Abstract
Equol [7-hydroxy-3-(4′-hydroxyphenyl)-chroman], an isoflavan produced by intestinal bacteria in response to soy isoflavone intake in some but not all humans, exhibits a wide range of biological properties. It exists as the diastereoisomers S-(-)equol and R-(+)equol. Intestinal bacteria produce exclusively S-(-)equol, which has selective affinity for estrogen receptor (ER)-β. The evidence is conflicting on whether there is an advantage to producing S-(-)equol in response to soy isoflavone intakes, but the ability to now synthesize these diastereoisomers opens the way for future clinical trials to directly examine their potential in a number of hormone-dependent conditions. In this review, the plasma and urinary pharmacokinetics of S-(-)equol and R-(+)equol are reviewed and summarized, and some of the more recent evidence supporting potential biological effects of S-(-)equol is considered.
Introduction
In Part 1 of this overview of equol (1), the history, chemistry, and factors that influence equol production were reviewed. Part 2 separately reviews the pharmacokinetics and the biological properties of equol that have led to the current interest in this unique isoflavone metabolite.
The hormonal effects of equol are well documented from early observations using estrogen bioassays. It was not until after the discovery of the first estrogen receptor (ER)-α (2) and the discovery that a second ER (ERβ) was present in specific tissues (3) that the relative affinity of equol for both receptors could be quantified (4–6). The results from these studies places the natural soy isoflavone metabolite, S-(-)equol, into a category of a selective ER modulator and consequently prompts many questions as to whether it could confer some specific benefits in hormone-related conditions.
The ability of both S-(-)equol and its diastereoisomer, R-(+) equol, to antagonize the in vivo actions of dihydrotestosterone (7) further makes equol a unique molecule with potential for the treatment or prevention of androgen-mediated conditions. For these reasons equol is currently attracting considerable interest as a potential pharmaceutical or nutraceutical agent. The following will review its pharmacology and biological effects.
Pharmacokinetics of equol
To our knowledge, data from the first pharmacokinetic study of equol was described in a single healthy adult female administered 25 mg of (±)equol given as a single oral bolus dose (8). The plasma (±)equol concentration appearance/disappearance curve suggested that equol differed in its pharmacokinetic behavior from the soy isoflavones daidzein and genistein. Most notably it had a much higher apparent bioavailability and slower clearance rate (8). This was confirmed in later studies when the plasma pharmacokinetics of S-(-)equol and R-(+)equol were compared in 3 healthy adults (6). More recently, using [13C]labeled tracers, the plasma and urinary pharmacokinetics of enantiomeric pure S-(-)equol and R-(+)equol were determined in 12 healthy adults (6 males, 6 females) (9). Both enantiomers were rapidly absorbed and reached peak plasma concentrations after 2–3 h when taken with a meal. In an evaluation of the pharmacokinetics of a new S-(-)equol–containing supplement (SE5-OH) given to 12 healthy postmenopausal women, the average peak plasma concentration was observed after 1–2 h when taken without a meal (10). The differences in the absorption rates of S-(-)equol between these 2 studies is explained by a meal-effect altering gastric emptying time and slowing the initial absorption rate. Such differences will influence peak plasma concentrations, as was evident from the much higher dose-adjusted Cmax values attained with the S-(-)equol supplement given without a meal compared with the pure compounds given with a meal (10). Therefore, in practice, the maximal effect of S-(-)equol is more likely to occur if it is administered before a meal. Independent of this difference, the pharmacokinetics of enantiomeric pure S-(-)equol was similar to that of the S-(-)equol supplement produced by the fermentation of soy germ isoflavones with Lactococcus garvieae (10). S-(-)equol has an terminal elimination half-life of 7–8 h in healthy adults and, therefore, steady-state levels will be more readily attained by dosing twice daily to minimize peaks and troughs in circulating concentrations. Within the constraints of small sample-sizes, data from all these studies suggested no obvious gender differences in the pharmacokinetics of S-(-)equol. Two interesting findings arose from a comparison of the pharmacokinetics of the [13C]labeled enantiomers. Racemic (±)[13C]equol showed slower absorption, attained lower peak plasma concentrations, and had lower systemic bioavailability compared with S-(-)[13C]equol and R-(+)[13C]equol. Also, the apparent systemic bioavailability of R-(+)[13C]equol was significantly greater than that of S-(-)[13C]equol (9).
S-(-)equol and R-(+)equol undergo little biotransformation in humans, save phase II metabolism by conjugation to glucuronic acid and to a minor extent sulfuric acid. S-(-)equol circulates in plasma and is excreted in urine as predominantly the 7-glucuronide conjugate (11–13). In this respect, its metabolism is similar to that of the soy isoflavones daidzein and genistein (14–18). Conjugation is extremely efficient in humans and takes place on first-pass absorption within the enterocyte and also the liver. Uridine diphosphate-5′-glucuronosyltransferase is widely distributed throughout the gastrointestinal tract (19) and it is probable that it is the uridine diphosphate-5′-glucuronosyltransferase 1A10 isoform that catalyzes glucuronidation, because this one conjugates genistein (15). For equol, its major route of elimination is by renal excretion into urine. The percent fractional elimination in urine after oral administration is extremely high and in some adults it can be close to 100% (9,10), which is far higher than that of daidzein (30–40%) and genistein (7–15%) (20,21). Recoveries averaged 82% when S-(-)equol was given as a supplement and 61.3 ± 19.5% for enantiomeric pure S-(-)[13C]equol. The bioavailability of R-(+)equol was higher than its diastereoisomer S-(-)equol based on the plasma pharmacokinetics and urinary recovery of the [13C] tracers (9). Overall, the very high bioavailability of S-(-)equol would indicate that relatively modest doses (10–30 mg twice a day) would result in high steady-state plasma concentrations in the range observed for plasma S-(-)equol derived from soy foods.
