Abstract
Context
A US Food and Drug Administration advisory has warned that antidepressants may be associated with an increased risk of suicidal thoughts and behaviors in adolescents. This prompted a meta-analysis of trials in adults that found no overall increase in risk, but individual agents could not be studied.
Objective
To assess the risk of suicide and suicide attempts associated with individual antidepressant agents.
Design
Cohort study of incident users of antidepressant agents.
Setting
Population-based health care utilization data of all residents of British Columbia, Canada, aged 18 years and older between January 1, 1997, and December 31, 2005.
Patients
British Columbia residents who had antidepressant therapy initiated and had a recorded diagnosis of depression.
Intervention
Initiation of various antidepressant medications.
Main Outcome Measures
Combined suicide death or hospitalization due to self-harm.
Results
In a population of 287 543 adults aged 18 years and older with antidepressant therapy initiated, we observed outcome rates ranging from 4.41/1000 person-years to 9.09/1000 person-years. Most events occurred in the first 6 months after treatment initiation. After extensive propensity score adjustment, we found no clinically meaningful variation in the risk of suicide and suicide attempt between antidepressant agents compared with fluoxetine hydrochloride initiation: citalopram hydrobromide, hazard ratio=1.00 (95% confidence interval, 0.63–1.57); fluvoxamine maleate, hazard ratio=0.98 (95% confidence interval, 0.63–1.51); paroxetine hydrochloride, hazard ratio=1.02 (95% confidence interval, 0.77–1.35); and sertraline hydrochloride, hazard ratio = 0.75 (95% confidence interval, 0.53–1.05). Compared with selective serotonin reuptake inhibitors as a drug class, other classes including serotonin-norepinephrine reuptake inhibitors, tricyclic agents, and other newer and atypical agents had a similar risk. Restriction to patients with no antidepressant use in the past 3 years further reduced apparent differences between groups.
Conclusions
Our finding of equal event rates across antidepressant agents supports the US Food and Drug Administration’s decision to treat all antidepressants alike in their advisory. Treatment decisions should be based on efficacy, and clinicians should be vigilant in monitoring after initiating therapy with any antidepressant agent.
Despite the widespread use of antidepressant medications, particularly selective serotonin reuptake inhibitors (SSRIs), there is inconsistent evidence that growth in antidepressant use has reduced the prevalence of suicidal ideation or suicide attempts during the past decade.1–3 In October 2004, the US Food and Drug Administration issued an advisory that anti-depressants may be associated with an increased risk of suicidal thoughts and behaviors in children and adolescents.4 These warnings were prompted by a meta-analysis of all available randomized trials of antidepressants in this age group, in which patients randomized to antidepressants had nearly twice the rate of suicidal ideation or behavior relative to those given placebo.5 These concerns prompted the US Food and Drug Administration to undertake a reanalysis of all its available antidepressant trials in adults as well. This meta-analysis and several subsequent analyses of short-term trials found no increased risk of suicidality in adult antidepressant users.6–9 However, the interpretation of this meta-analysis is limited by a number of factors including the brief duration of trials, few suicide attempts and almost no completed suicides, varying definitions of suicidality, noncomparable doses, and heterogeneous patient mixes.
Nonrandomized studies comparing users of different antidepressant classes—SSRIs, tricyclic agents (TCAs), serotonin-norepinephrine reuptake inhibitors,10–12 or any antidepressant users vs nonusers13—have reported small or no differences in suicides and suicide attempts. However, serious questions remain whether these studies had adequate statistical power or were adequately controlled for prescribing biases caused by preferential avoidance of TCAs in patients at high risk. A cohort study in the General Practice Research Database found higher rates of suicide attempts in venlafaxine hydrochloride users compared with SSRI and TCA users but also observed a higher burden of suicide risk factors in venlafaxine users, raising the possibility of preferential prescribing in high-risk patients and residual confounding.14 A nested case-control study in residents of Ontario, Canada, aged 66 years and older found that SSRIs were associated with a nearly 5-fold increased risk of suicide during the first month of treatment compared with other antidepressants, but it found no difference between classes during subsequent periods.15 The investigators also observed a higher risk of violent suicide among SSRI users. However, a study using postmortem data found that a lower proportion of suicides was violent among SSRI users than among non–antidepressant users.16
While raising important concerns, the US Food and Drug Administration’s meta-analyses and advisories have not provided patients, clinicians, or policy makers with adequate guidance on the treatment decisions they face, and non-randomized studies face criticism for channeling bias. In the current study, we sought to address whether the risk of suicide is equal across antidepressant classes and agents after adjustment for selection factors—or whether there are particular regimens with safety advantages that should be prescribed preferentially in adult populations.
METHODS
PATIENTS AND DATA SOURCE
We conducted a cohort study of all residents of British Columbia, Canada, aged 18 years and older who initiated use of an antidepressant medication between January 1, 1997, and December 31, 2005. Initiation was defined as filling an antidepressant prescription without having filled one in the preceding year. We considered only the first treatment episode during the study period for each patient for the analysis. We required evidence of depression as indicated by a diagnosis of depression recorded during 2 office visits or as a hospital discharge diagnosis during the 6 months prior to through 2 months after the initiation date (eTable 1, http://www.archgenpsychiatry.com). Depressed episodes of an existing bipolar disorder did not qualify for study entrance. We allowed subjects with diagnoses following the initiation date to be included in the cohort because some health care practitioners may prescribe an antidepressant as part of diagnosing the condition, in which case the diagnosis might follow the prescription. To ensure complete ascertainment of prior drug use, we required that subjects be residents of British Columbia as evidenced by being enrolled in the provincial Medical Services Plan during the year prior to initiation. Bupropion hydrochloride was not considered owing to its potential use for smoking cessation, and escitalopram oxalate was not considered because it was not marketed until December 2004, near the end of the study period.
Table 1.
Baseline Patient Characteristicsa
| Patient Characteristic |
SSRIsb | SSRIs, Total |
SNRIsb | MAOIsc | Other Newer and Atypical Agentsc |
TCAsc | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| Citalopram | Fluoxetine | Fluvoxamine | Paroxetine | Sertraline | ||||||
| Total users, No. | 45 522 | 22 207 | 9690 | 74 780 | 36 135 | 188 334 | 35 732 | 1751 | 28 316 | 33 410 |
| Demographic characteristics |
||||||||||
| Age, mean (SD), y |
46.7 (18.4) | 41.9 (14.9) | 48.0 (18.0) | 46.1 (17.6) | 46.3 (17.7) | 45.9 (17.6) | 43.3 (16.1) | 48.6 (17.4) | 47.3 (16.4) | 48.8 (16.4) |
| Female, No. (%) | 25749 (56.6) | 12779 (57.5) | 5466 (56.4) | 42 092 (56.3) | 21 861 (60.5) | 107 947 (57.3) | 18396 (51.5) | 995 (56.8) | 14 290 (50.5) | 20 001 (59.9) |
