Abstract
Cultivation of actinomycete strain CNQ-418, retrieved from a deep ocean sediment sample off the coast of La Jolla, California, has provided marinopyrroles A–F. Sharing just 98% 16S rRNA gene sequence identity with S. sannurensis, the strain likely represents a new Streptomyces species. The metabolites contain an unusual 1,3′-bipyrrole core decorated with several chlorine and bromine substituents and possess marked antibacterial activity against methicillin-resistant Staphylococcus aureus (MRSA). The congested N,C-biaryl bond establishes an axis of chirality that, for marinopyrroles A-E, is configurationally stable at room temperature. Moreover, the natural products are fashioned strictly in the M-configuration. The Paal-Knorr condensation was adapted for the synthesis of the 1,3′-bipyrrole core. Halogenation of this material with N-bromosuccinimide cleanly furnished the 4,4′,5,5′-tetrahalogenated core that characterizes this class of marine-derived metabolites.
Keywords: marine bacterial metabolites; antibiotics; 1,3′-bipyrrole; atropisomerism; axial chirality
INTRODUCTION
The capacity of pathogenic bacteria, such as methicillin-resistant Staphylococcus aureus (MRSA), to develop resistance to antimicrobial compounds requires the “persistent discovery” of novel structural pharmacophores to combat infection.1 Bacterial resistance has developed against even the glycopeptide antibiotics (vancomycin and teicoplanin), which are widely considered the drugs of “last resort”. The first fully vancomycin-resistant S. aureus (VRSA) strain was isolated from the clinic in 2002.2
Marine bacteria of the order Actinomycetales (actinomycetes) have received little attention for their ability to produce antibiotics, although since the 1940s a plethora of antibiotics have been isolated from actinomycetes collected from the soil. Initially, the extent of evolutionary divergence between terrestrial and marine actinomycete strains was underestimated. Since then, comparisons of 16S rRNA sequence data have shown that deep-ocean sediments harbor new actinomycete taxa, some of which are fully restricted to life in the sea.3 Cultivation of these strains in the laboratory has yielded many structurally unique metabolites of considerable biological interest.4
As described in a previous communication,5 cultivation of the marine Streptomyces strain CNQ-418 in a seawater-based medium provided (−)-marinopyrroles A (1) and B (2), axially chiral, densely-halogenated natural products composed of two salicyloyl substituents on a 1,3′-bipyrrole core (Chart 1). The requirement of seawater for growth of this strain indicates a significant degree of adaptation to the marine environment. Under optimized culturing conditions, described herein, (−)-marinopyrroles C (3), (−)-D (4), (−)-E (5), and (±)-F (6) were also isolated from the culture broth of CNQ-418. The most abundant metabolite, marinopyrrole A (1), was obtained in a 50 mg L−1 yield after HPLC purification. Compared to 1, (−)-marinopyrroles C-E (3–5) possess an additional halogen atom attached to either the bipyrrole nucleus or one of the two salicyloyl moieties. The structure of (±)-marinopyrrole F (6) includes a rare eight-membered ether ring. Each metabolite, except (±)-6, was isolated as a single atropo-enantiomer exhibiting configurational stability at room temperature.
Chart 1.
Marinopyrroles A-F (1–6)
In addition to the marinopyrroles, there are only three known examples of axially chiral bipyrroles—1,1′-bipyrrole 7,6a 2,2′-bipyrrole 86b and, most recently, 3,3′-bipyrrole 96c (Chart 2). Compounds 7–9 are synthetic in origin and require a resolution step, either fractional recrystallization of a brucine salt or chiral HPLC purification, to attain atropo-enantiomeric purity. Furthermore, of the three possible bipyrroles that involve bonding through nitrogen only achiral 1,2′-bipyrroles have previously been shown to occur in nature.7 As such, the marinopyrroles represent the first naturally-occurring 1,3′-bipyrroles. Perhaps for this reason, reports that describe the synthesis of 1,3′-bipyrroles are few.8
Chart 2.
Axially chiral bipyrroles 7–9
RESULTS AND DISCUSSION
Sequence comparison of 16S rRNA, a 1542 nucleotide constituent of the prokaryotic 30S ribosomal subunit, has become a standard method for phylogenetic analysis of new bacterial isolates.9 Strain CNQ-418 shares 98.1% 16S rRNA gene sequence identity with its nearest neighbor, Streptomyces sannurensis (GenBank accession number bankit EU116310). Given that the ribosomal machinery is ancient and highly-conserved, small deviations in this sequence often reflect considerable genetic divergence. Though there is disagreement over the exact percentage of 16S rRNA variance that constitutes a new species, less than 97–99% sequence identity has been proposed as a benchmark. As such, CNQ-418 likely represents a new Streptomyces species.
