Abstract
Background: Calcium is an essential cotherapy in osteoporosis treatment. The relative effectiveness of various calcium salts for this purpose is uncertain. Many older women with osteoporosis have phosphorus intakes of <70% of the Recommended Dietary Allowance.
Objective: Our objective was to test the hypothesis that calcium phosphate would better support anabolic bone building than would calcium carbonate.
Design: This study was a 12-mo, randomized, positive-comparator, 2-arm, single-blind clinical trial in 211 patients treated with teriparatide who consumed <1000 mg phosphorus/d. Participants were randomly assigned to receive, in addition to teriparatide and 1000 IU cholecalciferol, 1800 mg calcium/d as either tricalcium phosphate or calcium carbonate. The primary endpoints were changes in lumbar spine and total hip bone mineral densities (BMDs); secondary endpoints were changes in bone resorption biomarkers and serum and urine calcium and phosphorus concentrations.
Results: In the combined group, the lumbar spine BMD increased by 7.2%, and total hip BMD increased by 2.1% (P < 0.01 for both). However, there was no significant difference between calcium-treatment groups, and there were no significant between-group differences in serum calcium and phosphorus concentrations or in urine calcium concentrations. Bone resorption biomarkers increased in both groups, as expected with teriparatide, but the increases in the 2 calcium groups did not differ significantly.
Conclusions: Tricalcium phosphate and calcium carbonate appear to be approximately equally effective in supporting bone building with a potent anabolic agent; phosphate salt may be preferable in patients with restricted phosphorus intakes. This trial was registered at clinicaltrials.gov as NCT00074711.
INTRODUCTION
An adequate calcium intake, mostly in the form of calcium supplements, is recognized as essential cotherapy in the treatment of postmenopausal osteoporosis. All of the registration trials of currently approved bone-active agents involved calcium supplementation, and the prescribing guidelines for these agents specify calcium cotherapy. However, there have been few published studies that evaluated the various calcium salts available for this purpose. Bone mineral, the mass of which increases in successful osteoporosis therapy, is a complex salt of calcium and phosphate, and manifestly, both ions must be available if bone mass is to increase.
Generally, it is calcium that is the limiting nutrient in the diets of North American women, with phosphorus intake usually considered adequate. However, National Health and Nutrition Examination Survey data reveal that substantial fractions of older women have phosphorus intakes of <70% of the adult Recommended Dietary Allowance (1, 2). These women, if given supplemental calcium in the form of carbonate or citrate salts, would be expected to have reduced dietary phosphorus availability because of calcium binding of food phosphorus, as in the use of calcium salts as phosphate binders in end-stage renal disease (3). If such binding occurs to any appreciable extent in women being treated for osteoporosis, bone gain could be limited by phosphorus availability, representing a kind of geriatric counterpart of the corresponding phenomenon in low-birth-weight newborns.
Accordingly, we designed this study to test the hypothesis that a calcium-phosphate supplement would better support bone gain in osteoporotic patients than would calcium carbonate, with a specific focus on women with phosphorus intakes below the median for their age. We selected a treatment context that used teriparatide because, of all the available treatments for osteoporosis, this agent produces the greatest bone-mineral augmentation.
SUBJECTS AND METHODS
Design
The study was a randomized, 2-arm, single-blind, active-comparator trial, and each participant nominally received 1800 mg calcium/d either as the carbonate or the triphosphate salt, which was determined by random-number assignment upon formal entry into the trial. The calcium tablets each contained 600 mg calcium, and participants were instructed to take 3 tablets/d (one at each meal). In addition, each participant received 20 μg teriparatide/d by subcutaneous injection and 1000 IU vitamin D3/d by mouth. The primary design endpoints were changes in lumbar spine and total hip bone mineral densities (BMDs), and secondary endpoints were changes in bone resorption biomarkers and serum concentrations and urinary excretion of calcium, phosphorus, and creatinine.