| Income in Can$, No. (%) |
||||||||||
| <16000 | 7780 (18.1) | 4166 (20.4) | 1798 (20.5) | 13400 (19.3) | 6320 (18.9) | 33464 (19.1) | 6136 (18.4) | 266 (16.6) | 5042 (19.5) | 6731 (22.2) |
| 16 000–28 000 | 2007 (4.7) | 1005 (4.9) | 588 (6.7) | 3905 (5.6) | 1892 (5.7) | 9397 (5.4) | 1494 (4.5) | 91 (5.7) | 1315 (5.1) | 1886 (6.2) |
| >28 000 | 33 099 (77.2) | 15 258 (74.7) | 6388 (72.8) | 52 071 (75.1) | 25165 (75.4) | 131 981 (75.5) | 25 813 (77.2) | 1247 (77.7) | 19 501 (75.4) | 21 753 (71.6) |
| Comorbidities and health services use intensity |
||||||||||
| Distinct generic medications prescribed, mean (SD), No. |
5.5 (4.3) | 4.8 (3.7) | 5.7 (4.3) | 5.4 (4.1) | 5.4 (4.1) | 5.4 (4.1) | 4.9 (3.8) | 5.9 (4.5) | 5.7 (4.3) | 6.7 (4.6) |
| Comorbidity score, mean (SD) |
0.24 (0.85) | 0.14 (0.62) | 0.25 (0.85) | 0.19 (0.74) | 0.21 (0.78) | 0.21 (0.77) | 0.16 (0.69) | 0.31 (0.99) | 0.22 (0.80) | 0.26 (0.86) |
| Psychiatric visit with depression diagnosis, No. (%) |
804 (1.8) | 361 (1.6) | 225 (2.3) | 908 (1.2) | 627 (1.7) | 2925 (1.6) | 681 (1.9) | 100 (5.7) | 675 (2.4) | 473 (1.4) |
| Psychiatric hospitalization, No. (%) |
2297 (5.1) | 771 (3.5) | 626 (6.5) | 3574 (4.8) | 2181 (6.0) | 9449 (5.0) | 1694 (4.7) | 142 (8.1) | 1907 (6.7) | 1368 (4.1) |
| Recorded psychiatric disorders, No. (%)d |
||||||||||
| ADHD | 136 (0.3) | 78 (0.4) | 25 (0.3) | 177 (0.2) | 105 (0.3) | 521 (0.3) | 94 (0.3) | 9 (0.5) | 89 (0.3) | 113 (0.3) |
| Anxiety or sleep disorder |
608 (1.3) | 227 (1.0) | 198 (2.0) | 1269 (1.7) | 536 (1.5) | 2838 (1.5) | 575 (1.6) | 39 (2.2) | 549 (1.9) | 524 (1.6) |
| Dementia | 331 (0.7) | 48 (0.2) | 64 (0.7) | 312 (0.4) | 219 (0.6) | 974 (0.5) | 152 (0.4) | 19 (1.1) | 208 (0.7) | 96 (0.3) |
| Mania | 623 (1.4) | 143 (0.6) | 140 (1.4) | 694 (0.9) | 530 (1.5) | 2130 (1.1) | 569 (1.6) | 42 (2.4) | 489 (1.7) | 171 (0.5) |
| Psychotic disorder |
883 (1.9) | 238 (1.1) | 195 (2.0) | 921 (1.2) | 634 (1.8) | 2871 (1.5) | 543 (1.5) | 22 (1.3) | 673 (2.4) | 382 (1.1) |
| Substance abuse | 971 (2.1) | 547 (2.5) | 311 (3.2) | 1881 (2.5) | 987 (2.7) | 4697 (2.5) | 951 (2.7) | 51 (2.9) | 1404 (5.0) | 1087 (3.3) |
| Suicide attempt | 250 (0.6) | 119 (0.5) | 70 (0.7) | 403 (0.5) | 267 (0.7) | 1109 (0.6) | 236 (0.7) | 15 (0.9) | 231 (0.8) | 121 (0.4) |
| Use of ≥ 1 additional psychiatric medicationse |
13 973 (30.7) | 5453 (24.6) | 3761 (38.8) | 28785 (38.5) | 12190 (33.7) | 64162 (34.1) | 10723 (30.0) | 670 (38.3) | 10 819 (38.2) | 12672 (37.9) |
| Concurrent stimulant use |
99 (0.2) | 58 (0.3) | 18 (0.2) | 107 (0.1) | 64 (0.2) | 346 (0.2) | 83 (0.2) | 8 (0.5) | 47 (0.2) | 61 (0.2) |
Abbreviations: ADHD, attention-deficit/hyperactivity disorder; MAOIs, monoamine oxidase inhibitors; SNRIs, serotonin-norepinephrine reuptake inhibitors; SSRIs, selective serotonin reuptake inhibitors; TCAs, tricyclic agents.
Baseline covariates were assessed during the year prior to treatment initiation.
Citalopram indicates generic citalopram hydrobromide and Celexa; fluoxetine, generic fluoxetine hydrochloride and Prozac; fluvoxamine, generic fluvoxamine maleate and Luvox; paroxetine, generic paroxetine hydrochloride and Paxil; sertraline, generic sertraline hydrochloride and Zoloft; and SNRIs, generic venlafaxine hydrochloride and Effexor.
Class lists are as follows: for MAOIs, moclobemide, phenelzine sulfate, and tranylcypromine sulfate; for other newer and atypical agents, mirtazapine, nefazodone hydrochloride, and trazodone hydrochloride; and for TCAs, amitriptyline hydrochloride, amoxapine, clomipramine hydrochloride, desipramine hydrochloride, doxepin hydrochloride, imipramine hydrochloride, maprotiline hydrochloride, nortriptyline hydrochloride, protriptyline hydrochloride, and trimipramine maleate.
Psychiatric disorders were identified based on the presence of 1 inpatient diagnosis code for the disorder or 2 or more outpatient diagnosis codes.
Additional psychiatric medications exclude stimulants.
The PharmaNet database used to identify antidepressant initiators includes the name, dose, and dispensed quantity for all prescription drugs dispensed in British Columbia pharmacies. This information is entered by pharmacists via a province-wide network that assures minimal underreporting and misclassification17 and is recorded for all dispensings independent of the payer. The British Columbia Ministry of Health Services also maintains linkable data on all physician services and hospitalizations for all persons in its publicly funded health care system. Up to 25 diagnoses for hospital discharges and 1 diagnosis for each medical service are recorded, with good specificity and completeness.18 These administrative data were further linked to vital statistics data that include information on cause of death, including suicide.
Because all British Columbia residents are covered for all medical services by the provincial Medical Services Plan except for a small number of federal employees and because drug dispensings are recorded for all dispensed prescription medications regardless of payer, the study sample is representative of British Columbia’s adult population (about 3 million in 2005). All low-income residents, most elderly residents, and most other residents have drug insurance, with varying degrees of coverage.19
ANTIDEPRESSANT MEDICATION EXPOSURE
Antidepressant medications were classified into the following groups: SSRIs, including citalopram hydrobromide, fluoxetine hydrochloride, fluvoxamine maleate, paroxetine hydrochloride, and sertraline hydrochloride; serotonin-norepinephrine reuptake inhibitors, including venlafaxine; TCAs, including amitriptyline hydrochloride, amoxapine, clomipra-mine hydrochloride, desipramine hydrochloride, doxepin hydrochloride, imipramine hydrochloride, maprotiline hydrochloride, nortriptyline hydrochloride, protriptyline hydrochloride, and trimipramine maleate; other newer and atypical agents, including mirtazapine, nefazodone hydrochloride, and trazodone hydrochloride; and monoamine oxidase inhibitors, including moclobemide, phenelzine sulfate, and tranylcypromine sulfate. Duloxetine hydrochloride, a serotonin-norepinephrine re-uptake inhibitor, was not marketed in Canada during the study period.
Exposure status was assigned based on the initiated medication, and follow-up began on the initiation date. We determined the availability of antidepressant supply for each patient day by stringing together consecutive antidepressant dispensings for each patient based on dispensing dates and reported “days’ supply.”20 When a dispensing occurred before the previous dispensing should have run out, use of the new dispensing was assumed to begin the day after the end of the old dispensing. Each patient’s exposure risk window ended when the subject had been without antidepressant supply for 14 days. We routinely assess the plausibility of recorded days’ supply relative to quantity dispensed and recommended dosing, and we have found this information to be accurate.21 Sensitivity analyses were conducted to assess the effect of stacking prescriptions. While it is possible that some patients fill prescriptions without actually taking the medication, the patient cost-sharing requirements in British Columbia are likely to reduce the frequency with which this occurs, and prior studies have demonstrated good correspondence between pharmacy records and self-reported prescription drug use.22,23
Patients were censored at the date of the end of their exposure risk window, switching to or treatment augmentation with another antidepressant medication, emigration from the province, occurrence of a study outcome, death, or the end of the study period or the end of 1 year, whichever came first. While patients could have multiple treatment episodes, we included only the first in our analysis.
