Abstract
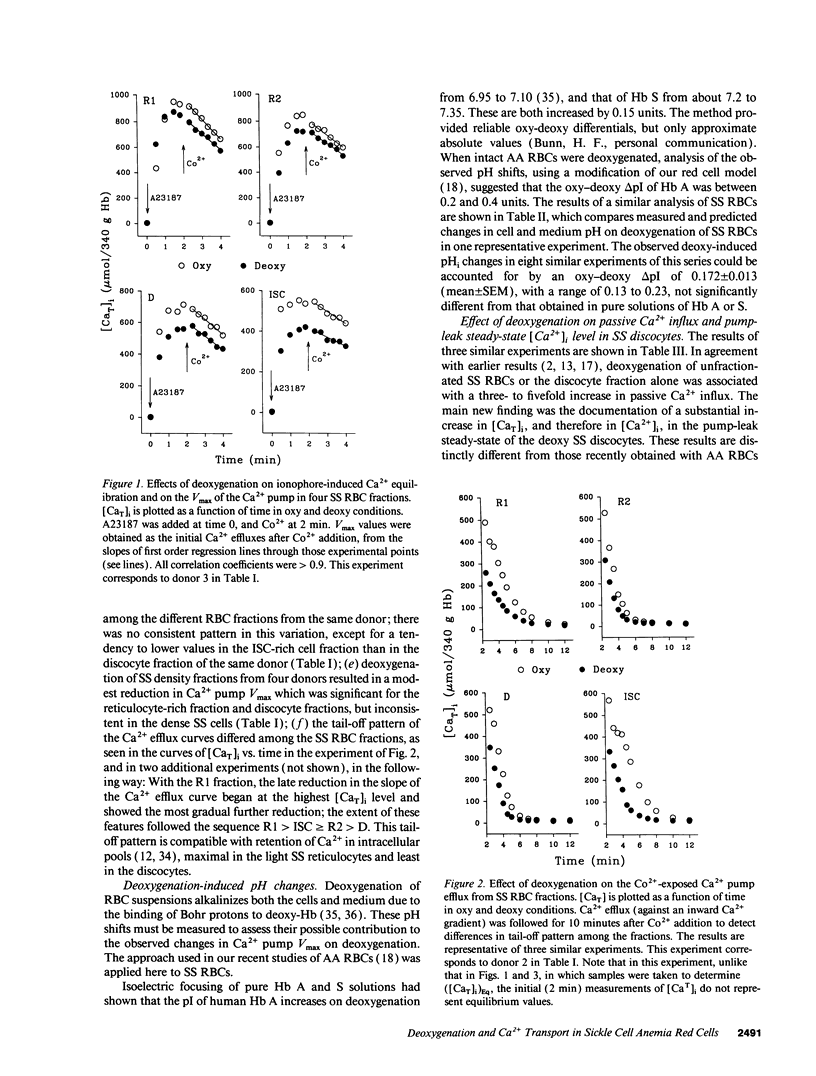
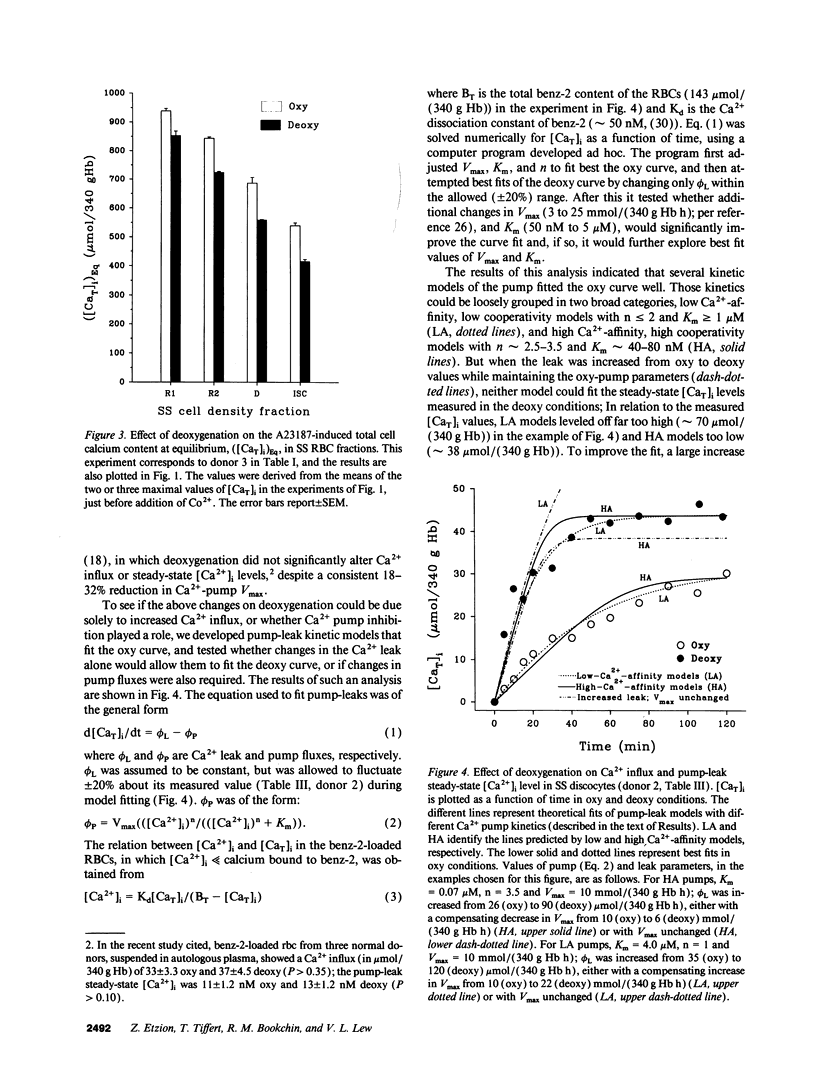
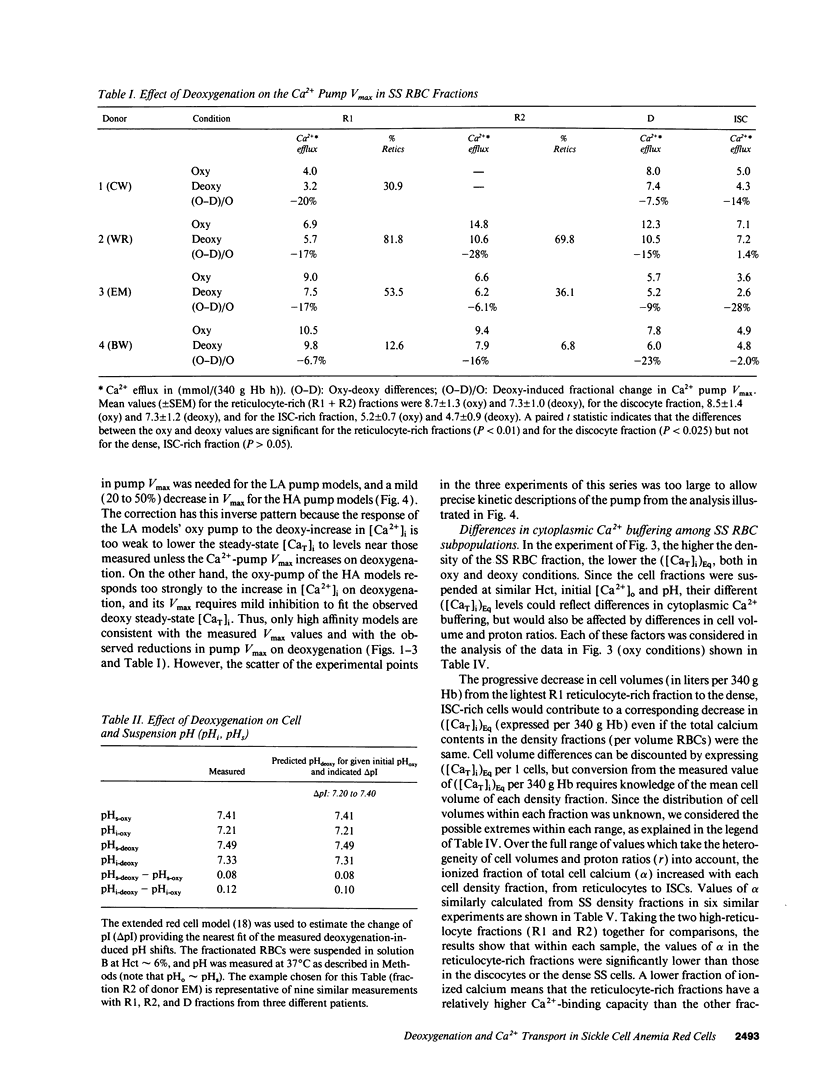
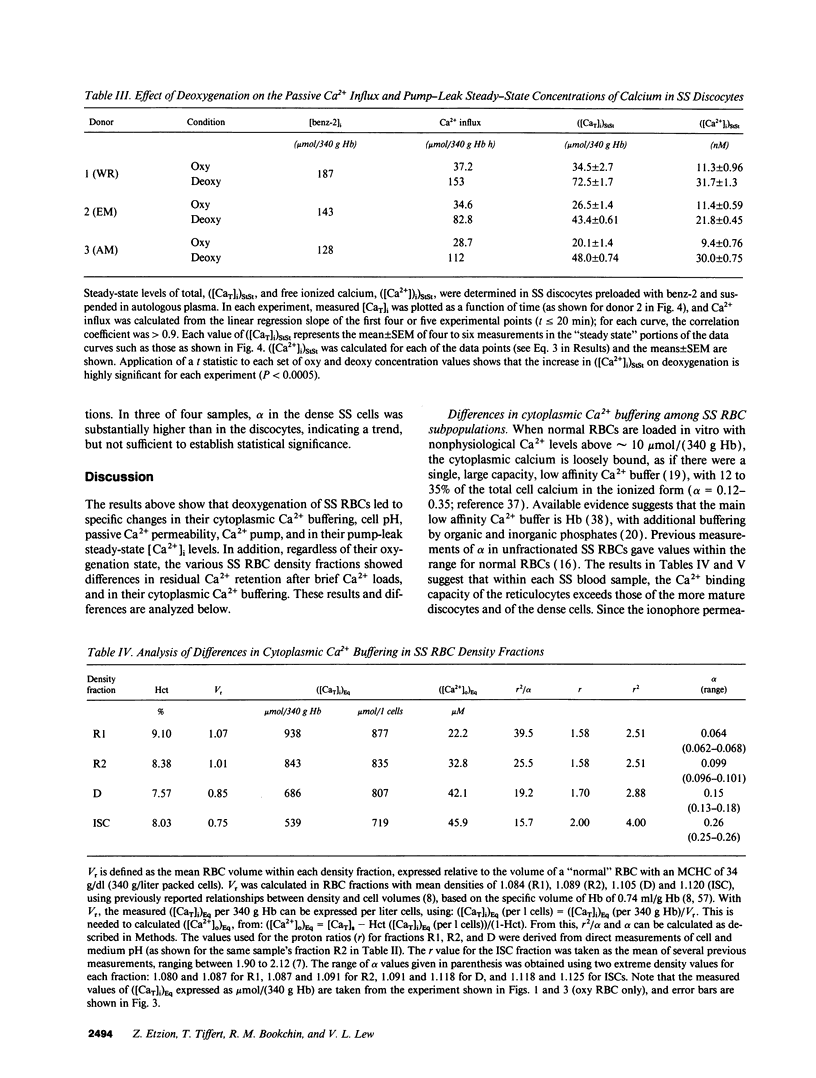
Elevated [Ca2+]i in deoxygenated sickle cell anemia (SS) red cells (RBCs) could trigger a major dehydration pathway via the Ca(2+)-sensitive K+ channel. But apart from an increase in calcium permeability, the effects of deoxygenation on the Ca2+ metabolism of sickle cells have not been previously documented. With the application of 45Ca(2+)-tracer flux methods and the combined use of the ionophore A23187, Co2+ ions, and intracellular incorporation of the Ca2+ chelator benz-2, in density-fractionated SS RBCs, we show here for the first time that upon deoxygenation, the mean [Ca2+]i level of SS discocytes was significantly increased, two- to threefold, from a normal range of 9.4 to 11.4 nM in the oxygenated cells, to a range of 21.8 to 31.7 nM in the deoxygenated cells, closer to K+ channel activatory levels. Unlike normal RBCs, deoxygenated SS RBCs showed a two- to fourfold increase in pump-leak Ca2+ turnover. Deoxygenation of the SS RBCs reduced their Ca2+ pump Vmax, more so in reticulocyte- and discocyte-rich than in dense cell fractions, and decreased their cytoplasmic Ca2+ buffering. Analysis of these results suggests that both increased Ca2+ influx and reduced Ca2+ pump extrusion contribute to the [Ca2+]i elevation.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Berger H., Jänig G. R., Gerber G., Ruckpaul K., Rapoport S. M. Interaction of haemoglobin with ions. Interactions among magnesium, adenosine 5'-triphosphate, 2,3-bisphosphoglycerate, and oxygenated and deoxygenated human haemoglobin under simulated intracellular conditions. Eur J Biochem. 1973 Oct 18;38(3):553–562. doi: 10.1111/j.1432-1033.1973.tb03090.x. [DOI] [PubMed] [Google Scholar]
- Bertles J. F., Milner P. F. Irreversibly sickled erythrocytes: a consequence of the heterogeneous distribution of hemoglobin types in sickle-cell anemia. J Clin Invest. 1968 Aug;47(8):1731–1741. doi: 10.1172/JCI105863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blostein R., Grafova E. Decrease in Na(+)-K(+)-ATPase associated with maturation of sheep reticulocytes. Am J Physiol. 1990 Aug;259(2 Pt 1):C241–C250. doi: 10.1152/ajpcell.1990.259.2.C241. [DOI] [PubMed] [Google Scholar]