Initial observations using proton NMR and UV/Vis spectroscopy revealed the largely aromatic character of marinopyrrole A (1). The presence of halogen atoms was concluded upon examining the LR-ESI mass spectrum [m/z (M+H)+: 509, 511, 513; m/z (M+Na)+: 531, 533, 535], which showed an isotope composition indicative of multiple chlorine substituents. Analysis of high-resolution mass spectral data indicated the molecular formula C22H12Cl4N2O4 [m/z (M–H)− calcd for C22H1135Cl4N2O4 506.9473, found 506.9447]. Analogues 2–6 displayed a similar UV profile. Marinopyrroles B (2) and E (5) possess an additional bromine atom (C22H11BrCl4N2O4), while marinopyrroles C (3) and D (4) have an additional chlorine atom (C22H11Cl5N2O4). Marinopyrrole F (6) contains an extra degree of unsaturation and analyzed for the molecular formula C22H11Cl3N2O4.
Planar Structure
Elucidation of the planar structure of marinopyrrole A (1) via NMR methods proved difficult. Only two salicyloyl substituents could be confidently defined by interpretation of COSY, HSQC, and HMBC NMR experiments (Figure 1). The presence of the ortho-hydroxyl functionality on each of these rings was concluded from the chemical shifts of the O-bearing aromatic carbons (δ 161.1 and 162.5) and from its reactivity with methylating and acylating reagents. Two sharp singlets at δ 11.18 and 10.42 were attributed to the peri-hydroxyl protons, and a broad 1H signal at δ 9.73 was assigned to an acidic N–H proton. A single aromatic singlet at δ 6.72, which showed a strong correlation to one of the carbonyl carbons (δ 186.8), as well as to carbons at δ 128.8 and 124.1, remained unassigned. With only two proton resonances available to map 2D-heteronuclear correlations, the structure of the central nitrogen-containing portion of the molecule, consisting of 14 contiguous quaternary carbon centers, could not be solved.10
Figure 1.

Partial NMR-based structure of marinopyrrole A (1)
The planar bipyrrole structure common to the marinopyrroles was finally defined by an X-ray crystal structure determination of marinopyrrole B (2). Slow evaporation of a pure solution of 2 in toluene at room temperature yielded crystals that were amenable to X-ray analysis. The X-ray structure showed that a polyhalogenated 1,3′-bipyrrole comprised the core of the molecule, a motif that no natural product to date has featured. Fortuitously—given the gamut of fruitless attempts to obtain an X-ray quality crystal from 1—the bromine atom in 2 facilitated the X-ray assignment of an M-configuration to the natural product (vide infra).
The bipyrrole skeleton having been revealed, the planar structures for marinopyrroles A (1) and C-D (3–5) were then readily determined by interpretation of 2D NMR and mass spectral data. Since the additional singlet at δ 6.72 in the proton spectrum of 1 showed a strong HMBC correlation to the C-6′ carbonyl group, it was positioned at C-3′ (see Figure 1). The HMBC correlation from H-3′ to the C-6′ ketone carbon was instrumental in uncovering the halogenation pattern in penta-halogenated analogues 3–5. Combined with the correlation from H-12′ to C-6′, the two salicyloyl substituents could be distinguished from each other (see Figure 1).
Marinopyrrole C (3) analyzed for the molecular formula C22H11Cl5N2O4. The carbon signal at δC 186.7 was assigned to C-6′ based on the HMBC correlation from H-3′ (δ 6.81). The other carbonyl signal at δC 184.5 was then assigned to C-6. An HMBC correlation from the proton doublet at δ 7.66 (H-12′) to C-6′ allowed entry into the adjacent salicyloyl spin system. Uninterrupted COSY correlations between the protons of this salicyloyl ring showed the absence of a substituent. Instead, HMBC and COSY correlations indicated that the chlorine atom was attached to the other salicyloyl ring at C-11 (δC 123.8).11
Marinopyrrole D (4) also analyzed for the molecular formula C22H11Cl5N2O4. The carbon signal at δC 185.3 was assigned to C-6′ based on the HMBC correlation from H-3′ (δ 6.69). The signal at δC 185.1 was then assigned to C-6. Here, an HMBC correlation from the proton signal at δ 7.32 (H-12′) to C-6′ allowed entry into the adjacent salicyloyl spin system. In this isomer, COSY and HMBC correlations showed that the chlorine substituent was attached to the same salicyloyl ring at C-11′ (δC 123.7). COSY correlations between protons of the other salicyloyl ring (H-9 to H-12) were uninterrupted.