STUDY END POINTS
Study outcomes included attempted and completed suicide. Suicide attempt was defined as a hospitalization with an International Classification of Diseases, Ninth Revision (ICD-9)24 external cause of injury code (E-code) of deliberate self-harm (E950.x-E958.x). Completed suicides were deaths with an ICD-9 E-code of E950.x-E958.x or an International Statistical Classification of Diseases, 10th Revision (ICD-10)25 diagnosis code of X60-X84 listed as the cause of death. These were identified using a vital statistics file that includes cause of death as reported on the patient’s death certificate. Violent suicide attempts (ICD-9 E-codes) and completed suicides (ICD-10 X-codes) were defined as those involving the following: hanging (E953.0; X70), gunshot or explosion (E955; X72-X75), jumping or lying in front of a moving object (E957, E958.0; X80, X81), stabbing or blunt trauma (E956; X78, X79), vehicle collision (E958.5, E958.6; X82), electrocution (E958.4; X83), and self-immolation (E958.1; X76). Assignment of an E-code is mandatory in Canada when a diagnosis of poisoning or injury is coded.26 E-codes were provided for more than 97% of injury hospitalizations and thus are considered complete in British Columbia, contrary to most US administrative data sources.27 Studies of the validity of the deliberate self-harm E-codes used in this study have reported a positive predictive value of 86% relative to the gold standard of suicide attempts as identified by medical record review28 and good agreement between coding and expert opinion on the cause and intent of injuries.29 Suicide death certificates are also accurate relative to the gold standard of medical record review, with a sensitivity of 90% and a specificity of 100%.30
PATIENT CHARACTERISTICS
Patient characteristics were assessed at treatment initiation based on medical claims during the year preceding cohort entry. These included age, sex, calendar year of cohort entry, adjusted family income status (<Can$16 000, Can$16 000-Can$28 000, and >Can$28 000 as defined by premium subsidy levels),31 enrollment in an income assistance plan, number of acute hospitalizations, Charlson Comorbidity Index score,32 and number of distinct generic entities prescribed. Psychiatric disorders were defined as the presence of 2 outpatient diagnoses or 1 inpatient diagnosis. These included anxiety or sleep disorders, mania and bipolar disorder, attention-deficit/hyperactivity disorder, substance abuse, psychotic disorder, delirium, personality disorders, and other mental disorders (hysteria, acute reactions to stress, sexual deviations, and disturbance of emotions specific to childhood or adolescence). Additional indicators of psychiatric severity were number of psychiatric hospitalizations, number of psychiatric visits with a depression diagnosis, suicide attempt in the past year, concurrent stimulant use, and number of psychiatric drug classes (antipsychotics, benzodiazepines, and other psychotropics) used simultaneously. Concurrent stimulant use was defined as having a stimulant supply available on the antidepressant initiation date. In addition to psychiatric comorbidities, we measured a number of general medical comorbidities, including malignant neoplasms, pain requiring opiates, poisoning or drug toxic effects, injury other than poisoning, peptic ulcer disease and gastrointestinal hemorrhage, stroke and transient ischemic attack, Parkinson disease, seizure disorders, urinary incontinence, cardiovascular disease, and chronic lung disease. A complete list of covariates and their definitions is provided in eTable 2.
Table 2.
Suicide and Attempted Suicide Counts and Rates During 1-Year Follow-up
| Suicides | Suicide Attempts | Composite Events | Violent Composite Eventsb |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Antidepressanta | Users, No. |
Person- Years |
No. | Event Rate/1000 Person-Years (95% CI) |
No. | Event Rate/1000 Person-Years (95% CI) |
No. | Event Rate/1000 Person-Years (95% CI) |
No. | Event Rate/1000 Person-Years (95% CI) |
| All Adults | ||||||||||
| SSRIs | ||||||||||
| Citalopram | 45 522 | 24 054 | 22 | 0.91 (0.57–1.38) | 86 | 3.58 (2.86–4.42) | 106 | 4.41 (3.61–5.33) | 21 | 0.87 (0.54–1.33) |
| Fluoxetine | 22 207 | 10 872 | 10 | 0.92 (0.44–1.69) | 69 | 6.35 (4.94–8.03) | 78 | 7.17 (5.67–8.95) | 18 | 1.65 (0.98–2.61) |
| Fluvoxamine | 9690 | 4182 | 4 | 0.95 (0.26–2.44) | 35 | 8.37 (5.83–11.64) | 38 | 9.09 (6.43–12.47) | 8 | 1.90 (0.82–3.75) |
| Paroxetine | 74 780 | 36 430 | 22 | 0.60 (0.38–0.91) | 226 | 6.20 (5.42–7.07) | 246 | 6.75 (5.94–7.65) | 45 | 1.23 (0.90–1.65) |
| Sertraline | 36 135 | 17 649 | 11 | 0.62 (0.31–1.11) | 83 | 4.70 (3.75–5.83) | 94 | 5.33 (4.30–6.52) | 15 | 0.85 (0.47–1.40) |
| Class | ||||||||||
| SSRIs, total | 188 334 | 93 187 | 69 | 0.74 (0.58–0.94) | 499 | 5.35 (4.90–5.85) | 562 | 6.03 (5.54–6.55) | 107 | 1.15 (0.94–1.38) |
| SNRIs | 35 732 | 18 900 | 15 | 0.79 (0.44–1.31) | 111 | 5.87 (4.83–7.07) | 124 | 6.56 (5.46–7.82) | 21 | 1.11 (0.69–1.69) |
| MAOIs | 1751 | 669 | 1 | 1.49 (0.04–8.32) | 3 | 4.48 (0.92–13.11) | 4 | 5.98 (1.63–15.31) | 1 | 1.49 (0.04–8.28) |
| Other newer and atypical agents | 28 316 | 12 318 | 14 | 1.14 (0.62–1.90) | 56 | 4.55 (3.43–5.90) | 69 | 5.60 (4.36–7.09) | 17 | 1.41 (0.82–2.26) |
| TCAs | 33 410 | 14 642 | 5 | 0.34 (0.11–0.80) | 82 | 5.60 (4.45–6.95) | 87 | 5.94 (4.76–7.33) | 9 | 0.62 (0.28–1.17) |
| Total (excluding SSRIs, total) | 287 543 | 139 717 | 104 | 0.74 (0.61–0.90) | 751 | 5.38 (5.00–5.77) | 846 | 6.06 (5.65–6.48) | 155 | 1.11 (0.94–1.30) |
| Treatment-Naive Adultsc | ||||||||||
| SSRIs | ||||||||||
| Citalopram | 43 698 | 23 112 | 22 | 0.95 (0.60–1.44) | 83 | 3.59 (2.86–4.45) | 103 | 4.46 (3.64–5.40) | 21 | 0.91 (0.56–1.39) |
| Fluoxetine | 10 953 | 5728 | 6 | 1.05 (0.38–2.28) | 28 | 4.89 (3.25–7.06) | 33 | 5.76 (3.97–8.09) | 10 | 1.74 (0.84–3.21) |
| Fluvoxamine | 4032 | 1858 | 2 | 1.08 (0.13–3.88) | 11 | 5.92 (2.96–10.59) | 13 | 7.00 (3.73–11.96) | 3 | 1.61 (0.33–4.70) |
| Paroxetine | 50 174 | 25 688 | 19 | 0.74 (0.44–1.15) | 129 | 5.02 (4.19–5.97) | 146 | 5.68 (4.80–6.68) | 29 | 1.13 (0.75–1.62) |
| Sertraline | 20 941 | 10 823 | 7 | 0.65 (0.26–1.33) | 46 | 4.25 (3.11–5.67) | 53 | 4.90 (3.67–6.41) | 8 | 0.74 (0.32–1.45) |
| Class | ||||||||||
| SSRIs, total | 129 798 | 67 208 | 56 | 0.83 (0.63–1.08) | 297 | 4.42 (3.93–4.95) | 348 | 5.18 (4.65–5.75) | 71 | 1.05 (0.82–1.33) |
| SNRIs | 30 321 | 16 528 | 12 | 0.73 (0.37–1.27) | 85 | 5.14 (4.11–6.36) | 95 | 5.75 (4.65–7.03) | 14 | 0.85 (0.46–1.42) |
| MAOIs | 454 | 190 | 0 | 0.00 (0.00–19.31) | 1 | 5.26 (0.13–29.32) | 1 | 5.26 (0.13–29.32) | 0 | 0.00 (0.00–19.31) |
| Other newer and atypical agents | 19 363 | 8820 | 11 | 1.25 (0.62–2.23) | 32 | 3.63 (2.48–5.12) | 43 | 4.88 (3.53–6.57) | 11 | 1.28 (0.64–2.29) |
| TCAs | 19 658 | 9277 | 3 | 0.32 (0.07–0.94) | 49 | 5.28 (3.91–6.98) | 52 | 5.61 (4.19–7.35) | 5 | 0.54 (0.18–1.27) |
| Total (excluding SSRIs, total) | 199 594 | 102 024 | 82 | 0.80 (0.64–1.00) | 464 | 4.55 (4.14–4.98) | 539 | 5.28 (4.85–5.75) | 101 | 0.99 (0.81–1.20) |
| Adults With No Prior Suicide Attempt | ||||||||||
| SSRIs | ||||||||||
| Citalopram | 45 272 | 23 938 | 21 | 0.88 (0.54–1.34) | 84 | 3.51 (2.80–4.34) | 103 | 4.30 (3.51–5.22) | 20 | 0.84 (0.51–1.29) |
| Fluoxetine | 22 088 | 10 823 | 10 | 0.92 (0.44–1.70) | 66 | 6.10 (4.72–7.76) | 75 | 6.93 (5.45–8.69) | 18 | 1.66 (0.98–2.62) |
| Fluvoxamine | 9620 | 4152 | 4 | 0.96 (0.26–2.46) | 34 | 8.19 (5.67–11.44) | 37 | 8.91 (6.27–12.28) | 8 | 1.92 (0.83–3.78) |
| Paroxetine | 74 377 | 36 262 | 18 | 0.50 (0.29–0.78) | 215 | 5.93 (5.16–6.78) | 231 | 6.37 (5.58–7.25) | 39 | 1.07 (0.76–1.47) |
| Sertraline | 35 868 | 17 532 | 11 | 0.63 (0.31–1.12) | 83 | 4.73 (3.77–5.87) | 94 | 5.36 (4.33–6.56) | 15 | 0.85 (0.48–1.41) |
| Class | ||||||||||
| SSRIs, total | 187 225 | 92 707 | 64 | 0.69 (0.53–0.88) | 482 | 5.20 (4.75–5.68) | 540 | 5.82 (5.34–6.34) | 100 | 1.08 (0.88–1.31) |
| SNRIs | 35 496 | 18 791 | 15 | 0.80 (0.45–1.31) | 107 | 5.69 (4.67–6.88) | 120 | 6.39 (5.29–7.64) | 20 | 1.06 (0.65–1.64) |
| MAOIs | 1736 | 664 | 1 | 1.50 (0.04–8.38) | 3 | 4.52 (0.93–13.20) | 4 | 6.02 (1.64–15.42) | 1 | 1.50 (0.04–8.34) |
| Other newer and atypical agents | 28 085 | 12 243 | 14 | 1.14 (0.62–1.92) | 54 | 4.41 (3.31–5.76) | 67 | 5.47 (4.24–6.95) | 17 | 1.42 (0.83–2.27) |
| TCAs | 33 289 | 14 597 | 5 | 0.34 (0.11–0.80) | 82 | 5.62 (4.47–6.97) | 87 | 5.96 (4.77–7.35) | 9 | 0.62 (0.28–1.17) |
| Total (excluding SSRIs, total) | 285 831 | 139 002 | 99 | 0.71 (0.58–0.87) | 728 | 5.24 (4.86–5.63) | 818 | 5.88 (5.49–6.30) | 147 | 1.06 (0.89–1.24) |
Abbreviations: CI, confidence interval; MAOIs, monoamine oxidase inhibitors; SNRIs, serotonin-norepinephrine reuptake inhibitors; SSRIs, selective serotonin reuptake inhibitors; TCAs, tricyclic agents.
