Abstract
Background
Chagas disease is common in Central and South America and the southern United States. The causative agent is Trypanosoma cruzi (T cruzi, Order Kinetoplastida, Family Trypanosomatidae), a kinetoplastid protozoan parasite of humans and other vertebrates. It is a serious public health issue and the leading cause of heart disease and cardiovascular death in Central and South America. In 1984 a colony baboon was discovered to be infected with T cruzi.
Methods
Since the initial diagnosis was made by microscopic observation of the amastigote forms of T. cruzi in myocardial fibers, T. cruzi amastigotes have been identified in three additional baboons.
Results
The primary findings were similar in all four baboons and were congestive heart failure with edema of dependent areas, hydrothorax, hydropericardium, and multifocal to diffuse lymphoplasmacytic myocarditis.
Conclusions
A baboon animal model of Chagas disease could contribute significantly to the development of therapies for the disease in humans.
Keywords: nonhuman primate, protozoa, animal model, heart, Trypanosoma cruzi
Introduction
Chagas disease (American trypanosomiasis) (11, 12) is a zoonotic disease common in Central and South America and the southern United States (29, 60). The causative agent of Chagas disease is Trypanosoma cruzi (T cruzi, Order Kinetoplastida, Family Trypanosomatidae), a kinetoplastid protozoan parasite of humans and other vertebrates.
Chagas disease is a serious public health issue—it is the leading cause of heart disease and cardiovascular death in Central and South America (24, 29, 30, 47, 52), and only malaria and schistosomiasis pose greater tropical disease burdens (61). The disease is estimated to affect 16–18 million people in Latin America, and 120 million people are at risk of becoming infected (62). About 27% of those infected will develop cardiac symptoms, and 9% of cases progress to gastrointestinal or peripheral nervous system involvement (60). There are no vaccines to prevent infection, and no safe and effective therapeutic options (15, 19, 42, 55, 56, 57). Serious concerns have been raised about the safety of domestic blood and tissue banks, and the risk of transfusion- and transplant-associated transmission of Chagas disease, as the number of infected individuals migrating to the US increases (10, 13, 23, 25, 36, 45, 46, 49, 50).
A number of cases of Chagas disease in humans and other animals have been reported in the United States, particularly in the south and especially in southern Texas and Louisiana (1, 4, 5, 6, 9, 13, 17, 18, 24, 39, 41). Natural (53) and experimental (40) T. cruzi infection has also been documented in multiple New World and Old World primate species. In 1984 a baboon from the outdoor primate colony at the Southwest National Primate Research Center at the Southwest Foundation for Biomedical Research (SPC) in San Antonio, TX, was found dead in its cage and subsequently determined to be infected with T. cruzi (21). Since this initial discovery, more than 182 baboons in the colony have tested seropositive for T. cruzi, and Chagas disease is now suspected in a number of baboon deaths. The primary route of infection has not been positively established, but in consequence of their largely outdoor housing the baboons are believed to become infected by catching and eating infected insects (2, 3, 14, 33, 48, 54). Based on retrospectively collected data on seropositivity to T. cruzi, the seroprevalence in the baboon colony is currently estimated at 2–3%.
In this report we summarize the major pathologic and histologic findings in four cases of confirmed Chagas disease in colony baboons. These cases exhibit considerable similarity in their gross, microscopic, and histologic features and illustrate clearly the similarity of chagasic pathology in baboon to that seen in human cases of the disease.
Parasite Cycle and Morphology
The most common mode of transmission of Chagas disease is via several hematophagous species of triatomine (reduviid) insect (Order Reduviidae, Family Hemiptera). Following a blood meal the insect defecates near the site of the bite, and the human host can become infected when the feces from a parasitized insect contaminate the bite wound or penetrate the body through broken skin or the mucosa of the mouth and eyes. Infection can also result from eating infected insects, and is probably a significant route of infection in nonhuman vertebrates such as the opossum and domestic dogs (3, 14, 33, 48, 54).
T. cruzi epimastigotes replicate in the gut of the insect vector and differentiate into metacyclic trypomastigotes. These are released with the feces of infected insects and enter the host through broken skin or mucosal membranes. The trypomastigotes appear in blood stains as long, slender, C– or S–shaped flagellates 15–20 μm in length. A single flagellum originates posteriorly, travels superiorly along the spindle-shaped body of the organism, and projects anteriorly as free flagellum (16, 20, 37). Trypomastigotes invade a variety of tissues in the vertebrate host and replicate as amastigotes, which are smaller, ovoid, aflagellate forms 2–5 μm in length. These proliferate intracellularly before differentiating into a variant form of trypomastigote and entering the bloodstream to infect additional cells, or to be taken up by another insect vector during a blood meal. In all forms a large, round central nucleus and a posteriorly located, elliptical- or bar-shaped kinetoplast are easily visualized (16, 21).
