Abstract
Equol, first isolated from equine urine in 1932 and identified 50 years later in human urine as a metabolite of the soy isoflavones, daidzin and daidzein, is produced by intestinal bacteria in some, but not all, adults. This observation led to the term equol-producers to define those adults that could make equol in response to consuming soy isoflavones and the hypothesis that the health benefits of soy-based diets may be greater in equol-producers than in equol nonproducers. By virtue of a chiral center, equol occurs as a diastereoisomer and intestinal bacteria are enantiospecific in synthesizing exclusively the S-(-)equol enantiomer, an enantiomer that has selective affinity for the estrogen receptor-β. Both enantiomers are of interest from a clinical and pharmacological perspective and are currently being developed as nutraceutical and pharmacological agents. The wide range of biological activities these enantiomers possess warrants their investigation for the treatment of a number of hormone-related conditions involving estrogen-dependent and androgen-related conditions. The following review describes the history, chemistry, and factors governing the intestinal bacterial formation of equol.
Introduction
It is now 57 years since the first report appeared describing a new phenolic compound in an estrogenic fraction of pregnant mare urine (1). It was suggested that the compound be given the name equol, after the equine source of the material. Efforts to obtain large-scale quantities of the compound led to the recognition that it was also present in appreciable amounts in the urine of stallions and nonpregnant mares and the conclusion that it was not associated with the presence of high estrogen states. During the autumn months, the amounts of equol declined and by winter it was impossible to isolate it from urine. The authors concluded that, “so far as can be determined, no dietary factor was the cause of this (seasonal) variation…” (2). It later became apparent that this was not the case when in SW Australia reports emerged of a catastrophic “failure to breed” associated with uterine abnormalities and endometriosis in sheep grazing on Trifolium subterranium clover (3). Reductions in sperm counts and motility were also documented in ewes (4). This clover disease, as it was so-called, was found to be the result of extremely high circulating concentrations of equol, formed by rumenal bacteria from the ingestion of large amounts of the methoxylated isoflavone, formononetin, abundant in several indigent species of clover (5–7). Equol was even found as a component in urinary calculi of sheep and cattle (8). Equol has since been reported to be present in the urine and/or plasma of many other animal species, including cows (9), hens (10–14), monkeys (15,16), chimpanzees (17,18), dogs (19), mice (20), rats (20–22), and pigs (16,23), but there are marked differences in the extent of metabolism of isoflavones into equol by these species. Rodents, e.g., very efficiently convert daidzin/daidzein to equol (24), whereas pigs and humans have been reported to do this less efficiently (16,24). In the decades leading to 1970, a great deal of work was performed defining the metabolism of isoflavones and biological actions of equol (6,25–28). While its estrogenic effects were well documented based upon field observations and classical bioassays, it was not until after the discovery of the first estrogen receptor (ER)5 in the mid-1960s (29) that the relative affinity of equol for the ER could be quantified. When the relative molar binding affinities of a number of phytoestrogens for sheep uterine ER were compared, equol was found to have much higher affinity than its precursor daidzein in competing with radioactive estradiol for binding to the cytosolic receptor (27), supporting the theory that it may be advantageous to be able to convert daidzein to equol (24).
There was little interest in equol for several decades until the chance discovery in 1980 of high concentrations of an unknown estrogen-like compound in rat urine (30) accompanying the mammalian lignans, enterolactone and enterodiol (31–33). At the time, it was referred to as compound 386/192, a notation for the molecular ion and base peak in the mass spectrum of its trimethylsilyl ether derivative. In common with most endogenous steroid hormones, including estrogens, it was conjugated predominantly to glucuronic acid and a lesser extent to sulfuric acid (34). Its presence in such high concentrations in rat urine fortuitously afforded a means of isolating sufficient quantities for structural elucidation studies by infrared spectroscopy, NMR, and GC-MS (35) and the subsequent confirmation that it was identical in chemical structure to the equol first isolated from pregnant mares urine in 1932 by Marrian et al. (1,2). This confirmation was made possible because one of us (K.D.R.S.) was gifted from the curator of the UK Medical Research Council's Steroid Reference Collection (the late Professor D.N. Kirk) the original 4.0 mg sample of equol isolated from pregnant mares urine by Marrian et al in 1932. Equol was also found to occur as a minor constituent in the urine of many adults. The link between equol and soy came about after a series of studies in which different plant-based foods were fed to rats maintained on a purified diet. The introduction of soy protein led to a huge increase in the urinary excretion of equol and following this observation, the soy isoflavone daidzin was isolated and shown to be a precursor to equol (35). It was also found that the introduction of soy protein to the diet led to an increased excretion of equol in some but not all adults (36), whereas in vitro incubation of cultured fecal flora from equol-producing individuals with either daidzein or soy protein resulted in the formation of equol (36). The finding of high concentrations of equol in the urine of adults consuming soy foods prompted the hypothesis that this nonsteroidal estrogen may be beneficial in the prevention and treatment of many hormone-dependent conditions (36).
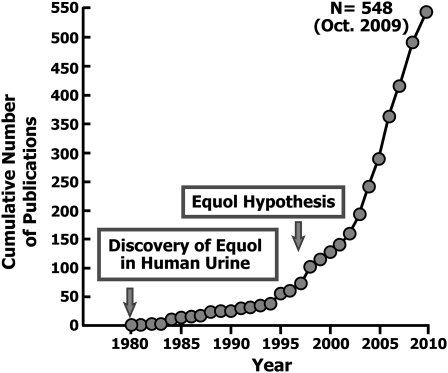
Progress in research studies of equol was hampered by the lack of sufficient amounts of the compound for biological and clinical testing and by the divergent interest and focus on genistein, the other soy-derived isoflavone that was shown to be an potent inhibitor of tyrosine protein kinases (37) and a compound that was readily available in bulk. Almost 20 y after the finding of equol in human urine, it was proposed that the efficiency with which adults convert daidzein to equol when consuming diets containing soy foods could enhance the clinical effectiveness of soy-based diets—the so called equol-hypothesis (24)—and this has driven a resurgence of interest in equol, as is evident from the almost exponential increase since 1980 in the number of publications cited by a PUBMED search of this as a keyword (Fig. 1). At the recent 8th International Soy Symposium held in Tokyo, Japan, for the first time an entire session was devoted to equol (38). This overview will focus on some of the key areas related to this unique molecule and is not intended to be a comprehensive review of the topic.
FIGURE 1 .
Cumulative number of publications on equol by year since its first identification in human urine.
Chemistry
Equol [7-hydroxy-3-(4′-hydroxyphenyl)-chroman], an isoflavan, belongs to the general class of compounds referred to as nonsteroidal estrogens. It has a molecular composition of C15H1403 and a molecular weight of 242.27 Daltons. The heterocyclic structure contains 2 reactive hydroxyls and 1 relatively inert and unreactive oxygen in the central furan ring. Physicochemically, it is nonpolar and relatively insoluble in solution, something that should be considered when conducting in vitro experiments, particularly at high concentrations. It is also extremely acid-labile and can readily be destroyed (>60%) in the general work-up of samples, particularly if acidic hydrolytic steps are used (39). Despite having 2 phenolic rings, it exhibits poor UV absorption characteristics, meaning that HPLC with UV detection is unsuitable for its measurement in most biological fluids. As a result of a chiral carbon at position C-3 of the molecule, equol exists in 2 enantiomeric forms, R-(+)equol and S-(-)equol, and the latter is the natural diastereoisomer produced by intestinal bacteria in the intestine of humans and rats (40). This makes it distinct from its precursor isoflavone, daidzein, and the 2 other major isoflavones of soy, genistein and glycitein (41). Equol can be readily synthesized from daidzein by catalytic hydrogenation, but this yields the (±)equol form (42) and it is the form that has been commercially available and mostly utilized in studies of its biological potency and properties. Indeed, unless otherwise stated, it can be assumed that all previously reported experiments used (±)equol and not the individual enantiomers. It cannot always be assumed that the racemate will behave in an identical manner to that of the individual enantiomers and this was recently shown to be the case for its pharmacokinetics (43). It is probable that biological effects may be underestimated when testing with the racemate and this may particularly hold true for binding affinities to receptors. The racemic mixture can be readily separated by chiral chromatography and the earliest studies used this approach to isolate sufficient amounts of each enantiomer to determine the estrogen binding affinities (40,44) and the pharmacokinetics (43). More recently, methods for the selective synthesis of S-(-)equol (45,46) and R-(+)equol (45) have been described. Methods for the synthesis of [13C]labeled isoflavone analogs (47–50) have been described that can represent suitable starting points for the preparation of stable-labeled [13C]equol for use as tracers in metabolic studies or for internal standards in stable-isotope dilution mass spectrometric assays (43). The synthesis of S-(-)equol and R-(+)equol by chiral chemistry (45) now affords the large-scale production of enantiomeric pure compounds for use in clinical and animal studies. Finally, the production of S-(-)equol from daidzein-rich soy germ by a specific equol-producing bacterium, Lactococcus garvieae (51), offers an alternative biological means to producing specifically S-(-)equol (52). With these breakthroughs, it is now possible to study in some detail the effects of equol in animals and humans and such studies will likely shed further light on the relevance of this metabolite to the clinical outcomes.