Citalopram indicates citalopram hydrobromide (Celexa); fluoxetine, fluoxetine hydrochloride (Prozac); fluvoxamine, fluvoxamine maleate (Luvox); paroxetine, paroxetine hydrochloride (Paxil); sertraline, sertraline hydrochloride (Zoloft); and SNRIs, venlafaxine hydrochloride.
Violent suicide attempts (International Classification of Diseases, Ninth Revision24 E-codes) and violent completed suicides (International Statistical Classification of Diseases, 10th Revision25 X-codes) were defined as follows: hanging (E953.0; X70), gunshot or explosion (E955; X72-X75), jumping or lying in front of a moving object (E957, E958.0; X80, X81), stabbing or blunt trauma (E956; X78, X79), vehicle collision (E958.5, E958.6; X82), electrocution (E958.4; X83), and self-immolation (E958.1; X76).
No antidepressant use in the past 3 years.
STATISTICAL ANALYSES
We used Cox proportional hazards regression to estimate the effect of antidepressant use on the composite outcome of attempted or completed suicide. Fluoxetine was used as the reference group for comparisons among the SSRIs, in keeping with a recent meta-analysis of antidepressant efficacy.33 For between-class comparisons, SSRIs—the most widely used class—served as the reference group. Owing to the anticipated small number of outcomes, we included all potential confounders in an exposure propensity score to control for confounding by indication.34 No variable selection was applied. After plotting and comparing the distribution of propensity scores for the patients in the exposed and reference groups, we truncated our study population to the area of overlap. To the extent that subjects with nonoverlapping propensity scores may have absolute clinical indications or contraindications for a particular treatment, a trimmed analysis removing these subjects may offer a more relevant estimate of treatment effects.35 Because relative risk estimates varied within deciles of propensity scores, we used exposure propensity score decile as a categorical variable in the Cox proportional hazards models.
In addition to constructing a propensity score including the described covariates, we constructed a high-dimensional propensity score including empirically identified covariates.36 This technique examines all dispensed drugs, recorded diagnoses, and performed procedures reported for a patient before the initiation of antidepressant therapy and identifies those codes that could potentially bias the exposure and outcome in question. All identified codes were sorted by their potential for confounding and were used to create a propensity score based on the top 500 identified variables in addition to the variables identified by us (eTable 2). This new technique has been shown to further improve adjustment for confounding.36 We consider results from this analysis to be the most completely adjusted and will base our conclusions mainly on these results.
In addition to the propensity score–adjusted analysis, we conducted a multivariate-adjusted analysis. This model was constructed using a backward selection algorithm to select covariates from the list in eTable 2 with the exclusion criteria set to 0.2 as recommended by simulation studies on variable selection in epidemiology.37 We conducted 2 subgroup analyses: one restricted to subjects without a prior suicide attempt and a second restricted to subjects who were treatment naive (defined as having no antidepressant use in the past 3 years with complete records during this period). In addition, we tested for effect measure modification by prior psychiatric hospitalization and prior suicide attempt.
In addition to the parametric analyses we have described, we plotted Kaplan-Meier curves for suicide attempt–free survival as a function of the duration of continuous use of the index antidepressant. We also created adjusted Kaplan-Meier plots by weighting each subject by the inverse of his or her probability for treatment as estimated in the propensity score analysis described earlier.
RESULTS
From January 1, 1997, to December 31, 2005, a total of 287 543 adults with a depression diagnosis initiated antidepressant therapy. As shown in Table 1, women accounted for 56.2% of the population. Substance abuse (2.8%), anxiety and sleep disorders (1.6%), psychotic disorder (1.6%), and other mental disorders (5.6%) were the most common psychiatric comorbidities. Among the population, 5.1% had been hospitalized for a psychiatric condition and 0.6% had a prior suicide attempt. The SSRIs were the most common class of medication prescribed, accounting for 65.5% of antidepressant use. Paroxetine and citalopram, the most commonly prescribed drugs, accounted for 39.7% and 24.2% of overall SSRI use, respectively. A total of 199 594 subjects (69.4%) had no antidepressant use in the past 3 years, composing a treatment-naive subgroup.
The mean follow-up was 0.49 person-years. A total of 89 826 subjects (31.2%) reached 1 year of follow-up; 197 681 subjects (68.8%) were censored before the end of 1 year. Antidepressant discontinuation was the most common reason for censoring (49.2%), followed by antidepressant switching or augmentation (10.6%), end of the study period (5.0%), nursing home admission (2.1%), emigration (0.8%), nonsuicide death (0.8%), and attempted suicide or suicide death (0.3%).