Serum and urine calcium concentrations were monitored at each study visit, and if the serum calcium concentration was >10.6 mg/dL or urine calcium:creatinine exceeded 0.4 g/g, the supplement regimen was altered and repeat specimens obtained. For elevations of serum calcium concentrations, teriparatide dosing was scheduled for bedtime, and the intake of calcium supplements was shifted to morning and noon. For elevations of urine calcium concentrations, calcium dosing was reduced from 1800 to 1200 mg/d, and in a few cases, it was reduced from 1800 to 600 mg/d (n = 10 for any reduction). Dose adjustments did not differ significantly between groups. For the intake of calcium-supplement tablets, compliance was assessed by pill count and reinforced by a daily dose check sheet; for teriparatide, compliance was assessed by a daily injection calendar. The calcium check sheet and the injection calendar were reviewed at each study visit. Compliance for teriparatide injections averaged 99.4%, compliance for vitamin D injections averaged 100%, and for compliance for calcium intake averaged 98.3%.
Before random assignment, otherwise eligible participants entered a 2-wk run-in period, before which they were instructed in the method and during which they gave themselves daily subcutaneous injections with the Lilly pen (Eli Lilly and Company, Indianapolis, IN) filled with sterile saline. They also took 1800 mg calcium as the carbonate. The purpose of the run-in period was to elicit problems before entry that might lead to dropout and, hence, to minimize sampling unit losses after being randomly assigned.
Participants
Participants were women with moderate to severe idiopathic, postmenopausal osteoporosis as indicated by a spine BMD T-score value at the hip or spine below –1.0 together with at least one prevalent nontraumatic fracture of a vertebral body. Other inclusion criteria were as follows: age of 60–85 y, body mass index (in kg/m2) <30, phosphorus intakes below the National Health and Nutrition Examination Survey median for their age (≈1000 mg/d), a serum creatinine concentration <1.3 mg/dL, a serum phosphorus concentration <3.6 mg/dL, and a serum uric acid concentration <7.5 mg/dL. Phosphorus intake was assessed by using a food-frequency questionnaire designed for this purpose and administered by a research dietitian. The food-frequency questionnaire effectively screened out individuals with high intakes of dairy, meat, and leavened cereal-grain products. Additional exclusion criteria were as follows: a history of non–skin cancer within the previous 5 y, a history of renolithiasis, use within the previous 2 y of bone-active agents of a >4-wk duration, current systemic corticosteroid therapy, and any endocrine disorder (other than stable treated hypothyroidism; n = 32) that might alter the bony response to teriparatide.
Participants were recruited from a database of research volunteers in our unit and by telephone solicitation. Participants were consented through a 2-stage process: the first for screening and the second for formal entry. The project was approved by the Creighton University Institutional Review Board, and each participant gave signed consent. Study progress and participant safety were monitored throughout the study by a Data Safety and Monitoring Board appointed by the National Institute of Arthritis and Musculoskeletal and Skin Diseases.
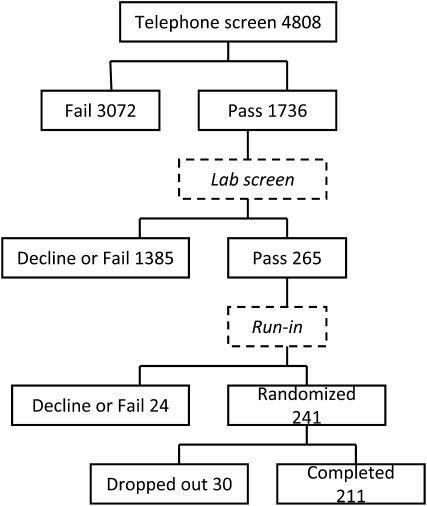
The flow of participants through the process is illustrated in Figure 1. More than 4800 potential participants were identified and screened by telephone interview, with 1736 selected for a face-to-face interview and laboratory screening. At the end of this process, 265 individuals were eligible for and agreed to enter into the run-in period. By far the largest reason for noneligibility at this stage was a serum phosphorus value above 3.6 mg/dL. Twenty-four of the eligible subjects declined or were rejected from the run-in period, which left 241 subjects who were randomly assigned to treatments. Of these 241 subjects, 211 of them finished the 12 mo of the study for a loss rate after random assignment of 12%.
FIGURE 1.
Numerical flow of potential participants through the solicitation, screening, run-in, and randomization phases of the trial.