Role of intestinal bacteria in the formation of S-(-)Equol
The early evidence in support an intestinal bacterial origin for equol in humans and animals was as follows: 1) germ-free animals fed a soy diet do not excrete equol in urine (30); 2) S-(-)equol is not excreted in the urine or found in the plasma of either newborn infants that lack a developed microflora (53) or in infants up to the age of 4-mo fed exclusively soy infant formula from early life (54,55); 3) incubation of soy, or daidzein with human fecal flora from adults that produce equol, leads to the formation of S-(-)equol (36,40); 4) some antibiotics will knockout the production of equol (18,56,57).
Even though it was known for decades that intestinal bacteria were responsible for the production of S-(-)equol, it is only in recent years that specific bacteria capable of converting daidzin/daidzein to S-(-)equol have been isolated and identified (Table 1). Interestingly, whereas it was reported that 1.59% of injected [14C]genistein was recovered as [14C]equol in domestic fowl (77), metabolic and pharmacokinetic studies in humans administered [13C]daidzein and [13C]genistein tracers show conclusively that in humans, S-(-)equol is formed from daidzein and not genistein (78). Although the major degradative pathway for genistein leads to p-ethylphenol, a phenol first found in goat urine (79) and 4-hydroxyphenyl-2-propionic acid in rats (80), recently it was shown that an anaerobic bacterium from mouse intestine could produce 5-hydroxy-equol from genistein by analogous reactions to those that yield equol from daidzein (66,74). All of these conversions are time dependent and slow, and in humans it takes 12–36 h for the appearance of [13C]equol in plasma after oral administration of [13C]daidzein (78), which is consistent with a colonic origin for its formation.
TABLE 1.
List of intestinal bacteria that cultured in vitro have been found to biotransform isoflavones to S-(-)equol or related intermediates
| Reference | Bacterium strain | Source | Reaction1 |
|---|---|---|---|
| Maruo et al., 2008 (58) | Adlercreutzia equolifaciens | Human | Daidzein → Equol |
| Minamida et al., 2006 (59) | Asaccharobacter celatus AHU1763 | Rat | Daidzein → Equol |
| Minamida et al, 2008 (60) | Asaccharobacter celatus gen,nov.sp. nov strain do03 | Rat | Daidzein → Equol |
| Ueno and Uchiyama, 2002 (61) | Bacteroides ovatus | Human | Daidzein → Equol |
| Tsangalis et al., 2002 (62) | Bifidobacterium | Human | Daidzein → Equol |
| Tsangalis et al., 2002 (62) | Bifidobacterium animalis | Pure culture | Daidzein → Equol |
| Raimondi et al., 2009 (63) | Bifidobacterium sp (22 strains) | Human | Daidzin → Daidzein |
| Hur et al., 2000 (64) | Clostridium sp HGH6 | Bovine | Daidzin → Dihydrodaidzein |
| Tamura et al., 2007 (65) | Clostridium-like bacterium | Human | Daidzin → Dihydrodaidzein |
| Matthies et al., 2008 (66) | Coriobacteriaceae sp MT1B9 | Mouse | Daidzein → Equol |
| Matthies et al., 2008 (66) | Coriobacteriaceae sp MT1B9 | Mouse | Genistein → 5-Hydroxy-equol |
| Wang et al., 2005 (67) | Eggerthella sp Julong 732 | Human | Dihydrodaidzein → Equol |
| Kim et al., 2009 (68) | Eggerthella sp Julong 732 | Human | Dihydrodaidzein → Equol |
| Yokoyama and Suzuki, 2008 (69) | Eggerthella sp YY7918 | Human | Daidzein → Equol |
| Decroos et al., 2005 (70) | Enterococcus faecium | Human | Daidzein → Equol |
| Tamura et al., 2007 (65) | Escherichia coli (HGH21 and HGH6) | Human | Daidzin → Daidzein |
| Yu et al., 2008 (71) | Eubacterium sp D1 and D2 | Pig | Daidzein → Equol |
| Decroos et al., 2005 (70) | Finegoldia magna | Human | Daidzein → Equol |
| Decroos et al., 2005 (70) | Lactobacillus mucosae | Human | Daidzein → Equol |
| Wang et al., 2006 (72) | Lactobacillus sp Niu-O16 | Human | Daidzein → Equol |
| Ishimi et al., 2008 (73) | Lactococcus garvieae (Lc 20-92) | Human | Daidzein → Equol |
| Ueno and Uchiyama, 2002 (61) | Ruminococcus productus | Human | Daidzein → Equol |
| Matthies et al., 2009 (74) | Slackia sp HE8 | Human | Daidzein → Equol |
| Matthies et al., 2009 (74) | Slackia sp HE9 | Human | Genistein → 5-Hydroxy-equol |
| Jin et al., 2009 (75) | Slackia equolifaciens (Strain DZE) | Human | Daidzein → Equol |
| Jin et al., 2008 (76) | Strain PUE | Human | Purarin → Daidzein |
| Ueno and Uchiyama, 2002 (61) | Streptococcus intermedius | Human | Daidzein → Equol |
| Decroos et al., 2005 (70) | Veillonella sp | Human | Daidzein → Equol |
| Decroos et al., 2005 (70) | Mixture of Lactobacillus mucosae EP12, Enterococcus faecium EP11, Finegoldia magna EP13, and Veillonella sp | Human | Daidzein → Equol |
In most cases the definitive structure of the equol product was not determined, but it can be assumed that in all cases the product is the S-(-)equol enantiomer.
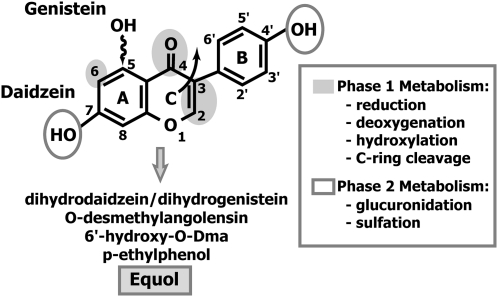
Biotransformations that take place after oral administration of soy isoflavones are summarized in Figure 2. The production of S-(-)equol from daidzin requires 3 key steps. Daidzin first undergoes hydrolysis to split the glucoside moiety and effect release of the bioavailable aglycon, daidzein. This step is crucial to all soy isoflavones, because the conjugated forms (glucosides) do not cross the enterocyte and are consequently not bioavailable (81). Hydrolysis is very efficient and begins in the proximal intestine by the action of brush border membrane β-glucosidases (82). Bacterial β-glucosidases are also capable of performing this hydrolysis and many of the common bacteria that reside in the intestinal tract do this (62,63,83). The efficiency of this initial hydrolytic step by brush border membrane glucosidases is also exemplified by the very high plasma concentrations of daidzein and genistein in infants fed soy infant formula (54,55) that have immature and undeveloped gut microflora (53). Daidzein is reduced to S-(-)equol through the intermediate dihydrodaidzein then converted by deoxygenation to yield S-(-)equol. Daidzein is also metabolized to O-desmethylangolensin as a result of cleavage across the hetercyclic ring (84), but this metabolite appears to be of little interest in that it has no known biological activity.