Endogenous estrogens, as with most hormones, circulate predominantly bound to albumin and sex hormone binding globulin (22) and also to α-fetoprotein (23). Less than 5% of estradiol is present in the free (unbound form), which is the fraction that is available for receptor occupancy. For equol it has been reported that 49.7% circulates in the free form, which is significantly higher than daidzein (18.7% free), its precursor (22). Thus, the biological activity of equol should be enhanced by its reduced binding to serum proteins and greater availability for receptor binding. In a dose-dependent manner, equol in vitro inhibits the binding of estradiol and testosterone for serum proteins (24).
Biological properties of equol
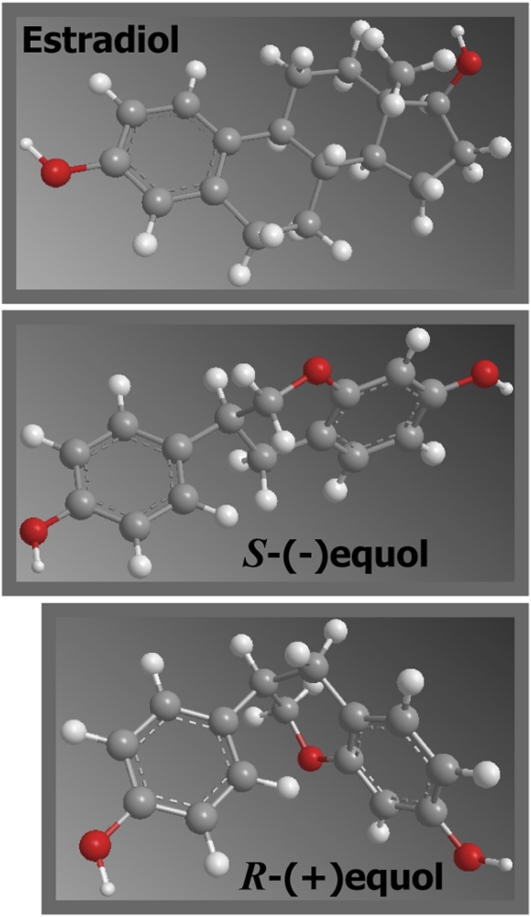
The diastereoisomers of equol share many similarities yet some significant differences in biological properties. The more planar-looking S-(-)equol enantiomer is strikingly similar in conformational structure to estradiol and, not surprisingly, this enantiomer binds to the ER (Fig. 1). When first isolated from equine urine in 1932, long before the discovery of the first ER (2), it was reported to have no estrogenic activity when injected into ovariectomized mice in doses up to 0.086 mg (25); however, its uterotrophic activity was later acknowledged. The earliest in vitro study of the binding of S-(-)equol, isolated from sheep urine showed it to have a relative molar binding affinity of 0.4 compared with estradiol, which was about 4 times the affinity of its precursor, daidzein (26). Later, 5 mg of equol, presumed to be the racemic form because it was chemically synthesized by methods at the time that were not enantioselective, when injected subcutaneously into 3-wk-old female rats increased uterine weight to the same extent as 0.005 mg of estradiol (27) and it was shown to antagonize the binding of estradiol to the ER. Others have reported similar relative binding affinities using a selection of different in vitro systems (28–30). It should, however, be pointed out that in most cases these early studies would have examined binding to ERα, because they predated the discovery of ERβ (3) and because this is the major ER subtype localized to the uterus (31). These early data are also possibly underestimated because of the use of racemic mixtures. Several more recent studies have since reported the binding characteristics of the individual enantiomers toward ERα and ERβ (4–6,32–34). S(-)equol produced from incubation of soy isoflavones with enteric bacteria when tested in competitive binding assays with human ERα and ERβ, and in a gene expression assay, was found to bind more strongly to ERβ than to ERα (4). The preferential binding of S(-)equol to ERβ has been confirmed in multiple studies (5,6,32) and indicates the molecule shares the characteristics of a selective ER modulator in this regard. However, S-(-)equol induces transcription either similarly, or more strongly, with ERα than with ERβ (4,5), as does R(+)equol (5), indicative of both being agonists. So the differential effects of 2 almost identical molecules on the ER subtypes is quite striking and shows how the presence of a chiral center in the molecule confers quite different biological properties.
FIGURE 1 .
A comparison of the conformation structures of estradiol, S-(-)equol, and R-(+)equol.
Given the present interest in S-(-)equol as a possible pharmaceutical or nutraceutical agent for a number of hormone-dependent disorders (9,10,35), the question of whether the ER agonist action could pose some risk for women with breast cancer or for those in high risk groups remains to be addressed (36–38). Recent studies using animal models of breast cancer have examined the role of equol on the growth of mammary tumors (39,40). In one model, S-(-)equol did not stimulate the growth of human ER positive MCF-7 cells transplanted into the athymic mouse (39). This important finding is in striking contrast to the marked stimulatory effect of the soy isoflavone genistein reported earlier by the same investigators in this same model (41), an observation that led to the issue of whether soy foods are safe for women with breast cancer. To date, there are no human data to support this concern, but 2 recent large prospective clinical studies of breast cancer survivors suggest that soy food consumption is associated with more favorable prognosis, in reducing risk of recurrence and improving survival (42,43). In a different animal model, S-(-)equol did not stimulate the growth of mammary tumors induced by the chemical carcinogen dimethylbenz[a]anthracene, but neither did it prove to be chemopreventive (40). R-(+)equol on the other hand was found in this same model to be potently chemopreventive (40). Combining data from these 2 animal models of breast cancer suggests that S-(-)equol should not increase risk for breast cancer and R-(+)equol could be a useful chemopreventive agent. If these animal data can be reliably extrapolated to humans, then the ability to make equol when consuming soy foods could be advantageous in reducing the risk of breast cancer.