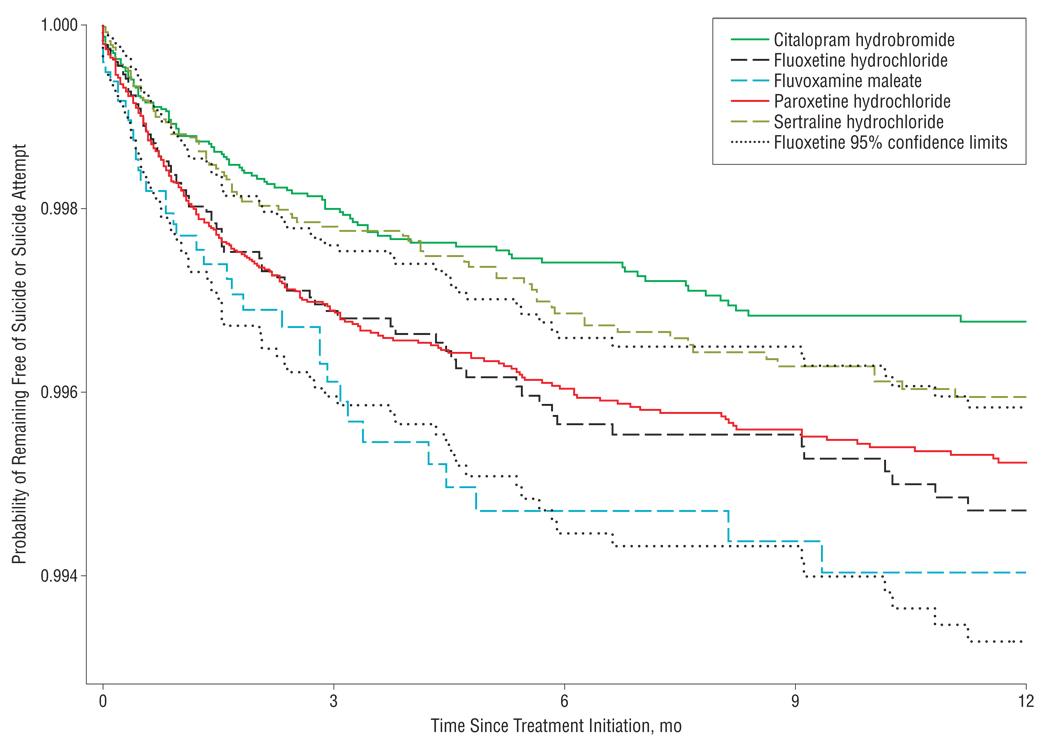
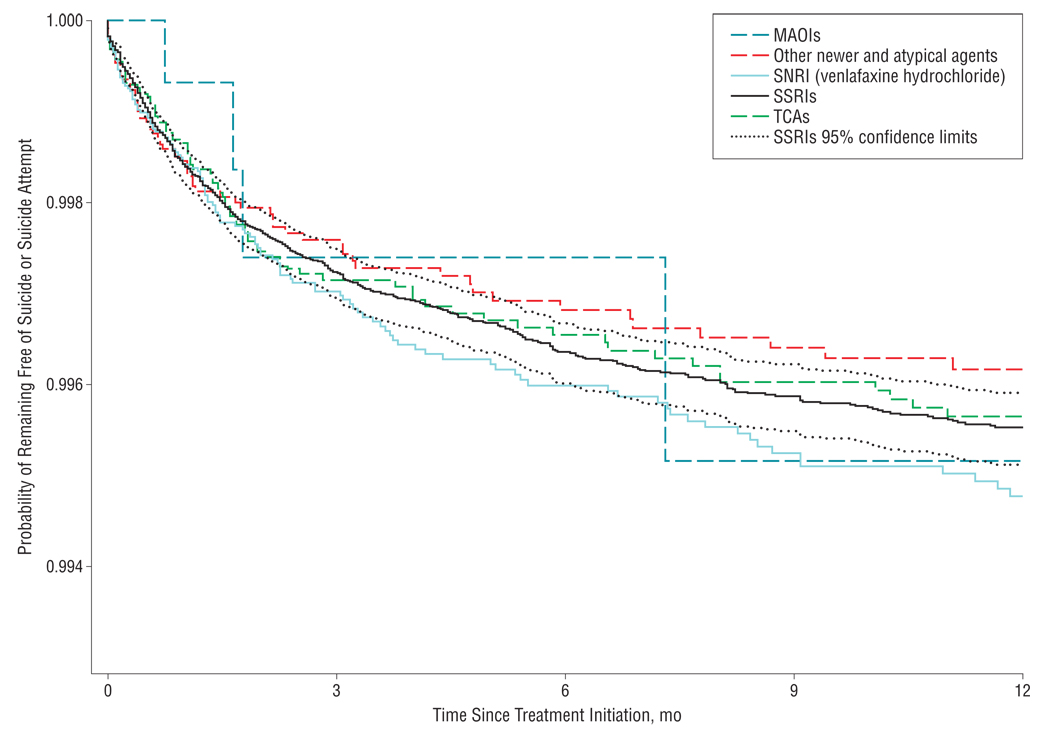
During the first year of antidepressant use, we identified a total of 846 adults who attempted suicide (751 adults) and/or completed suicide (104 adults), yielding an event rate of 6.06 attempted and completed suicides per 1000 person-years (95% confidence interval [CI], 5.65–6.48). The composite event rates in subgroups of treatment-naive subjects and subjects without a prior suicide attempt were similar to the overall rate (Table 2). Although the hazards were proportional throughout the 1-year follow-up period, most of the events happened in the first 6 months after initiation (Figure 1 and Figure 2). We identified a total of 155 violent suicides or suicide attempts during the follow-up period, resulting in a rate of 1.11 per 1000 person-years (95% CI, 0.94–1.30).
Figure 1.
Probability of remaining free of suicide or suicide attempt by selective serotonin reuptake inhibitor agent and time since treatment initiation.
Figure 2.
Probability of remaining free of suicide or suicide attempt by antidepressant class and time since treatment initiation. MAOIs indicates monoamine oxidase inhibitors; SNRI, serotonin-norepinephrine reuptake inhibitor; SSRIs, selective serotonin reuptake inhibitors; and TCAs, tricyclic agents.
As shown in Table 3, in unadjusted analyses citalopram was associated with a reduced risk of attempted or completed suicide compared with fluoxetine among all adults (hazard ratio [HR]=0.63; 95% CI, 0.47–0.85). This effect was attenuated to 0.86 (95% CI, 0.56–1.32) in a propensity score–adjusted analysis and to 1.00 (95% CI, 0.63–1.58) when the analysis was restricted to patients with no antidepressant use in the past 3 years. Sertraline was also associated with an apparent reduced risk in all adults in the propensity score–adjusted analysis (HR=0.72; 95% CI, 0.53–0.99). This effect was attenuated when the population was restricted to subjects with no antidepressant use in the 3 years prior to antidepressant initiation. Venlafaxine was associated with an increased risk of attempted or completed suicide after propensity score adjustment (HR=1.22; 95% CI, 1.00–1.49). This association was attenuated after restriction to subjects with no antidepressant use in the 3 years prior to antidepressant initiation (HR=1.16; 95% CI, 0.92–1.47). There were no significant differences among antidepressant classes. Truncating follow-up time at 6 months yielded results quantitatively similar to our 1-year analysis (eTable 3). Propensity score–weighted Kaplan-Meier plots of pairwise comparisons with fluoxetine are presented in eFigure 1, and pairwise comparisons of other antidepressant classes are presented in eFigure 2. The lack of major differences between survival plots is consistent with our findings in Table 3. The HRs were similarly proportional over time between agents and refute meaningful differences in time to suicidal acts after initiation of drug treatment.
Table 3.
Event Hazard Ratios for Composite and Violent Composite Outcomes During 1-Year Follow-up Comparing Other Selective Serotonin Reuptake Inhibitors vs Fluoxetine and Comparing Other Antidepressant Classes vs Selective Serotonin Reuptake Inhibitorsa
| HR (95% CI) | ||||||
|---|---|---|---|---|---|---|
| Composite Outcome | Violent Composite Outcome | |||||
| Antidepressantb | Unadjusted | Adjusted for Age, Sex, and Calendar Year |
Adjusted for Propensity Score Decilec |
Adjusted for High-Dimensional Propensity Score Deciled |
Adjusted for Propensity Score Decilec |
Adjusted for High-Dimensional Propensity Score Deciled |
| All Adults | ||||||
| SSRIs | ||||||
| Citalopram | 0.63 (0.47–0.85) | 0.97 (0.63–1.50) | 0.86 (0.56–1.32) | 1.00 (0.63–1.57) | 0.35 (0.16–0.77) | 0.40 (0.17–0.95) |
| Fluvoxamine | 1.22 (0.83–1.79) | 1.28 (0.86–1.90) | 1.09 (0.73–1.63) | 0.98 (0.63–1.51) | 1.14 (0.48–2.69) | 1.35 (0.55–3.35) |
| Paroxetine | 0.95 (0.74–1.22) | 1.12 (0.87–1.46) | 0.98 (0.75–1.28) | 1.02 (0.77–1.35) | 0.70 (0.40–1.23) | 0.81 (0.42–1.54) |
| Sertraline | 0.75 (0.55–1.01) | 0.86 (0.64–1.17) | 0.72 (0.53–0.99) | 0.75 (0.53–1.05) | 0.54 (0.27–1.09) | 0.53 (0.24–1.18) |
| Class | ||||||
| SNRIs | 1.12 (0.92–1.36) | 1.21 (0.99–1.48) | 1.22 (1.00–1.49) | 1.25 (1.01–1.54) | 0.94 (0.58–1.52) | 0.98 (0.58–1.66) |
| MAOIs | 0.87 (0.33–2.33) | 0.76 (0.28–2.05) | 0.76 (0.28–2.05) | 0.96 (0.31–3.01) | 1.15 (0.16–8.38) | No events |
| Other newer and atypical agents | 0.90 (0.70–1.16) | 0.94 (0.73–1.20) | 0.82 (0.63–1.07) | 0.76 (0.56–1.04) | 1.09 (0.65–1.83) | 1.03 (0.58–1.86) |
| TCAs | 0.97 (0.77–1.21) | 1.04 (0.83–1.31) | 1.04 (0.82–1.31) | 1.14 (0.88–1.47) | 0.57 (0.28–1.13) | 0.45 (0.19–1.03) |
| Treatment-Naive Adultse | ||||||
| SSRIs | ||||||
| Citalopram | 0.77 (0.52–1.15) | 1.00 (0.64–1.57) | 1.00 (0.63–1.58) | 1.08 (0.64–1.80) | 0.37 (0.16–0.82) | 0.36 (0.15–0.86) |
| Fluvoxamine | 1.17 (0.62–2.23) | 1.30 (0.67–2.51) | 1.15 (0.59–2.26) | 1.16 (0.57–2.34) | 0.93 (0.25–3.46) | 0.81 (0.21–3.20) |
| Paroxetine | 0.99 (0.68–1.44) | 1.08 (0.74–1.58) | 1.05 (0.71–1.55) | 1.21 (0.77–1.88) | 0.57 (0.27–1.19) | 0.70 (0.31–1.58) |
| Sertraline | 0.85 (0.55–1.32) | 0.98 (0.63–1.52) | 0.85 (0.54–1.34) | 0.79 (0.49–1.27) | 0.43 (0.17–1.10) | 0.39 (0.15–1.03) |
| Class | ||||||
| SNRIs | 1.14 (0.91–1.43) | 1.15 (0.91–1.45) | 1.16 (0.92–1.47) | 1.19 (0.93–1.52) | 0.74 (0.41–1.31) | 0.81 (0.43–1.52) |
| MAOIs | 0.93 (0.13–6.61) | 1.01 (0.14–7.21) | 0.95 (0.13–6.77) | No events | No events | No events |
| Other newer and atypical agents | 0.92 (0.67–1.26) | 0.99 (0.72–1.35) | 0.87 (0.62–1.22) | 0.74 (0.50–1.12) | 1.01 (0.53–1.92) | 0.82 (0.37–1.80) |
| TCAs | 1.08 (0.81–1.44) | 1.23 (0.92–1.65) | 1.25 (0.92–1.69) | 1.43 (1.02–2.00) | 0.53 (0.21–1.33) | 0.44 (0.14–1.42) |
| Adults With No Prior Suicide Attempt | ||||||
| SSRIs | ||||||
| Citalopram | 0.64 (0.48–0.86) | 0.96 (0.62–1.48) | 0.84 (0.54–1.30) | 0.93 (0.59–1.47) | 0.33 (0.15–0.73) | 0.38 (0.16–0.91) |
| Fluvoxamine | 1.24 (0.84–1.84) | 1.31 (0.88–1.95) | 1.10 (0.73–1.66) | 0.97 (0.62–1.52) | 1.14 (0.48–2.70) | 1.35 (0.55–3.33) |
| Paroxetine | 0.93 (0.72–1.20) | 1.09 (0.84–1.42) | 0.95 (0.72–1.25) | 0.95 (0.72–1.27) | 0.60 (0.33–1.07) | 0.76 (0.39–1.48) |
| Sertraline | 0.78 (0.58–1.06) | 0.90 (0.66–1.22) | 0.75 (0.55–1.02) | 0.69 (0.49–0.98) | 0.54 (0.27–1.09) | 0.55 (0.25–1.21) |
| Class | ||||||