FIGURE 2 .
Principal metabolic biotransformations of the soy isoflavones daidzein and genistein. The structure for daidzein is shown and genistein has the identical structure but with an additional hydroxyl group at position C-5 of the A-ring, highlighted by the wavy arrow.
Bacteria are enantioselective in metabolizing daidzein to exclusively S-(-)equol and not R-(+)equol (40,67,72). It is not completely clear whether the conversion of daidzein to S-(-)equol is performed by a single bacterium or whether there are distinctly different bacteria that execute these reactions, or both. The large variability in the levels of dihydrodaidzein and S-(-)equol in human urine (85) would indicate that there is more than a single bacterium responsible for producing S-(-)equol. Furthermore, the finding that certain antibiotics selectively inhibit the formation of equol but not dihydrodaidzein when human feces from equol-producers are incubated with daidzein also supports this contention (56). The list of intestinal bacteria that can produce equol in culture is ever increasing (Table 1); a number of strains have been isolated that perform only conversion of daidzin/daidzein to dihydrodaidzein whereas others appear to be able to completely convert daidzein to equol (Table 1). One in particular, Lactococcus garvieae, that is found in some Italian cheeses (51) and has been isolated from human feces (61,73) was shown to efficiently convert daidzin to S-(-)equol and has been used to produce the first natural S-(-)equol–containing nutraceutical (52,86). Whether demonstrating formation of S-(-)equol in culture can be considered representative of intraluminal colonic formation of S-(-)equol remains uncertain. It may, e.g. under specific conditions be possible to demonstrate conversion of daidzein to equol in vitro, yet such conditions may not necessarily be reflective of the intraluminal milleu in the human intestinal tract. For example, culturing human fecal flora from equol-producers under conditions that enhance fermentation, such as a high nonstarch polysaccharide medium, enhances conversion of daidzein to equol (87), as do hydrogen gas, butyrate, and proprionate (59,70). Despite the ability to manipulate equol production in vitro, human dietary intervention studies using prebiotics or probiotics have had little impact on its formation (88–92). Therefore, some caution is required in relying on in vitro fecal cultures from adults as a means of confirming equol-producer status (93). Such approaches should always be coupled with specific measurement of equol in urine (94,95). In the future, it is likely that molecular techniques in bacteriology may help to more accurately define equol-producer status.
Frequency of equol-producers
All animal species tested produce equol in response to consumption of isoflavones whether from soy protein or clover (16,23,24). Rodents in particular have very high plasma S-(-)equol concentrations, in part because most commercial rodent diets are formulated with soy protein (20,96). For this reason, careful consideration is needed when using rodents, particularly if the primary endpoints being examined are in any way influenced by estrogens, either directly or indirectly, through upstream signaling pathways with estrogen response elements (20,97–99). Frequently, there is a lack of awareness on the part of investigators of the type and composition of the diets being used by their animal facilities or by the vendors providing rodents for research studies. A high batch-to-batch variability in the isoflavone content of commercial rodent unpurified diet makes it impossible to control for the background level of these phytoestrogens (97).
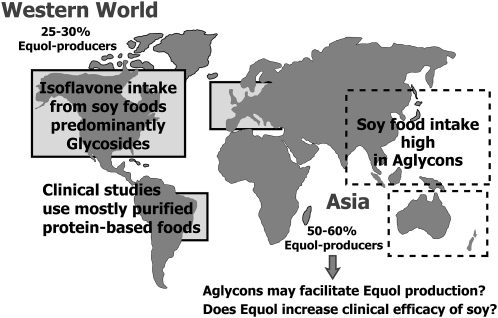
When the association between soy isoflavones and equol was first made, it was noted that not all healthy adults produced equol when challenged with soy protein (36). The first reported dietary intervention study of the 6 healthy adults fed 40 g of textured vegetable protein for 7 consecutive days found only 4/6 excreted equol in urine; the term equol-producers was thus coined. Many other studies of larger sample sizes have provided a consensus that only 25–30% of the adult population of Western countries produce S-(-)equol when fed soy foods containing isoflavones (94,100,101). This is significantly lower than the reported 50–60% frequency of equol-producers in adults from Japan, Korea, or China (102–105) or in Western adult vegetarians (95). The reasons for these differences are unclear but are important to understand if the hypothesis that the ability to produce equol when consuming soy foods (equol hypothesis) is advantageous in terms of enhancing health benefits can be clearly demonstrated (24,106). Thus far, the data are inconclusive, but this is partly because, to our knowledge, none of the clinical studies have preselected participants on the basis of equol-producer status but rather have retrospectively subanalyzed data from equol-producers, and most are underpowered. Significant differences in gene expression between equol-producers and equol nonproducers have been demonstrated in postmenopausal women exposed to an isoflavone supplement, with the most notably significant alterations in expression of a number of estrogen-responsive genes (107).
It would appear that equol-producer status is a relatively stable phenomenon, as evidenced from repeat testing over prolonged periods of time in adults (24,89,95,108). There have been many studies looking at associations between equol-producer status and dietary components, including fat and carbohydrate composition (93,101), PUFA (109), dairy intakes (110), lactose (111), green tea consumption (112), seaweed (113), and soy food intake (103,105,110), but no clear conclusions can be made. Prolonged soy food consumption appears not to be a factor driving equol formation (114). One possible explanation for the differences in the frequency of equol-producers among populations, we speculate, may be related to the type of soy foods consumed (115). There are marked differences in the isoflavone composition of Western and Asian soy foods (Fig. 3) (41). Asians consume a high proportion of isoflavone aglycons, because fermented soy foods account for about one-third of the total intake of soy foods (116,117). Based upon the typical 20–50 mg/d intake of total isoflavones by Asians, we estimate that 10–30 mg are ingested in the form of aglycons, which in most adults are absorbed faster than glycosides (43,102,118–121) and may be more easily converted to equol than glycosides. In a recent cholesterol-lowering dietary intervention study of a soy germ-enriched pasta containing predominantly isoflavones in the aglycon form, it was found that 69% of the patients were equol-producers (115), which is considerably higher than the 20–30% expected frequency for Westerners (95). Most dietary intervention studies of soy have used foods or products made from isolated soy proteins as the source of isoflavones (122) or soy isoflavone supplements that contain almost exclusively isoflavone glucosides (119). Isolated soy proteins are not commonly consumed in the Asian diet and this may explain the lower frequency of equol-producers in Western adults. Furthermore, if equol production does enhance the efficacy of soy foods (24,106), then this could explain the variable results obtained from recent studies of soy foods. Future studies of foods or supplements containing predominantly isoflavone aglycons may be more meaningful and should be considered as more closely mimicking the isoflavone composition of a typical Asian diet (115).
FIGURE 3 .
Differences in the type of soy foods consumed by Western and Asian populations may account for differences in the frequency of equol-producers.