While much of the interest in equol has centered on its estrogenic effects, equol enantiomers have a myriad of other biological properties with the potential to be of value in many clinical areas, including cancer, cardiovascular disease, osteoporosis, and menopausal symptoms (8); several of these areas are discussed below.
Uniquely, both S-(-)equol and R-(+)equol bind dihydrotestosterone and inhibit the in vivo stimulatory effect of this potent androgen on prostate growth (7). Neither S-(-)equol nor R-(+)equol bind to the androgen receptor, but its selective androgen-modulating activity, combined with S-(-)equol having selective affinity for ERβ, suggests that S-(-)equol may have potential in a number of androgen-mediated conditions, in particular prostate cancer treatment or prevention. The pharmaceutical industry has more recently turned its attention toward ERβ agonists in the search for the next generation of drugs to treat prostate cancer (44) and in this regard S-(-)equol may be a potential candidate. A small case control of prostate cancer patients from South Korea, Japan, and the US found a low frequency of equol-producers among the patients compared with age-matched controls (45), while in a separate Japanese study, the risk for prostate cancer was reported to show an inverse dose-response relationship with plasma equol concentrations (46).
Equol can be broadly classified as a polyphenol and due to the high number of π-electrons, it has hydrogen/donor properties and will scavenge free radicals. The in vitro antioxidant property of equol, presumed to be racemic equol, is well documented (47–50) and the antioxidant activities of the individual enantiomers should be similar. (±)Equol has the highest antioxidant activity of all the isoflavones that have been tested. To date there are no in vivo human data on the extent to which administering equol may influence lipid peroxidation, an important risk factor for atherosclerosis, but LDL oxidation by cultured monocyte/macrophages was shown to be inhibited by an antioxidant effect mediated through inhibition of superoxide radical production (51). The effect of equol on inhibition of nitric oxide (NO) production by inducible NO synthase gene expression in murine macrophages was reported as being mediated through upstream signaling pathways, specifically by Akt activation and downregulation of nuclear factor-κB activity (52); inducible NO synthase is implicated in the development of atherosclerosis. These findings are perhaps not unexpected, because genistein is antiinflammatory by an effect on reducing NO production (53). Several studies show equol to be a vasorelaxant, inducing endothelial and NO-dependent relaxation (54–60), suggesting equol may be helpful in reducing risk of cardiovascular disease. The isoflavone intermediate dihydrodaidzein and the closely related dehydroequol are also vasodilatory (55,61). No study has yet examined the vasodilatory actions of S-(-)equol or R-(-)equol separately and it is too early to know whether either enantiomer may be effective in the clinical arena. Studies of soy isoflavones have yielded mixed results with regard to the effects on endothelial function (62–69), but equol-producer status was not directly examined. In one recent clinical study of hypercholesterolemic patients, brachial artery-mediated vasodilatation was significantly greater in equol-producers compared with equol-nonproducers after 4 wk of dietary intervention with a soy isoflavone-containing food that resulted in a high proportion of equol-producers (70). Similar differential effects between equol-producers and nonproducers were observed in arterial stiffness in a study of postmenopausal women taking tibolone (71) and these translated into lower diastolic blood pressure (72). This was not the case in the former study (69). Because inflammation plays a key role in the onset of cardiovascular disease (73), it is possible, given equol's documented effect on the NO pathway, that it may act as an antiinflammatory agent. Serum high sensitivity C-reactive protein concentration, a surrogate marker of inflammation and cardiovascular risk, was shown in a recent study to be reduced in equol-producers by a soy isoflavone-containing food (70). In a recent study, equol and genistein, but not daidzein, modulated the inflammatory response in activated macrophages by inhibition of NO and prostaglandin E2 while regulating gene transcription of cytokines and inflammatory markers (74).
In vitro cell culture and animal studies have provided impressive data on the bone-trophic effects of isoflavones (75), but recent clinical studies of soy isoflavone supplementation in postmenopausal women have proved disappointing (76–80). None of these trials have prescreened for equol-producer status and randomized accordingly and all have used isoflavone mixtures of predominantly conjugated rather than aglycon forms. Interestingly, the aglycon genistein given at a dose of 54 mg/d for 3 y to postmenopausal women was reported to have impressive effects on bone, with increases in spine and hip bone mineral density (81). Studies from Japan show more favorable responses in measures of bone loss in those women who are equol-producers (82). Equol in its racemic form has been shown to have modest effects in preventing bone loss in animal models of osteoporosis (83–87) but has yet to be used in clinical trials. S-(-)equol is also being studied for its effects on reducing the incidence and frequency of menopausal symptoms (88), particularly hot flushes. Data from Japan have indicated that the severity of overall menopausal symptoms is significantly lower in women who are equol-producers treated with a soy isoflavone supplement (89).
In conclusion, S-(-)equol is a unique nonsteroidal estrogen that binds preferentially to ERβ and at the same time antagonizes the in vivo action of the potent androgen dihydrotestosterone. It occurs as 2 distinct diastereoisomers and both have properties that warrant their further investigation for the prevention and/or treatment of a number of estrogen- and androgen-mediated diseases or disorders as was first proposed in 1984 (90). The ability to now synthesize bulk quantities of enantiomeric pure S-(-)equol and R-(+)equol should permit future clinical studies to be conducted that will more clearly define the potential benefits of these diastereoisomers. More importantly, such direct studies of the pure compounds will enable a better understanding of the extent to which there are advantages to producing equol from soy foods, as has been proposed. If the equol hypothesis can be substantiated, then for those adults who are unable to produce equol due to a lack of intestinal equol-producing bacteria or some other factors, one option is to administer these enantiomers in the form of a nutraceutical or pharmaceutical. Major clinical studies are likely to emerge in the near future that will permit a better understanding of the potential value of equol in numerous clinical areas, not just those discussed above.
