Abstract
Full T cell activation requires TCR engagement (signal 1) in the context of costimulation (signal 2). Costimulation is required for maximal expression of effector cytokines and prevention of T cell anergy. It has become increasingly clear that another major function of costimulation is to up-regulate the metabolic machinery necessary for T cell function. In this report we demonstrate that anergic T cells are metabolically anergic, in that upon full stimulation (signals 1 plus 2) they fail to up-regulate the machinery necessary to support increased metabolism. These findings suggest that one mechanism responsible for the maintenance of T cell anergy is failure to up-regulate the metabolic machinery. Furthermore, we demonstrate that by blocking leucine, glucose, and energy metabolism, T cell activation is mitigated. Additionally, inhibition of these metabolic pathways during T cell activation leads to anergy in Th1-differentiated cells. Overall, our findings extend the role of T cell metabolism in regulating T cell function.
The response of a T cell to a particular Ag is determined by the context in which the Ag is encountered (1). In the two-signal model, TCR engagement (signal 1) results in full T cell activation only when recognition occurs in the setting of costimulation (signal 2). Signal 2 is typically delivered when B7 molecules on the surface of activated APCs engage CD28 on the surface of the T cell. In the absence of costimulation, Ag recognition fails to promote full cytokine release and proliferation. Furthermore, the T cells enter a state of hyporesponsiveness known as anergy. Anergic T cells fail to elaborate cytokines and proliferate even when stimulated under normally activating conditions (signals 1 plus 2) (2).
Upon activation, the bioenergetic demands of a T cell increase dramatically over the resting state. Activated T cells are highly anabolic and demonstrate a marked increase in glycolysis (3). T cells will employ glycolysis to generate ATP even in the presence of high levels of oxygen. With activation there is an increase in glucose and amino acid uptake and suppression of catabolism. While there has been much focus on the role of costimulation in promoting T cell effector function, only recently has its role in regulating metabolism become apparent. CD28-induced activation of Akt increases glucose uptake by promoting the translocation of the glucose transporter glut1 to the cell surface (4). CD28 stimulation also promotes the activation of mammalian target of rapamycin (mTOR)4 (5). mTOR, through its downstream substrates S6K1 and 4E-BP1, promotes translation and protein synthesis (6). Likewise, mTOR facilitates increased amino acid uptake by increasing the cell surface expression of amino acid transporters. T cell activation requires increased energy utilization, and, as such, a role for the energy sensor AMP kinase in regulating T cell function is becoming apparent (7). AMP kinase is activated under conditions of stress and leads to the inhibition of energy consuming processes. AICAR, an activator of AMP kinase, has been shown to suppress T cell activation (8).
Our laboratory is interested in understanding the signaling pathways that regulate T cell activation vs tolerance (9). During the course of our studies we have identified mTOR as playing a critical role in integrating costimulatory signals (5, 10). Our group and others have demonstrated that for Th1 T cells, inhibition of mTOR promotes T cell anergy, even in the presence of signals 1 plus 2 (11, 12). Since mTOR is a central regulator of protein synthesis and metabolism, we wanted to investigate the status of metabolic pathways in anergic T cells. Our data indicate that not only are anergic Th1 T cells unable to up-regulate metabolic machinery in response to signals 1 plus 2, but inhibition of metabolism can lead to T cell anergy.
Materials and Methods
Reagents and Abs
N-acetyl-leucine amide (NALA) was purchased from Bachem and dissolved in ethanol. 2-Deoxyglucose (2DG) and 5-aminoimidazole-4-carboxamide ribonucleoside (AICAR) were obtained from Sigma-Aldrich and dissolved in PBS. Rapamycin was purchased from Sigma-Aldrich and dissolved in ethanol. Phospho-S6K1 (Thr421/Ser424), phospho-Akt (S473), and total S6K1 were obtained from Cell Signaling Technology. Total Akt was obtained from Santa Cruz Biotechnology. Anti-rabbit IgG HRP-linked Ab was obtained from Amersham Biosciences.
Cell culture and mice
A.E7, a CD4+ Th1 clone specific for pigeon cytochrome c (PCC) peptide 81–104 (FAGIKKKAERADLIAYLKQATAK), was maintained as previously described (11). Briefly, A.E7 cells were maintained in 50% RPMI 1640 and 50% EHAA supplemented with 10% FBS, 2 mM glutamine, 50 μM 2-ME, 100 IU/ml penicillin, 100 μg/ml streptomycin, 250 ng/ml amphotericin B, and 50 μg/ml gentamicin (Invitrogen). To expand these cells, they were stimulated for 48 h with 5 μM PCC peptide (Sigma-Aldrich) and irradiated (3000 rad) B10.A mouse splenocytes (with a ratio of A.E7 cell/B10.A splenocyte as 1:10). Mouse rIL-2 (1 ng/ml; Invitrogen) was then added to the culture. After 10 days, live cells were isolated by a Ficoll gradient (Sigma-Aldrich). B10.A mice were purchased from Taconic. 5C.C7 mice, RAG2−/− mice with a transgenic TCR specific for PCC peptide 81–104, were obtained from Taconic. To generate previously activated 5C.C7 T cells, splenocytes from these mice were incubated with 5 μM peptide for 48 h and rested in IL-2 for 5 days. Live T cells were isolated by a Ficoll gradient. The studies have been reviewed and approved by the Institutional Animal Care and Use Committee at Johns Hopkins University.
Flow cytometry
For all analyses, cells were stained in 1×PBS, 2% FBS, 0.02% sodium azide solution. Abs to CD4, CD98, and CD71 were purchased from BD Biosciences. Live cells were stained for 15 min at 4°C and fixed in 4% PFA. Cells were analyzed using a FACSCalibur flow cytometer (BD Biosciences) and CellQuest software.
Cytokine and lactate detection
Cytokines were measured from cell culture supernatants using the Ready-SET-Go! murine IFN-γ and IL-2 kits from eBioscience. Lactate was measured from supernatants using the EnzyChrom L-lactate assay kit from BioAssay Systems.