The NMR spectra for marinopyrrole E (5), C22H11BrCl4N2O4, were similar to the spectra for marinopyrrole D (4). As before, the signals at δC 185.3 and δC 185.1 were assigned to C-6′ and C-6. COSY and HMBC correlations showed that the halogen atom was attached to the lower salicyloyl ring at C-11′ (δC 120.0).
Elucidation of the planar structure of marinopyrrole F (6), C22H11Cl3N2O4, required more analysis. In the 1H NMR spectrum of 6, a low field peri-hydroxyl proton signal, one of two, was conspicuously missing, and the chemical shift of the C-6 carbonyl carbon was shifted 10 ppm upfield (δC 175.8) compared to 1–5. Formation of this trichloro analogue, we thought, may arise from intramolecular displacement of the chlorine atom at C-4 or C-5′ in 1 by the pendant C-8 or C-8′ phenolic group. An HMBC NMR correlation from H-3′ to an aromatic carbon at δ 145.8 suggested attachment of C-5′ to an electronegative oxygen atom. Reaction of the C-8 phenolic group via intramolecular cyclization would also account for the upfield shift of C-6, since the hydrogen bond between the peri-proton and the C-6 carbonyl group would no longer exist. In addition, this putative cyclization step is consistent with the observation that nucleophilic aromatic substitution readily occurs at C-5′ in 1 with several external nucleophiles (vide infra). An X-ray structure verified our proposed structure of marinopyrrole F (6) as an eight-membered ether derivative of 1 (Figure 2).
Figure 2.
ORTEP drawing of marinopyrrole F (6)
Absolute Configuration
X-Ray analysis of marinopyrrole B (2) allowed for assignment of an M-configuration to this metabolite. The circular dichroism spectrum of 2, when compared to the spectra of the other metabolites, showed that marinopyrroles A (1), C (3), D (4), and E (5) were also M-configured. Each CD spectrum showed negative first and positive second Cotton effects in the salicyloyl-absorbing region (λ 300–400 nm) (see Supporting Information). This pattern suggested a negative exciton coupling between the salicyloyl substituents of 1–5, agreeing with an M-configuration about the biaryl bond. The enantiopurity of metabolites 1–5 was confirmed using chiral HPLC (Chiralcel® OD-H), which indicated that biosynthetic formation of the N,C-biaryl bond proceeds in a highly atropo-selective manner.
Although metabolites 1–5 are fashioned by nature in enantiopure form, the molecules could be racemized at elevated temperatures.12 Heating marinopyrrole A (1) and B (2) in toluene at 120 °C over 20 h was sufficient for complete racemization. Interestingly, racemization of marinopyrroles 3–5 either required prolonged heating in 1,2-dichlorobenzene at 150 °C (as in 3) or could not be noticeably accomplished at all (as in 4 and 5), even at 180 °C. It appears that the steric influence of the halogen substituents at C-11 and C-11′ significantly raises the energetic barrier for rotation about the biaryl bond.
In contrast, marinopyrrole F (6) was isolated in racemic form. The material produced no measurable optical rotation and lacked the CD Cotton effects apparent in 1–5. Although formation of the oxacycle in 6 may proceed without stereoselection, another possibility is that this biaryl bond itself is not configurationally stable at room temperature. To ascertain this, a sample of enantio-enriched 6 was collected using chiral HPLC. Analysis of this material, stored in methanol for 18 h at room temperature, showed complete racemization. Apparently, the fused ether ring lowers the barrier for atropisomerism in 6. The optical purity degrades at room temperature, despite the sterically-hindered environment still present about the biaryl bond.
Reactivity
Monodeoxypyoluteorin (10)13 was also isolated from cultures of actinomycete strain CNQ-418, providing direct evidence for the biosynthetic origin of marinopyrrole A (1). The structure and antibacterial activity of pyoluteorin14a–c and the related pyrrolomycins14d–f are well-documented. We inferred, however, that the 1,3′-bipyrrole structure of the marinopyrroles would exhibit reactivity that is distinct from its simpler pyrrole congeners. A distinct mode of reactivity might point toward a unique target or mechanism-of-action. In order to probe the inherent reactivity of the marinopyrrole structure and define structure-activity relationships, a semisynthetic study of marinopyrrole A (1) was undertaken.

Manipulation of the polar salicyloyl ester functionality on the molecule’s periphery was first examined (Scheme 1). Both phenolic groups of marinopyrrole A (1) were cleanly acetylated to provide 11 via treatment with acetic anhydride. The natural product was per-methylated using dimethylsulfate to afford 12. Finally, as a testament to the strong hydrogen bonding interaction between each carbonyl group and its peri-proton, the pyrrole nitrogen could be selectively methylated in a cold ethereal solution of diazomethane to give compound 13. Yields of 13 were much lower when the reaction was carried out in methanol, a solvent that likely disrupts the peri-hydrogen bonding.