Diagnosis of Chagas Disease
Microscopic detection of T. cruzi organisms in the blood or tissues of infected subjects has long been the gold standard for diagnosing Chagas disease (32). Although an animal may be seropositive for T. cruzi, or suggestive sub-clinical lymphocytic myocarditis may be observed histologically, direct observation of the infectious organism in the tissues is considered crucial for establishing that the cardiomyopathy is actually chagasic.
During the acute stage of infection, trypomastigotes can frequently be detected in the peripheral circulation and morphologic criteria for identifying the organisms as T. cruzi can be applied (8, 37). Visual identification of the parasite is greatly facilitated by the presence of the central nucleus and bar-shaped kinetoplast (16). Observation of the kinetoplast is considered diagnostic for T. cruzi, and aids in differentiating T. cruzi from the morphologically similar intracellular protozoa Sarcocystis spp. and Toxoplasma spp. (27, 38).
In the intermediate and chronic stages of the disease the trypomastigotes effectively disappear from the peripheral circulation and are extremely difficult to detect microscopically. Ideally, sectioning of infected tissues will reveal intracellular nests of kinetoplastid amastigotes, otherwise positive determination of infection with T. cruzi generally requires the application of several diagnostic techniques, such as microscopic examination of blood smears, serological assays, xenodiagnosis, and PCR-based assays for direct detection and quantification of parasite DNA (7, 31, 32, 34, 35, 44).
Materials and Methods
The baboons described in this report were housed at the SPC in San Antonio, TX. SPC is located on a 332-acre campus in southern Texas and currently maintains a colony of about 3600–3700 baboons, as well as smaller numbers of primates such as chimpanzees, macaques, and marmosets. The colony was initiated in 1958 with founder baboons captured in the wild in Kenya and Tanzania (22, 59), and the present colony comprises 5–6 generations of animals. Regular introduction of new, unrelated founder baboons helps to maintain or increase overall genetic diversity in the colony. Most of the baboons in the present colony are P.h. anubis (olive baboons), P.h. cynocephalus (yellow), and their hybrids, but there are also small numbers of P.h. hamadryas (sacred) and P.h. ursinus (Chacma).
All animals at SPC are cared for in strict compliance with the Guide for the Care and Use of Laboratory Animals [National Research Council, 1996], the Animal Welfare Act, and the Institutional Animal Care and Use Committee of the SPC.
Housing
Except as required for colony management or special projects, baboons are housed in large, multi-animal enclosures with some degree of outdoor exposure. About 300–700 baboons are maintained in each of two circular, 6-acre, open-air corrals having dirt floors and sheet-metal walls (22). The remaining baboons are mostly housed in various outdoor, but sheltered, gang-cage facilities having concrete floors and open walls of chain-linked fencing material. The baboons are fed a standard diet (SWF Primate Diet 3715; Harlan-Teklad, Madison, WI, USA) ad libitum.
Pathology
Complete necropsies were done on all the baboons and extensive tissue samples were collected, fixed in 10% neutral-buffered formalin, processed conventionally, embedded in paraffin, sectioned at 5 μm, and stained with hematoxylin and eosin (H & E). Standard techniques of light microscopy were used to search tissue sections for nests of the amastigote form of T. cruzi. A positive diagnosis of Chagas disease was made if any intra- or extracellular forms of the parasite could be microscopically visualized, and identified as T. cruzi according to morphological criteria.
Serology
Serum samples from three of the case report baboons were assessed for T. cruzi seropositivity. One of these animals had offspring; serum samples from all three offspring were also assessed for T. cruzi seropositivity. Seropositivity to T. cruzi was assessed using an in vitro enzyme immunoassay (EIA) for the qualitative detection of antibody to T. cruzi (Abbott Laboratories, Abbott Park, IL). This assay does not cross-react with the nonpathogenic trypanosome T. rangeli, which is transmitted by some of the same vectors as T. cruzi [38]. Additional serologic tests included Enzyme Linked Immunosorbent Assay (ELISA), using T. cruzi antibody; IVD Research, Inc., Carlsbad, CA, and Bio-Manguinhos, Ministry of Health, Oswaldo Cruz Foundation, Rio de Janeiro and Indirect Immunofluorescence Assay (IFA) (using immobilized T. cruzi), Bio-Manguinhos, Ministry of Health, Oswaldo Cruz Foundation, Rio de Janeiro.