| SNRIs | 1.13 (0.93–1.38) | 1.22 (0.99–1.49) | 1.24 (1.01–1.52) | 1.22 (0.98–1.51) | 0.97 (0.59–1.58) | 0.97 (0.56–1.67) |
| MAOIs | 0.91 (0.34–2.44) | 0.80 (0.30–2.15) | 0.79 (0.30–2.13) | 0.97 (0.31–3.04) | 1.22 (0.17–8.87) | No events |
| Other newer and atypical agents | 0.92 (0.71–1.18) | 0.95 (0.74–1.23) | 0.83 (0.63–1.09) | 0.82 (0.60–1.11) | 1.16 (0.69–1.95) | 1.15 (0.65–2.04) |
| TCAs | 1.01 (0.80–1.27) | 1.09 (0.87–1.37) | 1.07 (0.84–1.35) | 1.14 (0.88–1.49) | 0.59 (0.30–1.17) | 0.46 (0.20–1.07) |
Abbreviations: CI, confidence interval; HR, hazard ratio; MAOIs, monoamine oxidase inhibitors; SNRIs, serotonin-norepinephrine reuptake inhibitors; SSRIs, selective serotonin reuptake inhibitors; TCAs, tricyclic agents.
Outcomes indicate attempted and completed suicide. The reference group for the SSRI comparisons is fluoxetine hydrochloride (Prozac); the reference group for the antidepressant drug class comparisons is SSRIs. The MAOIs were not investigated owing to the small numbers of exposed subjects.
Citalopram indicates citalopram hydrobromide (Celexa); fluvoxamine, fluvoxamine maleate (Luvox); paroxetine, paroxetine hydrochloride (Paxil); sertraline, sertraline hydrochloride (Zoloft); and SNRIs, venlafaxine hydrochloride.
The propensity score includes demographic, psychiatric, and general clinical covariates listed in eTable 2.
The high-dimensional propensity score includes the 500 empirically identified covariates most likely to be confounders as well as the demographic, psychiatric, and clinical covariates listed in eTable 2.
No antidepressant use in the past 3 years.
Citalopram appeared to be associated with a lower risk of violent suicides and suicide attempts (HR=0.35; 95% CI, 0.16–0.77). This apparent effect was not attenuated by restriction to treatment-naive subjects or to subjects without prior suicide attempts.
We did not find evidence of effect measure modification by prior psychiatric hospitalization.
COMMENT
In a population of 287 543 adults aged 18 years and older initiating antidepressant therapy in British Columbia between January 1, 1997, and December 31, 2005, we observed no clinically meaningful variation in the risk of suicide and suicide attempt by the type of antidepressant initiated. Most events occurred in the first 6 months after treatment initiation. We observed some variation in the effect size between individual agents to the extent that 95% CIs were not overlapping when comparing an-tidepressants using conventional propensity score methods. Effect sizes became more similar after restriction to subjects without any antidepressant use in the past 3 years; this population is much closer to a population of firsttime antidepressant users and an analysis within this population is less confounded by prior treatment experience and progression of the underlying condition, providing a more valid estimate of the causal treatment effects.
Our results are consistent with the findings of several other recent observational studies that reported small or no differences in suicides and suicide attempts between antidepressant classes10–12 and a meta-analysis of randomized controlled trial data that found no difference in suicide attempt rates between SSRI and TCA users.38 Similar to a cohort study using General Practice Research Database data, we found a higher rate of suicidal acts in venlafaxine users compared with SSRI users, even after adjustment for measured confounders. This effect was attenuated in secondary analyses restricted to treatment-naive users, suggesting some residual confounding in our primary analysis.14 In contrast to a case-control study in Ontario residents aged 66 years and older,15 we did not observe an increased risk of violent suicide among SSRI initiators relative to initiators of other antidepressants. This same article reported that SSRIs were associated with a nearly 5-fold increased risk of suicide during the first month of treatment compared with other antidepressants but found no difference between classes during subsequent periods. While our adjusted analyses did not specifically compare suicide rates between agents during the first month of treatment, our HRs appeared to be proportional over time and we did not observe an overall increase in the risk of suicide among patients treated with SSRIs relative to patients treated with other antidepressants.
During the 9-year study period, the suicide risk among all British Columbia residents aged 18 years and older averaged around 0.16 suicide deaths per 1000 people.39,40 The rate we observed after initiation of antidepressant therapy was almost 5 times higher, reflecting the current depressed state and greater degree of psychiatric co-morbidity in our population.
One of the major strengths of our study is that the large, stable study population allowed us to look at a variety of medications and at important subgroups. We had an adequate sample size to restrict our study to new initiators of antidepressants with a recorded depression diagnosis. An incident user design reduces the likelihood of missing early adverse events, allows for an evaluation of risks over time, ensures that the assessment of patient baseline characteristics is uninfluenced by any effects of antidepressant treatment, and reduces the likelihood that current treatment assignment is influenced by past drug-related experiences such as adverse effects and refractory symptoms. To have clearly identified exposure groups, we compared monotherapies with each other and censored patient follow-up as soon as the patient switched drugs or augmented therapy. This analytic strategy makes treatment groups more comparable with regard to initial health state and avoids analytic difficulties associated with comparing patients who escalate or change treatment in response to treatment failure or adverse effects vs those who do not. This will reduce the generalizability of our findings to patients receiving monotherapy, but the improvement in validity outweighs this reduction in generalizability. Similarly, we limited the follow-up time for the primary analysis to 12 months. Most events happened during that period, and longer observation would lead to increasingly selected populations in later months. Such patients would by definition not switch or discontinue their initial antidepressant for a 12-month period and their depression is likely well controlled without drug intolerance.