Although the type of soy foods consumed may influence equol production, the overriding factors governing equol production remain: 1) the requirement of specific equol-producing bacteria; 2) the presence of a source of substrate(s), which for soy is daidzin or daidzein; and 3) having optimum intraluminal conditions in terms of redox potential for the reactions to take place. Clearly, if the bacteria are lacking, then it is not possible to produce equol, but the equol-producing bacteria may also be present but inactive. It is only possible to be an equol-producer when consuming soy foods or products containing isoflavones. The very low urinary or plasma equol concentrations reported in many large population-based studies of Westerners (123–127) are merely an indication of the fact that soy foods are not consumed on a regular basis, rather than a reflection of the true frequency of equol-producers in these populations. Therefore, to tease out an equol-producer, it is crucial to challenge with sufficient amounts of substrate for conversion to equol and it is necessary to allow sufficient transit time for the substrate to reach the distal gastrointestinal tract and colon where the bacterial reactions take place. These practical considerations have been discussed in detail previously (94,95). The standard approach is to feed a soy isoflavone-containing food, such as soymilk, or an isoflavone supplement, such as soy germ, that is preferentially high in daidzein (128) for 3 consecutive days to attain a steady state (94,95,108,129). Urine is then collected and daidzein and S-(-)equol measured. We recently opted to define an equol-producer based on the ratio of equol:daidzein (95), because this reflects the product/precursor relationship and overcomes the drawbacks of using absolute urinary (or plasma) equol concentrations (36,94,101,130), which are subject to considerable variation due to inter-individual differences in isoflavone pharmacokinetics (78,102,119,120,131–133). Defining an equol-producer by the equol:daidzein ratio also circumvents the need for accurately timed 24-h urine collections.
Acknowledgments
This manuscript was written and approved by both authors.
Published in a supplement to The Journal of Nutrition. Presented at the “Equol, Soy, and Menopause Research Leadership Conference”, held in Washington, DC, June 16, 2009. The supplement coordinator for this supplement is Kara Lewis, Life Sciences Research Organization (LSRO) Senior Staff Scientist. The supplement is the responsibility of the guest editors to whom the Editor of The Journal of Nutrition has delegated supervision of both technical conformity to the published regulations of The Journal of Nutrition and general oversight of the scientific merit of each article. Publication costs for this supplement were defrayed in part by the payment of page charges. This publication must therefore be hereby marked “advertisement” in accordance with 18 USC section 1734 solely to indicate this fact. The Guest Editor for this supplement is Neil Shay. Guest Editor disclosure: Neil Shay declares no conflict of interest. Supplement Coordinator disclosure: Kara Lewis is currently under contract with and receives compensation from the supplement sponsor. She was also compensated for attending and organizing the Equol, Soy, and Menopause Research Leadership Conference and for organizing, writing, editing, or reviewing, and collection of supplemental manuscripts. The opinions expressed in this publication are those of the authors and are not attributable to the sponsors or the publisher, Editor, or Editorial Board of The Journal of Nutrition.
Author disclosures K. D. R. Setchell was supported by funding from the NIH (grant nos. R01AT-003313 and R01AT-002190) and has intellectual property on equol enantiomers, including patents licensed by Cincinnati Children's Hospital Medical Center, Cincinnati, OH, to industry and is a consultant to Otsuka Pharmaceuticals Company, Tokyo, Japan. C. Clerici, no conflict of interest.
Abbreviations used: BBM, brush border membrane; ER, estrogen receptor; ISP, isolated soy proteins; RMB, relative molar binding.
References
- 1.Marrian GF, Haslewood GA. Equol, a new inactive phenol isolated from the ketohydroxyoestrin fraction of mares' urine. Biochem J. 1932;26:1227–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Marrian GF, Beall D. The constitution of equol. Biochem J. 1935;29:1586–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bennetts HW, Underwood EJ, Shier FL. A specific breeding problem of sheep on subterranean clover pastures in Western Australia. Aust J Agric Res. 1946;22:131–8. [DOI] [PubMed] [Google Scholar]
- 4.Lightfoot RJ, Croker KP, Neil HG. Failure of sperm transport in relation to ewe infertility following prolonged grazing on oestrogenic pastures. Aust J Agric Res. 1968;18:755–65. [Google Scholar]
- 5.Biggers JD, Curnow DH. Oestrogenic activity of subterranean clover. I. The oestrogenic activity of genistein. Biochem J. 1954;58:278–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pope GS. The importance of pasture plant oestrogens in the reproduction and lactation of grazing animals. Dairy Sci Abstr. 1954;16:334–55. [Google Scholar]
- 7.Braden A, Hart N, Lamberton J. The estrogenic activity and metabolism of certain isoflavones in sheep. Aust J Agric Res. 1967;18:335–48. [Google Scholar]
- 8.Nottle MC. Composition of some urinary calculi of ruminants in Western Australia. Res Vet Sci. 1976;21:309–17. [PubMed] [Google Scholar]
- 9.Klyne W, Wright AA. Steroids and other lipids of pregnant cow's urine. J Endocrinol. 1959;18:32–45. [DOI] [PubMed] [Google Scholar]
- 10.Common RH, Ainsworth L. Identification of equol in the urine of the domestic fowl. Biochim Biophys Acta. 1961;53:403–4. [DOI] [PubMed] [Google Scholar]
- 11.Chang HH, Robinson AR, Common RH. Excretion of radioactive diadzein and equol as monosulfates and disulfates in the urine of the laying hen. Can J Biochem. 1975;53:223–30. [DOI] [PubMed] [Google Scholar]
- 12.Chang HH, Robinson AR, Chan AH, Common RH. Radioactive conversion products of intramuscularly injected [4-14C]formononetin including sulfates in the urine of hens. Can J Biochem. 1977;55:50–5. [DOI] [PubMed] [Google Scholar]
- 13.Saitoh S, Sato T, Harada H, Matsuda T. Biotransformation of soy isoflavone-glycosides in laying hens: intestinal absorption and preferential accumulation into egg yolk of equol, a more estrogenic metabolite of daidzein. Biochim Biophys Acta. 2004;1674:122–30. [DOI] [PubMed] [Google Scholar]
