Abstract
Background:
Doppler echocardiographic studies in patients with β-Thalassemia Major (β-TM) had shown different patterns of left ventricle (LV) systolic and diastolic dysfunctions.
Aim:
This cross-sectional study was designed to study the LV systolic and diastolic function in patients with β-TM using Pulsed Doppler (PD) Echocardiogram and assess the QTc interval and QT dispersion (QTd) on 12 leads ECG.
Method:
All patients were evaluated clinically as well as by echocardiography and 12 leads ECG. The study included patients with β-TM (n = 38, age 15.7 ± 8.9 years), compared with an age-matched healthy control group (n = 38, age 15.9 ± 8.9 years).
Results:
In 38 patients with β-TM Compared with healthy control group, The QTc interval and the QTd dispersion on ECG were increased with no significant difference mode echo showed that β-TM patients have thicker LV septal wall index (0.659 ± 0.23 vs. 0.446 ± 0.219 cm/M2, P < 0.001), posterior wall index (0.659 ± 0.235 vs. 0.437 ± 0.214 cm/M2, P < 0.01), and larger LVEDD index is (3.99 ± 0.48 vs. 2.170 ± 0.57 cm/M2. P < 0.05). Pulsed Doppler showed high LV trans-mitral E wave velocity index (70.818 ± 10.139 vs. 57.532 ± 10.139, P < 0.05) and E/A ratio (1.54 vs.1.23, P < 0.01). The duration of deceleration time index (DT) and isovolumic relaxation time index (IVRT) were significantly shorter in patients with β-TM (150.234 ± 20.0.23 vs. 167.123 ± 167.123 ± 19.143 msec/M2, P < 0.01) and (60.647 ± 6.77 vs. 75.474 ± 5.83 msec/M2, P < 0.001), respectively. The tricuspid valve velocity in patients with β-TM was significantly higher than controls (2.993 ± 0.569 vs. 1.93 ± 0.471 m/sec, respectively, P < 0.01), with calculated pulmonary artery pressure of 2.4 times the control (36.0 vs. 14.8 mmHg). However, the LVEF% or fractional shortening were not significantly different.
Conclusion:
In this study, β-thalassemia major patients compared with controls have differences of QT dispersion and corrected QT interval that is of no statistical significance. A significantly thicker LV wall and LV diastolic filling indices are suggestive of restrictive diastolic pattern. These data indicate that LV diastolic abnormalities compromised initially in patients with β-thalassemia major.
Keywords: beta-thalassemia major, QT dispersion, pulsed echo Doppler, Bahrain
Introduction
Beta-Thalassemia Major (β-TM) is a genetic blood disorder caused by reduced synthesis of β-globin chain. The consequences of chronic anemia include growth retardation, bone marrow expansion, extramedullary hematopoiesis, splenomegaly, greater intestinal iron absorption, hypercoagulability and higher susceptibility to infection.1,2 Patients are usually maintained on periodic blood transfusion regimens to prevent complications and survive into adulthood. Because of the hemolysis and repeated blood transfusion, β-TM is associated with iron overload,3 The iron deposition could adversely affect both the structure and function of the heart. Previous studies indicated that patients with β-TM develop ventricular systolic dysfunction leading to congestive heart failure.4
The National Student Screening Project in Bahrain indicated that the prevalence of β-TM in high school students is 0.09%, with β-thalassaemia trait representing 2.9% of the sample population.5 Similar findings were observed in a study targeting subjects seeking premarital screening in Bahrain.6
Echocardiographic changes in patients with β-TM had not been previously studied in Bahrain. Evaluation of systolic and diastolic function by echo pulsed Doppler can assist in staging the disease progression and may help to prevent further cardiac damage by applying intense chelating regimens and combination therapy.7 The left Ventricle (LV) diastolic filling measured by pulsed wave Doppler echocardiography reflect the diastolic filling characteristics of the LV and depends on various factors including LV ventricle relaxation, compliance, systolic function and loading conditions.8 LV Diastolic filling pattern has been classified into normal, restrictive and abnormal relaxation pattern on the basis of early filling E wave and late filling A wave on LV trans-mitral diastolic filling.9,10
QT dispersion (QTd), defined as maximum QT interval minus minimum QT interval, was proposed as an index of ventricular recovery times to distinguish myocardium that is homogenous from myocardium that display non-homogenicity in the repolarization time of myocardium.11 The normal range of QTd value is 10–70 ms with mean 29 ± 26 ms in young age.12 QTd had been shown to increase in a variety of cardiac diseases including acute myocardial infarction (MI), post MI, hypertrophic cardio-myopathy, left ventricle hypertrophy of hypertension, heart failure, idiopathic dilated cardiomyopathy and Long QT syndrome indicating general repolarization abnormality in this group of patients.13–16