Anergy model
To induce anergy, T cells were treated with plate-bound anti-CD3 (3 μg/ml, 2C11; BD Biosciences). To activate, T cells were stimulated with plate-bound anti-CD3 plus soluble anti-CD28 (2 μg/ml, 37.51; eBioscience). After 16 h, the cells were washed three times with PBS to remove Abs and rested in media for 5–7 days. Upon rechallenge, 5 × 104 T cells were stimulated with 5 × 105 irradiated B10.A splenocytes and PCC peptide (0 – 0.5 μM), and proliferation was measured by thymidine incorporation after 48 h. To determine IL-2 production upon rechallenge, 4 × 105 treated cells were stimulated with plate-bound anti-CD3 (1 μg/ml) plus soluble anti-CD28 (2 μg/ml), supernatants were collected after 24 h, and IL-2 was measured by ELISA (eBioscience).
Immunoblot analysis
T cells were harvested and washed with ice-cold PBS. The cells were lysed with whole cell lysis buffer containing 20 mM Tris (pH 7.5), 150 mM NaCl, 1 mM EDTA, 1 mM EGTA, 1% Triton X-100, 2.5 mM sodium pyrophosphate, 1 mM β-glycerolphosphate, 1 mM sodium orthovanadate, 1 mM PMSF, and 1×protease inhibitor cocktail (Roche). After 20 min of incubation on ice, cell lysates were spun at 15,000 × g for 5 min, and supernatant was collected. Protein concentration was assessed using a BCA protein assay kit (Pierce). Next, equal amount of cell lysates (20 – 40 μg) were loaded onto NuPAGE minigels (Invitrogen), separated by SDS-PAGE, and transfered to nitrocellulose or polyvinylidene difluoride membrane (Invitrogen). The membrane was then blocked with TBS with 5% nonfat dry milk and 0.1% Tween 20 and probed with indicated Abs according to the manufacturer’s instructions.
Results
The induction of anergy is associated with incomplete up-regulation of metabolic machinery
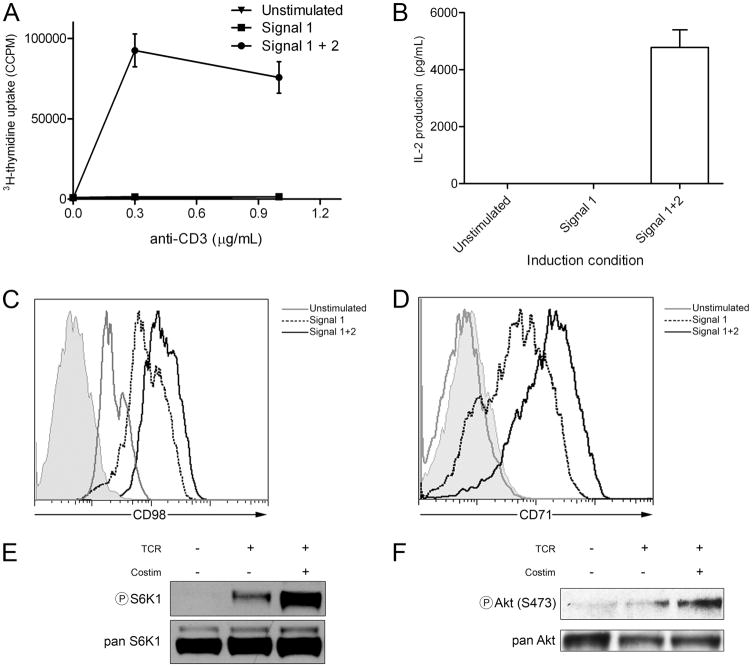
When the CD4+, Th1, PCC-specific T cell clone A.E7 is stimulated with anti-CD3 in the absence of costimulation, such cells demonstrate a marked decrease in their proliferative capacity and ability to produce IL-2 (Fig. 1, A and B). From a metabolic perspective, A.E7 T cells stimulated with signal 1 alone demonstrate decreased up-regulation of the amino acid transporter CD98 (Fig. 1C). Likewise, they do not maximally up-regulate the transferrin receptor (CD71), which is responsible for the uptake of iron (Fig. 1D). mTOR activation, which is critical for cellular metabolism, proceeds by two downstream signaling complexes: TORC1, which is associated with the adaptor protein raptor, and TORC2, which is associated with the adaptor protein rictor (13). Downstream TORC1 signaling leads to the phosphorylation of S6K1, while TORC2 activation leads to phosphorylation of Akt at S473. Stimulation with anti-CD3 alone fails to fully up-regulate TORC1 activity as determined by the phosphorylation of S6K1 (Fig. 1E). Likewise, as has been previously shown, Akt activity (14), which plays a role in glucose metabolism in T cells, fails to become fully up-regulated by signal 1 alone (Fig. 1F). Thus, when stimulated with signal 1 alone (anti-CD3), A.E7 T cell clones not only fail to be fully activated but also demonstrate defects in the metabolic machinery necessary for glucose, amino acid, and iron metabolism.
FIGURE 1.
Costimulation is required for full T cell activation and up-regulation of metabolic machinery. A, A.E7 T cells were left un-stimulated or treated with anti-CD3 alone (signal 1) or anti-CD3 plus anti-CD28 (signals 1 plus 2) for 16 h, then interrogated for proliferation by thymidine incorporation. B, Supernatants from A were interrogated for IL-2 by ELISA. C, CD98 cell surface expression of cells treated as in A by FACS. The shaded tracing indicates the iso-type control for stimulated T cells. D, As in C, but for CD71. E, Resting A.E7 T cells were left unstimulated or stimulated through the TCR (anti-CD3) or TCR and costimulation (anti-CD3 plus anti-CD28) for 3 h and interrogated for phospho-S6K1. F, As in E, but for phospho-Akt (S473). Total enzyme (pan) probes are included as loading controls. Data are representative of three independent experiments.