Scheme 1.
“Capping” the polar functionality in 1a
a Reagents and conditions: (a) Ac2O, DMAP, pyr., rt, 62%; (b) Me2SO4, K2CO3, Me2CO, rt, quant.; (c) CH2N2, ether, 0 °C, 86%. DMAP = 4-(dimethylamino)pyridine
The highly-halogenated bipyrrole structure of marinopyrrole A (1) and its analogues suggested that the molecules may act as electrophiles. Accordingly, 1 was treated with oxygen, sulfur, and nitrogen-containing nucleophiles (Scheme 2). Under basic conditions and elevated temperatures, 1 reacted with methanol (1 to 14), N-acetylcysteamine (1 to 15), and dimethylamine (1 to 16) via an aromatic substitution reaction.15 The displacement of chloride by these nucleophiles occurred exclusively at C-5′, as illustrated by analysis of 2D HMBC experiments (Table 2). The mutual HMBC correlation from H-3′ and H-14′ in 14–16 to C-5′ established the site of substitution. As expected, the carbon resonance of C-5′ was greatly influenced by the presence of the newly-attached heteroatom, shifting C-5′ to δC 146.7 in 14, to δC 131.6 in 15, and to δC 143.8 in 16. The C-5′ chemical shift in methyl ether 14 (δC 146.7) was in close agreement with the shift in phenyl ether 6 (δ 145.8).
Scheme 2.
Nucleophilic aromatic substitution at C-5′a
a Reagents and conditions: (a) K2CO3, MeOH, 65 °C, 42% (63% b.o.r.s.m); (b) N-acetylcysteamine, K2CO3, DMF, 80 °C, 55%; (c) NMe2, THF, 65 °C, 42%; (d) DMA, 145 °C, 62%; (e) NMe2, THF, 55 °C, quant. DMF = N,N-dimethylformamide, DMA = N,N-dimethylacetamide. b.o.r.s.m. = based on recovered starting material.
Table 2.
Selected 1H and 13C NMR spectral data for 14–16 (CDCl3)
 | ||||||
|---|---|---|---|---|---|---|
| 14 (X = O) | 15 (X = S) | 16 (X = N) | ||||
| position | δCa | δH, mult.b | δCa | δH, mult.b | δCa | δH, mult.c |
| 3′ | 122.4 | 6.66, s | 119.9 | 6.82, s | 122.0 | 6.69, s |
| 5′ | 146.7 | 131.6 | 143.8 | |||
| 6′ | 186.3 | 187.6 | 186.6 | |||
| 14′ | 60.9 | 3.79, s | 35.4 | 2.37, m 2.67, m |
41.5 | 2.30, s |
75 MHz.
500 MHz.
600 MHz.
The tendency for aromatic substitution at C-5′ was further demonstrated when, after prolonged heating at 120 °C in N,N-dimethylacetamide (DMA), marinopyrrole A (1) was converted to marinopyrrole F (6) (see Scheme 2). Subsequent treatment of 6 with dimethylamine effected, in quantitative yield, conversion to 16. Thus, in addition to 1, ether 6 can also function as an effective arylating agent. Monodeoxypyoluteorin and its derivatives, notably, do not participate in nucleophilic aromatic substitution under similar conditions.
Lastly, when subjected to elevated temperatures in pyridine, marinopyrrole A (1) reacted with the ε-amine of N-α-benzyloxycarbonyl-lysine methyl ester hydrochloride to give imine 17 (Scheme 3). The adduct was isolated as a mixture of two imine conformers after purification by reversed-phase HPLC.16,17 HMBC correlations from H-14 [δ 3.32 (1H), 3.17 (2H), and 3.02 (1H)] to carbonyl signals at δC 161.9 and 161.5 showed the presence of an imine. Several other HMBC correlations revealed that imine formation occurred exclusively at C-6. First, HMBC correlations from H-12 (δ 7.09 and 7.00) to C-6 were evident.18 In addition, the H-3′ singlets at δ 6.66 and 6.53, one from each imine conformer, and the H-12′ doublets at δ 7.75 and 7.40 showed HMBC correlations to the intact C-6′ ketone (δC 185.9 and 187.1). The mechanism by which the molecule targets actin and elicits cytotoxicity in eukaryotic cells, detailed in a previous publication by our group,19 may indeed depend upon imine formation between 1 and a key amine-containing residue of the target.
Scheme 3.