The prognostic value of QTd had been evaluated in patients with end stage renal disease patients requiring hemodialysis, and in patients with diabetes mellitus.17,18
β-TM patients require repeated transfusion of packed cell (PC), lysis of transfused packed cell releases much iron which is not excretable. Iron deposition in the body organs especially in the heart, the kidney and the brain are the main reasons for these organ failure causing death during second decade.19 It has been shown that ventricular wall thickening may be altered by pathological iron deposition.20 The iron deposition may affect the ventricular recovery times due to inhomogencity in the repolarization time with altered QT interval and QTd.
So far the impact of iron deposition on the QTd interval in patients with thalssemia major has not been evaluated and we are determined to know whether OT interval and QTd alteration in this group of patients. Thus, the aim of this study is assess the QTc interval and QTd using 12 leads Electrocardiogram (ECG) and pulsed Doppler echocardiogram in patients with β-TM in Bahrain.
Subjects and Methods
This study included 38 patients with transfusion-dependent β-TM and 38 patients with no thalassemia were used as a control group. The study was conducted during the interval from January 2009 to June 2009. A constitutional ethical approval was obtained for the study.
Selection was consecutive from patients who are on regular follow-up in Pediatric Hematology clinic at Salmaniya Medical Complex (SMC) in Bahrain.
Patients were included if they had a follow-up for more than 3 years at SMC, with a confirmed diagnosis of β-TM based on electrophoresis. Each patient had received blood transfusions every three weeks to maintain hemoglobin levels above 9 g/dl since infancy. All patients had also been receiving desferrioxamine as a chelating therapy (25–50 mg/kg body weight by subcutaneous infusion with an infusion pump for 8–12 h) regularly for at least 1 year. In the control group, patients were all with no valve disease, normal LV function and negative blood tests for thalassemia. They were selected from the pool of healthy patients who are referred for evaluation of systolic murmur and turned to have normal echo.
The range of age was counted on the selection to match the study patients. The blood samples in the control group were withdrawn one week after the performance of echo and twelve leads ECG.
Patients were excluded if they have end stage renal disease with creatinine clearance of <30% of normal, severe liver disease, diabetes mellitus, hypoparathyroidism, advanced heart failure, hypertrophic cardiomyopathy or if the patient is taking active cardiac medications.
Each patient in the study had a clinical and hematological data file including duration of disease, cardiovascular assessment for pulse rate, jugular venous pressure wave, apex beat, blood pressure, heart sounds and presence or absence of murmur, ankle edema and body weight. Data of the blood hemoglobin, serum ferritin and creatinine concentrations were all extracted.
Twelve leads ECG
All patients in the β-TM and the control group had 12 leads ECG, the QT interval corrected for Heart rate was performed using Bazett21 formula in which the K value modified by Shipley22 (is 0.397 for men and 0.415 for women). The twelve leads ECG recorded on each patient at 25 mm/s. Three consecutive cycles were measured in each of the chest leads (V1–V6) and from the three values a mean of QT was calculated in chest leads V1–V6. The QT dispersion was defined as the difference between maximum and minimum QT. The methods of measurement were as reported previously by Pye et al.23
Doppler echocardiography
All patients in the study group and the control group had the echo examination by 2.5–5 MHz transducer, using HP E33 echo machine. The echocardiographic measurements were done by the an echo technician who is not aware of the clinical status of the patients the data were reviewed by a second technician blinded of the first results and an average of the two reading was tabulated for the 2-D and Doppler results the Doppler velocities and other echo findings were taken as an average of at least three cardiac cycles, according to the criteria of the American Society of Echocardiography. 24 Each patient enrolled in the study had echocardiographic measurements including M Mode, 2D echo, color flow, systolic and diastolic myocardial indexes.