Case Reports
Serodiagnostic and histopathologic data have shown that colony baboons at the SPC become infected with T. cruzi and develop clinically evident Chagas disease. Infected animals manifest a chronic-stage symptomatology and may eventually die from the disease, typically from congestive heart failure (43, 58). A decisive diagnosis of Chagas disease, however, is frequently elusive. Among the colony animals that have tested seropositive for T. cruzi and are believed to be infected, blood smears have never revealed the extracellular trypomastigotes. In four exceptional cases, however, organisms morphologically consistent with T. cruzi were confidently identified in the heart tissues of the infected animals, pointing conclusively to a diagnosis of Chagas disease. All four cases died of spontaneous, natural causes between 1984 and 2002.
Case 1
This is the initial case of chagasic pathology recognized in the baboon colony at the SPC, and reported by Gleiser (21). A female P.h. cynocephalus, age 3.6 mo, was discovered dead in its cage. No external gross lesions were recognized. Findings included pleural effusions, ascites, lymphoplasmacytic myocarditis with occasional neutrophils, and Individual myofiber necrosis. Numerous amastigotic nests were seen in myocardial fibers and an inflammatory cell response was clearly associated with ruptured myofibers. Unfortunately, no archived blood or tissues from this animal are available for retrospective analysis.
Case 2
The animal was a male P.h. anubis aged 6.6 yr, that had been on and off its feed for several weeks. Following a natural death, fresh blood was noted around the mouth and perianal areas, and the posterior subcutaneous tissue of both thighs was markedly edematous. The thoracic and peritoneal cavities contained an excess of clear, reddish-brown fluid, and the pericardial sac contained similar clotted material. A chronic, diffuse, multifocal nonsuppurative inflammatory reaction was present in the heart. Inflammatory infiltrate comprising primarily lymphocytes and plasma cells was associated with fibrosis and necrotic myocardial fibers.
Clusters of elliptical organisms morphologically consistent with T. cruzi were observed in many myocardial fibers. Lymphocytic foci were present in esophageal skeletal muscle. Pulmonary vasculature was markedly congested and edematous, with a diffuse proteinaceous fluid accumulation in the alveolar spaces. The liver showed chronic passive congestion with severe centrilobular necrosis. Other changes included interstitial nephritis with lymphocytes and multifocal nephrocalcinosis. Also noted were lymphocytic infiltrates of the smooth muscle and lamina propria in the urinary bladder wall. A blood sample collected at age 2.7 yr was seronegative for T. Cruzi by EIA.
Case 3
A female P.h. anubis aged 10.4 yr was discovered dead in a transfer cage following hospitalization for mastitis. Gross examination revealed a thick, white, plaque-like lesion on the heart left ventricle near the apex, sufficiently severe to have been the cause of death. Microscopically there were multifocal lymphoplasmacytic myocardial infiltrates with associated myocardial necrosis. The inflammation was especially prominent in the endocardium and left ventricle, and organisms morphologically consistent with T. cruzi were identified in a single cardiac muscle fiber. Other changes included lymphoplasmacytic cholangiohepatitis. A blood sample collected at age 5.2 yr was seronegative for T. Cruzi by both ELISA and IFA. This animal was dam to three viable offspring (2 F, 1 M), all of which have since died, but on the basis of the most recent sample from each (collected at ages 3.9, 10.2, and 11.5 yr), all offspring of this animal were seronegative for T. Cruzi, one by EIA and ELISA and the other two by EIA, ELISA and IFA.
Case 4
A male P.h. anubis/P.h. cynocephalus hybrid, aged 8.7 yr, presented with pitting edema and well-defined necrosis of the lower left leg. The left hand appeared paralyzed. Heart rate was elevated with a gallop rhythm. Hematuria and semenuria were noted, the scrotum was markedly distended, and the bladder contained a hypoechoic substance. The clinical signs were consistent with deep vein thrombosis or similar vascular obstruction, infarction, and septicemia, and the animal was euthanized.