Anergic T cells fail to up-regulate metabolic machinery upon rechallenge
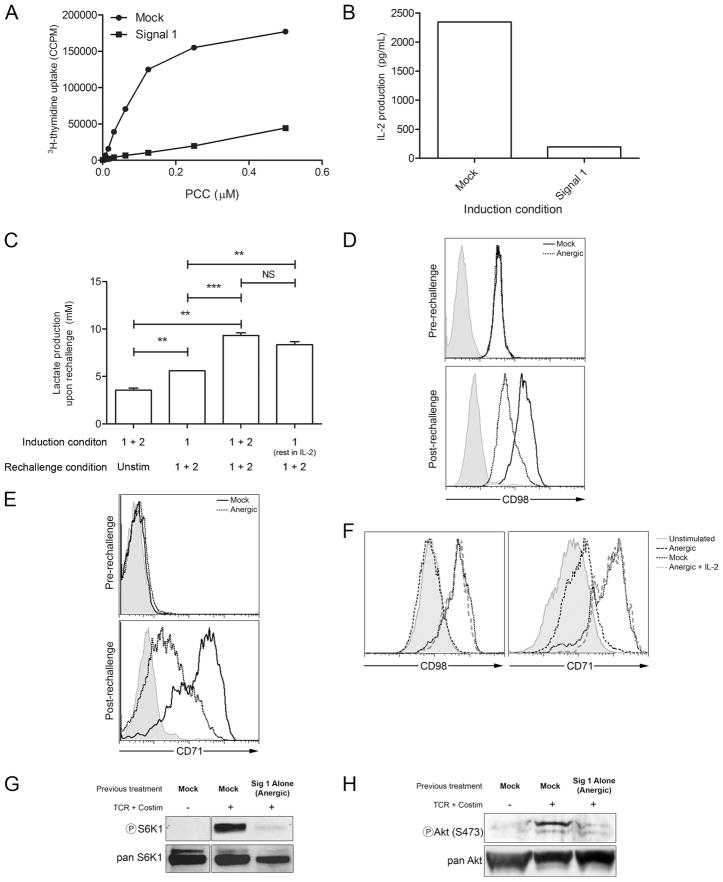
When A.E7 cells are stimulated with anti-CD3, rested, and then rechallenged with full stimulation, such cells are hyporesponsive; they are anergic. As we and others have previously shown, full stimulation of anergic Th1 T cells act functionally as if they were only stimulated with anti-CD3 alone. Since anti-CD3 alone is insufficient to support T cell metabolism, we next examined the regulation of metabolic machinery in the anergic cells. A.E7 T cells were left unstimulated or rendered anergic with anti-CD3 stimulation alone, rested in fresh media for 7 days, and rechallenged. Upon rechallenge such cells fail to produce IL-2 and proliferate (Fig. 2, A and B). Next, we sought to assess the metabolic potential of anergic verus nonanergic cells. Since it has been shown that the glycolytic pathway plays a critical role in providing energy for activated T cells, we interrogated anergic and nonanergic T cells for their ability to produce lactate upon rechallenge (14). T cells that had been given initially full stimulation produce a basal level of lactate while resting (first bar of Fig. 2C). Upon activation with signals 1 plus 2, anergic T cells (cells that had previously been activated with signal 1 alone) produce slightly higher levels of lactate when compared with the unstimulated cells (Fig. 2C). On the other hand, T cells that had been activated with signals 1 plus 2 (nonanergic T cells) and then rechallenged 7 days later with signals 1 plus 2 produced more lactate than did both the unstimulated cells (resting cells vs activated, p < 0.01) and the rechallenged anergic cells (anergic vs activated, p < 0.001). It has previously been demonstrated that culturing anergic T cells in IL-2 reverses anergy (15). Thus, we wanted to determine whether IL-2 also reversed the metabolic defects seen in anergic cells. Indeed, cells that had been previously rendered anergic with signal 1 alone and then cultured in IL-2 demonstrated high levels of lactate production (anergic vs anergic plus IL-2, p < 0.01; anergic plus IL-2 vs activated, p > 0.10), consistent with the fact that IL-2 reverses anergy.
FIGURE 2.
Upon rechallenge with signals 1 plus 2, anergic T cells fail to not only produce IL-2 and proliferate but up-regulate metabolic machinery. A, Mock-treated and anergic (signal 1) A.E7 T cells were rechallenged with APCs plus PCC peptide for 48 h. Cells were then interrogated for proliferation by thymidine uptake. B, Mock-treated and anergic (signal 1) T cells were rechallenged with anti-CD3 and anti-CD28 for 16 h and interrogated for IL-2 by ELISA. C, Supernatants from rechallenged T cells were tested for L-lactate production. For these experiments some cells were left unstimulated, and some anergic T cells were rescued with high-dose IL-2. D, CD98 cell surface expression of cells treated as in B by FACS. The shaded tracing indicates the isotype control for the stimulated T cells that were initially mock treated. E, As in D, but for CD71. F, As in D and E, but some anergic cells were rescued with IL-2, G, Mock-treated or anergic A.E7 T cells were left unstimulated or were stimulated for 3 h with anti-CD3 and anti-CD28; lysates were made and probed for phospho-S6K1 by immunoblot. H, As in G, but for phospho-Akt (S473). Total enzyme (pan) probes are included as loading controls. Data are representative of three independent experiments. Statistics were performed using the Student’s t test.**, p < 0.01; ***, p < 0.001; NS, p < 0.05.
In as much as the anergic cells failed to mount a strong glycolytic response, we wanted to examine their ability to up-regulate other aspects of metabolism upon rechallenge. As such, anergic and nonanergic (“mock” treated during induction) T cells were assayed for their ability to up-regulate the amino acid transporter CD98 and the transferrin receptor CD71 (Fig. 2, D and E). Previously, we have demonstrated that clonotypic T cells with low CD71 expression could be used as a crude way to identify anergic T cells in vivo (5). In both cases, in the resting state, these receptors were equivalently expressed between the nonanergic and anergic T cells. When the cells were then rechallenged with full stimulation (anti-CD3 plus anti-CD28), the nonanergic (mock) T cells up-regulated both CD98 and CD71 (Fig. 2, D and E). However, when the anergic T cells were rechallenged with full stimulation (anti-CD3 plus anti-CD28) they failed to fully up-regulate CD98 and CD71 (Fig. 2, D and E). Alternatively, when anergic cells are rescued with high-dose IL-2 (2 ng/ml) during the rest period, these cells regain their metabolic activity and their ability to up-regulate these metabolic receptors (Fig. 2F). Note that the up-regulation of both CD98 and CD71 in the anergic cells rescued in IL-2 overlaps with that of the rechallenged nonanergic (mock-treated) cells. Likewise, anergic T cells failed to fully activate TORC1 as measured by S6K1 phosphorylation and TORC2 as measured by Akt S473 phosphorylation (Fig. 2, G and H). Thus, in addition to being functionally hyporesponsive, anergic T cells are hyporesponsive in terms of their ability to up-regulate metabolic machinery. From a bioenergetic perspective, anergic T cells are metabolically anergic as well.