The echo parameters including the left ventricle (LV) septal wall thickness, posterior LV wall thickness, LV cavity dimension in systole, and LV dimension in diastole were all measured using the M mode tracing. LV fractional shortening (FS) and LV ejection fraction percentage (LVEF%) were measured using Teichholz formula: V = {7.0/(2.4 + D)*D3}. The presence of valve diseases and both the left and right atrial areas were all assessed in the apical four chamber view.
The left ventricle diastolic filling indices included early diastolic wave (E wave), late diastolic wave (A wave), deceleration time of E wave (DT), isovolumic relaxation time (IVRT) and E wave to A wave (E/A) ratio. The tricuspid valve velocity in systole as an index of pulmonary artery pressure was calculated using tricuspid velocity jet in modified Bernoulli equation {Δ p RV-RA = 4(VTR)2}.25
Statistical analysis
All data were entered and analyzed using the Statistical Package of Social Sciences (SPSS) version 17.0. Data are presented as mean ± SD. Unpaired student t-test was used to analyze the differences between the variables in the control group and patients with β-TM. The QTd and echo Doppler findings were adjusted for Body Surface Area (BSA) in both groups. Differences between groups were considered statistically significant at a probability value of < 0.05.
Results
The demographic data on the β-TM patients and the healthy controls are summarized in Table 1. The β-TM group had significantly lower body weight and height (P < 0.001) and body surface area of 1.15 ± 0.2 vs. 1.01 ± 0.2, P < 0.05) compared with the control group.
Table 1.
Demographic characteristics of control (n = 38) and thalassemia patients (n = 38) in the study population. Data are presented as mean ± SD. P value < 0.05 is considered statistically significant.
| Parameter | Control | Thalassemia | P-value |
|---|---|---|---|
| Age (Years) | 15.79 ± 8.94 | 15.92 ± 8.92 | 0.92 |
| Male | 22 (57%) | 23 (60%) | 0.75 |
| Weight (kg) | 33.00 ± 7.25 | 25.6 ± 8.30 | 0.034* |
| Height (cm) | 144.3 ± 9.62 | 133.4 ± 9.64 | 0.001* |
| BSA | 1.15 ± 0.2 | 1.02 ± 0.2 | 0.001* |
| Systolic pressure (mmHg) | 123.34 ± 12.15 | 128.16 ± 12.03 | 0.108 |
| Diastolic pressure (mmHg) | 69.61 ± 7.06 | 70.71 ± 8.23 | 0.101 |
| Heart rate (BPM) | 67.58 ± 7.26 | 92 ± 6.99 | 0.001* |
| Serum ferritin (ug/l) | 230 ± 22 | 1628.6 ± 243.8 | 0.001* |
| Serum creatinine (umol/l) | 80.8 ± 10.12 | 75.23 ± 11.13 | 0.076 |
| Hemoglobin gm/dl | 10.2 ± 0.5 | 9.5 ± 0.4 | 0.065 |
= (P < 0.05).
The mean age was 15.7 ± 8.9 year (range: 7–25) and 57% male, while the mean age of the control group was 15.9 ± 8.3 year (range; 6–24) There was no significant difference in the gender distribution between the two groups. The systolic and diastolic pressures values were comparable in both groups, but the heart rate was significantly higher by 27% in β-TM group. The serum ferritin level was significantly higher in the β-TM group compared with the control group (P < 0.001). No significant differences in serum creatinine and hemoglobin level between both groups.