Note added in proof: Work by Setchell et al (9) has shown that the unconjugated fraction of [2-13C]S-(-)equol accounted for only 0.10 ± 0.05% of the total [2-13C]S-(-)equol in plasma following oral administration of 20 mg of the [13C]equol tracer to 12 healthy adults.
Acknowledgments
The manuscript was written and approved by both authors.
Published in a supplement to The Journal of Nutrition. Presented at the “Equol, Soy, and Menopause Research Leadership Conference”, held in Washington, DC, June 16, 2009. The supplement coordinator for this supplement is Kara Lewis, Life Sciences Research Organization (LSRO) Senior Staff Scientist. The supplement is the responsibility of the guest editors to whom the Editor of The Journal of Nutrition has delegated supervision of both technical conformity to the published regulations of The Journal of Nutrition and general oversight of the scientific merit of each article. Publication costs for this supplement were defrayed in part by the payment of page charges. This publication must therefore be hereby marked "advertisement" in accordance with 18 USC section 1734 solely to indicate this fact. The Guest Editor for this supplement is Neil Shay. Guest Editor disclosure: Neil Shay declares no conflict of interest. Supplement Coordinator disclosure: Kara Lewis is currently under contract with and receives compensation from the supplement sponsor. She was also compensated for attending and organizing the Equol, Soy, and Menopause Research Leadership Conference and for organizing, writing, editing, or reviewing, and collection of supplemental manuscripts. The opinions expressed in this publication are those of the authors and are not attributable to the sponsors or the publisher, Editor, or Editorial Board of The Journal of Nutrition.
Author disclosures: K. D. R. Setchell was supported by funding from the NIH (grant nos. R01AT-003313 and R01AT-002190) and has intellectual property on equol enantiomers, including patents licensed by Cincinnati Children's Hospital Medical Center, Cincinnati, OH to industry and is a consultant to Otsuka Pharmaceuticals Company, Tokyo, Japan. C. Clerici, no conflict of interest.
References
- 1.Setchell KDR, Clerici C. Equol: history, chemistry and formation. J Nutr. 2010;140:1355–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jensen EV. On the mechanism of estrogen action. Perspect Biol Med. 1962;6:47–59. [DOI] [PubMed] [Google Scholar]
- 3.Kuiper GG, Enmark E, Pelto-Huikko M, Nilsson S, Gustafsson JA. Cloning of a novel receptor expressed in rat prostate and ovary. Proc Natl Acad Sci USA. 1996;93:5925–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Morito K, Hirose T, Kinjo J, Hirakawa T, Okawa M, Nohara T, Ogawa S, Inoue S, Muramatsu M, et al. Interaction of phytoestrogens with estrogen receptors α and β. Biol Pharm Bull. 2001;24:351–6. [DOI] [PubMed] [Google Scholar]
- 5.Muthyala RS, Ju YH, Sheng S, Williams LD, Doerge DR, Katzenellenbogen BS, Helferich WG, Katzenellenbogen JA. Equol, a natural estrogenic metabolite from soy isoflavones: convenient preparation and resolution of R- and S-equols and their differing binding and biological activity through estrogen receptors alpha and beta. Bioorg Med Chem. 2004;12:1559–67. [DOI] [PubMed] [Google Scholar]
- 6.Setchell KDR, Clerici C, Lephart ED, Cole SJ, Heenan C, Castellani D, Wolfe BE, Nechemias-Zimmer L, Brown NM, et al. S-equol, a potent ligand for estrogen receptor beta, is the exclusive enantiomeric form of the soy isoflavone metabolite produced by human intestinal bacterial flora. Am J Clin Nutr. 2005;81:1072–9. [DOI] [PubMed] [Google Scholar]
- 7.Lund TD, Munson DJ, Haldy ME, Setchell KDR, Lephart ED, Handa RJ. Equol is a novel anti-androgen that inhibits prostate growth and hormone feedback. Biol Reprod. 2004;70:1188–95. [DOI] [PubMed] [Google Scholar]
- 8.Setchell KDR, Brown NM, Lydeking-Olsen E. The clinical importance of the metabolite equol-a clue to the effectiveness of soy and its isoflavones. J Nutr. 2002;132:3577–84. [DOI] [PubMed] [Google Scholar]
- 9.Setchell KDR, Zhao X, Jha P, Heubi JE, Brown NM. The pharmacokinetic behavior of the soy isoflavone metabolite S- (-)equol and its diastereoisomer R-(+)equol in healthy adults determined by using stable-isotope-labeled tracers. Am J Clin Nutr. 2009;90:1029–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Setchell KDR, Zhao X, Shoaf SE, Ragland K. The pharmacokinetics of S- (-)equol administered as SE5-OH tablets to healthy postmenopausal women. J Nutr. 2009;139:2037–43. [DOI] [PubMed] [Google Scholar]
- 11.Axelson M, Setchell KDR. Conjugation of lignans in human urine. FEBS Lett. 1980;122:49–53. [DOI] [PubMed] [Google Scholar]