- Bookchin R. M., Lew V. L. Effect of a 'sickling pulse' on calcium and potassium transport in sickle cell trait red cells. J Physiol. 1981 Mar;312:265–280. doi: 10.1113/jphysiol.1981.sp013628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bookchin R. M., Lew V. L. Progressive inhibition of the Ca pump and Ca:Ca exchange in sickle red cells. Nature. 1980 Apr 10;284(5756):561–563. doi: 10.1038/284561a0. [DOI] [PubMed] [Google Scholar]
- Bookchin R. M., Ortiz O. E., Lew V. L. Evidence for a direct reticulocyte origin of dense red cells in sickle cell anemia. J Clin Invest. 1991 Jan;87(1):113–124. doi: 10.1172/JCI114959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bookchin R. M., Ortiz O. E., Somlyo A. V., Somlyo A. P., Sepulveda M. I., Hockaday A., Lew V. L. Calcium-accumulating inside-out vesicles in sickle cell anemia red cells. Trans Assoc Am Physicians. 1985;98:10–20. [PubMed] [Google Scholar]
- Bookchin R. M., Raventos C., Lew V. L. Abnormal vesiculation and calcium transport by 'one-step' inside-out vesicles from sickle cell anemia red cells. Comparisons with transport by intact cells. Prog Clin Biol Res. 1981;55:163–182. [PubMed] [Google Scholar]
- Bunn H. F., McDonough M. Asymmetrical hemoglobin hybrids. An approach to the study of subunit interactions. Biochemistry. 1974 Feb 26;13(5):988–993. doi: 10.1021/bi00702a024. [DOI] [PubMed] [Google Scholar]
- Bunn H. F., Ransil B. J., Chao A. The interaction between erythrocyte organic phosphates, magnesium ion, and hemoglobin. J Biol Chem. 1971 Sep 10;246(17):5273–5279. [PubMed] [Google Scholar]
- Bureau M., Banerjee R. Structure-volume relationships in hemoglobin. A densitometric and dilatometric study of the oxy leads to deoxy transformation. Biochimie. 1976;58(4):403–407. doi: 10.1016/s0300-9084(76)80249-0. [DOI] [PubMed] [Google Scholar]
- Corash L. M., Piomelli S., Chen H. C., Seaman C., Gross E. Separation of erythrocytes according to age on a simplified density gradient. J Lab Clin Med. 1974 Jul;84(1):147–151. [PubMed] [Google Scholar]