Gross examination revealed thrombosis of the medial and distal portions of the femoral artery, with secondary degeneration and necrosis of the supported skeletal muscle. The right kidney was almost totally infarcted and necrotic. The lungs were massively congested with abundant dark eosinophilic proteinaceous material in alveolar spaces. Histologically the myocardium had focal to diffuse collections of mixed inflammatory infiltrates, and rare myofibers contained protozoal organisms consistent with T. cruzi (Figure 1). A blood sample collected at necropsy (8.7 yr) was seropositive for T. cruzi by EIA, ELISA and IFA.
Figure 1.
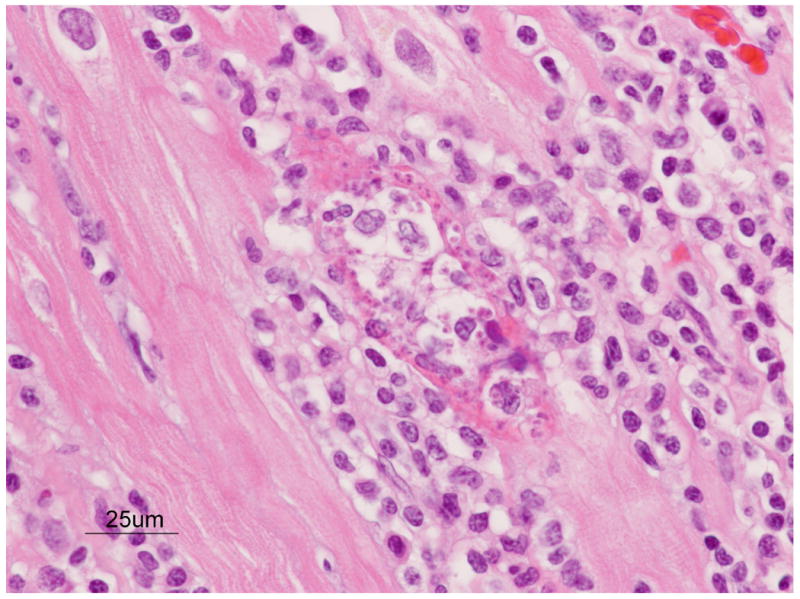
Focal mononuclear inflammation and myocarditis in a nine year old male baboon. Note the lymphoplasmatic cell infiltrates, separation of myocardial fibers, necrosis, and nest of T. cruzi amastigotes within one myofiber. H&E stain.
Discussion
Since the initial case report of T. cruzi infection in a baboon at the SPC (21), three additional cases of Chagas disease in the baboon colony have been confirmed by microscopic visualization of the infectious organism, and in one case by serology. The new cases have many features in common with the index case, and collectively these four cases now define the clinical and histopathologic presentation of chagasic pathology in the baboon.
In each case, necropsy disclosed pathology consistent with Chagas disease, and organisms morphologically consistent with T. cruzi were identified microscopically. The primary findings were congestive heart failure with associated edema of dependent areas, severe multifocal to diffuse myocarditis, extensive lymphocytic and plasmacytic myocardial infiltration, hydrothorax and hydropericardium. Heart failure was indicated by the consistent association of chagasic pathology with congestion and necrosis of the liver and congestion and edema of the lung. All four cases were essentially identical with respect to T. cruzi-induced primary gross and histologic expression of the disease, and differed only in the severity of lesions.
The logistics of controlling the disease in a large outdoor baboon colony of nearly 4000 baboons is difficult. The best techniques are to establish a good vector control program, practice good managemant and clinical care, cull infected baboons and use therapy, as a last option, for valuable animals. Therapy is generally not practical, as it expensive and requires maintainence of individual animals in the hospital indefinitely and repeated sedation of an animals on a regular schedule for the administration of chemotherapeutic agents. Additionally, once an infected baboon has entered the chronic stage of the disease chemotherapy is generally ineffectual (15, 19, 28, 51, 55, 56, 57). Baboons that are seropositive for T. cruzi are monitored closely, however, and an extensive panel of tissue specimens is collected when any of these animals die. Special precautions against accidental infection (26) are taken when personnel must work with seropositive animals.
Our present understanding of Chagas disease in the colony baboons at SPC is derived entirely from retrospective data, and is therefore limited in various irremediable ways. There are many aspects of the disease process, from exposure to infection, seroconversion, and pathogenesis, that are not easily investigated with such data and will require dedicated prospective studies. Nevertheless, the concordance of chagasic pathology in baboons to that observed in humans suggests that the baboon can be developed as a nonhuman primate animal model for Chagas disease in humans (43, 58). Baboons can become infected naturally with T. cruzi, and infected animals exhibit the disease progression and pathologic changes, including EEG abnormalities, seen in human cases of Chagas disease (63, 64, 65). In many cases the infected animals develop frank Chagas disease and die of cardiac-related causes. A nonhuman primate animal model with these features could contribute significantly to the development of therapies for Chagas disease in humans.