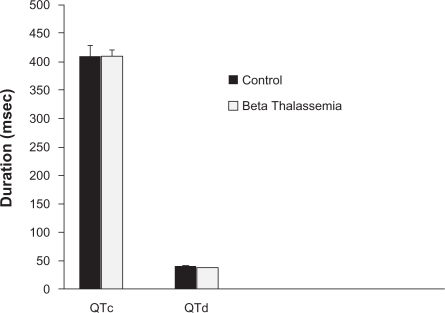
Figure 1 showed the value of QTc and QTd in both groups. There was no significant difference in QTc between both groups. The QTc in the β-TM group was 409 ± 23.034 ms vs. 402 ± 24.023 ms in the control group (P = 0.087). The QT d was slightly longer in the β-TM group compared with the control with no statistical significance (41 ± 22.014 ms vs. 37 ± 20.164 ms, respectively, P = 0.078).
Figure 1.
The mean QT interval (QTc) and QT dispersion (QTd) in milliseconds in patients with beta thalassemia major (β-TM) and control healthy group.
Echo doppler findings
Table 2 summarizes the echo Doppler findings in both control and β-TM groups. The β-TM group showed significantly higher LV wall thickness manifested by significantly thicker posterior wall and interventricular septum (P < 0.001 in both). LV dimensions at the end of systole (ESD) and diastole (EDD) were significantly higher in β-TM compared with control group (P = 0.035 and P < 0.001, respectively). However, no significant difference in left ventricle fractional shortening (FS) and ejection fraction (LVEF%) between the two groups were observed. These data indicate compensated normal systolic function in the β-TM group.
Table 2.
Echocardiographic findings in control (n = 38) and thalassemia patients (n = 38) in the study population. Data are presented as mean ± SEM per body surface area (m2). P value < 0.05 is considered statistically significant.
| Parameter | Control | Thalassemia | P-value |
|---|---|---|---|
| IVS/BSA (cm/M2) | 0.446 ± 0.219 | 0.659 ± 0.26 | <0.001* |
| PW/BSA (cm/M2) | 0.437 ± 0.214 | 0.659 ± 0.235 | <0.001* |
| LVEDD/BSA (mm/M2) | 2.170 ± 0.57 | 3.995 ± 0.48 | <0.035* |
| LVESD/BSA (mm/M2) | 2.217 ± 0.45 | 2.40 ± 0.54 | <0.001* |
| FS (%) | 31.329 ± 3.810 | 33.878 ± 4.944 | 0.107 |
| LVEF (%) | 57.656 ± 6.641 | 58.454 ± 6.504 | 0.103 |
| E wave/BSA (cm/sec) | 57.532 ± 12.959 | 70.818 ± 10.139 | <0.027* |
| A wave/BSA (cm/sec) | 46.330 ± 9.851 | 44.646 ± 7.776 | 0.109 |
| E/A ratio | 1.233 ± 0.27 | 1.54 ± 0.235 | <0.021 |
| DT/BSA (msec) | 167.123 ± 19.143 | 150.234 ± 20.023 | <0.01 |
| IVRT/BSA (msec) | 75.474 ± 5.83 | 60.647 ± 6.770 | <0.01 |
| Tricuspid velocity/BSA (m/sec) | 1.453 ± 0.471 | 2.993 ± 0.569 | <0.01 |
Abbreviations: IVS, interventricular septal thickness; Pw, posterior wall thickness; LVEDD, left ventricle end diastolic diameter; LVESD, left ventricle end systolic diameter; FS, fractional shortening; EF, left ventricle ejection fraction; E wave, mitral E wave peak velocity; A wave, mitral A wave peak velocity; DT, E wave deceleration time; IVRT, isovolumic relaxation time; TR, tricuspid velocity.
Compared with controls, the β-TM group showed a significantly higher E wave (P = 0.027), higher E/A ratio (P = 0.021), shorter deceleration time of E wave (P < 0.01) and shorter isovolumic relaxation time (IVRT) (P < 0.01). These data indicate diastolic dysfunction of restrictive pattern in β-TM group.