- DUNHAM E. T., GLYNN I. M. Adenosinetriphosphatase activity and the active movements of alkali metal ions. J Physiol. 1961 Apr;156:274–293. doi: 10.1113/jphysiol.1961.sp006675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dagher G., Lew V. L. Maximal calcium extrusion capacity and stoichiometry of the human red cell calcium pump. J Physiol. 1988 Dec;407:569–586. doi: 10.1113/jphysiol.1988.sp017432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eaton J. W., Skelton T. D., Swofford H. S., Kolpin C. E., Jacob H. S. Elevated erythrocyte calcium in sickle cell disease. Nature. 1973 Nov 9;246(5428):105–106. doi: 10.1038/246105a0. [DOI] [PubMed] [Google Scholar]
- Ferreira H. G., Lew V. L. Use of ionophore A23187 to measure cytoplasmic Ca buffering and activation of the Ca pump by internal Ca. Nature. 1976 Jan 1;259(5538):47–49. doi: 10.1038/259047a0. [DOI] [PubMed] [Google Scholar]
- Flatman P. W., Lew V. L. Excess magnesium converts red cell (sodium+potassium) ATPase to the potassium phosphatase. J Physiol. 1980 Oct;307:1–8. doi: 10.1113/jphysiol.1980.sp013419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flatman P. W., Lew V. L. The magnesium dependence of sodium-pump-mediated sodium-potassium and sodium-sodium exchange in intact human red cells. J Physiol. 1981 Jun;315:421–446. doi: 10.1113/jphysiol.1981.sp013756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flatman P. W. The effect of buffer composition and deoxygenation on the concentration of ionized magnesium inside human red blood cells. J Physiol. 1980 Mar;300:19–30. doi: 10.1113/jphysiol.1980.sp013148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- García-Sancho J., Lew V. L. Detection and separation of human red cells with different calcium contents following uniform calcium permeabilization. J Physiol. 1988 Dec;407:505–522. doi: 10.1113/jphysiol.1988.sp017428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- García-Sancho J., Lew V. L. Heterogeneous calcium and adenosine triphosphate distribution in calcium-permeabilized human red cells. J Physiol. 1988 Dec;407:523–539. doi: 10.1113/jphysiol.1988.sp017429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- García-Sancho J., Lew V. L. Properties of the residual calcium pools in human red cells exposed to transient calcium loads. J Physiol. 