Measurements
BMD was measured at the lumbar spine and total hip on a Hologic Delphi instrument (Hologic, Bedford, MA) at baseline and 3, 6, and 12 mo. In our hands, this instrument had a long-term precision of ≈1.5% at both the hip and spine in individuals >55 y of age. The measurements at baseline and at the end of study were duplicated (with subjects getting off and on the scanning table between paired measurements). For purposes of determining the primary endpoint, the duplicate measurements at 0 and 12 mo were each averaged to yield single beginning and ending values. The primary outcome variable was the difference in BMD across this 12-mo period. Lateral spine radiographs were obtained on entry and at the end of the study and read individually by one of us (RRR).
Urine specimens were second void, fasting, morning samples. Serum calcium and creatinine concentrations were measured by Creighton Medical Laboratories (Omaha, NE) on a Roche Integra system (Basel, Switzerland) with enzymatic colorimetric and orthocresophthalein complexone methods, respectively. Urinary N-telopeptide (NTx) concentrations were measured by ARUP with a chemiluminescent assay. Serum and urine phosphorus concentrations were measured by an ammonium molybdate reaction on a Chiron Express Plus System (Bayer, Tarrytown, NY), urine calcium concentrations were measured by atomic absorption spectrophometry on a Perkin-Elmer AA Analyst 100 spectrometer (Perkin-Elmer, Norwalk, CT), urine hydroxyproline concentrations were measured by a modification of the Bergman and Loxley method (4), and urine creatinine concentrations were measured by a modification of the Jaffe method on the Chiron Express Plus System (Gilford Instruments, Oberlin, OH).
Statistical analyses
Data were assembled in an Access database (Microsoft Corp, Redmond, WA) and analyzed with SAS version 9.1 (SAS Institute Inc, Cary, NC). Within groups, BMD measurements and serum and urine biomarkers were compared at baseline and at 12 mo by using a paired t test. Between groups, changes at 12 mo from baseline in BMD measurements and serum and urine biomarkers were compared by using the Satterthwaite (unpooled variances) t test. In addition, BMD measurements were analyzed with repeated-measures analysis of variance, with the visit (4 levels), treatment group (2 levels), and visit-by-group interaction effects always included in the model. This allowed for the inclusion of covariates such as baseline biomarkers and age to evaluate whether these more complex models revealed an effect-of-treatment group. Ordinary type-III F tests were used to evaluate the significance of between-subject variables such as treatment group in these analyses.
RESULTS
Baseline data
The baseline data, divided by treatment assignment, are shown in Table 1. The 2 groups were well matched and comparable in most important respects. The sole exceptions were for the bone resorption biomarkers (NTx:creatinine and hydroxyproline:creatinine) which, by chance, were ≈10% higher in the group randomly assigned to phosphate supplementation than in the group randomly assigned to carbonate supplementation. Both differences were of only borderline significance (0.10 > P > 0.05).
TABLE 1.
Baseline characteristics by group1
| Carbonate |
Phosphate |
||||
| Variable | n | Values | n | Values | P |
| Age (y) | 106 | 70.07 ± 6.342 | 105 | 70.09 ± 7.02 | 0.98 |
| Weight (kg) | 106 | 70.56 ± 15.63 | 105 | 72.15 ± 16.37 | 0.47 |
| Height (m) | 106 | 1.60 ± 0.06 | 105 | 1.60 ± 0.06 | 0.81 |
| BMI (kg/m2) | 106 | 27.65 ± 5.90 | 105 | 28.37 ± 6.43 | 0.40 |
| Hip BMD (g/cm2) | 106 | 0.78 ± 0.10 | 105 | 0.76 ± 0.11 | 0.24 |
| Spine BMD (g/cm2) | 106 | 0.86 ± 0.12 | 105 | 0.88 ± 0.12 | 0.24 |
| Prevalent fractures | 106 | 33 | 105 | 33 | 1.00 |
| sPhos (mg/dL) | 106 | 3.25 ± 0.32 | 105 | 3.30 ± 0.27 | 0.26 |
| scrt (mg/dL) | 106 | 0.816 ± 0.15 | 105 | 0.81 ± 0.17 | 0.64 |
| sCa (mg/dL) | 106 | 9.614 ± 0.42 | 105 | 9.68 ± 0.47 | 0.29 |
| NTx (nmol BCE/mmol) | 106 | 31.27 ± 14.34 | 105 | 34.86 ± 15.96 | 0.08 |
| catocrt (g/g) | 106 | 0.16 ± 0.09 | 105 | 0.136 ± 0.10 | 0.10 |
| ohptocrt (μmol/mmol) | 106 | 16.19 ± 5.89 | 105 | 18.07 ± 7.86 | 0.06 |
| ptocrt (g/g) | 106 | 0.57 ± 0.20 | 105 | 0.56 ± 0.22 | 0.89 |
BMD, bone mineral density; sPhos, serum phosphorus; scrt, serum creatinine; sCa, serum calcium; NTx, urinary N-telopeptide; BCE, bone collagen equivalents; catocrt, urinary calcium:creatinine ratio; ohprtocrt, urine hydroxyproline:creatinine ratio; ptocrt, urinary phosphorus:creatinine ratio.