Imine formation at C-6
Marinopyrrole A (1) is constitutionally and configurationally stable to a variety of both strongly acidic conditions (e.g., trifluoroacetic acid in dichloromethane) and strongly basic conditions (e.g., 6 N aqueous sodium hydroxide in methanol), but can be racemized at temperatures above 100 °C. Although the conversion of 1 to compounds 11–16 was accomplished under less rigorous conditions, the enantiopurity of many of these derivatives eroded. The optical purity of the material was maintained when reaction occurred distal to the biaryl bond, as in the formation of 13. When the reaction proceeded at a site ortho to the biaryl bond, however, the products were obtained either in diminished enantiopurity or in racemic form, as in 14 (0% ee), 15 (76% ee), and 16 (0% ee).
Synthetic Studies
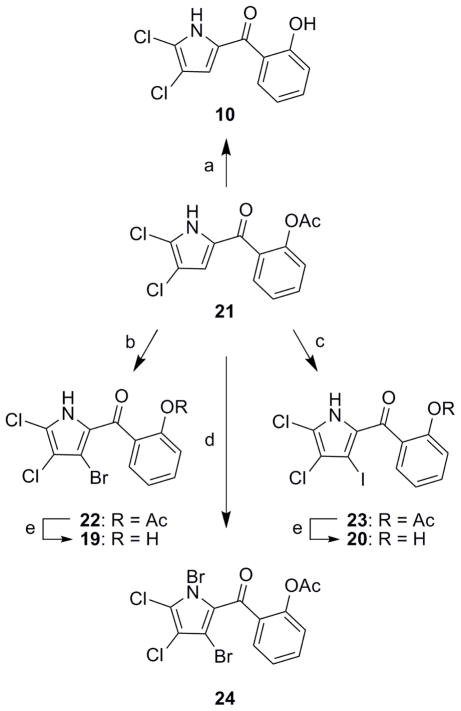
Although the preparation of N-phenyl azoles via Ullman coupling is well documented, direct construction of an N,C-linked bipyrrole in this way has not been previously demonstrated.20,21 A total synthesis of marinopyrrole A (1) that featured Ullman coupling of two pyrroles would, therefore, be novel (Scheme 4). In this reaction, a copper (I) salt catalyzes carbon-nitrogen bond formation between monodeoxypyoluteorin (10) and C-3-iodo or -bromo monodeoxypyoluteorin (19,20). Chelation both of diamine ligands (e.g., N,N-dimethylethane-1,2-diamine) and the pendant carbonyl functionality in 10 should promote oxidative addition of organocopper (I) species 18 to the copper (III) intermediate.
Scheme 4.
Proposed Ullman coupling for the synthesis of (±)-1
A short sequence consisting of Friedel-Crafts acylation of pyrrole with 2-methoxybenzoyl chloride, demethylation, acetylation, and selective chlorination of the pyrrole provided O-acetyl monodeoxypyoluteorin (21) (Scheme 5).13b The phenol functionality was liberated in aqueous acid to give 10, which agreed in all respects with the natural material obtained from the culture of strain CNQ-418. To prepare a coupling partner for the Ullman reaction, 21 was converted to 22 in 57% yield using one equivalent of N-bromosuccinimide (NBS). Acetate 22 afforded 19, the structure of which was verified with an X-ray crystal structure determination (See Supporting Information). Similarly, 21 reacted with N-iodosuccinimide (NIS) in 65% yield to give 23, which after deprotection yielded 20.22 N-Bromination to form 24 occurred when excess NBS was employed.23
Scheme 5.
Synthesis of monodeoxypyoluteorin (10) and 3-halo analoguesa
a Reagents and conditions: (a) aq. HCl, MeOH, rt, 83%; (b) NBS (1 equiv), DCE, 90 °C, 57%; (c) NIS, DCE, 90 °C, 65%; (d) NBS (2 equiv), DCE, 90 °C, 82%; (e) aq. HCl, MeOH, rt, 19: 94%, 20: 94%. NBS = N-bromosuccinimide, NIS = N-iodosuccinimide, DCE = 1,2-dichloroethane.
Attempts to couple 3-halo pyrroles 19,20,22,23 to either 10 or 21, and effect formation of the key biaryl bond, proved fruitless. Several modern methods were employed.20 Presumably, the reaction was not attainable due to unfavorable steric interactions between the substituents ortho to the site of bond formation. Indeed, the efficiency of transition metal-catalyzed Ullman coupling reactions described in the literature is largely reduced by the presence of one or more ortho-substituents. Even di-ortho-substituted biaryls are often inaccessible or require forceful conditions (infinite concentrations and high catalyst loading).20a Consequently, the goal to prepare a tetra-ortho-substituted biaryl compound, such as marinopyrrole A, in this way may be too lofty.