1988 Dec;407:541–556. doi: 10.1113/jphysiol.1988.sp017430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- García-Sancho J. Pyruvate prevents the ATP depletion caused by formaldehyde or calcium-chelator esters in the human red cell. Biochim Biophys Acta. 1985 Feb 28;813(1):148–150. doi: 10.1016/0005-2736(85)90357-8. [DOI] [PubMed] [Google Scholar]
- Gerber G., Berger H., Jänig G. R., Rapoport S. M. Interaction of haemoglobin with ions. Quantitative description of the state of magnesium, adenosine 5'-triphosphate, 2,3-bisphosphoglycerate, and human haemoglobin under simulated intracellular conditions. Eur J Biochem. 1973 Oct 18;38(3):563–571. doi: 10.1111/j.1432-1033.1973.tb03091.x. [DOI] [PubMed] [Google Scholar]
- Harrison D. G., Long C. The calcium content of human erythrocytes. J Physiol. 1968 Dec;199(2):367–381. doi: 10.1113/jphysiol.1968.sp008658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kosk-Kosicka D., Bzdega T. Activation of the erythrocyte Ca2+-ATPase by either self-association or interaction with calmodulin. J Biol Chem. 1988 Dec 5;263(34):18184–18189. [PubMed] [Google Scholar]
- Kosk-Kosicka D., Bzdega T., Wawrzynow A., Scaillet S., Nemcek K., Johnson J. D. Erythrocyte Ca2(+)-ATPase: activation by enzyme oligomerization versus by calmodulin. Adv Exp Med Biol. 1990;269:169–174. doi: 10.1007/978-1-4684-5754-4_28. [DOI] [PubMed] [Google Scholar]
- Lauf P. K., Bauer J., Adragna N. C., Fujise H., Zade-Oppen A. M., Ryu K. H., Delpire E. Erythrocyte K-Cl cotransport: properties and regulation. Am J Physiol. 1992 Nov;263(5 Pt 1):C917–C932. doi: 10.1152/ajpcell.1992.263.5.C917. [DOI] [PubMed] [Google Scholar]
- Lauf P. K. K+:Cl- cotransport: sulfhydryls, divalent cations, and the mechanism of volume activation in a red cell. J Membr Biol. 1985;88(1):1–13. doi: 10.1007/BF01871208. [DOI] [PubMed] [Google Scholar]
- Lew V. L., Bookchin R. M. Role of reticulocyte transport heterogeneity in the generation of mature sickle cells with different volumes. Biochem Soc Trans. 1992 Nov;20(4):797–800. doi: 10.1042/bst0200797. [DOI] [PubMed] [Google Scholar]