Mean ± SD (all such values).
Median.
Outcome data
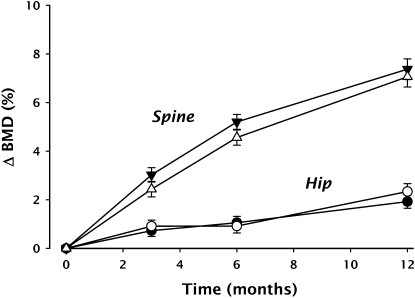
The BMD data are shown in Table 2 as the change in areal density (g/cm2) from baseline at 3, 6, and 12 mo for total hip and spine separated by treatment assignment, and the time course of percentage changes at the 2 skeletal sites by treatment groups is shown in Figure 2. BMD increased in a clearly linear fashion at the hip and nearly so at the spine. Changes were significant at both measurement sites at all time points relative to baseline (P < 0.01), but there was no significant difference between the 2 treatment groups.
TABLE 2.
Change in bone mineral density over 12 mo (g/cm2) by group1
| Carbonate |
Phosphate |
|||
| Time | n | Mean ± SEM | n | Mean ± SEM |
| Hip | ||||
| 3 mo | 105 | 0.00552 ± 0.00194 | 105 | 0.00654 ± 0.00186 |
| 6 mo | 106 | 0.00801 ± 0.00197 | 104 | 0.00702 ± 0.00213 |
| 12 mo | 106 | 0.01450 ± 0.00203 | 105 | 0.01680 ± 0.00227 |
| Spine | ||||
| 3 mo | 104 | 0.0248 ± 0.00242 | 105 | 0.0216 ± 0.00260 |
| 6 mo | 106 | 0.0433 ± 0.00248 | 104 | 0.0395 ± 0.00266 |
| 12 mo | 106 | 0.0607 ± 0.00336 | 105 | 0.0609 ± 0.00356 |
There were no significant differences between groups for any of the variables.
FIGURE 2.
Time course of percentage change from baseline in bone mineral density (BMD) for the total hip and lumbar spine at 3, 6, and 12 mo of treatment with teriparatide. Open symbols represent data from the calcium phosphate group, and closed symbols represent data from the calcium carbonate group. Error bars = 1 SEM.
The changes across the 12-mo treatment period in pertinent serum, urine, and bone-resorption biomarker variables by treatment group are shown in Table 3. Neither group experienced a change in serum calcium concentrations; however, urine calcium concentrations (expressed as the calcium-to-creatinine ratio) rose as expected: by 0.011 (41%) in the carbonate group and by 0.021 (64%) in the phosphate group. Both elevations were significantly different from a zero change when expressed relative to baseline, but the between-group difference was not significant.
TABLE 3.