Acknowledgments
The authors gratefully acknowledge the assistance of Marie Silva and Antonio Perez for histopathology support. This research was supported in part by National Institutes of Health grants R01 RR016347 and P51 RR013986, and by facilities constructed with support from Research Facilities Improvement Program Grants C06 RR015456 and C06 RR014578 from the National Center for Research Resources, National Institutes of Health.
References
- 1.Anon. Found: two cases of Chagas disease. Texas Health Bulletin. 1956;9:11–13. [Google Scholar]
- 2.Argañaraz ER, Hubbard GB, Ramos LA, Ford AL, Nitz N, Leland MM, Vandeberg JL, Teixeira AR. Blood-sucking lice may disseminate Trypanosoma cruzi infection in baboons. Rev Inst Med Trop S Paulo. 2001;43:271–276. doi: 10.1590/s0036-46652001000500007. [DOI] [PubMed] [Google Scholar]
- 3.Barretto MP, Ribeiro RD, Neto FMB. Reservoirs and wild vectors of Trypanosoma cruzi. LXVIII: infection of mammals by oral route. Rev Bras Biol. 1978;38:455–459. [PubMed] [Google Scholar]
- 4.Beard CB, Pye G, Steurer FJ, Rodriguez R, Campman R, Peterson AT, Ramsey J, Wirtz RA, Robinson LE. Chagas disease in a domestic transmission cycle, southern Texas, USA. Emerg Infect Dis. 2003;9:103–105. doi: 10.3201/eid0901.020217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Betz TG. Chagas' disease investigation. Tex Prev Dis News. 1984;31:1–4. [Google Scholar]
- 6.Bradley KK, Bergman DK, Woods JP, Crutcher JM, Kirchhoff LV. Prevalence of American trypanosomiasis (Chagas disease) among dogs in Oklahoma. J Am Vet Med Assoc. 2000;217:1853–1857. doi: 10.2460/javma.2000.217.1853. [DOI] [PubMed] [Google Scholar]
- 7.Britto C, Cardoso MA, Vanni CMM, Hasslocher-Moreno A, Xavier SS, Oelemann W, Santoro A, Pirmez C, Morel CM, Wincker P. Polymerase chain reaction detection of Trypanosoma cruzi in human blood samples as a tool for diagnosis and treatment evaluation. Parasitology. 1995;110:241–247. doi: 10.1017/s0031182000080823. [DOI] [PubMed] [Google Scholar]
- 8.Bullock BC, Wolf RH, Clarkson TB. Myocarditis associated with trypanosomiasis in a cebus monkey (Cebus albifrons) J Am Vet Med Assoc. 1967;151:920–922. [PubMed] [Google Scholar]
- 9.Burkholder JE, Allison TC, Kelly VP. Trypanosoma cruzi (Chagas) (Protozoa: Kinetoplastida) in invertebrate, reservoir, and human hosts of the lower Rio Grande valley of Texas. J Parasitol. 1980;66:305–311. [PubMed] [Google Scholar]
- 10.CDC. Chagas disease after organ transplantation—United States, 2001. MMWR. 2002;51:210–212. [PubMed] [Google Scholar]
- 11.Chagas C. Neue Trypanosomen. Vorläufige Mitteilung. Archiv für Schiffs- und Tropen-Hygiene. 1909;13:120–122. [Google Scholar]
- 12.Chagas C. Über eine neue Trypanosomiasis des Menschen. Zweite vorläufige Mitteilung. Archiv für Schiffs- und Tropen-Hygiene. 1909;13:351–353. [Google Scholar]
- 13.Cimo PL, Luper WE, Scouros MA. Transfusion-associated Chagas' disease in Texas: report of a case. Tex Med. 1993;89:48–50. [PubMed] [Google Scholar]
- 14.daSilva NN, Clausell DT, Nolibos H, deMello AL, Ossanai J, Rapone T, Snell T. Epidemic outbreak of Chagas disease probably due to oral contamination [Portuguese] Rev Inst Med trop São Paulo. 1968;10:265–276. [PubMed] [Google Scholar]
- 15.deCastro SL. The challenge of Chagas' disease chemotherapy: an update of drugs assayed against Trypanosoma cruzi. Acta Trop. 1993;53:83–98. doi: 10.1016/0001-706x(93)90021-3. [DOI] [PubMed] [Google Scholar]