Intermolecular Ullman coupling between monodeoxypyoluteorin (10) and 3-halo substrates was often preceded by intramolecular reaction of the organocopper species with the pendant phenol. The acetate functionality in 22 and 23 was unstable to the reaction conditions, and the phenol groups, once liberated, engaged in intramolecular coupling to furnish a 1H-chromeno[3,2-b]pyrrol-9-one. Under optimized conditions for the production of chromene 25, bromide 19 was subjected to copper (I) iodide and potassium carbonate at 110 °C (Scheme 6). A 41% yield of 25 was thus obtained after HPLC purification. The preparation of 1H-chromeno[3,2-b]pyrrol-9-ones has been described only once before, employing a reductive cyclization of a nitro-coumarin with triethylphosphite.24 Unlike monodeoxypyoluteorin (10) and its 3-halo analogues, chromene 25 displayed little activity against methicillin-resistant S. aureus and human colon cancer cells (HCT-116).
Scheme 6.
1H-chromeno[3,2-b]pyrrol-9-one 25 via intramolecular Ullman reaction
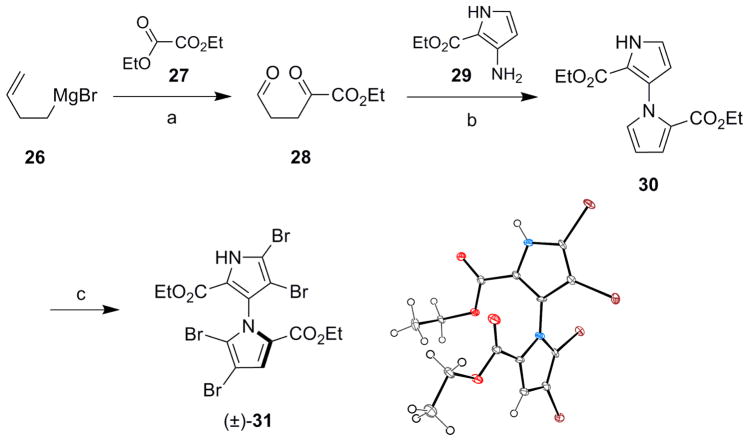
Although we could not effect coupling of two salicyloyl-substituted pyrrole monomers to form marinopyrrole A, we remained interested in the synthesis of the 1,3′-bipyrrole core. 1,3′-Bipyrroles have been previously synthesized according to one of two methods: (1) condensation of a 1,4-diketo compound onto a 3-aminopyrrole (Paal-Knorr reaction) and (2) reaction of a β-pyrroyl-α,β-unsaturated ester with tosylmethyl isocyanide (TosMIC).8 Adopting a strategy based on the former method, Grignard reagent 26 was treated with oxalate 27 at low temperature to give the product resulting from mono-addition, as described in the literature (Scheme 7).25 Ozonolysis then provided aldehyde 28. This material was condensed with 3-aminopyrrole 2926 to furnish bipyrrole 30, the structure of which was confirmed through a series of 2D NMR experiments (see Supporting Information). Brominated bipyrrole 31 was obtained via treatment with NBS, though tetrachlorination of 30 using excess NCS could not be accomplished. The structure of 31 was confirmed by X-ray analysis. Tetrabromide 31 should exist as a racemic mixture of two configurationally stable atropo-enantiomers at room temperature, but this could not be strictly confirmed by chiral chromatography.
Scheme 7.
Synthesis of the tetrahalogenated 1,3′-bipyrrole core of the marinopyrrolesa
a Reagents and conditions: (a) 1. 27, THF, −78 °C, 90%; 2. O3, −78 °C, then Me2S; (b) 29, THF, PTSA, 23% (two steps); (c) NBS, DCE, 90 °C, 86%. PTSA = p-toluenesulfonic acid, NBS = N-bromosuccinimide, DCE = 1,2-dichloroethane
Antibiotic Properties
The marinopyrroles A–C (1–3), obtained in sufficient quantity and purity for testing, displayed significant activity against methicillin-resistant Staphylococcus aureus (MRSA) with MIC90 values of less than 1 μg mL−1 (Table 3). Marinopyrrole F (6) and derivatives 11–16, encompassing modifications of 1 either at a polar functionality on the periphery or at core halogen substituents, were much less active against MRSA. The loss in activity of 11–13 reflects the importance of the hydrogen bonding capacity of the salicyloyl hydroxyl groups and the N–H functionality. Methylation of the latter functionality proved particularly detrimental to activity. The requirement of a free N–H group for activity against MRSA may point toward a crucial hydrogen-bonding interaction between the molecule and its bacterial target. The loss in antimicrobial activity of 6, 15, and 16 may reflect the central role that the C-5′ chlorine substituent plays in the mechanism of antibiotic action. Ether 14 retained much of the activity characteristic of metabolites 1–3, presumably, since the C-5′ methoxy substituent can also function as an effective leaving group in nucleophilic aromatic substitution reactions. Remarkably, none of the semisynthetic derivatives were more active against MRSA than the natural products. Advanced intermediates 30 and 31 showed little antimicrobial activity and cytotoxicity, pointing to the importance of the salicyloyl substituents.