The ratio of E wave velocity of the mitral to the tissue Doppler of the septal mitral annulus (E/e− ratio) was significantly higher in the β-TM group compared to the control group (P < 0.01), and the tricuspid valve velocity of right ventricle was significantly higher (P < 0.01). In the β-TM group compared with the control, using Bernoulli equation for the tricuspid valve velocity, the calculated pulmonary artery pressure was 2.4 times the control (36.0 vs. 14.8 mmHg, respectively); the data is not shown in the table.
Finally, the left and right atrial areas were significantly larger in β-TM group compared to the control (P < 0.01). The color flow and continuous flow of the LV in the β-TM group showed mild mitral regurgitation in nine patients and three mild aortic regurgitation.
Discussion
In this study, patients with β-TM were evaluated with pulse Doppler echocardiogram for diastolic filling of left ventricle (LV) and 12 leads ECG for evaluation of QT interval and QT dispersion compared with age-matched controls. The main findings are compared with controls, the diastolic indices of LV in β-TM patients showed higher early diastolic filling of LV and high E/A ratio suggesting restrictive diastolic pattern and thus stiff LV wall. These findings are in keeping with another study by Yaprak et al3,26 who demonstrated that β-TM patients (n = 63) had significantly higher E wave, E/A ratio, and lower A wave velocity, suggesting restrictive pattern in 54% in the study population; no correlation was found with hemoglobin level. Similarly, it has been reported that transmitral diastolic filling measured by Doppler in patients with β-TM (n = 32, none of them had heart failure) exhibits a restrictive pattern (Spirit P et al27). This was also in agreement with a previous report that high E/A ratio is the most common finding in patients with β-TM.28
The LVEF% and fractional shortening were normal with no difference between patients with β-TM and the control group. The normal LVEF% indicates that β-TM patients are having minimal deleterious effect of myocardial iron overload on myocardial systolic function and that was evident by the optimal level of serum ferritin in the study population. This may also explain the absence of heart failure and absence of significant valve diseases in the current study population. In one report it was shown that the overall cardiovascular prognosis was good if the serum ferritin is below 2500 ng/dl; the low ferritin of <1000 ng/dl was associated with normal LVEF% while high level was associated with low LVEF%.29 In another study, however, right sided heart failure was reported in 16% of β-TM patients with high serum ferritin of >2500 ng/ml.30 Therefore, the iron overload in the current study appears to mediate the impaired diastolic function with the development of pulmonary hypertension leading to stiffness of the myocardial wall. So it seems in this population and in keeping with others myocardial disease passes through a stage of impaired relaxation before development of systolic dysfunction.31 With mean pre-transfusion hemoglobin concentration of 9.5 gm/dl in the β-TM group, there is an increase dimension of LV cavity and LV volume in systole and diastole as well as LV wall thickness. The increase of the volume load in this population may be a reflection of Frank Starling mechanism and may be due the increase of heart rate, this was observed by others who related the increment of LV volume to factors mediated by chronic anemia.32,33
The ECG heart rate corrected QT interval and the QT dispersion were slightly higher in the TM group compared with the control group with no statistical difference.
This ECG finding may indicate the mild impact of iron deposition in the myocardium in this group of patients and perhaps a long-term follow up for serum ferritin and QT dispersion may be warranted to evaluate the temporal relationship between these two factors. The QTd was significantly increased in patients with coronary artery disease, end stage renal disease and in diabetes mellitus.16–18
Findings from the current study is in keeping with previous reports indicating that patients with β-TM have non significant increased of QTd compared with controls.34
However in this study, the increment of LV wall thickness and LV cavity dimensions, both can possibly lead to increase of the heterogenicity of repolarization and prolongation of QT dispersion. The significant increment of QTd in β-TM patients may be due to early disease involvement or the effective chelating therapy and transfusion regimen or both.