- 12.Axelson M, Kirk DN, Farrant RD, Cooley G, Lawson AM, Setchell KDR. The identification of the weak oestrogen equol [7-hydroxy-3- (4′-hydroxyphenyl)chroman] in human urine. Biochem J. 1982;201:353–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Axelson M, Sjovall J, Gustafsson BE, Setchell KDR. Soya: a dietary source of the non-steroidal oestrogen equol in man and animals. J Endocrinol. 1984;102:49–56. [DOI] [PubMed] [Google Scholar]
- 14.Sfakianos J, Coward L, Kirk M, Barnes S. Intestinal uptake and biliary excretion of the isoflavone genistein in rats. J Nutr. 1997;127:1260–8. [DOI] [PubMed] [Google Scholar]
- 15.Doerge DR, Chang HC, Churchwell MI, Holder CL. Analysis of soy isoflavone conjugation in vitro and in human blood using liquid chromatography-mass spectrometry. Drug Metab Dispos. 2000;28:298–307. [PubMed] [Google Scholar]
- 16.Shelnutt SR, Cimino CO, Wiggins PA, Ronis MJJ, Badger TM. Pharmacokinetics of the glucuronide and sulfate conjugates of genistein and daidzein in men and women after consumption of a soy beverage. Am J Clin Nutr. 2002;76:588–94. [DOI] [PubMed] [Google Scholar]
- 17.Shelnutt SR, Cimino CO, Wiggins PA, Badger TM. Urinary pharmacokinetics of the glucuronide and sulfate conjugates of genistein and daidzein. Cancer Epidemiol Biomarkers Prev. 2000;9:413–9. [PubMed] [Google Scholar]
- 18.Ronis MJ, Little JM, Barone GW, Chen G, Radominska-Pandya A, Badger TM. Sulfation of the isoflavones genistein and daidzein in human and rat liver and gastrointestinal tract. J Med Food. 2006;9:348–55. [DOI] [PubMed] [Google Scholar]
- 19.Strassburg CP, Manns MP, Tukey RH. Expression of the UDP-glucuronosyltransferase 1A locus in human colon. Identification and characterization of the novel extrahepatic UGT1A8. J Biol Chem. 1998;273:8719–26. [DOI] [PubMed] [Google Scholar]
- 20.Setchell KDR, Brown NM, Desai P, Zimmer-Nechemias L, Wolfe BE, Brashear WT, Kirschner AS, Cassidy A, Heubi JE. Bioavailability of pure isoflavones in healthy humans and analysis of commercial soy isoflavone supplements. J Nutr. 2001;131:S1362–75. [DOI] [PubMed] [Google Scholar]
- 21.Setchell KDR, Faughnan MS, Avades T, Zimmer-Nechemias L, Brown NM, Wolfe BE, Brasheer WT, Desai P, Oldfield MF, Botting NP, Cassidy A. Comparing the pharmacokinetics of daidzein and genistein with the use of 13C-labeled tracers in premenopausal women. Am J Clin Nutr. 2003;77:411–9. [DOI] [PubMed] [Google Scholar]
- 22.Nagel SC, vom Saal FS, Welshons WV. The effective free fraction of estradiol and xenoestrogens in human serum measured by whole cell uptake assays: physiology of delivery modifies estrogenic activity. Proc Soc Exp Biol Med. 1998;217:300–9. [DOI] [PubMed] [Google Scholar]
- 23.Garreau B, Vallette G, Adlercreutz H, Wahala K, Makela T, Benassayag C, Nunez EA. Phytoestrogens: new ligands for rat and human α-fetoprotein. Biochim Biophys Acta. 1991;1094:339–45. [DOI] [PubMed] [Google Scholar]
- 24.Martin ME, Haourigui M, Pelissero C, Benassayag C, Nunez EA. Interactions between phytoestrogens and human sex steroid binding protein. Life Sci. 1996;58:429–36. [DOI] [PubMed] [Google Scholar]
- 25.Marrian GF, Haslewood GA. Equol, a new inactive phenol isolated from the ketohydroxyoestrin fraction of mares' urine. Biochem J. 1932;26:1227–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shutt DA, Cox RI. Steroid and phyto-oestrogen binding to sheep uterine receptors in vitro. J Endocrinol. 1972;52:299–310. [DOI] [PubMed] [Google Scholar]
- 27.Tang BY, Adams NR. Effect of equol on oestrogen receptors and on synthesis of DNA and protein in the immature rat uterus. J Endocrinol. 1980;85:291–7. [DOI] [PubMed] [Google Scholar]
- 28.Thompson MA, Lasley BL, Rideout BA, Kasman LH. Characterization of the estrogenic properties of a nonsteroidal estrogen, equol, extracted from urine of pregnant macaques. Biol Reprod. 1984;31:705–13. [DOI] [PubMed] [Google Scholar]
- 29.Markiewicz L, Garey J, Adlercreutz H, Gurpide E. In vitro bioassays of non-steroidal phytoestrogens. J Steroid Biochem Mol Biol. 1993;45:399–405. [DOI] [PubMed] [Google Scholar]
- 30.Branham WS, Dial SL, Moland CL, Hass BS, Blair RM, Fang H, Shi L, Tong W, Perkins RG, et al. Phytoestrogens and mycoestrogens bind to the rat uterine estrogen receptor. J Nutr. 2002;132:658–64. [DOI] [PubMed] [Google Scholar]
- 31.Kuiper GG, Carlsson B, Grandien K, Enmark E, Haggblad J, Nilsson S, Gustafsson JA. Comparison of the ligand binding specificity and transcript tissue distribution of estrogen receptors α and β. Endocrinology. 1997;138:863–70. [DOI] [PubMed] [Google Scholar]
- 32.Kostelac D, Rechkemmer G, Briviba K. Phytoestrogens modulate binding response of estrogen receptors α and β to the estrogen response element. J Agric Food Chem. 2003;51:7632–5. [DOI] [PubMed] [Google Scholar]
- 33.Matsumura A, Ghosh A, Pope GS, Darbre PD. Comparative study of oestrogenic properties of eight phytoestrogens in MCF7 human breast cancer cells. J Steroid Biochem Mol Biol. 2005;94:431–43. [DOI] [PubMed] [Google Scholar]