- Lew V. L., Freeman C. J., Ortiz O. E., Bookchin R. M. A mathematical model of the volume, pH, and ion content regulation in reticulocytes. Application to the pathophysiology of sickle cell dehydration. J Clin Invest. 1991 Jan;87(1):100–112. doi: 10.1172/JCI114958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lew V. L., Hockaday A., Sepulveda M. I., Somlyo A. P., Somlyo A. V., Ortiz O. E., Bookchin R. M. Compartmentalization of sickle-cell calcium in endocytic inside-out vesicles. Nature. 1985 Jun 13;315(6020):586–589. doi: 10.1038/315586a0. [DOI] [PubMed] [Google Scholar]
- Lew V. L., Tsien R. Y., Miner C., Bookchin R. M. Physiological [Ca2+]i level and pump-leak turnover in intact red cells measured using an incorporated Ca chelator. Nature. 1982 Jul 29;298(5873):478–481. doi: 10.1038/298478a0. [DOI] [PubMed] [Google Scholar]
- Noguchi C. T., Schechter A. N. Sickle hemoglobin polymerization in solution and in cells. Annu Rev Biophys Biophys Chem. 1985;14:239–263. doi: 10.1146/annurev.bb.14.060185.001323. [DOI] [PubMed] [Google Scholar]
- Noguchi C. T., Torchia D. A., Schechter A. N. Intracellular polymerization of sickle hemoglobin. Effects of cell heterogeneity. J Clin Invest. 1983 Sep;72(3):846–852. doi: 10.1172/JCI111055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orringer E. P., Mattern W. D. Formaldehyde-induced hemolysis during chronic hemodialysis. N Engl J Med. 1976 Jun 24;294(26):1416–1420. doi: 10.1056/NEJM197606242942602. [DOI] [PubMed] [Google Scholar]
- Ortiz O. E., Lew V. L., Bookchin R. M. Calcium accumulated by sickle cell anemia red cells does not affect their potassium (86Rb+) flux components. Blood. 1986 Mar;67(3):710–715. [PubMed] [Google Scholar]
- Ortiz O. E., Lew V. L., Bookchin R. M. Deoxygenation permeabilizes sickle cell anaemia red cells to magnesium and reverses its gradient in the dense cells. J Physiol. 1990 Aug;427:211–226. doi: 10.1113/jphysiol.1990.sp018168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pressman B. C. Biological applications of ionophores. Annu Rev Biochem. 1976;45:501–530. doi: 10.1146/annurev.bi.45.070176.002441. [DOI] [PubMed] [Google Scholar]
- Rhoda M. D., Apovo M., Beuzard Y., Giraud F. Ca2+ permeability in deoxygenated sickle cells. Blood. 1990 Jun 15;75(12):2453–2458. [PubMed] [Google Scholar]
