analysis and review of longer-term outcomes. Arch Intern Med. 2006;166:2314–2321. doi: 10.1001/archinte.166.21.2314. [DOI] [PubMed] [Google Scholar]
- 55.Thielke SM, Fan MY, Sullivan M, Unutzer J. Pain limits the effectiveness of collaborative care for depression. Am J Geriatr Psychiatry. 2007;15:699–707. doi: 10.1097/JGP.0b013e3180325a2d. [DOI] [PubMed] [Google Scholar]
- 56.Kroenke K, Shen J, Oxman TE, Williams JW, Jr, Dietrich AJ. Impact of pain on the outcomes of depression treatment: results from the RESPECT trial. Pain. 2008;134:209–215. doi: 10.1016/j.pain.2007.09.021. [DOI] [PubMed] [Google Scholar]
- 57.Lin EH, Katon W, Von Korff M, et al. Effect of improving depression care on pain and functional outcomes among older adults with arthritis: a randomized controlled trial. JAMA. 2003;290:2428–2429. doi: 10.1001/jama.290.18.2428. [DOI] [PubMed] [Google Scholar]
- 58.Dobscha SK, Corson K, Perrin NA, et al. Collaborative care for chronic pain in primary care: a clustered randomized trial. JAMA. 2009;301:1242–1252. doi: 10.1001/jama.2009.377. [DOI] [PubMed] [Google Scholar]
- 59.Stewart WF, Ricci JA, Chee E, Hahn SR, Morganstein D. Cost of lost productive work time among US workers with depression. JAMA. 2003;289:3135–3144. doi: 10.1001/jama.289.23.3135. [DOI] [PubMed] [Google Scholar]
- 60.Stewart WF, Ricci JA, Chee E, Morganstein D, Lipton R. Lost productive time and cost due to common pain conditions in the US workforce. JAMA. 2003;290:2443–2454. doi: 10.1001/jama.290.18.2443. [DOI] [PubMed] [Google Scholar]
- 61.Jung AC, Staiger T, Sullivan M. The efficacy of selective serotonin reuptake inhibitors for the management of chronic pain. J Gen Intern Med. 1997;12:384–389. doi: 10.1046/j.1525-1497.1997.00064.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Lynch ME. Antidepressants as analgesics: a review of randomized controlled trials. J Psychiatry Neurosci. 2001;26:30–36. [PMC free article] [PubMed] [Google Scholar]
- 63.Krebs EE, Gaynes BN, Gartlehner G, et al. Treating the physical symptoms of depression with second-generation antidepressants: a systematic review and meta-analysis. Psychosomatics. 2008;49:191–198. doi: 10.1176/appi.psy.49.3.191. [DOI] [PubMed] [Google Scholar]
- 64.Rubenstein LV. Improving care for depression: there's no free lunch. Ann Intern Med. 2006;145:544–546. doi: 10.7326/0003-4819-145-7-200610030-00013. [DOI] [PubMed] [Google Scholar]
- 65.Callahan CM. Depression in primary care: encouragement and caution for the business case. J Gen Intern Med. 2006;21:1125–1127. doi: 10.1111/j.1525-1497.2006.00603.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Katon WJ, Schoenbaum M, Fan MY, et al. Cost-effectiveness of improving primary care treatment of late-life depression. Arch Gen Psychiatry. 2005;62:1313–1320. doi: 10.1001/archpsyc.62.12.1313. [DOI] [PubMed] [Google Scholar]
- 67.Unutzer J, Katon WJ, Fan MY, et al. Long-term cost effects of collaborative care for late-life depression. Am J Manag Care. 2008;14:95–100. [PMC free article] [PubMed] [Google Scholar]
- 68.Kroenke K, Swindle R. Cognitive-behavioral therapy for somatization and symptom syndromes: a critical review of controlled clinical trials. Psychother Psychosom. 2000;69:205–215. doi: 10.1159/000012395. [DOI] [PubMed] [Google Scholar]
- 69.Wang PS, Simon GE, Avorn J, et al. Telephone screening, outreach, and care management for depressed workers and impact on clinical and work productivity outcomes: a randomized controlled trial. JAMA. 2007;298:1401–1411. doi: 10.1001/jama.298.12.1401. [DOI] [PMC free article] [PubMed] [Google Scholar]