- 34.Carreau C, Flouriot G, Bennetau-Pelissero C, Potier M. Respective contribution exerted by AF-1 and AF-2 transactivation functions in estrogen receptor α induced transcriptional activity by isoflavones and equol: consequence on breast cancer cell proliferation. Mol Nutr Food Res. 2009;53:652–8. [DOI] [PubMed] [Google Scholar]
- 35.Yee S, Burdock GA, Kurata Y, Enomoto Y, Narumi K, Hamada S, Itoh T, Shimomura Y, Ueno T. Acute and subchronic toxicity and genotoxicity of SE5-OH, an equol-rich product produced by Lactococcus garvieae. Food Chem Toxicol. 2008;46:2713–20. [DOI] [PubMed] [Google Scholar]
- 36.Messina MJ, Persky V, Setchell KDR, Barnes S. Soy intake and cancer risk: a review of the in vitro and in vivo data. Nutr Cancer. 1994;21:113–31. [DOI] [PubMed] [Google Scholar]
- 37.Messina M, Barnes S, Setchell KDR. Phyto-oestrogens and breast cancer. Lancet. 1997;350:971–2. [DOI] [PubMed] [Google Scholar]
- 38.Messina MJ, Loprinzi CL. Soy for breast cancer survivors: a critical review of the literature. J Nutr. 2001;131:S3095–108. [DOI] [PubMed] [Google Scholar]
- 39.Ju YH, Fultz J, Allred KF, Doerge DR, Helferich WG. Effects of dietary daidzein and its metabolite, equol, at physiological concentrations on the growth of estrogen-dependent human breast cancer (MCF-7) tumors implanted in ovariectomized athymic mice. Carcinogenesis. 2006;27:856–63. [DOI] [PubMed] [Google Scholar]
- 40.Brown NM, Belles CA, Lindley SL, Zimmer-Nechemias LD, Zhao X, Witte DP, Kim M-O, Setchell KDR. The chemopreventive action of equol enatiomers in a chemically-induced animal model of breast cancer. Carcinogenesis.2010;31:886–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ju YH, Allred CD, Allred KF, Karko KL, Doerge DR, Helferich WG. Physiological concentrations of dietary genistein dose-dependently stimulate growth of estrogen-dependent human breast cancer (MCF-7) tumors implanted in athymic nude mice. J Nutr. 2001;131:2957–62. [DOI] [PubMed] [Google Scholar]
- 42.Shu XO, Zheng Y, Cai H, Gu K, Chen Z, Zheng W, Lu W. Soy food intake and breast cancer survival. JAMA. 2009;302:2437–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Guha N, Kwan ML, Quesenberry CP Jr, Weltzien EK, Castillo AL, Caan BJ. Soy isoflavones and risk of cancer recurrence in a cohort of breast cancer survivors: the Life After Cancer Epidemiology study. Breast Cancer Res Treat. 2009;118:395–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Imamov O, Lopatkin NA, Gustafsson JA. Estrogen receptor beta in prostate cancer. N Engl J Med. 2004;351:2773–4. [DOI] [PubMed] [Google Scholar]
- 45.Akaza H, Miyanaga N, Takashima N, Naito S, Hirao Y, Tsukamoto T, Fujioka T, Mori M, Kim WJ, et al. Comparisons of percent equol producers between prostate cancer patients and controls: case-controlled studies of isoflavones in Japanese, Korean and American residents. Jpn J Clin Oncol. 2004;34:86–9. [DOI] [PubMed] [Google Scholar]
- 46.Ozasa K, Nakao M, Watanabe Y, Hayashi K, Miki T, Mikami K, Mori M, Sakauchi F, Washio M, et al. Serum phytoestrogens and prostate cancer risk in a nested case-control study among Japanese men. Cancer Sci. 2004;95:65–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Arora A, Nair MG, Strasburg GM. Antioxidant activities of isoflavones and their biological metabolites in a liposomal system. Arch Biochem Biophys. 1998;356:133–41. [DOI] [PubMed] [Google Scholar]
- 48.Mitchell JH, Gardner PT, McPhail DB, Morrice PC, Collins AR, Duthie GG. Antioxidant efficacy of phytoestrogens in chemical and biological model systems. Arch Biochem Biophys. 1998;360:142–8. [DOI] [PubMed] [Google Scholar]
- 49.Chung JE, Kim SY, Jo HH, Hwang SJ, Chae B, Kwon DJ, Lew YO, Lim YT, Kim JH, et al. Antioxidant effects of equol on bovine aortic endothelial cells. Biochem Biophys Res Commun. 2008;375:420–4. [DOI] [PubMed] [Google Scholar]
- 50.Choi EJ. Evaluation of equol function on anti- or prooxidant status in vivo. J Food Sci. 2009;74:H65–71. [DOI] [PubMed] [Google Scholar]
- 51.Hwang J, Wang J, Morazzoni P, Hodis HN, Sevanian A. The phytoestrogen equol increases nitric oxide availability by inhibiting superoxide production: an antioxidant mechanism for cell-mediated LDL modification. Free Radic Biol Med. 2003;34:1271–82. [DOI] [PubMed] [Google Scholar]
- 52.Kang JS, Yoon YD, Han MH, Han SB, Lee K, Park SK, Kim HM. Equol inhibits nitric oxide production and inducible nitric oxide synthase gene expression through down-regulating the activation of Akt. Int Immunopharmacol. 2007;7:491–9. [DOI] [PubMed] [Google Scholar]
- 53.Salzman AL, Preiser JC, Setchell KDR, Szabo C. Isoflavone-mediated inhibition of tyrosine kinase: a novel antiinflammatory approach. J Med Food. 1999;2:179–81. [DOI] [PubMed] [Google Scholar]