Twelve-month changes in metabolic variables by group1
| Carbonate |
Phosphate |
||||
| Variable | n | Mean ± SEM | n | Mean ± SEM | P2 |
| sPhos (mg/dL) | 104 | +0.245 ± 0.0423 | 103 | +0.139 ± 0.0454 | 0.09 |
| scrt (mg/dL) | 106 | +0.051 ± 0.0123 | 105 | −0.001 ± 0.012 | <0.003 |
| sCa (mg/dL) | 106 | +0.001 ± 0.047 | 105 | −0.048 ± 0.041 | 0.44 |
| NTx (nmol BCE/mmol) | 106 | +17.1 ± 2.793 | 105 | +16.0 ± 3.423 | 0.80 |
| catocrt (g/g) | 106 | +0.011 ± 0.0103 | 105 | +0.021 ± 0.0103 | 0.50 |
| ohprtocrt (μmol/mmol) | 106 | +6.26 ± 0.9623 | 105 | +5.61 ± 1.253 | 0.68 |
| ptocrt (g/g) | 106 | −0.053 ± 0.028 | 104 | +0.073 ± 0.0253 | <0.001 |
sPhos, serum phosphorus; scrt, serum creatinine; sCa, serum calcium; NTx, urinary N-telopeptide; BCE, bone collagen equivalents; catocrt, urinary calcium:creatinine ratio; ohprtocrt, urinary hydroxyproline:creatinine ratio; ptocrt, urinary phosphorus:creatinine ratio.
Between-group comparisons.
Within-group comparisons: 3P < 0.01, 4P < 0.05.
Serum phosphorus concentrations rose significantly in both groups (although by only 8% and 5%, respectively). The difference between the groups was not significant and, perhaps of greater interest, the rise in the phosphate group was not greater from that in the carbonate group. The carbonate group experienced a small (6.25%) but significant rise (P < 0.005) in serum creatinine concentrations, whereas the phosphate group exhibited no change.
The bone-resorption biomarkers both increased significantly, as expected, across the 12 mo of treatment, but the difference between the 2 treatment groups was not significant (P > 0.60 for both NTx:creatinine and hydroxyproline:creatinine). If anything, the rise in the phosphate supplement was smaller than for the carbonate-salt supplement (62% compared with 77% for NTx:creatinine and 45% compared with 55% for hydroxyproline:creatinine).
Adverse events
There were no serious adverse events with the exception of a single cardiac death judged to be unrelated to the study or its interventions. There were no new fractures. Similarly, whereas there were numerous minor events, there was no preponderance of adverse events, either globally or by body systems, in either supplement group.
DISCUSSION
This study showed that both calcium phosphate and calcium carbonate fully supported the bone-building effect of teriparatide and that the phosphate salt was not inferior to the carbonate salt. Although such comparative data have largely been unavailable, it is known that calcium-phosphate salts produce the effects generically attributed to calcium (5–8). Thus, we conclude that either salt can be used with equal assurance of efficacy in the management of patients with osteoporosis. In this respect, the calcium density of tricalcium phosphate (at ≈37.5%) is nearly as high as for calcium carbonate (at 40%). Thus, the tablet size and number should be comparable for the 2 salts.
Apart from the salt itself, the calcium-supplement dose used in our trial was substantially larger than the 1000-mg dose used in the teriparatide treatment trial by Neer et al (9), and the change in BMD at both measurement sites was larger as well. When compared on an annualized basis, we saw an 18% greater increase than Neer et al (9) at the spine and a 30% greater increase at the hip.
The metabolic changes produced in this study are also worthy of some comment, largely for what they did not show. The absence of a rise in serum calcium concentrations that could not be handled by dose timing, especially in spite of larger than average calcium-supplement doses, is reassuring, particularly as hypercalcemia is generally considered a risk with teriparatide therapy.
In addition to its calcium content, the phosphate supplement added a considerable phosphorus load that amounted to ≈2 g/d, in some cases tripling the phosphorus intakes of the phosphate group. These women handled the extra phosphorus well. Serum phosphorus concentrations did not rise appreciably, whereas urine phosphorus concentrations, as reflected in the phosphorus-to-creatinine ratio, rose by a mean of 26%. This is in contrast with the carbonate group, which experienced no rise in urine phosphorus concentrations. Typically, absorption of phosphorus from animal-based foods ranges from 60% to 80% of intake, and most absorbed phosphorus would be spilled into the urine. The relatively small rise in urine phosphorus concentrations in the phosphate group suggests that the amount of absorbed phosphorus was relatively low in this study, probably because most of it was complexed with the calcium in the salt. That also means that diet phosphorus was spared and was available to support the increase in BMD.