The prolongation of QT interval is associated with an increased risk of a characteristic life threatening cardiac arrhythmia and hence in this study the increment of QT interval may herald such late outcome.15
The magnitude of E wave velocity is governed by the initial left ventricle pressure and shown to be directly related to it.34 In this study, the decreased DT time of E wave was mostly related to the amplitude of the E wave and is due to the impaired relaxation of LV. The IVRT was also shortened reflecting the impaired LV relaxation pattern, probably due to iron overload stiffness of LV wall. In a previous report, it was shown that this period of time is influenced by many factors such as heart rate, BSA and age; so these factors need to be considered and adjusted.35
There were significantly higher velocity across the tricuspid valve in the β-TM group indicates early development of pulmonary hypertension in the β-TM group. This finding indicates the presence of early right ventricular dysfunction even before the development of LV dysfunction that was normal in this study. Pulmonary hypertension in the β-TM patients may be due to pulmonary diffusion defect, hypoxia and possibly airway obstruction due to iron overload.36–38
Conclusion
We conclude from this study that β-thalassemia patients who are receiving periodic transfusions and chelation therapy have normal systolic function, larger left ventricle dimensions, abnormal LV relaxation pattern suggestive of restrictive pattern, higher LV end-diastolic pressure. The QTc interval and the QT dispersion were not significantly increased in β-TM patients. These data suggests that diastolic abnormalities appears initially in patients with β-thalassemia.
Footnotes
Disclosures
This manuscript has been read and approved by all authors. This paper is unique and is not under consideration by any other publication and has not been published elsewhere. The authors and peer reviewers of this paper report no conflicts of interest.
References
- 1.Fujita S. Congenital hemolytic anemia–hemoglobin abnormality–thalassemia. Nippon Rinsho. 1996;54:2454–9. [PubMed] [Google Scholar]
- 2.Fosburg MT, Nathan DG. Treatment of Cooley’s anemia. Blood. 1990;76:435–44. [PubMed] [Google Scholar]
- 3.3-Ehlers KH, Levin AR, Markenson AL, Marcus JR, Klein AA, Hilgartner MW, et al. Longitudinal study of cardiac function in thalassemia major. Ann N Y Acad Sci. 1980;344:397–404. doi: 10.1111/j.1749-6632.1980.tb33678.x. [DOI] [PubMed] [Google Scholar]
- 4.4-Hahalis G, Manolis AS, Apostolopoulos D, Alexopoulos D, Vagenakis AG, Zoumbos NC. Right ventricular cardiomyopathy in beta-thalassaemia major. Eur Heart J. 2002;23:147–56. doi: 10.1053/euhj.2001.2709. [DOI] [PubMed] [Google Scholar]
- 5.Al-Arrayed S, Hafadh N, Amin S, Al-Mukhareq H, Sanad H. Student screening for inherited blood disorders in Bahrain. Eastern Mediterranean Health Journal. 2003;9(3):344–52. [PubMed] [Google Scholar]
- 6.Al-Arrayed SS, Hafadh N, Al-Serafi S. Premarital counseling: an experience from Bahrain. Eastern Mediterranean Health Journal. 1997;3:415–9. [Google Scholar]
- 7.Gharzuddine WS, Kazma HK, Nuwayhid IA, et al. Doppler characterization of left ventricular diastolic function in beta-thalassaemia major. Evidence for an early stage of impaired relaxation. European Journal of Echocardiography. 2002;3:47–51. doi: 10.1053/euje.2001.0114. [DOI] [PubMed] [Google Scholar]