Inhibition of metabolic pathways mitigates T cell function
Our data demonstrate that when anergic T cells are stimulated with full stimulation (signals 1 plus 2), they fail to completely up-regulate metabolic machinery necessary for amino acid, glucose, and iron metabolism. Our findings raised the possibility that the failure of anergic cells to fully up-regulate metabolic machinery was not just a consequence of anergy but perhaps contributed to the functional anergic phenotype. For example, whereas a hallmark of T cell clonal anergy is the inability to produce IL-2 and IFN-γ upon rechallenge, our data suggested that the block in the production of these two cytokines may be enforced in part by the inability of anergic cells to adequately increase metabolism. Thus, we wanted to determine the effect of limiting amino acids, glucose, and energy on IL-2 and IFN-γ production.
To examine this, we examined the effect of blocking metabolic pathways on T cell activation. We first tested whether signals 1 plus 2 could activate T cells in the presence of amino acid deprivation. The branched-chain amino acid leucine is the most abundant amino acid in dietary proteins. Furthermore, the inability of the liver to catabolize leucine prevents its “first-pass” loss, allowing leucine to act as a nutrient signal (16). Alteration in leucine abundance would have a considerable impact on amino acid-derived signaling.
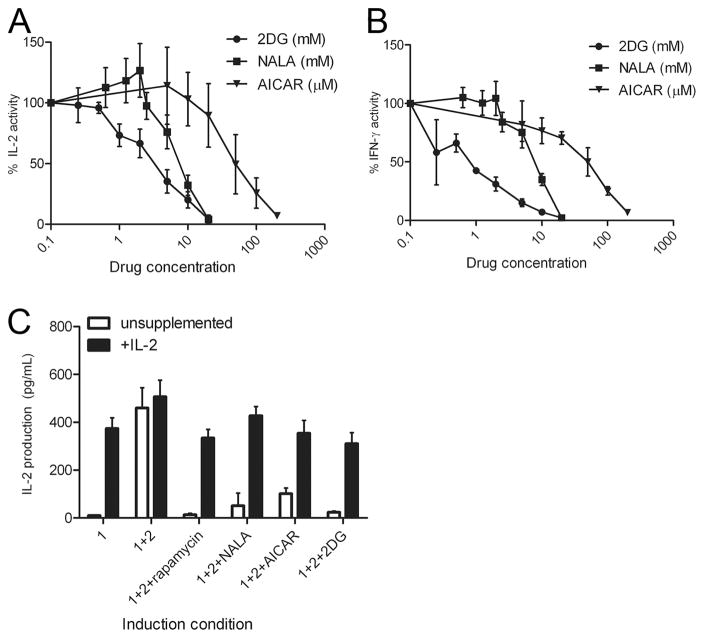
To elucidate the role of leucine in T cell activation, we stimulated T cells in the presence of the leucine antagonist NALA. NALA mimics leucine depletion by acting as a competitive antagonist (17). Primary 5C.C7 T cells were stimulated for 2 days with peptide and then expanded for an additional 5 days in IL-2 to generate previously activated primary Th1 cells. These cells were rechallenged with anti-CD3 plus anti-CD28 in the presence of increasing doses of NALA. From a technical perspective, stimulating the T cells with Abs enabled us to eliminate a potential affect of leucine antagonism on APCs. By functionally decreasing available leucine with NALA, there is a dose-dependent inhibition of both proliferation (data not shown), IL-2, and IFN-γ production (Fig. 3, A and B). Notably, the decrease in function was not associated with a decrease in viability as measured by trypan blue (data not shown). Thus, in nonanergic cells, limiting amino acid metabolism leads to a decrease in Th1 cytokine production. Intimately linked to the availability of nutrients is energy in the form of ATP. The status of cellular energy stores is sensed by AMP-activated protein kinase (AMPK) (18). Energy insufficiency increases the ratio of AMP/ATP, leading to an interaction between AMP and a regulatory subunit of AMPK. This association induces a conformational change in AMPK that allows AMPK to be phosphorylated and activated by its upstream protein kinases (19). Thus, activation of AMPK signals the cells that their energy supply is insufficient. Here, we employed a well-known activator of AMPK, AICAR, to imitate energy deprivation (18). As with NALA, AICAR inhibited IL-2 and IFN-γ production in a dose-dependent fashion (Fig. 3, A and B). Once again, the decrease in function was not associated with an increase in cell death.
FIGURE 3.
Metabolic inhibition leads to decreased T cell function. Previously activated (Th1) 5C.C7 T cells were stimulated in varying concentrations of 2DG (mM), NALA (mM), or AICAR (μM). Supernatants were then interrogated for (A) IL-2 or (B) IFN-γ. Data are normalized values from three independent experiments. Error bars indicate SD. C, Metabolic inhibition induces anergy even in the presence of costimulation. A.E7 T cells were stimulated 16 h with anti-CD3 alone or anti-CD3 plus anti-CD28 in the presence of rapamycin (200 nM), NALA (2 mM), AICAR (10 μM), or 2DG (500 μM). The cells were washed and rested without drug for 7 days in unsupplemented media or media containing 1 ng/ml murine IL-2. The cells were then rechallenged for 16 h with anti-CD3, anti-CD28, without any drugs, and interrogated for IL-2 production by ELISA. Error bars indicate SD. Data are representative of three independent experiments.
Anergic T cells displayed a decrease in Akt activation, which is crucial for the full up-regulation of glucose metabolism (4). As such, we wanted to determine the effect of inhibiting glucose metabolism on T cell function. 2DG, an antagonist of glucose, is able to compete with glucose for the same transporters on the cell surface, and hexokinase, which catalyzes the first step of glycolysis in the cytoplasm (21). However, the product, 2-deoxy-glucose-6-phosphate, cannot be further processed. Therefore, 2DG inhibits both the uptake and utilization of glucose. When 5C.C7-derived Th1 cells were fully activated (anti-CD3 plus anti-CD28) in the presence of increasing concentrations of 2DG, we observed a decrease in IL-2 and IFN-γ production that was not associated with increased death (Fig. 3, A and B). When compared with leucine and energy depletion, inhibiting glucose metabolism appeared to be a more potent means of inhibiting T cell function. Overall, these data demonstrate that limiting branched-chain amino acid, energy, and glucose in Th1 T cells can inhibit the production of Th1 effector cytokines.