- 14.Saitoh S, Sato T, Harada H, Takita T. Transfer of soy isoflavone into the egg yolk of chickens. Biosci Biotechnol Biochem. 2001;65:2220–5. [DOI] [PubMed] [Google Scholar]
- 15.Molteni A, Brizio-Molteni L, Persky V. In vitro hormonal effects of soybean isoflavones. J Nutr. 1995;125:S751–6. [DOI] [PubMed] [Google Scholar]
- 16.Gu L, House SE, Prior RL, Fang N, Ronis MJ, Clarkson TB, Wilson ME, Badger TM. Metabolic phenotype of isoflavones differ among female rats, pigs, monkeys, and women. J Nutr. 2006;136:1215–21. [DOI] [PubMed] [Google Scholar]
- 17.Adlercreutz H, Musey PI, Fotsis T, Bannwart C, Wahala K, Makela T, Brunow G, Hase T. Identification of lignans and phytoestrogens in urine of chimpanzees. Clin Chim Acta. 1986;158:147–54. [DOI] [PubMed] [Google Scholar]
- 18.Blair RM, Appt SE, Franke AA, Clarkson TB. Treatment with antibiotics reduces plasma equol concentration in cynomolgus monkeys (Macaca fascicularis). J Nutr. 2003;133:2262–7. [DOI] [PubMed] [Google Scholar]
- 19.Juniewicz PE, Pallante MS, Moser A, Ewing LL. Identification of phytoestrogens in the urine of male dogs. J Steroid Biochem. 1988;31:987–94. [DOI] [PubMed] [Google Scholar]
- 20.Brown NM, Setchell KDR. Animal models impacted by phytoestrogens in commercial chow: implications for pathways influenced by hormones. Lab Invest. 2001;81:735–47. [DOI] [PubMed] [Google Scholar]
- 21.Nilsson A. Demethylation of the plant oestrogen biochanin A in the rat. Nature. 1961;192:358. [DOI] [PubMed] [Google Scholar]
- 22.Axelson M, Sjovall J, Gustafsson BE, Setchell KDR. Origin of lignans in mammals and identification of a precursor from plants. Nature. 1982;298:659–60. [DOI] [PubMed] [Google Scholar]
- 23.Lundh T. Metabolism of estrogenic isoflavones in domestic animals. Proc Soc Exp Biol Med. 1995;208:33–9. [DOI] [PubMed] [Google Scholar]
- 24.Setchell KDR, Brown NM, Lydeking-Olsen E. The clinical importance of the metabolite equol-a clue to the effectiveness of soy and its isoflavones. J Nutr. 2002;132:3577–84. [DOI] [PubMed] [Google Scholar]
- 25.Shutt D, Braden A. The significance of equol in relation to the oestrogenic responses in sheep ingesting clover with a high formononetin content. Aust J Agric Res. 1968;19:545–53. [Google Scholar]
- 26.Lindsay DR, Kelly RW. The metabolism of phyto-oestrogens in sheep. Aust Vet J. 1970;46:219–22. [DOI] [PubMed] [Google Scholar]
- 27.Shutt DA, Cox RI. Steroid and phyto-oestrogen binding to sheep uterine receptors in vitro. J Endocrinol. 1972;52:299–310. [DOI] [PubMed] [Google Scholar]
- 28.Shutt DA. The effects of plant oestrogens on animal reproduction. Endeavour. 1976;35:110–3. [DOI] [PubMed] [Google Scholar]
- 29.Jensen EV. On the mechanism of estrogen action. Perspect Biol Med. 1962;6:47–59. [DOI] [PubMed] [Google Scholar]
- 30.Axelson M, Setchell KDR. The excretion of lignans in rats: evidence for an intestinal bacterial source for this new group of compounds. FEBS Lett. 1981;123:337–42. [DOI] [PubMed] [Google Scholar]
- 31.Setchell KDR, Lawson AM, Mitchell FL, Adlercreutz H, Kirk DN, Axelson M. Lignans in man and in animal species. Nature. 1980;287:740–2. [DOI] [PubMed] [Google Scholar]
- 32.Setchell KDR, Lawson AM, Conway E, Taylor NF, Kirk DN, Cooley G, Farrant RD, Wynn S, Axelson M. The definitive identification of the lignans trans-2,3-bis (3-hydroxybenzyl)-gamma-butyrolactone and 2,3-bis(3-hydroxybenzyl)butane-1,4-diol in human and animal urine. Biochem J. 1981;197:447–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Setchell KDR, Lawson AM, Borriello SP, Harkness R, Gordon H, Morgan DM, Kirk DN, Adlercreatz H, Anderson LC, et al. Lignan formation in man: microbial involvement and possible roles in relation to cancer. Lancet. 1981;2:4–7. [DOI] [PubMed] [Google Scholar]
- 34.Axelson M, Setchell KDR. Conjugation of lignans in human urine. FEBS Lett. 1980;122:49–53. [DOI] [PubMed] [Google Scholar]
- 35.Axelson M, Kirk DN, Farrant RD, Cooley G, Lawson AM, Setchell KDR. The identification of the weak oestrogen equol [7-hydroxy-3- (4′-hydroxyphenyl)chroman] in human urine. Biochem J. 1982;201:353–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Setchell KDR, Borriello SP, Hulme P, Kirk DN, Axelson M. Nonsteroidal estrogens of dietary origin: possible roles in hormone-dependent disease. Am J Clin Nutr. 1984;40:569–78. [DOI] [PubMed] [Google Scholar]
- 37.Akiyama T, Ishida J, Nakagawa S, Ogawara H, Watanabe S, Itoh N, Shibuya M, Fukami Y. Genistein, a specific inhibitor of tyrosine-specific protein kinases. J Biol Chem. 1987;262:5592–5. [PubMed] [Google Scholar]
- 38.Messina M, Watanabe S, Setchell KDR. Report on the 8th International Symposium on the Role of Soy in Health Promotion and Chronic Disease Prevention and Treatment. J Nutr. 2009;139:S796–802. [DOI] [PubMed] [Google Scholar]
- 39.Chang HS, Robinson AR, Common RH. Lability of equol to acidic hydrolysis procedures. Anal Biochem. 1975;63:290–2. [DOI] [PubMed] [Google Scholar]
- 40.Setchell KDR, Clerici C, Lephart ED, Cole SJ, Heenan C, Castellani D, Wolfe BE, Nechemias-Zimmer L, Brown NM, et al. S-equol, a potent ligand for estrogen receptor beta, is the exclusive enantiomeric form of the soy isoflavone metabolite produced by human intestinal bacterial flora. Am J Clin Nutr. 2005;81:1072–9. [DOI] [PubMed] [Google Scholar]
- 41.Coward L, Barnes N, Setchell KDR, Barnes S. Genistein and daidzein, and their β-glycosides conjugates: anti-tumour isoflavones in soybean foods from American and Asian diets. J Agric Food Chem. 1993;41:1961–7. [Google Scholar]
- 42.Lamberton JA, Suares H, Watson KG. Catalytic hydrogenation of isoflavones. The preparation of (±)equol and related isoflavones. Aust J Chem. 1978;31:455–7. [Google Scholar]
- 43.Setchell KDR, Zhao X, Jha P, Heubi JE, Brown NM. The pharmacokinetic behavior of the soy isoflavone metabolite S- (-)equol and its diastereoisomer R-(+)equol in healthy adults determined by using stable-isotope-labeled tracers. Am J Clin Nutr. 2009;90:1029–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Muthyala RS, Ju YH, Sheng S, Williams LD, Doerge DR, Katzenellenbogen BS, Helferich WG, Katzenellenbogen JA. Equol, a natural estrogenic metabolite from soy isoflavones: convenient preparation and resolution of R- and S-equols and their differing binding and biological activity through estrogen receptors alpha and beta. Bioorg Med Chem. 2004;12:1559–67. [DOI] [PubMed] [Google Scholar]
- 45.Setchell KDR, Sirokin V, Girindus America Inc., Children's Hospital Medical Center, assignees. Method for enantioselective hydrogenation of chromenes. United States patent US 7528267. 2009. Nov 5.