- 54.Gimenez I, Lou M, Vargas F, Alvarez-Guerra M, Mayoral JA, Martinez RM, Garay RP, Alda JO. Renal and vascular actions of equol in the rat. J Hypertens. 1997;15:1303–8. [DOI] [PubMed] [Google Scholar]
- 55.Chin-Dusting JP, Fisher LJ, Lewis TV, Piekarska A, Nestel PJ, Husband A. The vascular activity of some isoflavone metabolites: implications for a cardioprotective role. Br J Pharmacol. 2001;133:595–605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Walker HA, Dean TS, Sanders TA, Jackson G, Ritter JM, Chowienczyk PJ. The phytoestrogen genistein produces acute nitric oxide-dependent dilation of human forearm vasculature with similar potency to 17β-estradiol. Circulation. 2001;103:258–62. [DOI] [PubMed] [Google Scholar]
- 57.Mahn K, Borras C, Knock GA, Taylor P, Khan IY, Sugden D, Poston L, Ward JP, Sharpe RM, et al. Dietary soy isoflavone induced increases in antioxidant and eNOS gene expression lead to improved endothelial function and reduced blood pressure in vivo. FASEB J. 2005;19:1755–7. [DOI] [PubMed] [Google Scholar]
- 58.Joy S, Siow RC, Rowlands DJ, Becker M, Wyatt AW, Aaronson PI, Coen CW, Kallo I, Jacob R, et al. The isoflavone equol mediates rapid vascular relaxation: Ca2+-independent activation of endothelial nitric-oxide synthase/Hsp90 involving ERK1/2 and Akt phosphorylation in human endothelial cells. J Biol Chem. 2006;281:27335–45. [DOI] [PubMed] [Google Scholar]
- 59.Jackman KA, Woodman OL, Chrissobolis S, Sobey CG. Vasorelaxant and antioxidant activity of the isoflavone metabolite equol in carotid and cerebral arteries. Brain Res. 2007;1141:99–107. [DOI] [PubMed] [Google Scholar]
- 60.Cheng C, Wang X, Weakley SM, Kougias P, Lin PH, Qizhi Y, Chen C. Soybean isoflavonoid equol blocks ritonavir-Induced endothelial dysfunction in porcine pulmonary arteries and human pulmonary artery endothelial cells. J Nutr. 2010;140:12–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Chin-Dusting JP, Boak L, Husband A, Nestel PJ. The isoflavone metabolite dehydroequol produces vasodilatation in human resistance arteries via a nitric oxide-dependent mechanism. Atherosclerosis. 2004;176:45–8. [DOI] [PubMed] [Google Scholar]
- 62.Teede HJ, Dalais FS, Kotsopoulos D, Liang YL, Davis S, McGrath BP. Dietary soy has both beneficial and potentially adverse cardiovascular effects: a placebo-controlled study in men and postmenopausal women. J Clin Endocrinol Metab. 2001;86:3053–60. [DOI] [PubMed] [Google Scholar]
- 63.Steinberg FM, Guthrie NL, Villablanca AC, Kumar K, Murray MJ. Soy protein with isoflavones has favorable effects on endothelial function that are independent of lipid and antioxidant effects in healthy postmenopausal women. Am J Clin Nutr. 2003;78:123–30. [DOI] [PubMed] [Google Scholar]
- 64.Lissin LW, Oka R, Lakshmi S, Cooke JP. Isoflavones improve vascular reactivity in post-menopausal women with hypercholesterolemia. Vasc Med. 2004;9:26–30. [DOI] [PubMed] [Google Scholar]
- 65.Petri Nahas E, Nahas Neto J, De Luca L, Traiman P, Pontes A, Dalben I. Benefits of soy germ isoflavones in postmenopausal women with contraindication for conventional hormone replacement therapy. Maturitas. 2004;48:372–80. [DOI] [PubMed] [Google Scholar]
- 66.Hermansen K, Hansen B, Jacobsen R, Clausen P, Dalgaard M, Dinesen B, Holst JJ, Pedersen E, Astrup A. Effects of soy supplementation on blood lipids and arterial function in hypercholesterolaemic subjects. Eur J Clin Nutr. 2005;59:843–50. [DOI] [PubMed] [Google Scholar]
- 67.Colacurci N, Chiantera A, Fornaro F, de Novellis V, Manzella D, Arciello A, Chiantera V, Improta L, Paolisso G. Effects of soy isoflavones on endothelial function in healthy postmenopausal women. Menopause. 2005;12:299–307. [DOI] [PubMed] [Google Scholar]
- 68.Kreijkamp-Kaspers S, Kok L, Bots ML, Grobbee DE, Lampe JW, van der Schouw YT. Randomized controlled trial of the effects of soy protein containing isoflavones on vascular function in postmenopausal women. Am J Clin Nutr. 2005;81:189–95. [DOI] [PubMed] [Google Scholar]
- 69.Hall WL, Vafeiadou K, Hallund J, Bugel S, Koebnick C, Reimann M, Ferrari M, Branca F, Talbot D, et al. Soy-isoflavone-enriched foods and inflammatory biomarkers of cardiovascular disease risk in postmenopausal women: interactions with genotype and equol production. Am J Clin Nutr. 2005;82:1260–8. [DOI] [PubMed] [Google Scholar]
- 70.Clerici C, Setchell KDR, Battezzati PM, Pirro M, Giuliano V, Asciutti S, Castellani D, Nardi E, Sabatino G, et al. Pasta naturally enriched with isoflavone aglycons from soy germ reduces serum lipids and improves markers of cardiovascular risk. J Nutr. 2007;137:2270–8. [DOI] [PubMed] [Google Scholar]
- 71.Tormala R, Appt S, Clarkson TB, Groop PH, Ronnback M, Ylikorkala O, Mikkola TS. Equol production capability is associated with favorable vascular function in postmenopausal women using tibolone; no effect with soy supplementation. Atherosclerosis. 2008;198:174–8. [DOI] [PubMed] [Google Scholar]