Inhibition of metabolism induces anergy even in the presence of costimulation
T cells stimulated with signal 1 alone fail to proliferate, produce IL-2, and are anergic upon rechallenge. The induction of anergy was associated with inhibition of the metabolic machinery. Likewise, our data in Fig. 3, A and B, demonstrate that inhibition of metabolism leads to inhibition of T cell function. In the next series of experiments we tested if inhibition of the metabolic machinery promoted the induction of anergy. Specifically, we sought to determine whether stimulation of A.E7 T cells in the presence of NALA, 2DG, or AICAR resulted in anergy. To investigate this, A.E7 cells were stimulated with signal 1 alone or signals 1 plus 2 in the absence or presence of a metabolic inhibitor (NALA, 2DG, or AICAR). Sixteen hours later, the cells were washed extensively to remove anti-CD3 and anti-CD28 Abs and the inhibitors, and then rested in fresh unsupplemented media for 7 days. The cells that were initially treated with signal 1 alone were rendered anergic, as they produced less IL-2 upon full rechallenge (Fig. 3C). In contrast, the cells that were stimulated with signals 1 plus 2 during induction remained active upon rechallenge, secreting a large amount of IL-2. The cells that were treated with metabolic inhibitors in the earlier stimulation were hyporesponsive upon rechallenge in the absence of the inhibitors; they were rendered anergic. Thus, metabolic deficiency promoted anergy even in the presence of costimulation.
Interestingly, we observed that anergy could be induced at doses of the metabolic inhibitors (NALA (2 mM), AICAR (10 μM), or 2DG (500 μM)) that had minimal affect on cytokine production (note that at these doses in Fig. 3, A and B, there is minimal inhibition of IL-2 and IFN-γ). However, these low doses of the inhibitors were still able to induce a state of anergy such that upon rechallenge (in the absence of the inhibitors) the cells failed to produce IL-2. That is, even though the inhibitor-treated cells produced copious amounts of IL-2 during the initial stimulation they were still rendered anergic and failed to produce IL-2 upon rechallenge 7 days later (Fig. 3C). Thus, the metabolic inhibitors were able to induce anergy even in the presence of IL-2, suggesting that they were acting downstream of IL-2R signaling.
A hallmark of anergy is its ability to be reversed by addition of IL-2. We sought to test whether T cells rendered anergic by metabolic inhibition could be rescued by IL-2. A.E7 T cells were stimulated with signal 1 alone, signals 1 plus 2, or signals 1 plus 2 with drug as previously described. Cells were washed and rested in either unsupplemented media or media containing 1 ng/ml murine IL-2. Seven days later, the cells were all rechallenged with signals 1 plus 2 and assessed for IL-2 production. While cells receiving signal 1 alone are rendered anergic, those cells that received IL-2 during the rest period were rescued (Fig. 3C). Furthermore, cells rendered anergic by metabolic inhibition were also rescued by addition of IL-2. Thus, although metabolic inhibition can promote the induction of anergy even in the presence of costimulation and IL-2 production, cells rendered anergic in the presence of metabolic inhibitors can be rescued from anergy by being cultured in IL-2 in the absence of the inhibitors.
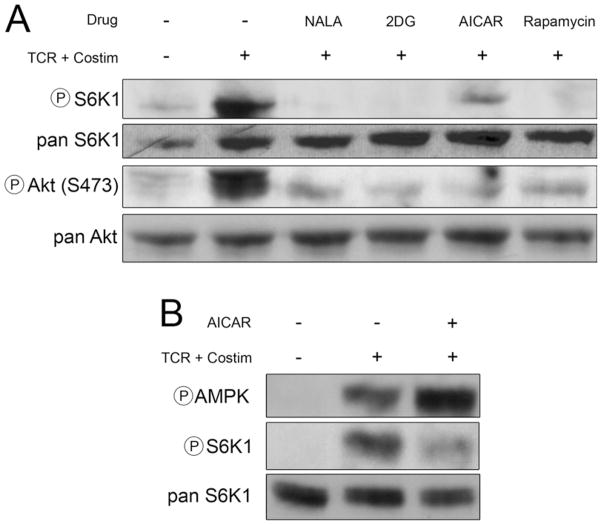
Previously, we and others have shown that for Th1 cells, TCR engagement in the absence of mTOR activation leads to anergy (Fig. 3C, signals 1 plus 2 plus rapamycin) (11, 12). Thus, we hypothesized that the ability of the metabolic inhibitors to induce anergy might be due to their ability to inhibit mTOR. 5C.C7 T cells were stimulated with anti-CD3 plus anti-CD28 in the presence of rapamycin, NALA, 2DG, and AICAR. Extracts from these cells were interrogated for mTOR activity by immunoblot analysis (Fig. 4A). TORC1 activity results in the phosphorylation of S6K1 and, in addition to rapamycin, each of the inhibitors (at the doses that induce anergy) blocks S6K1 phosphorylation. TORC2 activation results in the phosphorylation of Akt at S473. Each of the inhibitors blocked phosphorylation of Akt at S473, demonstrating their ability to block TORC2. For AICAR, we hypothesized that this inhibition of mTOR was mediated through the activation of AMPK. Unstimulated T cells display essentially no phosphorylation of AMPK (Fig. 4B). Stimulation with signals 1 plus 2 leads to a slight increase in AMPK phosphorylation but a robust increase in mTOR activation as measured by phosphorylation of S6K1. On the other hand, as has been previously described, AICAR markedly enhances AMPK phosphorylation and inhibits mTOR (Fig. 4B) (20). Thus, mechanistically, the ability of these compounds to induce anergy correlates with their ability to inhibit mTOR activation.
FIGURE 4.
Full T cell stimulation in the presence of metabolic inhibition leads to tolerance induction via mTOR inhibition. A, NALA, 2DG, and AICAR mimic starvation signals in an mTOR-dependent fashion. Previously activated 5C.C7 T cells were left unstimulated or stimulated with anti-CD3 plus anti-CD28 (TCR + Costim) in the presence or absence of NALA (2 mM), 2DG (500 μM), AICAR (10 μM), or rapamycin (200 nM) for 3 h, then lysed and interrogated for phospho-S6K1 and phospho-Akt (S473). Total enzyme (pan) probes are included as loading controls. B, 5C.C7 T cells were left unstimulated or were stimulated with anti-CD3 and anti-CD28 (TCR + Costim) with or without AICAR (10 μM); lysates were made and probed for phospho-AMPK, phospho-S6K1, and pan S6K1 by immunoblot. Immunoblots are representative of at least three independent experiments.