- Schatzmann H. J. Dependence on calcium concentration and stoichiometry of the calcium pump in human red cells. J Physiol. 1973 Dec;235(2):551–569. doi: 10.1113/jphysiol.1973.sp010403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simonsen L. O., Gomme J., Lew V. L. Uniform ionophore A23187 distribution and cytoplasmic calcium buffering in intact human red cells. Biochim Biophys Acta. 1982 Nov 22;692(3):431–440. doi: 10.1016/0005-2736(82)90394-7. [DOI] [PubMed] [Google Scholar]
- TOSTESON D. C., CARLSEN E., DUNHAM E. T. The effects of sickling on ion transport. I. Effect of sickling on potassium transport. J Gen Physiol. 1955 Sep 20;39(1):31–53. doi: 10.1085/jgp.39.1.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TOSTESON D. C. The effects of sickling on ion transport. II. The effect of sickling on sodium and cesium transport. J Gen Physiol. 1955 Sep 20;39(1):55–67. doi: 10.1085/jgp.39.1.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiffert T., Etzion Z., Bookchin R. M., Lew V. L. Effects of deoxygenation on active and passive Ca2+ transport and cytoplasmic Ca2+ buffering in normal human red cells. J Physiol. 1993 May;464:529–544. doi: 10.1113/jphysiol.1993.sp019649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiffert T., Garcia-Sancho J., Lew V. L. Irreversible ATP depletion caused by low concentrations of formaldehyde and of calcium-chelator esters in intact human red cells. Biochim Biophys Acta. 1984 Jun 13;773(1):143–156. doi: 10.1016/0005-2736(84)90559-5. [DOI] [PubMed] [Google Scholar]
- Tiffert T., Spivak J. L., Lew V. L. Magnitude of calcium influx required to induce dehydration of normal human red cells. Biochim Biophys Acta. 1988 Aug 18;943(2):157–165. doi: 10.1016/0005-2736(88)90547-0. [DOI] [PubMed] [Google Scholar]
- Wiley J. S., Shaller C. C. Selective loss of calcium permeability on maturation of reticulocytes. J Clin Invest. 1977 Jun;59(6):1113–1119. doi: 10.1172/JCI108735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williamson P., Puchulu E., Penniston J. T., Westerman M. P., Schlegel R. A. Ca2+ accumulation and loss by aberrant endocytic vesicles in sickle erythrocytes. J Cell Physiol. 1992 Jul;152(1):1–9. doi: 10.1002/jcp.1041520102. [DOI] [PubMed] [Google Scholar]