Table 3.
| compd | MRSAa (MIC90) | HCT-116b (IC50) |
|---|---|---|
| (–)-1 | 0.31 | 4.5 |
| (–)-2 | 0.63 | 5.3 |
| (–)-3 | 0.16 | 0.21 |
| (±)-6 | 3.1 | 2.9 |
| 11 | 1.6 | 0.42 |
| 12 | NSA | NSA |
| 13 | NSA | NSA |
| 14 | 0.78 | 1.1 |
| 15 | 6.3 | 7.2 |
| 16 | 1.6 | 4.4 |
MRSA = methicillin-resistant Staphylococcus aureus. Positive control: vancomycin (MIC90 = 0.20–0.39 μg mL−1) and penicillin G (MIC90 = 6.3–12 μg mL−1).
HCT-116 is a human colon cancer cell line. Positive control: etoposide (IC50 = 0.29–2.9 μg mL−1). NSA = No Significant Activity (> 8 μg mL−1).
Many of the MRSA-active compounds also displayed significant cytotoxicity toward the human colon cancer line, HCT-116, with IC50’s predominantly between 1 and 5 μg mL −1 (see Table 3). The therapeutic window for the marinopyrroles, therefore, may be too narrow for the treatment of bacterial infection in humans. That the marinopyrroles, given their inherent cytotoxicity, may function as effective cancer chemotherapy agents remains an issue to be explored.
CONCLUSION
Herein we report the final structure assignments and reactivity of a family of densely-halogenated, axially chiral metabolites from a marine actinomycete strain identified as a member of the genus Streptomyces (strain CNQ-418). The marinopyrroles are antibiotics of an unprecedented structure class, containing the first naturally-occurring 1,3′-bipyrrole. Except for (±)-marinopyrrole F (6), the metabolites are M-configured and configurationally stable at room temperature. Given the difficulty associated with the transition metal-catalyzed formation of tetra-ortho-substituted biaryls in chemical synthesis, the biosynthetic mechanism by which this coupling occurs for the marinopyrroles is, necessarily, of considerable interest. That the N,C-biaryl bond is formed in a completely atropo-selective manner is likewise appealing. As such, elucidation of the biosynthetic gene cluster responsible for marinopyrrole production may set the stage for a biomimetic total synthesis and even elicit the development of novel biaryl coupling reactions with larger scope.
EXPERIMENTAL SECTION
Cultivation of CNQ-418
The strain was cultured in 2.8 L Fernbach flasks (20 × 1 L) in a seawater-based medium (per 1 L seawater: 10 g starch, 4 g yeast extract, 2 g peptone, 1 g CaCO3, 40 mg Fe2(SO4)3•4 H2O, 100 mg KBr) and shaken at 230 rpm at 27 °C.
Isolation and purification of the marinopyrroles
After 17 days of cultivation, sterilized XAD-18 resin (20 g L−1) was added, and the resin was collected 24 h later by filtration, washed with deionized water, and eluted with acetone. The acetone was removed under reduced pressure, and the resulting aqueous layer was extracted with ethyl acetate (3 × 600 mL). The combined extracts were dried, filtered, and concentrated. The crude extract was loaded onto silica gel (20 g) and fractionated on a silica column (80 × 60 mm, h x w) using a stepwise gradient (0.5% EtOAc in hexanes, 30% EtOAc in hexanes, EtOAc, 10% MeOH in EtOAc, MeOH). The fractions were concentrated to give F1 (81.6 mg), F2 (1.34 g), F3 (199.8 mg), F4 (144.8 mg), and F5 (422.4 mg). A portion of F2 (134 mg) was dissolved in MeOH (3.0 mL), and the marinopyrroles were purified by reversed-phase HPLC (80% MeOH in H2O, 0.2% TFA). Pure marinopyrrole A (1) (92.1 mg) was thus obtained. Further purification by reversed-phase HPLC (65–68% CH3CN in H2O, 0.2% TFA) provided, in order of elution, marinopyrrole F (6) (2.7 mg), C (3) (1.2 mg), B (2) (2.0 mg), D (4) (1.8 mg), E (5) (0.5 mg).