Nonrandomized studies using health care utilization data are particularly scrutinized for their limited control of confounding and their potential for misclassifying diagnoses.41 Confounding would occur if certain antidepressants were more likely to be given to patients with a greater background risk of suicide. We therefore controlled for sociodemographic, clinical, and health care utilization factors likely to be independent predictors of suicidality using traditional multivariate and high-dimensional propensity score techniques. Although we could show that with increasing adjustment (including high-dimensional propensity score adjustment36) point estimates generally moved closer to the null result, our ability to fully adjust for mental health status is limited by the measurement and reporting of mental health conditions as ICD-9 diagnosis codes on insurance claims to the provincial government. Well-validated behavioral risk factors such as impulsivity and hopelessness, environmental factors such as access to lethal means, and family history of completed suicide42 would not be measured in claims data. Random misclassification of confounders in health care utilization databases leads to incomplete adjustment of confounding bias.43 For example, our finding that the apparent increased risk of suicide among venlafaxine users was attenuated when we restricted our analysis to subjects with no antidepressant use in the past 3 years suggests that there may have been residual confounding by the presence of treatment-resistant depression or other suicide risk factors in our initial analysis. Other studies have documented that venlafaxine tends to be prescribed to people with past SSRI treatment failure44,45 and with a greater burden of suicide risk factors.46
There are several limitations inherent in our definition of completed and attempted suicide outcomes. It is known that suicides are underreported by coroners, with an estimated 10% of suicides misclassified as other injuries in one US study.30 However, the specificity of suicide as a cause of death has been shown to be extremely high.30 Relative risk estimates are unbiased if outcomes are assessed with 100% specificity, even if sensitivity is far lower owing to nondifferential misclassification.47 While underreporting of suicide deaths is likely nondifferential, there is a possibility that patients treated with specific agents or having specific histories might attract closer scrutiny for potential suicide. Decedents using TCAs, which are known to be fatal in overdose, might be more likely to receive a suicide diagnosis. To make our analyses comparable to those presented by Juurlink et al,15 we analyzed the sub–end point of violent completed and attempted suicide. Because there is some evidence that suicide mechanism is not coded with perfect accuracy and intent is coded with greater accuracy for injuries due to poisoning than those due to firearms29 and because of the smaller number of violent events, we place the greatest weight on our overall findings.
In terms of the validity of our suicide attempt definition, intentional self-harm E-codes do not distinguish between suicidality and self-harm without suicidal intent despite their widespread use in research and surveillance to identify suicide attempts. However, a study of the validity of deliberate self-harm E-codes to identify suicide attempts reported a positive predictive value of 86% for these codes relative to the gold standard of medical record review.28 Ascertainment of intentional self-harm admissions within the British Columbia hospital discharge database is likely to be nearly complete as this database has an E-coding completeness rate greater than 95% for the years evaluated and does not have the E-coding completeness problems documented in some US claims databases.27 However, we did not have data on suicide attempts by patients who were seen in an emergency department, treated, and released without an inpatient admission. An analysis of 2006 US Web-Based Injury Statistics Query and Reporting System48 data showed that 70% of patients with nonfatal self-harm cases seen in the emergency department were admitted to the hospital, suggesting that our definition had a 70% sensitivity for nonfatal intentional self-harm. Our inclusion of only more severe attempts that require at least 1 night in the hospital is likely to increase the specificity.
Despite these limitations, our finding of equal event rates across antidepressant agents supports the US Food and Drug Administration’s decision to treat all antidepressants alike in their advisory. Treatment decisions should be based on efficacy, and clinicians should be vigilant in monitoring after initiating therapy with any antidepressant agent.
Acknowledgments
Funding/Support: This work was supported by grant RO1-MH078708 from the National Institute of Mental Health.
Footnotes
Author Contributions: Dr Schneeweiss had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Financial Disclosure: None reported.
Disclaimer: The views expressed in this article do not necessarily represent the views of the National Institute of Mental Health, the National Institutes of Health, the Department of Health and Human Services, or the US government.
Online-Only Material: The eTables and eFigures are available at http://www.archgenpsychiatry.com.
REFERENCES
- 1.Kessler RC, Berglund P, Borges G, Nock M, Wang PS. Trends in suicide ideation, plans, gestures, and attempts in the United States, 1990–1992 to 2001–2003. JAMA. 2005;293(20):2487–2495. doi: 10.1001/jama.293.20.2487. [DOI] [PubMed] [Google Scholar]
- 2.Baldessarini RJ, Tondo L, Strombom IM, Dominguez S, Fawcett J, Licinio J, Oquendo MA, Tollefson GD, Valuck RJ, Tohen M. Ecological studies of antidepressant treatment and suicidal risks. Harv Rev Psychiatry. 2007;15(4):133–145. doi: 10.1080/10673220701551102. [DOI] [PubMed] [Google Scholar]
- 3.Isacsson G, Holmgren A, Osby U, Ahlner J. Decrease in suicide among the individuals treated with antidepressants: a controlled study of antidepressants in suicide, Sweden 1995–2005. Acta Psychiatr Scand. 2009;120(1):37–44. doi: 10.1111/j.1600-0447.2009.01344.x. [DOI] [PubMed] [Google Scholar]
- 4.US Food and Drug Administration. [Accessed February 1, 2005];Summary minutes of the CDER Psychopharmacologic Drugs Advisory Committee and the FDA Pediatric Advisory Committee, September 13–14, 2004. http://www.fda.gov/ohrms/dockets/ac/04/minutes/2004-4065M1_Final.htm.
- 5.Hammad TA, Laughren T, Racoosin J. Suicidality in pediatric patients treated with antidepressant drugs. Arch Gen Psychiatry. 2006;63(3):332–339. doi: 10.1001/archpsyc.63.3.332. [DOI] [PubMed] [Google Scholar]
- 6.US Food and Drug Administration. [Accessed May, 2007];Overview for December 13 Meeting of Psychopharmacologic Drugs Advisory Committee (PDAC) http://www.fda.gov/ohrms/dockets/ac/06/briefing/2006-4272b1-01-FDA.pdf.
- 7.Gunnell D, Saperia J, Ashby D. Selective serotonin reuptake inhibitors (SSRIs) and suicide in adults: meta-analysis of drug company data from placebo controlled, randomised controlled trials submitted to the MHRA’s safety review. BMJ. 2005;330(7488):385. doi: 10.1136/bmj.330.7488.385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Saperia J, Ashby D, Gunnell D. Suicidal behaviour and SSRIs: updated meta-analysis. BMJ. 2006;332(7555):1453. doi: 10.1136/bmj.332.7555.1453-a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hammad TA, Laughren TP, Racoosin JA. Suicide rates in short-term randomized controlled trials of newer antidepressants. J Clin Psychopharmacol. 2006;26(2):203–207. doi: 10.1097/01.jcp.0000203198.11453.95. [DOI] [PubMed] [Google Scholar]
- 10.Jick H, Kaye J, Jick S. Antidepressants and the risk of suicidal behaviors. JAMA. 2004;292(3):338–343. doi: 10.1001/jama.292.3.338. [DOI] [PubMed] [Google Scholar]
- 11.Martinez C, Rietbrock S, Wise L, Ashby D, Chick J, Moseley J, Evans S, Gunnell D. Antidepressant treatment and the risk of fatal and non-fatal self-harm in first episode depression: nested case-control study. BMJ. 2005;330(7488):389. doi: 10.1136/bmj.330.7488.389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Simon GE, Savarino J, Operskalski B, Wang PS. Suicide risk during antidepressant treatment. Am J Psychiatry. 2006;163(1):41–47. doi: 10.1176/appi.ajp.163.1.41. [DOI] [PubMed] [Google Scholar]
- 13.Valuck RJ, Libby AM, Sills MR, Giese AA, Allen RR. Antidepressant treatment and risk of suicide attempt by adolescents with major depressive disorder: a propensity-adjusted retrospective cohort study. CNS Drugs. 2004;18(15):1119–1132. doi: 10.2165/00023210-200418150-00006. [DOI] [PubMed] [Google Scholar]
- 14.Rubino A, Roskell N, Tennis P, Mines D, Weich S, Andrews E. Risk of suicide during treatment with venlafaxine, citalopram, fluoxetine, and dothiepin: retrospective cohort study. BMJ. 2007;334(7587):242. doi: 10.1136/bmj.39041.445104.BE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Juurlink DN, Mamdani MM, Kopp A, Redelmeier DA. The risk of suicide with selective serotonin reuptake inhibitors in the elderly. Am J Psychiatry. 2006;163(5):813–821. doi: 10.1176/ajp.2006.163.5.813. [DOI] [PubMed] [Google Scholar]
- 16.Fazel S, Grann M, Ahlner J, Goodwin G. Suicides by violent means in individuals taking SSRIs and other antidepressants: a postmortem study in Sweden, 1992–2004. J Clin Psychopharmacol. 2007;27(5):503–506. doi: 10.1097/jcp.0b013e31814ce3ef. [DOI] [PubMed] [Google Scholar]
- 17.British Columbia Ministry of Health Services. [Accessed October 17, 2008];PharmaNet. http://www.health.gov.bc.ca/pharmacare/pharmanet/netindex.html.