The rise in serum creatinine concentrations on the carbonate supplement was small and of a very uncertain clinical significance. Perhaps more reassuring was the absence of any change in the phosphate group, which indicated that the increased phosphorus load was well handled without any hint of deterioration in kidney function.
The rise in resorption biomarkers is what would be expected from teriparatide, which, in addition to its anabolic effect, produces most of the usual physiologic effects of parathyroid hormone. Moreover, the fact that the rise in NTx and hydroxyproline in the phosphate group was not higher than in the carbonate group indicates the safety of phosphate salt and of high phosphorus intakes in this group of women.
Finally, although the primary hypothesis was not supported in this study, this fact does not mean that there are not patients with osteoporosis who have sufficiently low dietary phosphorus intakes such that they would be better off with a calcium-phosphate supplement. We attempted to confine our study population to such individuals (by limiting inclusion to those with low dairy intakes and those with serum phosphorus concentrations <3.6 mg/dL). Nevertheless, we had a healthy volunteer effect in our recruitment and, for the most part, enrolled relatively well-nourished individuals. Hence, although these results do not show the superiority of a calcium-phosphate supplement, it remains possible that those patients with osteoporosis who have inadequate phosphorus intakes would be better served with calcium phosphate. Such a source would be safe for them.
Acknowledgments
The authors' responsibilities were as follows—RPH: was the principal investigator; RRR: managed the clinical care issues and radiographic assessment; PW: managed the data and performed the statistical analyses; JML: was the clinical coordinator; and all authors: contributed to the manuscript. None of the authors had a financial relationship to disclose.
REFERENCES
- 1.Alaimo K, McDowell MA, Briefel RR, et al. Dietary intake of vitamins, minerals, and fiber of persons 2 months and over in the United States: Third National Health and Nutrition Examination Survey, phase 1, 1988-91. Advance data from vital and health statistics; no. 258. Hyattsville, MD: National Center for Health Statistics, 1994 [PubMed] [Google Scholar]
- 2.Heaney RP. Phosphorus nutrition and the treatment of osteoporosis. Mayo Clin Proc 2004;79:91–7 [DOI] [PubMed] [Google Scholar]
- 3.Hutchison AJ. Oral phosphate binders. Kidney Int 2009;75:906–14 [DOI] [PubMed] [Google Scholar]
- 4.Bergman L, Loxley R. The determination of hydroxyproline in urine hydrolysates. Chim Acta 1970;27:347–9 [DOI] [PubMed] [Google Scholar]
- 5.Chapuy MC, Arlot ME, Duboeuf F, et al. Vitamin D3 and calcium to prevent hip fractures in elderly women. N Engl J Med 1992;327:1637–42 [DOI] [PubMed] [Google Scholar]
- 6.Rüegsegger P, Keller A, Dambacher MA. Comparison of the treatment effects of ossein-hydroxyapatite compound and calcium carbonate in osteoporotic females. Osteoporos Int 1995;5:30–4 [DOI] [PubMed] [Google Scholar]
- 7.Pines A, Raafat H, Lynn AH, Whittington J. Clinical trial of microcrystalline hydroxyapatite compound (Ossopan) in the prevention of osteoporosis due to corticosteroid therapy. Curr Med Res Opin 1984;8:734–42 [DOI] [PubMed] [Google Scholar]
- 8.Epstein O, Kato Y, Dick R, Sherlock S. Vitamin D, hydroxyapatite, and calcium gluconate in treatment of cortical bone thinning in postmenopausal women with primary biliary cirrhosis. Am J Clin Nutr 1982;36:426–30 [DOI] [PubMed] [Google Scholar]
- 9.Neer RM, Arnaud CD, Zanchetta JR, et al. Effect of parathyroid hormone (1-34) on fractures and bone mineral density in postmenopausal women with osteoporosis. N Engl J Med 2001;344:1434–41 [DOI] [PubMed] [Google Scholar]