- 16.de Souza W. A short review on the morphology of Trypanosoma cruzi: From 1909 to 1999. Mem Inst Oswaldo Cruz. 1999;94:17–36. doi: 10.1590/s0074-02761999000700003. [DOI] [PubMed] [Google Scholar]
- 17.Diaz JH. Chagas disease in the United States: a cause for concern in Louisiana? J La State Med Soc. 2007;159(1):21–3. 25–9. [PubMed] [Google Scholar]
- 18.Dorn PL, Perniciaro L, Yabsley MJ, Roellig DM, Balsamo G, Diaz J, Wesson D. Autochthonous transmission of Trypanosoma cruzi, Louisiana. Emerg Infect Dis. 2007;13(4):605–7. doi: 10.3201/eid1304.061002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fairlamb AH. Future prospects for the chemotherapy of Chagas' disease. Medicina (B Aires) 1999;59:179–187. [PubMed] [Google Scholar]
- 20.Garcia LS. Diagnostic Medical Parasitology. 4th. ASM Press; Washington, D.C.: 2001. [Google Scholar]
- 21.Gleiser CA, Yaeger RG, Ghidoni JJ. Trypanosoma cruzi infection in a colony-born baboon. J Am Vet Med Assoc. 1986;189:1225–1226. [PubMed] [Google Scholar]
- 22.Goodwin WJ, Coelho AM., Jr Development of a large scale baboon breeding program. Lab Anim Sci. 1982;32:672–676. [PubMed] [Google Scholar]
- 23.Grant IH, Gold JWM, Wittner M, Tanowitz HB, Nathan C, Mayer K, Reich L, Wollner N, Steinherz L, Ghavimi F, O'Reilly RJ, Armstrong D. Transfusion-associated acute Chagas disease acquired in the United States. Ann Intern Med. 1989;111:849–851. doi: 10.7326/0003-4819-111-10-849. [DOI] [PubMed] [Google Scholar]
- 24.Hagar JM, Rahimtoola SH. Chagas' heart disease in the United States. N Engl J Med. 1991;325:763–768. doi: 10.1056/NEJM199109123251103. [DOI] [PubMed] [Google Scholar]
- 25.Hanford EJ, Zhan FB, Lu Y, Giordano A. Chagas disease in Texas: recognizing the significance and implications of evidence in the literature. Soc Sci Med. 2007;65(1):60–79. doi: 10.1016/j.socscimed.2007.02.041. [DOI] [PubMed] [Google Scholar]
- 26.Herwaldt BL. Laboratory-acquired parasitic infections from accidental exposures. Clin Microbiol Rev. 2001;14:659–688. doi: 10.1128/CMR.14.3.659-688.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kasa TJ, Lathrop GD, Dupuy HJ, Bonney CH, Toft IIJD. An endemic focus of Trypanosoma cruzi infection in a subhuman primate research colony. J Am Vet Med Assoc. 1977;171:850–854. [PubMed] [Google Scholar]
- 28.Kinnamon KE, Poon BT, Hanson WL, Waits VB. In pursuit of drugs for American trypanosomiasis: evaluation of some “standards” in a mouse model. Proc Soc Exp Biol Med. 1997;216:424–428. doi: 10.3181/00379727-216-44192. [DOI] [PubMed] [Google Scholar]
- 29.Kirchhoff LV. American trypanosomiasis (Chagas' disease) – a tropical disease now in the United States. New Engl J Med. 1993;329:639–644. doi: 10.1056/NEJM199308263290909. [DOI] [PubMed] [Google Scholar]
- 30.Kirchhoff LV. Current public health concerns. In: Tyler K, Miles M, editors. American Trypanosomiasis World Class Parasites. Kluwer Academic Publishers; Boston, MA: 2003. pp. 157–162. [Google Scholar]
- 31.Kirchhoff LV, Votava JR, Ochs DE, Moser DR. Comparison of PCR and microscopic methods for detecting Trypanosoma cruzi. J Clin Microbiol. 1996;34:1171–1175. doi: 10.1128/jcm.34.5.1171-1175.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.López-Antuñano FJ, Rangel-Flores H, Ramos C. Diagnosis of Chagas' disease. Revista Latinoamericana de Microbiología. 2000;42:121–129. [Google Scholar]