- 46.Heemstra JM, Kerrigan SA, Doerge DR, Helferich WG, Boulanger WA. Total synthesis of (S)-equol. Org Lett. 2006;8:5441–3. [DOI] [PubMed] [Google Scholar]
- 47.Wahala K, Hase T, Adlercreutz H. Synthesis and labeling of isoflavone phytoestrogens, including daidzein and genistein. Proc Soc Exp Biol Med. 1995;208:27–32. [DOI] [PubMed] [Google Scholar]
- 48.Whalley JL, Bond TJ, Botting NP. Synthesis of 13C labelled daidzein and formononetin. Bioorg Med Chem Lett. 1998;8:2569–72. [DOI] [PubMed] [Google Scholar]
- 49.Baraldi PG, Spalluto G, Cacciari B, Romagnoli R, Setchell KDR. Chemical synthesis of [13C]daidzein. J Med Food. 1999;2:99–102. [DOI] [PubMed] [Google Scholar]
- 50.Whalley JL, Oldfield MF, Botting NP. Synthesis of [4-13C]-isoflavonoid phytoestrogens. Tetrahedron. 2000;56:455–60. [Google Scholar]
- 51.Fortina MG, Ricci G, Foschino R, Picozzi C, Dolci P, Zeppa G, Cocolin L, Manachini PL. Phenotypic typing, technological properties and safety aspects of Lactococcus garvieae strains from dairy environments. J Appl Microbiol. 2007;103:445–53. [DOI] [PubMed] [Google Scholar]
- 52.Yee S, Burdock GA, Kurata Y, Enomoto Y, Narumi K, Hamada S, Itoh T, Shimomura Y, Ueno T. Acute and subchronic toxicity and genotoxicity of SE5-OH, an equol-rich product produced by Lactococcus garvieae. Food Chem Toxicol. 2008;46:2713–20. [DOI] [PubMed] [Google Scholar]
- 53.Rotimi VO, Duerden BI. The development of the bacterial flora in normal neonates. J Med Microbiol. 1981;14:51–62. [DOI] [PubMed] [Google Scholar]
- 54.Setchell KDR, Zimmer-Nechemias L, Cai J, Heubi JE. Exposure of infants to phyto-oestrogens from soy-based infant formula. Lancet. 1997;350:23–7. [DOI] [PubMed] [Google Scholar]
- 55.Setchell KDR, Zimmer-Nechemias L, Cai J, Heubi JE. Isoflavone content of infant formulas and the metabolic fate of these phytoestrogens in early life. Am J Clin Nutr. 1998;68:S1453–61. [DOI] [PubMed] [Google Scholar]
- 56.Atkinson C, Berman S, Humbert O, Lampe JW. In vitro incubation of human feces with daidzein and antibiotics suggests interindividual differences in the bacteria responsible for equol production. J Nutr. 2004;134:596–9. [DOI] [PubMed] [Google Scholar]
- 57.Halm BM, Franke AA, Ashburn LA, Hebshi SM, Wilkens LR. Oral antibiotics decrease urinary isoflavonoid excretion in children after soy consumption. Nutr Cancer. 2008;60:14–22. [DOI] [PubMed] [Google Scholar]
- 58.Maruo T, Sakamoto M, Ito C, Toda T, Benno Y. Adlercreutzia equolifaciens gen. nov., sp. nov., an equol-producing bacterium isolated from human faeces, and emended description of the genus Eggerthella. Int J Syst Evol Microbiol. 2008;58:1221–7. [DOI] [PubMed] [Google Scholar]
- 59.Minamida K, Tanaka M, Abe A, Sone T, Tomita F, Hara H, Asano K. Production of equol from daidzein by gram-positive rod-shaped bacterium isolated from rat intestine. J Biosci Bioeng. 2006;102:247–50. [DOI] [PubMed] [Google Scholar]
- 60.Minamida K, Ota K, Nishimukai M, Tanaka M, Abe A, Sone T, Tomita F, Hara H, Asano K. Asaccharobacter celatus gen. nov., sp. nov., isolated from rat caecum. Int J Syst Evol Microbiol. 2008;58:1238–40. [DOI] [PubMed] [Google Scholar]
- 61.Ueno T, Uchiyama S. Identification of the specific intestinal bacteria capable of metabolising soy isoflavone to equol. Ann Nutr Metab. 2002;45:114. [Google Scholar]
- 62.Tsangalis D, Ashton JF, McGill AEJ, Shah NP. Enzymic transformation of isoflavone phytoestrogens in soymilk by β-glucosidase-producing Bifidobacteria. J Food Sci. 2002;67:3104–13. [Google Scholar]
- 63.Raimondi S, Roncaglia L, De Lucia M, Amaretti A, Leonardi A, Pagnoni UM, Rossi M. Bioconversion of soy isoflavones daidzin and daidzein by Bifidobacterium strains. Appl Microbiol Biotechnol. 2009;81:943–50. [DOI] [PubMed] [Google Scholar]
- 64.Hur HG, Lay JO Jr, Beger RD, Freeman JP, Rafii F. Isolation of human intestinal bacteria metabolizing the natural isoflavone glycosides daidzin and genistin. Arch Microbiol. 2000;174:422–8. [DOI] [PubMed] [Google Scholar]
- 65.Tamura M, Tsushida T, Shinohara K. Isolation of an isoflavone-metabolizing, Clostridium-like bacterium, strain TM-40, from human faeces. Anaerobe. 2007;13:32–5. [DOI] [PubMed] [Google Scholar]
- 66.Matthies A, Clavel T, Gutschow M, Engst W, Haller D, Blaut M, Braune A. Conversion of daidzein and genistein by an anaerobic bacterium newly isolated from the mouse intestine. Appl Environ Microbiol. 2008;74:4847–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Wang XL, Hur HG, Lee JH, Kim KT, Kim SI. Enantioselective synthesis of S-equol from dihydrodaidzein by a newly isolated anaerobic human intestinal bacterium. Appl Environ Microbiol. 2005;71:214–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kim M, Kim SI, Han J, Wang XL, Song DG, Kim SU. Stereospecific biotransformation of dihydrodaidzein into (3S)-equol by the human intestinal bacterium Eggerthella strain Julong 732. Appl Environ Microbiol. 2009;75:3062–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Yokoyama S, Suzuki T. Isolation and characterization of a novel equol-producing bacterium from human feces. Biosci Biotechnol Biochem. 2008;72:2660–6. [DOI] [PubMed] [Google Scholar]
- 70.Decroos K, Vanhemmens S, Cattoir S, Boon N, Verstraete W. Isolation and characterisation of an equol-producing mixed microbial culture from a human faecal sample and its activity under gastrointestinal conditions. Arch Microbiol. 2005;183:45–55. [DOI] [PubMed] [Google Scholar]
- 71.Yu ZT, Yao W, Zhu WY. Isolation and identification of equol-producing bacterial strains from cultures of pig faeces. FEMS Microbiol Lett. 2008;282:73–80. [DOI] [PubMed] [Google Scholar]
- 72.Wang XL, Kim HJ, Kang SI, Kim SI, Hur HG. Production of phytoestrogen S-equol from daidzein in mixed culture of two anaerobic bacteria. Arch Microbiol. 2007;187:155–60. [DOI] [PubMed] [Google Scholar]
- 73.Ishimi Y, Oka J, Tabata I, Ohtomo T, Ezaki J, Ueno T, Uchiyama S, Toda T, Uehara M, et al. Effects of soybean isoflavones on bone health and its safety in post-menopausal Japanese women. J Clin Biochem Nutr. 2008;43 (Suppl 1):48–52. [Google Scholar]
- 74.Matthies A, Blaut M, Braune A. Isolation of a human intestinal bacterium capable of daidzein and genistein conversion. Appl Environ Microbiol. 2009;75:1740–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Jin JS, Kitahara M, Sakamoto M, Hattori M, Benno Y. Slackia equolifaciens sp. nov., a human intestinal bacterium capable of producing equol. Int J Syst Evol Microbiol. Epub 2009. Sep 4. [DOI] [PubMed]
- 76.Jin JS, Nishihata T, Kakiuchi N, Hattori M. Biotransformation of C-glucosylisoflavone puerarin to estrogenic (3S)-equol in co-culture of two human intestinal bacteria. Biol Pharm Bull. 2008;31:1621–5. [DOI] [PubMed] [Google Scholar]
- 77.Cayen MN, Carter AL, Common RH. The conversion of genistein to equol in the fowl. Biochim Biophys Acta. 1964;86:56–64. [DOI] [PubMed] [Google Scholar]
- 78.Setchell KDR, Faughnan MS, Avades T, Zimmer-Nechemias L, Brown NM, Wolfe BE, Brashear WT, Desai P, Oldfield MF, et al. Comparing the pharmacokinetics of daidzein and genistein with the use of 13C-labeled tracers in premenopausal women. Am J Clin Nutr. 2003;77:411–9. [DOI] [PubMed] [Google Scholar]
- 79.Grant JK. p-Ethylphenylsulphuric acid in goat urine. Biochem J. 1948;43:523–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Coldham NG, Howells LC, Santi A, Montesissa C, Langlais C, King LJ, Macpherson DD, Sauer MJ. Biotransformation of genistein in the rat: elucidation of metabolite structure by product ion mass fragmentology. J Steroid Biochem Mol Biol. 1999;70:169–84. [DOI] [PubMed] [Google Scholar]
- 81.Setchell KDR, Brown NM, Zimmer-Nechemias L, Brashear WT, Wolfe BE, Kirschner AS, Heubi JE. Evidence for lack of absorption of soy isoflavone glycosides in humans, supporting the crucial role of intestinal metabolism for bioavailability. Am J Clin Nutr. 2002;76:447–53. [DOI] [PubMed] [Google Scholar]
- 82.Day AJ, DuPont MS, Ridley S, Rhodes M, Rhodes MJ, Morgan MR, Williamson G. Deglycosylation of flavonoid and isoflavonoid glycosides by human small intestine and liver beta-glucosidase activity. FEBS Lett. 1998;436:71–5. [DOI] [PubMed] [Google Scholar]
- 83.Steer TE, Johnson IT, Gee JM, Gibson GR. Metabolism of the soybean isoflavone glycoside genistin in vitro by human gut bacteria and the effect of prebiotics. Br J Nutr. 2003;90:635–42. [DOI] [PubMed] [Google Scholar]
- 84.Bannwart C, Adlercreutz H, Fotsis T, Wahala K, Hase T, Brunow G. Identification of O-desmethylangolensin, a metabolite of daidzein and of matairesinol, one likely plant precursor of the animal lignan enterolactone in human urine. Finn Chem Lett. 1984;120–5.