- 8.Nishaimura RA, Schwartz RS, Tajik AJ, Holmes DR. Non invasive measurement of rate of the left ventricular relaxation by Doppler echocardiography: validation with simultaneous cardiac catheterization. Circulation. 1993;88:146–55. doi: 10.1161/01.cir.88.1.146. [DOI] [PubMed] [Google Scholar]
- 9.Aessopos A, Deftereos S, Tsironi M, et al. Predictive echo-Doppler indices of left ventricular impairment in B-thalassemic patients. Ann Hematol. 2007;86:429–34. doi: 10.1007/s00277-007-0257-y. [DOI] [PubMed] [Google Scholar]
- 10.Choong CY, Herrman HC, Weyman AE, Fifer MA. Preload dependent Doppler derived indexes of left ventricle diastolic function in human. J Am Coll Cardiol. 1987;10:800–8. doi: 10.1016/s0735-1097(87)80273-5. [DOI] [PubMed] [Google Scholar]
- 11.Kautzner J, Malik M. QT dispersion and its clinical utility. Pacing Clin Electrophysiology. 1997;20:2625. doi: 10.1111/j.1540-8159.1997.tb06112.x. [DOI] [PubMed] [Google Scholar]
- 12.Malik M, Batchvarov VN. Measurment, interpretation and clinical potential of QT dispersion. J AM coll cardiol. 2000;36:1749. doi: 10.1016/s0735-1097(00)00962-1. [DOI] [PubMed] [Google Scholar]
- 13.Ashkaga T, Nishizzaki M, Arietta M, et al. Effect of dipyridamole on QT dispersion in vasospastic angina pectoris. Am J Cardiol. 1999;84:807. doi: 10.1016/s0002-9149(99)00441-5. [DOI] [PubMed] [Google Scholar]
- 14.Yi G, Elliott P, McKenna W, et al. QT dispersion and the risk factors for sudden cardiac death in patients with hypertrophic cardiomyopathy. Am J Cardiol. 1998;82:1514. doi: 10.1016/s0002-9149(98)00696-1. [DOI] [PubMed] [Google Scholar]
- 15.Haverkamp W, Breithardt G, Camm AJ, et al. Report on a policy conference of European society of cardiology. The potential for QT prorogation and proarrhythmia by non-antiarrhythmic drugs: clinical and regulatory implications. Eur Heart J. 2000;21:1216. doi: 10.1053/euhj.2000.2249. [DOI] [PubMed] [Google Scholar]
- 16.Galinier M, Vialette JC, Fourcade J, et al. QT interval dispersion as a predictor of arrhythmic events in congestive cardiac failure. Importance of aetiology. Eur Heart J. 1998;19:1054. doi: 10.1053/euhj.1997.0865. [DOI] [PubMed] [Google Scholar]
- 17.WuVc, Lin LY, Wu KD. QT interval dispersion in dialysis patients. Nephrology (carlton) 2005;10(2):109–12. doi: 10.1111/j.1440-1797.2005.00391.x. [DOI] [PubMed] [Google Scholar]
- 18.Langen KJ, Ziegler D, Weise F, et al. Evaluation of QT interval length, QT dispersion and myocardial miodobenzylguanidine uptake in insulin dependent diabetic patients with and without autonomic neuropathy. Clinic Sci (Lond) 1997;93(4):325–33. doi: 10.1042/cs0930325. [DOI] [PubMed] [Google Scholar]
- 19.Honig GR. Hematologic disorders (Thalassemia syndrome) In: Behrman RE, Kliegman RM, jenson Hb, editors. Nelson Textbook of pediatrics. 16th Ed. Phildelphia: W.B. Saunders Company; 2000. pp. 1484–7. [Google Scholar]
- 20.Foseca SF, kimura EY, Kerbauy J. Assessment of iron status in individual with heterozyotic b-thalassemia. Rev Assoc Med Bras. 1995;4193:203–6. [PubMed] [Google Scholar]
- 21.Bazett HC. An analysis of the time–relations of electrocardiograms. Heart. 1920;7:353. [Google Scholar]
- 22.Shipley RA, Hallaran WR. The four lead electrocardiogram in two hundred normal men and women. Am Heart J. 1936;11:325. [Google Scholar]