- 18.Williams JI, Young W. Inventory of Studies on the Accuracy of Canadian Health Administrative Databases. Toronto, ON: Institute for Clinical Evaluative Sciences; 1996. [Google Scholar]
- 19.Pharmaceutical Services Division, Ministry of Health Services, Government of British Columbia. [Accessed October 17, 2008];BC PharmaCare Annual Performance Report 2005. http://www.health.gov.bc.ca/pharmacare/pdf/APROnline.pdf.
- 20.Dormuth CR, Glynn RJ, Neumann P, Maclure M, Brookhart AM, Schneeweiss S. Impact of two sequential drug cost-sharing policies on the use of inhaled medications in older patients with chronic obstructive pulmonary disease or asthma. Clin Ther. 2006;28(6):964–978. doi: 10.1016/j.clinthera.2006.06.007. discussion 962–963. [DOI] [PubMed] [Google Scholar]
- 21.Dormuth CR, Schneeweiss S, Brookhart MA, Carney G, Bassett K, Adams S, Wright JM. Frequency and predictors of tablet splitting in statin prescriptions: a population-based analysis. Open Med. 2008;2(3):e5–e13. [PMC free article] [PubMed] [Google Scholar]
- 22.Kwon A, Bungay KM, Pei Y, Rogers WH, Wilson IB, Zhou Q, Adler DA. Antide-pressant use: concordance between self-report and claims records. Med Care. 2003;41(3):368–374. doi: 10.1097/01.MLR.0000053019.79054.B6. [DOI] [PubMed] [Google Scholar]
- 23.Johnson RE, Vollmer WM. Comparing sources of drug data about the elderly. J Am Geriatr Soc. 1991;39(11):1079–1084. doi: 10.1111/j.1532-5415.1991.tb02872.x. [DOI] [PubMed] [Google Scholar]
- 24.World Health Organization. Geneva, Switzerland: World Health Organization; International Classification of Diseases, Ninth Revision (ICD-9) 1977
- 25.World Health Organization. Geneva, Switzerland: World Health Organization; International Statistical Classification of Diseases, 10th Revision (ICD-10) 1992
- 26.Canadian Institute for Health Information. Abstracting Manual. Ottawa, ON: Canadian Institute for Health Information; 1999. [Google Scholar]
- 27.Clark DE, DeLorenzo MA, Lucas FL, Wennberg DE. Epidemiology and short-term outcomes of injured Medicare patients. J Am Geriatr Soc. 2004;52(12):2023–2030. doi: 10.1111/j.1532-5415.2004.52560.x. [DOI] [PubMed] [Google Scholar]
- 28.Iribarren C, Sidney S, Jacobs DR, Jr, Weisner C. Hospitalization for suicide attempt and completed suicide: epidemiological features in a managed care population. Soc Psychiatry Psychiatr Epidemiol. 2000;35(7):288–296. doi: 10.1007/s001270050241. [DOI] [PubMed] [Google Scholar]
- 29.LeMier M, Cummings P, West TA. Accuracy of external cause of injury codes reported in Washington State hospital discharge records. Inj Prev. 2001;7(4):334–338. doi: 10.1136/ip.7.4.334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Moyer LA, Boyle CA, Pollock DA. Validity of death certificates for injury-related causes of death. Am J Epidemiol. 1989;130(5):1024–1032. doi: 10.1093/oxfordjournals.aje.a115403. [DOI] [PubMed] [Google Scholar]
- 31.Warburton RN. Takeup of income-tested health-care premium subsidies: evidence and remedies for British Columbia. Can Tax J. 2005;53(1):1–28. [Google Scholar]
- 32.Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40(5):373–383. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 33.Cipriani A, Furukawa TA, Salanti G, Geddes JR, Higgins JP, Churchill R, Watanabe N, Nakagawa A, Omori IM, McGuire H, Tansella M, Barbui C. Comparative efficacy and acceptability of 12 new-generation antidepressants: a multiple-treatments meta-analysis. Lancet. 2009;373(9665):746–758. doi: 10.1016/S0140-6736(09)60046-5. [DOI] [PubMed] [Google Scholar]
- 34.Braitman LE, Rosenbaum PR. Rare outcomes, common treatments: analytic strategies using propensity scores. Ann Intern Med. 2002;137(8):693–695. doi: 10.7326/0003-4819-137-8-200210150-00015. [DOI] [PubMed] [Google Scholar]
- 35.Schneeweiss S, Patrick AR, Stürmer T, Brookhart MA, Avorn J, Maclure M, Rothman KJ, Glynn RJ. Increasing levels of restriction in pharmacoepidemiologic database studies of elderly and comparison with randomized trial results. Med Care. 2007;45((10)) suppl 2:S131–S142. doi: 10.1097/MLR.0b013e318070c08e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Schneeweiss S, Rassen JA, Glynn RJ, Avorn J, Mogun H, Brookhart MA. High-dimensional propensity score adjustment in studies of treatment effects using health care claims data. Epidemiology. 2009;20(4):512–522. doi: 10.1097/EDE.0b013e3181a663cc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Maldonado G, Greenland S. Simulation study of confounder-selection strategies. Am J Epidemiol. 1993;138(11):923–936. doi: 10.1093/oxfordjournals.aje.a116813. [DOI] [PubMed] [Google Scholar]
- 38.Fergusson D, Doucette S, Glass KC, Shapiro S, Healy D, Hebert P, Hutton B. Association between suicide attempts and selective serotonin reuptake inhibitors: systematic review of randomised controlled trials [published correction appears in BMJ. 2005;330(7492):653] BMJ. 2005;330(7488):396. doi: 10.1136/bmj.330.7488.396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.British Columbia Coroners Service. [Accessed November 18, 2008];Suicide statistics, 1997–2004. http://www.pssg.gov.bc.ca/coroners/publications/docs/stats-suicide-1997-2004.pdf.
- 40.BCStats. [Accessed November 18, 2008];British Columbia population by selected age groups. http://www.bcstats.gov.bc.ca/data/pop/pop/project/bc0806tab3.csv.
- 41.Schneeweiss S, Avorn J. A review of uses of health care utilization databases for epidemiologic research on therapeutics. J Clin Epidemiol. 2005;58(4):323–337. doi: 10.1016/j.jclinepi.2004.10.012. [DOI] [PubMed] [Google Scholar]
- 42.Hawton K, van Heeringen K. Suicide. Lancet. 2009;373(9672):1372–1381. doi: 10.1016/S0140-6736(09)60372-X. [DOI] [PubMed] [Google Scholar]
- 43.Greenland S, Robins J. Confounding and misclassification. Am J Epidemiol. 1985;122(3):495–506. doi: 10.1093/oxfordjournals.aje.a114131. [DOI] [PubMed] [Google Scholar]
- 44.Stahl SM, Grady MM, Moret C, Briley M. SNRIs: their pharmacology, clinical efficacy, and tolerability in comparison with other classes of antidepressants. CNS Spectr. 2005;10(9):732–747. doi: 10.1017/s1092852900019726. [DOI] [PubMed] [Google Scholar]
- 45.Rush AJ, Trivedi MH, Wisniewski SR, Stewart JW, Nierenberg AA, Thase ME, Ritz L, Biggs MM, Warden D, Luther JF, Shores-Wilson K, Niederehe G, Fava M. STAR*D Study Team. Bupropion-SR, sertraline, or venlafaxine-XR after failure of SSRIs for depression. N Engl J Med. 2006;354(12):1231–1242. doi: 10.1056/NEJMoa052963. [DOI] [PubMed] [Google Scholar]
- 46.Mines D, Hill D, Yu H, Novelli L. Prevalence of risk factors for suicide in patients prescribed venlafaxine, fluoxetine, and citalopram. Pharmacoepidemiol Drug Saf. 2005;14(6):367–372. doi: 10.1002/pds.1095. [DOI] [PubMed] [Google Scholar]
- 47.Kelsey JL, Whittemore AS, Evans AS, Thompson WD. Methods in Observational Epidemiology. 2nd ed. New York, NY: Oxford University Press; 1996. [Google Scholar]
- 48.Centers for Disease Control and Prevention. [Accessed February 12, 2008];Web-Based Injury Statistics Query and Reporting System (WISQARS), 2006. http://www.cdc.gov/ncipc/wisqars.