- 85.Kelly GE, Joannou GE, Reeder AY, Nelson C, Waring MA. The variable metabolic response to dietary isoflavones in humans. Proc Soc Exp Biol Med. 1995;208:40–3. [DOI] [PubMed] [Google Scholar]
- 86.Ishiwata N, Melby MK, Mizuno S, Watanabe S. New equol supplement for relieving menopausal symptoms: randomized, placebo-controlled trial of Japanese women. Menopause. 2009;16:141–8. [DOI] [PubMed] [Google Scholar]
- 87.Setchell KDR, Cassidy A. Dietary isoflavones: biological effects and relevance to human health. J Nutr. 1999;129:S758–67. [DOI] [PubMed] [Google Scholar]
- 88.Uehara M, Ohta A, Sakai K, Suzuki K, Watanabe S, Adlercreutz H. Dietary fructooligosaccharides modify intestinal bioavailability of a single dose of genistein and daidzein and affect their urinary excretion and kinetics in blood of rats. J Nutr. 2001;131:787–95. [DOI] [PubMed] [Google Scholar]
- 89.Lampe JW, Skor HE, Li S, Wahala K, Howald WN, Chen C. Wheat bran and soy protein feeding do not alter urinary excretion of the isoflavan equol in premenopausal women. J Nutr. 2001;131:740–4. [DOI] [PubMed] [Google Scholar]
- 90.Nettleton JA, Greany KA, Thomas W, Wangen KE, Adlercreutz H, Kurzer MS. Plasma phytoestrogens are not altered by probiotic consumption in postmenopausal women with and without a history of breast cancer. J Nutr. 2004;134:1998–2003. [DOI] [PubMed] [Google Scholar]
- 91.Greany KA, Nettleton JA, Wangen KE, Thomas W, Kurzer MS. Probiotic consumption does not enhance the cholesterol-lowering effect of soy in postmenopausal women. J Nutr. 2004;134:3277–83. [DOI] [PubMed] [Google Scholar]
- 92.Bonorden MJ, Greany KA, Wangen KE, Phipps WR, Feirtag J, Adlercreutz H, Kurzer MS. Consumption of Lactobacillus acidophilus and Bifidobacterium longum do not alter urinary equol excretion and plasma reproductive hormones in premenopausal women. Eur J Clin Nutr. 2004;58:1635–42. [DOI] [PubMed] [Google Scholar]
- 93.Gardana C, Canzi E, Simonetti P. The role of diet in the metabolism of daidzein by human faecal microbiota sampled from Italian volunteers. J Nutr Biochem. 2009;20:940–7. [DOI] [PubMed] [Google Scholar]
- 94.Lampe JW, Karr SC, Hutchins AM, Slavin JL. Urinary equol excretion with a soy challenge: influence of habitual diet. Proc Soc Exp Biol Med. 1998;217:335–9. [DOI] [PubMed] [Google Scholar]
- 95.Setchell KDR, Cole SJ. Method of defining equol-producer status and its frequency among vegetarians. J Nutr. 2006;136:2188–93. [DOI] [PubMed] [Google Scholar]
- 96.Thigpen JE, Setchell KDR, Ahlmark KB, Locklear J, Spahr T, Caviness GF, Goelz MF, Haseman JK, Newbold RR, et al. Phytoestrogen content of purified, open- and closed-formula laboratory animal diets. Lab Anim Sci. 1999;49:530–6. [PubMed] [Google Scholar]
- 97.Thigpen JE, Setchell KDR, Padilla-Banks E, Haseman JK, Saunders HE, Caviness GF, Kissling GE, Grant MG, Forsythe DB. Variations in phytoestrogen content between different mill dates of the same diet produces significant differences in the time of vaginal opening in CD-1 mice and F344 rats but not in CD Sprague-Dawley rats. Environ Health Perspect. 2007;115:1717–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Thigpen JE, Haseman JK, Saunders HE, Setchell KDR, Grant MG, Forsythe DB. Dietary phytoestrogens accelerate the time of vaginal opening in immature CD-1 mice. Comp Med. 2003;53:607–15. [PubMed] [Google Scholar]
- 99.Thigpen JE, Setchell KDR, Saunders HE, Haseman JK, Grant MG, Forsythe DB. Selecting the appropriate rodent diet for endocrine disruptor research and testing studies. ILAR J. 2004;45:401–16. [DOI] [PubMed] [Google Scholar]
- 100.Atkinson C, Frankenfeld CL, Lampe JW. Gut bacterial metabolism of the soy isoflavone daidzein: exploring the relevance to human health. Exp Biol Med (Maywood). 2005;230:155–70. [DOI] [PubMed] [Google Scholar]
- 101.Rowland IR, Wiseman H, Sanders TA, Adlercreutz H, Bowey EA. Interindividual variation in metabolism of soy isoflavones and lignans: influence of habitual diet on equol production by the gut microflora. Nutr Cancer. 2000;36:27–32. [DOI] [PubMed] [Google Scholar]
- 102.Watanabe S, Yamaguchi M, Sobue T, Takahashi T, Miura T, Arai Y, Mazur W, Wahala K, Adlercreutz H. Pharmacokinetics of soybean isoflavones in plasma, urine and feces of men after ingestion of 60 g baked soybean powder (kinako). J Nutr. 1998;128:1710–5. [DOI] [PubMed] [Google Scholar]
- 103.Arai Y, Uehara M, Sato Y, Kimira M, Eboshida A, Adlercreutz H, Watanabe S. Comparison of isoflavones among dietary intake, plasma concentration and urinary excretion for accurate estimation of phytoestrogen intake. J Epidemiol. 2000;10:127–35. [DOI] [PubMed] [Google Scholar]
- 104.Akaza H, Miyanaga N, Takashima N, Naito S, Hirao Y, Tsukamoto T, Fujioka T, Mori M, Kim WJ, et al. Comparisons of percent equol producers between prostate cancer patients and controls: case-controlled studies of isoflavones in Japanese, Korean and American residents. Jpn J Clin Oncol. 2004;34:86–9. [DOI] [PubMed] [Google Scholar]
- 105.Song KB, Atkinson C, Frankenfeld CL, Jokela T, Wahala K, Thomas WK, Lampe JW. Prevalence of daidzein-metabolizing phenotypes differs between Caucasian and Korean American women and girls. J Nutr. 2006;136:1347–51. [DOI] [PubMed] [Google Scholar]
- 106.Lampe JW. Is equol the key to the efficacy of soy foods? Am J Clin Nutr. 2009;89:S1664–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Niculescu MD, Pop EA, Fischer LM, Zeisel SH. Dietary isoflavones differentially induce gene expression changes in lymphocytes from postmenopausal women who form equol as compared with those who do not. J Nutr Biochem. 2007;18:380–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Frankenfeld CL, Atkinson C, Thomas WK, Goode EL, Gonzalez A, Jokela T, Wahala K, Schwartz SM, Li SS, Lampe JW. Familial correlations, segregation analysis, and nongenetic correlates of soy isoflavone-metabolizing phenotypes. Exp Biol Med (Maywood). 2004;229:902–13. [DOI] [PubMed] [Google Scholar]
- 109.Bolca S, Possemiers S, Herregat A, Huybrechts I, Heyerick A, De Vriese S, Verbruggen M, Depypere H, De Keukeleire D, et al. Microbial and dietary factors are associated with the equol producer phenotype in healthy postmenopausal women. J Nutr. 2007;137:2242–6. [DOI] [PubMed] [Google Scholar]