- 23.Pye M, Quinn AC, Cobbe SM. QT interval dispersion: a non–invasive marker of susceptibility to arrhythmia in patients with sustained ventricular arrhythmia? Br Heart J. 1994;71:511–4. doi: 10.1136/hrt.71.6.511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sahn DJ, De Maria A, Kisslo J, Weyman A. The committee on M mode standardization of the American Society of Echocardiography: results of survey of echocardiographic measurements. Circulation. 1978;58:1072–10. doi: 10.1161/01.cir.58.6.1072. [DOI] [PubMed] [Google Scholar]
- 25.Yock PG, Popp RL. Noninvasive estimation of right ventricle systolic pressure by Doppler ultrasound in patients with tricuspid regurgitation. Circulation. 1984;70:657–62. doi: 10.1161/01.cir.70.4.657. [DOI] [PubMed] [Google Scholar]
- 26.Yaprak I, Aksit S, Ozturk C, Bakiler AR, Dorak C, Turker M. Left ventricle diastolic abnormalities in children with beta-thalassemia major: a Doppler echocardiographic study. Turk J Pediatr. 1988;40(2):201–9. [PubMed] [Google Scholar]
- 27.Spirit P, Lupi G, Melevendi C, Veccio C. Restrictive diastolic abnormalities identified by Doppler echocardiography in patients with thalassemia major. Circulation. 1990;82(1):88–94. doi: 10.1161/01.cir.82.1.88. [DOI] [PubMed] [Google Scholar]
- 28.Bosi G, Crepaz R, Gamberini MR, et al. Left ventricle remodeling and systolic and diastolic function in young adult with beta thalassemia major: a Doppler echocardiographic assessment and correlation with hematological data. Heart. 2003;89(7):762–6. doi: 10.1136/heart.89.7.762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Iarussi D, Di Salvo G, Pergola V, et al. Pulsed Doppler tissue imaging and myocardial function in thalassemia major. Heart Vessels. 2003;18:1–6. doi: 10.1007/s003800300000. [DOI] [PubMed] [Google Scholar]
- 30.Kremastinos DT, Tsiapras DP, Tsetsos GA, et al. Left ventricular diastolic Doppler characteristics in β-thalassemia major. Circulation. 1993:1127–35. doi: 10.1161/01.cir.88.3.1127. [DOI] [PubMed] [Google Scholar]
- 31.Duke M, Abeimann WH. The haemodynamic response to chronic anemia. Circulation. 1969;34:503–15. doi: 10.1161/01.cir.39.4.503. [DOI] [PubMed] [Google Scholar]
- 32.Oliviehan NF, Nathan DG, Macmillan JH, et al. Survival in medically treated patients with homozygous beta-thalassemia. N Eng J Med. 1994;331:57–8. doi: 10.1056/NEJM199409013310903. [DOI] [PubMed] [Google Scholar]
- 33.Weyman A. Principles and Practice of echocardiography. 2nd edition. Philadelphia: Lea and Febiger; 1990. [Google Scholar]
- 34.Kocharian A, Dalir Rooyfard M, aghanouri R. Prolonged dispersion of QT and QTc in thalassemia major patients. Acta Medica Iranica. 2003;41:233–7. [Google Scholar]
- 35.Bulck FA, Mott MG, Martin RP. Left ventricle diastolic function in children measured by Doppler echocardiography normal values and relation with growth. Br Heart J. 1995;73:334–9. doi: 10.1136/hrt.73.4.334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Stewart RA, Joshi J, Alexander N, Nihoyanannopoulos P, Oakley CM. Adjustment for the influence of age and heart rate on Doppler measurement of left ventricle filling. Br Heart J. 1992;68:608–12. doi: 10.1136/hrt.68.12.608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cooper DM, Mansell AL, Weiner MA, et al. Low lung capacity and hypoxemia in children with thalassemia major. Ann Rev Respir Dis. 1980;121:639–46. doi: 10.1164/arrd.1980.121.4.639. [DOI] [PubMed] [Google Scholar]
- 38.Grant GP, Mansell AL, Graziano JH, Mellins RB. The effect of Transfusion on lung capacity, diffusion capacity and arterial oxygen saturation in patients with thalassemia major. Pediatr Res. 1986;20:20–3. doi: 10.1203/00006450-198601000-00005. [DOI] [PubMed] [Google Scholar]