Discussion
Our observations demonstrate that the T cell metabolic machinery is regulated in anergy and that regulation of T cell metabolism contributes to decreased effector function of anergic T cells. Phenotypically, anergic T cells fail to maximally express effector cytokines. In this report we demonstrate that the ability to up-regulate metabolic machinery is also inhibited in anergy. Since impaired T cell metabolism prevents full T cell effector function, our data raise the possibility that anergy is in part maintained by mitigating the up-regulation of T cell metabolic machinery. Clearly, our report does not exhaustively examine all of the metabolic changes that occur upon T cell activation. We have shown that glycolysis, as measured by lactate production, up-regulation of the amino acid transport CD98, up-regulation of the transferrin receptor CD71, Akt activation necessary for full glucose metabolism, and mTOR activation necessary for protein translation are all inhibited in anergic cells stimulated with signals 1 plus 2. As such, we propose that the term anergy (the inability to respond upon rechallenge) can be applied to not only a lack of cytokine production and proliferation in Th1 cells but also to a lack of up-regulation of the metabolic machinery. Future investigation will determine the full scope of metabolic anergy.
Additionally, we demonstrate that blocking leucine, glucose, and energy metabolism not only acutely inhibits T cell function but also promotes the induction of T cell anergy in Th1 T cells. Thus, activation of T cells under limiting nutritional conditions can promote long-term tolerance. Along these lines, it has recently been reported that infectious tolerance is in part maintained via the consumption of essential amino acids and the subsequent inhibition of mTOR (22). Indeed, our finding that pharmacologic inhibition of metabolism with NALA, AICAR, and 2DG can inhibit the production of Th1 cytokines is consistent with the notion that inhibition of metabolism (by either depleting nutrients or failing to up-regulate the metabolic machinery) can directly inhibit T cell function.
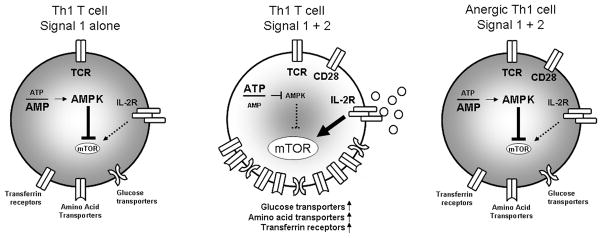
The two-signal model provides the framework for understanding the acquired immune response. To elicit full T cell activation, Ag recognition (signal 1) must occur concomitantly with costimulation (signal 2), as in CD28 ligation (1). Furthermore, for Th1 cells, signal 1 in the absence of signal 2 leads to T cell anergy (2). The consequence of TCR signaling in the presence or absence of costimulation has typically been evaluated by the generation of various effector cytokines. TCR engagement in the absence of costimulation leads to markedly diminished IL-2 production, and anergic T cells fail to produce IL-2 upon full stimulation. Thompson and colleagues have proposed that an additional critical role for costimulation is to promote an increase in the metabolic machinery necessary for the enhanced metabolic demand associated with T cell activation (14, 23). For example, CD28 stimulation regulates glucose metabolism that enables T cells to meet the increased energy requirements associated with activation. Importantly, these studies indicate that the increase in T cell metabolism is not merely a consequence of increased activation but rather a necessary step to facilitate activation (“fuel feeds function”) (3, 24). In their model (Fig. 5, left and center panels), the ability of costimulation to promote the induction of full activation is dependent on the up-regulation of the necessary metabolic machinery. In studying T cell anergy, we have noted that cytokines that are most dependent on costimulation (e.g., IL-2) are the same cytokines that are inhibited in anergy (25). We hypothesized that anergic T cells would display the same profile of metabolic regulation as cells stimulated with signal 1 alone. As seen in Figs. 1 and 2, amino acid, glucose, and iron metabolism are all similarly regulated by signals 1 and 2 and in anergic and nonanergic T cells. Thus, we propose that anergic T cells are metabolically anergic in that even in the presence of costimulation, they fail to up-regulate the requisite metabolic machinery to support full T cell activation (Fig. 5, right panel).
FIGURE 5.
Anergic T cells are metabolically anergic. Thompson and colleagues have proposed that when a T cell receives signal 1 alone, it fails to fully up-regulate the metabolic machinery necessary to support T cell activation (left panel) (3). As such, a major function of costimulation is to facilitate metabolic function necessary to fuel the T cell response. We propose that just as anergic T cells fail to produce IL-2 upon rechallenge with full stimulation (signals 1 plus 2), they also fail to up-regulate the metabolic machinery such as the transferrin receptor, amino acid transporters, and glucose transporters necessary to promote a full effector response (right panel). IL-2 has the ability to activate mTOR; however, in the absence of signal 2 (left panel) or when a cell is anergic (right panel), very little IL-2 is produced. In the presence of costimulation (middle panel) AMPK is modestly activated (see Fig. 4B); however, this is not enough to inhibit mTOR in the setting of full activation. Thus, in the absence of energy, glucose, or amino acids mTOR is inhibited, resulting in the induction of anergy. Likewise, a dearth of these nutrients or the inability to import and metabolize these nutrients can directly inhibit T cell function.
Upon activation, the bioenergetic demands of the T cell increase dramatically (3). Blocking the ability to meet these demands inhibits T cell function (Fig. 3). The leucine analog NALA inhibited T cell function. Previously, NALA had been shown to block proliferation in Jurkat cells by inhibiting mTOR (17). The ability of NALA to not only inhibit T cell function but also promote T cell anergy is thus consistent with our previous findings that TCR engagement in the absence of mTOR activation leads to anergy (11). The ability of 2DG to inhibit T cell activation is consistent with other reports demonstrating the ability of this glucose analog to inhibit T cell function (21). As with NALA, we demonstrate that 2DG can inhibit mTOR function and thus promote anergy. Also, we show that by mimicking energy depletion we can both acutely inhibit T cell function and promote anergy by inhibiting mTOR. These findings are consistent with previous findings demonstrating the ability of AICAR to inhibit IL-2 production in Jurkat T cells (8) and mitigate experimental autoimmune encephalomyelitis (26). On the other hand, recently it has been shown that AMPK is rapidly activated in a Ca2+-dependent fashion upon initial TCR engagement (27). Indeed, we see an increase in AMPK phosphorylation with full T cell activation (Fig. 4B). In light of the ability of AMPK to inhibit mTOR, the significance of the immediate activation of AMPK upon TCR engagement is unclear. Perhaps, the initial burst of AMPK activity regulates energy metabolism independently of mTOR inhibition. Consistent with this is the fact that despite the modest increase in AMPK upon activation with signals 1 plus 2, there did not appear to be a concomitant decrease in mTOR activation (Fig. 4B).