- 110.Nagata C, Ueno T, Uchiyama S, Nagao Y, Yamamoto S, Shibuya C, Kashiki Y, Shimizu H. Dietary and lifestyle correlates of urinary excretion status of equol in Japanese women. Nutr Cancer. 2008;60:49–54. [DOI] [PubMed] [Google Scholar]
- 111.Tamura A, Shiomi T, Hachiya S, Shigematsu N, Hara H. Low activities of intestinal lactase suppress the early phase absorption of soy isoflavones in Japanese adults. Clin Nutr. 2008;27:248–53. [DOI] [PubMed] [Google Scholar]
- 112.Miyanaga N, Akaza H, Takashima N, Nagata Y, Sonoda T, Mori M, Naito S, Hirao Y, Tsukamoto T, et al. Higher consumption of green tea may enhance equol production. Asian Pac J Cancer Prev. 2003;4:297–301. [PubMed] [Google Scholar]
- 113.Teas J, Hurley TG, Hebert JR, Franke AA, Sepkovic DW, Kurzer MS. Dietary seaweed modifies estrogen and phytoestrogen metabolism in healthy postmenopausal women. J Nutr. 2009;139:939–44. [DOI] [PubMed] [Google Scholar]
- 114.Vedrine N, Mathey J, Morand C, Brandolini M, Davicco MJ, Guy L, Remesy C, Coxam V, Manach C. One-month exposure to soy isoflavones did not induce the ability to produce equol in postmenopausal women. Eur J Clin Nutr. 2006;60:1039–45. [DOI] [PubMed] [Google Scholar]
- 115.Clerici C, Setchell KDR, Battezzati PM, Pirro M, Giuliano V, Asciutti S, Castellani D, Nardi E, Sabatino G, et al. Pasta naturally enriched with isoflavone aglycons from soy germ reduces serum lipids and improves markers of cardiovascular risk. J Nutr. 2007;137:2270–8. [DOI] [PubMed] [Google Scholar]
- 116.Wakai K, Egami I, Kato K, Kawamura T, Tamakoshi A, Lin Y, Nakayama T, Wada M, Ohno Y. Dietary intake and sources of isoflavones among Japanese. Nutr Cancer. 1999;33:139–45. [DOI] [PubMed] [Google Scholar]
- 117.Somekawa Y, Chiguchi M, Ishibashi T, Aso T. Soy intake related to menopausal symptoms, serum lipids, and bone mineral density in postmenopausal Japanese women. Obstet Gynecol. 2001;97:109–15. [DOI] [PubMed] [Google Scholar]
- 118.Izumi T, Piskula MK, Osawa S, Obata A, Tobe K, Saito M, Kataoka S, Kubota Y, Kikuchi M. Soy isoflavone aglycones are absorbed faster and in higher amounts than their glucosides in humans. J Nutr. 2000;130:1695–9. [DOI] [PubMed] [Google Scholar]
- 119.Setchell KDR, Brown NM, Desai P, Zimmer-Nechemias L, Wolfe BE, Brashear WT, Kirschner AS, Cassidy A, Heubi JE. Bioavailability of pure isoflavones in healthy humans and analysis of commercial soy isoflavone supplements. J Nutr. 2001;131:S1362–75. [DOI] [PubMed] [Google Scholar]
- 120.Zubik L, Meydani M. Bioavailability of soybean isoflavones from aglycone and glucoside forms in American women. Am J Clin Nutr. 2003;77:1459–65. [DOI] [PubMed] [Google Scholar]
- 121.Setchell KDR, Zhao X, Shoaf SE, Ragland K. The pharmacokinetics of S- (-)equol administered as SE5-OH tablets to healthy postmenopausal women. J Nutr. 2009;139:2037–43. [DOI] [PubMed] [Google Scholar]
- 122.Setchell KDR, Cole SJ. Variations in isoflavone levels in soy foods and soy protein isolates and issues related to isoflavone databases and food labeling. J Agric Food Chem. 2003;51:4146–55. [DOI] [PubMed] [Google Scholar]
- 123.Valentin-Blasini L, Sadowski MA, Walden D, Caltabiano L, Needham LL, Barr DB. Urinary phytoestrogen concentrations in the U.S. population (1999–2000). J Expo Anal Environ Epidemiol. 2005;15:509–23. [DOI] [PubMed] [Google Scholar]
- 124.Peeters PH, Slimani N, van der Schouw YT, Grace PB, Navarro C, Tjonneland A, Olsen A, Clavel-Chapelon F, Touillaud M, et al. Variations in plasma phytoestrogen concentrations in European adults. J Nutr. 2007;137:1294–300. [DOI] [PubMed] [Google Scholar]
- 125.Ward H, Chapelais G, Kuhnle GG, Luben R, Khaw KT, Bingham S. Breast cancer risk in relation to urinary and serum biomarkers of phytoestrogen exposure in the European Prospective into Cancer-Norfolk cohort study. Breast Cancer Res. 2008;10:R32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Travis RC, Spencer EA, Allen NE, Appleby PN, Roddam AW, Overvad K, Johnsen NF, Olsen A, Kaaks R, et al. Plasma phyto-oestrogens and prostate cancer in the European Prospective Investigation into Cancer and Nutrition. Br J Cancer. 2009;100:1817–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Goodman MT, Shvetsov YB, Wilkens LR, Franke AA, Le Marchand L, Kakazu KK, Nomura AM, Henderson BE, Kolonel LN. Urinary phytoestrogen excretion and postmenopausal breast cancer risk: the multiethnic cohort study. Cancer Prev Res (Phila Pa). 2009;2:887–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Murphy PA, Barua K, Hauck CC. Solvent extraction selection in the determination of isoflavones in soy foods. J Chromatogr B Analyt Technol Biomed Life Sci. 2002;777:129–38. [DOI] [PubMed] [Google Scholar]
- 129.Atkinson C, Newton KM, Aiello Bowles EJ, Lehman CD, Stanczyk FZ, Westerlind KC, Li L, Lampe JW. Daidzein-metabolizing phenotypes in relation to mammographic breast density among premenopausal women in the United States. Breast Cancer Res Treat. 2009;116:587–94. [DOI] [PubMed] [Google Scholar]
- 130.Zhao JH, Sun SJ, Arao Y, Oguma E, Yamada K, Horiguchi H, Kayama F. Identification of equol producers in a Japanese population by high-performance liquid chromatography with coulometric array for determining serum isoflavones. Phytomedicine. 2006;13:304–9. [DOI] [PubMed] [Google Scholar]
- 131.King RA, Bursill DB. Plasma and urinary kinetics of the isoflavones daidzein and genistein after a single soy meal in humans. Am J Clin Nutr. 1998;67:867–72. [DOI] [PubMed] [Google Scholar]
- 132.Shelnutt SR, Cimino CO, Wiggins PA, Badger TM. Urinary pharmacokinetics of the glucuronide and sulfate conjugates of genistein and daidzein. Cancer Epidemiol Biomarkers Prev. 2000;9:413–9. [PubMed] [Google Scholar]
- 133.Bloedon LT, Jeffcoat AR, Lopaczynski W, Schell MJ, Black TM, Dix KJ, Thomas BF, Albright C, Busby MG, et al. Safety and pharmacokinetics of purified soy isoflavones: single-dose administration to postmenopausal women. Am J Clin Nutr. 2002;76:1126–37. [DOI] [PubMed] [Google Scholar]