- 33.Mendez MLC, Torres BN, Aguilar RA. The oral route: an access port for Trypanosoma cruzi. Rev Latinoam Microbiol. 1992;34:39–42. [PubMed] [Google Scholar]
- 34.Mortara RA, daSilva S, Patricio FR, Higuchi ML, Lopes ER, Gabbai AA, Carnevale P, Rocha A, Ferreira MS, Souza MM, deFranco MF, Turcato G, Jr, Neto BHF. Imaging Trypanosoma cruzi within tissues from chagasic patients using confocal microscopy with monoclonal antibodies. Parasitol Res. 1999;85:800–808. doi: 10.1007/s004360050636. [DOI] [PubMed] [Google Scholar]
- 35.Moser DR, Kirchhoff LV, Donelson JE. Detection of Trypanosoma cruzi by DNA amplification using the polymerase chain reaction. J Clin Microbiol. 1989;27:1477–1482. doi: 10.1128/jcm.27.7.1477-1482.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nickerson P, Orr P, Schroeder ML, Sekla L, Johnston JB. Transfusion-associated Trypanosoma cruzi infection in a non-endemic area. Ann Intern Med. 1989;111:851–853. doi: 10.7326/0003-4819-111-10-851. [DOI] [PubMed] [Google Scholar]
- 37.Nogueira N, Coura JR. American trypanosomiasis (Chagas' disease) In: Warren KS, Mahmoud AA, editors. Tropical and Geographical Medicine chapter 30. McGraw-Hill; New York: 1984. pp. 253–269. [Google Scholar]
- 38.Pan AA, Rosenberg GB, Hurley MK, Schock GJ, Chu VP, Aiyappa A. Clinical evaluation of an EIA for the sensitive and specific detection of serum antibody to Trypanosoma cruzi (Chagas' disease) J Infect Dis. 1992;165:585–8. doi: 10.1093/infdis/165.3.585. [DOI] [PubMed] [Google Scholar]
- 39.Pentima MCD, Hwang LY, Skeeter CM, Edwards MS. Prevalence of antibody to Trypanosoma cruzi in pregnant Hispanic women in Houston. Clin Infect Dis. 1999;28:1281–1285. doi: 10.1086/514790. [DOI] [PubMed] [Google Scholar]
- 40.Philipp MT, Purcell JE. Modeling parasitic diseases in nonhuman primates: malaria, Chagas' disease, and filariasis. In: Wolfe-Coote S, editor. The Laboratory Primate. Academic Press; San Diego CA: 2005. pp. 91–103. [Google Scholar]
- 41.Pung OJ, Banks CW, Jones DN, Krissinger MW. Trypanosoma cruzi in wild raccoons, opossums, and triatomine bugs in southeast Georgia, U.S.A. J Parasitol. 1995;81:324–326. [PubMed] [Google Scholar]
- 42.Raasi A, Luquetti AO. Specific treatment for Trypanosoma cruzi infection (Chagas disease) In: Tyler K, Miles M, editors. American Trypanosomiasis World Class Parasites. Kluwer Academic Publishers; Boston, MA: 2003. pp. 117–125. [Google Scholar]
- 43.Ramos L, Hubbard GB, Argañaraz ER, Ford AL, VandeBerg JL, Teixeira ARL. Animal model of Chagas' disease: natural Trypanosoma cruzi infection of baboons reared in captivity. Mem Inst Oswaldo Cruz. 1998;93:96. [Google Scholar]
- 44.Russomando G, Figueredo A, Almirón M, Sakamoto M, Morita K. Polymerase chain reaction-based detection of Trypanosoma cruzi DNA in serum. J Clin Microbiol. 1992;30:2864–2868. doi: 10.1128/jcm.30.11.2864-2868.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Schmunis GA. Chagas' disease and blood transfusion. Prog Clin Biol Res. 1985;182:127–145. [PubMed] [Google Scholar]
- 46.Schmunis GA. Trypanosoma cruzi, the etiologic agent of Chagas' disease: status in the blood supply in endemic and nonendemic countries. Transfusion. 1991;31:547–557. doi: 10.1046/j.1537-2995.1991.31691306255.x. [DOI] [PubMed] [Google Scholar]
- 47.Schofield CJ, Dias JCP. The Southern Cone initiative against Chagas disease. Adv Parasitol. 1999;42:1–27. doi: 10.1016/s0065-308x(08)60147-5. [DOI] [PubMed] [Google Scholar]