Finally, our studies demonstrate that inhibition of mTOR by targeting diverse upstream signaling pathways can all promote anergy. Previously, our group and others have demonstrated the ability of rapamycin to promote T cell tolerance (5, 11, 12, 28). Our new studies raise the possibility that inhibitors of metabolic pathways might prove to be novel inducers of T cell tolerance. Interestingly, whereas higher doses of the metabolic inhibitors blocked cytokine production, their ability to induce anergy was seen at doses that did not inhibit T cell function. For example, the metabolic inhibitors were able to induce anergy at doses that do not inhibit IL-2 production. It has been shown that IL-2 prevents/reverses anergy in part by activating mTOR (29). In this context our observations are consistent with the notion that (at low doses) the metabolic inhibitors promoted anergy by blocking IL-2-induced mTOR activation.
It is becoming clear that controlling nutrient availability can regulate immune responses. This appears to be particularly true when it comes to amino acids. Mellor and Munn have demonstrated that expression of IDO by macrophages and dendritic cells can inhibit T cell proliferation and function by degrading extracellular tryptophan (30). Mechanistically, the IDO-expressing APCs activate the GCN2 pathway in the responding T cells, which can both inhibit their function and even promote T cell anergy (31). Alternatively, it has been shown that dendritic cells can promote T cell activation by taking up environmental cysteine and providing T cells with the amino acid cysteine (32). It remains to be determined if in vivo-specific mechanisms are induced that regulate immune responses by controlling the availability of glucose. Notably, however, tumors are uniquely adapted to rapid growth in part by optimally competing for nutrients. As such, the immediate tumor microenvironment can be depleted of vital nutrients. The tumor microenvironment has been shown to inhibit T cell function and promote tumor Ag-specific tolerance (33). Thus, by limiting metabolic constituents, tumors may inhibit antitumor responses and induce tumor-specific tolerance.
Acknowledgments
We thank members of the Powell, Drake, and Pardoll laboratories for technical assistance and reagents.
Footnotes
This work was supported in part by National Institutes of Health Grants R01CA098109 and R01CA14227 and by an Ajinomoto Pilot Project grant.
Abbreviations used in this paper: mTOR, mammalian target of rapamycin; NALA, N-acetyl-leucine amide; 2DG, 2-deoxyglucose; AICAR, 5-aminoimidazole-4-carbox-amide ribonucleoside; PCC, pigeon cytochrome c; AMPK, AMP-activated protein kinase.
Disclosures
The authors have no financial conflicts of interest.
Publisher's Disclaimer: “This is an author-produced version of a manuscript accepted for publication in The Journal of Immunology (The JI). The American Association of Immunologists, Inc. (AAI), publisher of The JI, holds the copyright to this manuscript. This version of the manuscript has not yet been copyedited or subjected to editorial proofreading by The JI; hence, it may differ from the final version published in The JI (online and in print). AAI (The JI) is not liable for errors or omissions in this author-produced version of the manuscript or in any version derived from it by the U.S. National Institutes of Health or any other third party. The final, citable version of record can be found at www.jimmunol.org.”
References
- 1.Schwartz RH. Costimulation of T lymphocytes: the role of CD28, CTLA-4, and B7/BB1 in interleukin-2 production and immunotherapy. Cell. 1992;71:1065–1068. doi: 10.1016/s0092-8674(05)80055-8. [DOI] [PubMed] [Google Scholar]
- 2.Schwartz RH. T cell anergy. Annu Rev Immunol. 2003;21:305–334. doi: 10.1146/annurev.immunol.21.120601.141110. [DOI] [PubMed] [Google Scholar]
- 3.Fox CJ, Hammerman PS, Thompson CB. Fuel feeds function: energy metabolism and the T-cell response. Nat Rev Immunol. 2005;5:844–852. doi: 10.1038/nri1710. [DOI] [PubMed] [Google Scholar]
- 4.Frauwirth KA, Riley JL, Harris MH, Parry RV, Rathmell JC, Plas DR, Elstrom RL, June CH, Thompson CB. The CD28 signaling pathway regulates glucose metabolism. Immunity. 2002;16:769–777. doi: 10.1016/s1074-7613(02)00323-0. [DOI] [PubMed] [Google Scholar]
- 5.Zheng Y, Collins SL, Lutz MA, Allen AN, Kole TP, Zarek PE, Powell JD. A role for mammalian target of rapamycin in regulating T cell activation versus anergy. J Immunol. 2007;178:2163–2170. doi: 10.4049/jimmunol.178.4.2163. [DOI] [PubMed] [Google Scholar]
- 6.Harris TE, Lawrence JC., Jr TOR signaling. Sci STKE. 2003;2003:re15. doi: 10.1126/stke.2122003re15. [DOI] [PubMed] [Google Scholar]
- 7.Tokunaga C, Yoshino K, Yonezawa K. mTOR integrates amino acid-and energy-sensing pathways. Biochem Biophys Res Commun. 2004;313:443–446. doi: 10.1016/j.bbrc.2003.07.019. [DOI] [PubMed] [Google Scholar]
- 8.Jhun BS, Oh YT, Lee JY, Kong Y, Yoon KS, Kim SS, Baik HH, Ha J, Kang I. AICAR suppresses IL-2 expression through inhibition of GSK-3 phosphorylation and NF-AT activation in Jurkat T cells. Biochem Biophys Res Commun. 2005;332:339–346. doi: 10.1016/j.bbrc.2005.04.126. [DOI] [PubMed] [Google Scholar]
- 9.Powell JD, Zheng Y. Dissecting the mechanism of T-cell anergy with immunophilin ligands. Curr Opin Investig Drugs. 2006;7:1002–1007. [PubMed] [Google Scholar]
- 10.Allen A, Zheng Y, Gardner L, Safford M, Horton MR, Powell JD. The novel cyclophilin binding compound, sanglifehrin A, disassociates G1 cell cycle arrest from tolerance induction. J Immunol. 2004;172:4797–4803. doi: 10.4049/jimmunol.172.8.4797. [DOI] [PubMed] [Google Scholar]