- 72.Tormala RM, Appt S, Clarkson TB, Tikkanen MJ, Ylikorkala O, Mikkola TS. Individual differences in equol production capability modulate blood pressure in tibolone-treated postmenopausal women: lack of effect of soy supplementation. Climacteric. 2007;10:471–9. [DOI] [PubMed] [Google Scholar]
- 73.Ross R. Atherosclerosis: an inflammatory disease. N Engl J Med. 1999;340:115–26. [DOI] [PubMed] [Google Scholar]
- 74.Blay M, Espinel AE, Delgado MA, Baiges I, Blade C, Arola L, Salvado J. Isoflavone effect on gene expression profile and biomarkers of inflammation. J Pharm Biomed Anal. 2010;51:382–90. [DOI] [PubMed] [Google Scholar]
- 75.Setchell KDR, Lydeking-Olsen E. Dietary phytoestrogens and their effect on bone: evidence from in vitro and in vivo, human observational, and dietary intervention studies. Am J Clin Nutr. 2003;78:S593–609. [DOI] [PubMed] [Google Scholar]
- 76.Ma DF, Qin LQ, Wang PY, Katoh R. Soy isoflavone intake increases bone mineral density in the spine of menopausal women: meta-analysis of randomized controlled trials. Clin Nutr. 2008;27:57–64. [DOI] [PubMed] [Google Scholar]
- 77.Brink E, Coxam V, Robins S, Wahala K, Cassidy A, Branca F. Long-term consumption of isoflavone-enriched foods does not affect bone mineral density, bone metabolism, or hormonal status in early postmenopausal women: a randomized, double-blind, placebo controlled study. Am J Clin Nutr. 2008;87:761–70. [DOI] [PubMed] [Google Scholar]
- 78.Liu J, Ho SC, Su YX, Chen WQ, Zhang CX, Chen YM. Effect of long-term inervention of soy isoflavones on bone mineral density in women: a meta-analysis of randomized controll trials. Bone. 2009;44:948–53. [DOI] [PubMed] [Google Scholar]
- 79.Wong WW, Lewis RD, Steinberg FM, Murray MJ, Cramer MA, Amato P, Young RL, Barnes S, Ellis KJ, et al. Soy isoflavone supplementation and bone mineral density in menopausal women: a 2-y multicenter clinical trial. Am J Clin Nutr. 2009;90:1433–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Alekel DL, Van Loan MD, Koehler KJ, Hanson LN, Stewart JW, Hanson KB, Kurzer MS, Peterson CT. The Soy Isoflavones for Reducing Bone Loss (SIRBL) Study: a 3-y randomized controlled trial in postmenopausal women. Am J Clin Nutr. 2010;91:218–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Marini H, Minutoli L, Polito F, Bitto A, Altavilla D, Atteritano M, Gaudio A, Mazzaferro S, Frisina A, et al. Effects of the phytoestrogen genistein on bone metabolism in osteopenic postmenopausal women: a randomized trial. Ann Intern Med. 2007;146:839–47. [DOI] [PubMed] [Google Scholar]
- 82.Wu J, Oka J, Ezaki J, Ohtomo T, Ueno T, Uchiyama S, Toda T, Uehara M, Ishimi Y. Possible role of equol status in the effects of isoflavone on bone and fat mass in postmenopausal Japanese women: a double-blind, randomized, controlled trial. Menopause. 2007;14:866–74. [DOI] [PubMed] [Google Scholar]
- 83.Ward WE, Kim S, Chan D, Fonseca D. Serum equol, bone mineral density and biomechanical bone strength differ among four mouse strains. J Nutr Biochem. 2005;16:743–9. [DOI] [PubMed] [Google Scholar]
- 84.Fujioka M, Uehara M, Wu J, Adlercreutz H, Suzuki K, Kanazawa K, Takeda K, Yamada K, Ishimi Y. Equol, a metabolite of daidzein, inhibits bone loss in ovariectomized mice. J Nutr. 2004. t;134:2623–7. [DOI] [PubMed] [Google Scholar]
- 85.Mathey J, Mardon J, Fokialakis N, Puel C, Kati-Coulibaly S, Mitakou S, Bennetau-Pelissero C, Lamothe V, Davicco MJ, et al. Modulation of soy isoflavones bioavailability and subsequent effects on bone health in ovariectomized rats: the case for equol. Osteoporos Int. 2007;18:671–9. [DOI] [PubMed] [Google Scholar]
- 86.Rachon D, Seidlová-Wuttke D, Vortherms T, Wuttke W. Effects of dietary equol administration on ovariectomy induced bone loss in Sprague-Dawley rats. Maturitas. 2007;58:308–15. [DOI] [PubMed] [Google Scholar]
- 87.Legette LL, Martin BR, Shahnazari M, Lee WH, Helferich WG, Qian J, Waters DJ, Arabshahi A, Barnes S, et al. Supplemental dietary racemic equol has modest benefits to bone but has mild uterotropic activity in ovariectomized rats. J Nutr. 2009;139:1908–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Ishiwata N, Melby MK, Mizuno S, Watanabe S. New equol supplement for relieving menopausal symptoms: randomized, placebo-controlled trial of Japanese women. Menopause. 2009;16:141–8. [DOI] [PubMed] [Google Scholar]
- 89.Jou HJ, Wu SC, Chang FW, Ling PY, Chu KS, Wu WH. Effect of intestinal production of equol on menopausal symptoms in women treated with soy isoflavones. Int J Gynaecol Obstet. 2008;102:44–9. [DOI] [PubMed] [Google Scholar]
- 90.Setchell KDR, Borriello SP, Hulme P, Kirk DN, Axelson M. Nonsteroidal estrogens of dietary origin: possible roles in hormone-dependent disease. Am J Clin Nutr. 1984;40:569–78. [DOI] [PubMed] [Google Scholar]