- 48.Shikanai-Yasuda MA, Marcondes CB, Guedes LA, Siqueira GS, Barone AA, Dias JCP, Neto VA, Tolezano JE, Peres BA, Arruda ER, Jr, Lopes MH, Shiroma M, Chapadeiro E. Possible oral transmission of acute Chagas' disease in Brazil. Rev Inst Med trop São Paulo. 1991;33:351–357. doi: 10.1590/s0036-46651991000500003. [DOI] [PubMed] [Google Scholar]
- 49.Skolnick A. Does influx from endemic areas mean more transfusion-associated Chagas' disease? J Am Med Assoc. 1989;262:1433. [PubMed] [Google Scholar]
- 50.Skolnick A. Deferral aims to deter Chagas' parasite. J Am Med Assoc. 1991;265:173. [PubMed] [Google Scholar]
- 51.Stoppani AOM. Quimioterapia de la enfermedad de Chagas. Medicina (B Aires) 1999;59:147–165. [PubMed] [Google Scholar]
- 52.Tanowitz HB, Kirchhoff LV, Simon D, Morris SA, Weiss LM, Wittner M. Chagas' disease. Clin Microbiol Rev. 1992;5:400–419. doi: 10.1128/cmr.5.4.400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Toft JD, II, Eberhard ML. Parasitic diseases. In: Bennett BT, Abee CE, Henrickson R, editors. Nonhuman Primates in Biomedical Research Diseases. Academic Press; San Diego CA: 1998. pp. 115–116. [Google Scholar]
- 54.Travi BL. Infeccion natural por Trypanosoma cruzi en Cebus apella. Bol Primatol Arg. 1985;3:33–35. [Google Scholar]
- 55.Urbina JA. Chemotherapy of Chagas' disease: the how and the why. J Mol Med. 1999;77:332–338. doi: 10.1007/s001090050359. [DOI] [PubMed] [Google Scholar]
- 56.Urbina JA. Rational approaches to specific chemotherapy of Chagas disease. In: Tyler K, Miles M, editors. American Trypanosomiasis World Class Parasites. Kluwer Academic Publishers; Boston, MA: 2003. pp. 127–135. [Google Scholar]
- 57.Urbina JA, Docampo R. Specific chemotherapy of Chagas disease: controversies and advances. Trends Parasitol. 2003;19:495–501. doi: 10.1016/j.pt.2003.09.001. [DOI] [PubMed] [Google Scholar]
- 58.VandeBerg JL, Ford AL, Teixeira ARL, Hubbard GB. Epidemiology of seropositivity for Trypanosoma cruzi in baboons maintained in Texas. Mem Inst Oswaldo Cruz. 2000;95:77. [Google Scholar]
- 59.Williams-Blangero S. Recent trends in genetic research on captive and wild nonhuman primate populations. Yearb Phys Anthropol. 1991;34:69–96. [Google Scholar]
- 60.World Health Organization. Chagas disease. Progress towards elimination of transmission, Argentina. Wkly Epidemiol Rec. 1996;71:12–15. [PubMed] [Google Scholar]
- 61.World Health Organization. Chagas disease. Interruption of transmission. Wkly Epidemiol Rec. 1997;72:1–4. [PubMed] [Google Scholar]
- 62.World Health Organization. Chagas disease, Venezuela, 1999. Wkly Epidemiol Rec. 1999;74:290–292. [PubMed] [Google Scholar]
- 63.Zabalgoitia M, Ventura J, Anderson L, Carey KD, Williams JT, VandeBerg JL. Morphologic and functional characterization of Chagasic heart disease in non-human primates. Am J Trop Med Hyg. 2003;68:248–252. [PubMed] [Google Scholar]
- 64.Zabalgoitia M, Ventura J, Anderson L, Williams JT, Carey KD, VandeBerg JL. Electrocardiographic findings in naturally acquired chagasic heart disease in nonhuman primates. J Electrocardiol. 2003;36:155–160. doi: 10.1054/jelc.2003.50019. [DOI] [PubMed] [Google Scholar]
- 65.Zabalgoitia M, Ventura J, Lozano JL, Anderson L, Carey KD, Hubbard GB, Williams JT, VandeBerg JL. Myocardial contrast echocardiography in assessing microcirculation in baboons with Chagas disease. Microcirculation. 2004;11:271–278. doi: 10.1080/10739680490425976. [DOI] [PubMed] [Google Scholar]