- 11.Powell JD, Lerner CG, Schwartz RH. Inhibition of cell cycle progression by rapamycin induces T cell clonal anergy even in the presence of costimulation. J Immunol. 1999;162:2775–2784. [PubMed] [Google Scholar]
- 12.Vanasek TL, Khoruts A, Zell T, Mueller DL. Antagonistic roles for CTLA-4 and the mammalian target of rapamycin in the regulation of clonal anergy: enhanced cell cycle progression promotes recall antigen responsiveness. J Immunol. 2001;167:5636–5644. doi: 10.4049/jimmunol.167.10.5636. [DOI] [PubMed] [Google Scholar]
- 13.Sabatini DM. mTOR and cancer: insights into a complex relationship. Nat Rev Cancer. 2006;6:729–734. doi: 10.1038/nrc1974. [DOI] [PubMed] [Google Scholar]
- 14.Frauwirth KA, Thompson CB. Regulation of T lymphocyte metabolism. J Immunol. 2004;172:4661–4665. doi: 10.4049/jimmunol.172.8.4661. [DOI] [PubMed] [Google Scholar]
- 15.Beverly B, Kang SM, Lenardo MJ, Schwartz RH. Reversal of in vitro T cell clonal anergy by IL-2 stimulation. Int Immunol. 1992;4:661–671. doi: 10.1093/intimm/4.6.661. [DOI] [PubMed] [Google Scholar]
- 16.Suryawan A, Jeyapalan A, Orellana RA, Wilson FA, Nguyen HV, Davis TA. Leucine stimulates protein synthesis in skeletal muscle of neonatal pigs by enhancing mTORC1 activation. Am J Physiol. 2008;295:E868–E875. doi: 10.1152/ajpendo.90314.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hidayat S, Yoshino K, Tokunaga C, Hara K, Matsuo M, Yonezawa K. Inhibition of amino acid-mTOR signaling by a leucine derivative induces G1 arrest in Jurkat cells. Biochem Biophys Res Commun. 2003;301:417–423. doi: 10.1016/s0006-291x(02)03052-8. [DOI] [PubMed] [Google Scholar]
- 18.Reiter AK, Bolster DR, Crozier SJ, Kimball SR, Jefferson LS. Repression of protein synthesis and mTOR signaling in rat liver mediated by the AMPK activator aminoimidazole carboxamide ribonucleoside. Am J Physiol. 2005;288:E980–E988. doi: 10.1152/ajpendo.00333.2004. [DOI] [PubMed] [Google Scholar]
- 19.Hay N, Sonenberg N. Upstream and downstream of mTOR. Genes Dev. 2004;18:1926–1945. doi: 10.1101/gad.1212704. [DOI] [PubMed] [Google Scholar]
- 20.Kimball SR. Interaction between the AMP-activated protein kinase and mTOR signaling pathways. Med Sci Sports Exerc. 2006;38:1958–1964. doi: 10.1249/01.mss.0000233796.16411.13. [DOI] [PubMed] [Google Scholar]
- 21.Cham CM, Driessens G, O’Keefe JP, Gajewski TF. Glucose deprivation inhibits multiple key gene expression events and effector functions in CD8+ T cells. Eur J Immunol. 2008;38:2438–2450. doi: 10.1002/eji.200838289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cobbold SP, Adams E, Farquhar CA, Nolan KF, Howie D, Lui KO, Fairchild PJ, Mellor AL, Ron D, Waldmann H. Infectious tolerance via the consumption of essential amino acids and mTOR signaling. Proc Natl Acad Sci USA. 2009;106:12055–12060. doi: 10.1073/pnas.0903919106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jacobs SR, Herman CE, Maciver NJ, Wofford JA, Wieman HL, Hammen JJ, Rathmell JC. Glucose uptake is limiting in T cell activation and requires CD28-mediated Akt-dependent and independent pathways. J Immunol. 2008;180:4476–4486. doi: 10.4049/jimmunol.180.7.4476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jones RG, Thompson CB. Revving the engine: signal transduction fuels T cell activation. Immunity. 2007;27:173–178. doi: 10.1016/j.immuni.2007.07.008. [DOI] [PubMed] [Google Scholar]
- 25.Powell JD. The induction and maintenance of T cell anergy. Clin Immunol. 2006;120:239–246. doi: 10.1016/j.clim.2006.02.004. [DOI] [PubMed] [Google Scholar]
- 26.Nath N, Giri S, Prasad R, Salem ML, Singh AK, Singh I. 5-Aminoimidazole-4-carboxamide ribonucleoside: a novel immunomodulator with therapeutic efficacy in experimental autoimmune encephalomyelitis. J Immunol. 2005;175:566–574. doi: 10.4049/jimmunol.175.1.566. [DOI] [PubMed] [Google Scholar]
- 27.Tamas P, Hawley SA, Clarke RG, Mustard KJ, Green K, Hardie DG, Cantrell DA. Regulation of the energy sensor AMP-activated protein kinase by antigen receptor and Ca2+ in T lymphocytes. J Exp Med. 2006;203:1665–1670. doi: 10.1084/jem.20052469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Colombetti S, Benigni F, Basso V, Mondino A. Clonal anergy is maintained independently of T cell proliferation. J Immunol. 2002;169:6178–6186. doi: 10.4049/jimmunol.169.11.6178. [DOI] [PubMed] [Google Scholar]
- 29.Powell JD, Bruniquel D, Schwartz RH. TCR engagement in the absence of cell cycle progression leads to T cell anergy independent of p27(Kip1) Eur J Immunol. 2001;31:3737–3746. doi: 10.1002/1521-4141(200112)31:12<3737::aid-immu3737>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- 30.Mellor AL, Munn DH. IDO expression by dendritic cells: tolerance and tryptophan catabolism. Nat Rev Immunol. 2004;4:762–774. doi: 10.1038/nri1457. [DOI] [PubMed] [Google Scholar]
- 31.Munn DH, Sharma MD, Baban B, Harding HP, Zhang Y, Ron D, Mellor AL. GCN2 kinase in T cells mediates proliferative arrest and anergy induction in response to indoleamine 2,3-dioxygenase. Immunity. 2005;22:633–642. doi: 10.1016/j.immuni.2005.03.013. [DOI] [PubMed] [Google Scholar]
- 32.Angelini G, Gardella S, Ardy M, Ciriolo MR, Filomeni G, Di Trapani G, Clarke F, Sitia R, Rubartelli A. Antigen-presenting dendritic cells provide the reducing extracellular microenvironment required for T lymphocyte activation. Proc Natl Acad Sci USA. 2002;99:1491–1496. doi: 10.1073/pnas.022630299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gajewski TF. Failure at the effector phase: immune barriers at the level of the melanoma tumor microenvironment. Clin Cancer Res. 2007;13:5256–5261. doi: 10.1158/1078-0432.CCR-07-0892. [DOI] [PubMed] [Google Scholar]