Marinopyrrole A (1)
UV/Vis (CH3CN/H2O): λmax = 270, 324, 348 nm; [α]D −69 (c 0.39, MeOH); IR (film): ν̃ = 1623, 1595 cm−1; 1H NMR: see Table 1; 13C NMR: 186.8, 185.9, 162.5, 161.1, 136.3, 136.2, 131.9, 130.3, 128.8, 124.1, 123.8, 123.1, 120.4, 120.3, 119.1, 118.9, 118.9, 118.7, 118.4, 117.8, 112.7, 110.7; HRMS (ESI-TOF): m/z (M–H)− calcd for C22H1135Cl4N2O4 506.9473, found 506.9447; m/z (M+Na)+ calcd for C22H1235Cl4N2O4Na 530.9449, found 530.9439.
Table 1.
1H and 13C NMR spectral data for marinopyrroles A-F (1–6)
| A (1) | C22H12Cl4N2O4 in CDCl3 | B (2) | C22H11BrCl4N2O4 in CDCl3 | C (3) | C22H11Cl5N2O4 in CDCl3 | D (4) | C22H11Cl5N2O4 in CDCl3 | E (5) | C22H11BrCl4N2O4 in CDCl3 | F (6) | C22H11Cl3N2O4 in DMSO-d6 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| position | δCa | δH, mult. (J, Hz)b | δCa | δH, mult. (J, Hz)b | δCc | δH, mult. (J, Hz)b | δCc | δH, mult. (J, Hz)b | δCc | δH, mult. (J, Hz)b | δCa | δH, mult. (J, Hz)b |
| 6 | 185.9 | – | 185.3 | – | 184.5 | – | 185.1 | – | 185.1 | – | 175.8 | – |
| 8 | 161.1 | – | 161.4 | – | 159.4 | – | 161.0 | – | 161.0 | – | 157.0 | – |
| 9 | 117.8 | 6.92, md | 118.1 | 6.96, d (8.2) | 118.9 | 6.87, d (9.0) | 118.0 | 6.94, mf | 118.4 | 6.96, d (7.8) | 123.1 | 7.78, mh |
| 10 | 136.2 | 7.35, t (7.8) | 136.4 | 7.42, m | 135.5 | 7.27, m | 136.3 | 7.40, t (7.8) | 136.0 | 7.40, t (7.8) | 135.9 | 7.83, mh |
| 11 | 118.7 | 6.52, t (7.8) | 118.6 | 6.58, m | 123.8 | – | 118.8 | 6.55, t (7.8) | 119.1 | 6.55, t (7.8) | 127.7 | 7.55, t (7.8) |
| 12 | 130.3 | 7.48, d (7.8) | 130.0 | 7.51, me | 129.6 | 7.58, d (2.4) | 129.9 | 7.33, mg | 129.7 | 7.32, d (7.8) | 132.7 | 8.06, d (7.8) |
| 3′ | 120.3 | 6.72, s | –j | – | 120.9 | 6.81, s | 120.4 | 6.69, s | 120.6 | 6.68, s | 120.4 | 6.95, s |
| 6′ | 186.8 | – | 188.5 | – | 186.7 | – | 185.3 | – | 185.3 | – | 183.8 | – |
| 8′ | 162.5 | – | 162.5 | – | 162.4 | – | 160.7 | – | 161.2 | – | 158.2 | – |
| 9′ | 118.4 | 7.03, d (7.8) | 117.9 | 7.00, d (8.2) | 118.3 | 7.03, d (7.8) | 120.1 | 6.96, mf | 120.4 | 6.91, d (9.0) | 117.0 | 6.91, mi |
| 10′ | 136.3 | 7.51, t (7.8) | 137.3 | 7.51, me | 136.1 | 7.52, t (7.8) | 136.3 | 7.44, d (9.0) | 139.0 | 7.56, d (9.0) | 134.0 | 7.44, t (7.8) |
| 11′ | 119.1 | 6.90, md | 119.2 | 6.85, m | 118.9 | 6.92, t (7.8) | 123.7 | – | 120.0 | – | 118.8 | 6.94, mi |
| 12′ | 131.9 | 7.58, d (7.8) | 134.2 | – | 131.1 | 7.66, d (7.8) | 130.3 | 7.32, mg | 133.4 | 7.44, d (2.6) | 130.9 | 7.67, d (7.8) |
| −NH, OH | 9.73, br s | 9.55, br s | 9.51, br s | 9.58, br s | 9.55, br s | 10.27, s | ||||||
| 10.42, s | 10.39, s | 10.26, s | 10.31, s | 10.31, s | 13.77, s | |||||||
| 11.18, s | 11.10, s | 11.06, s | 11.03, s | 11.05, s | ||||||||
75 MHz.
500 MHz.
Carbon chemical shifts were based on HSQC and HMBC data (500 MHz).
Overlapping signals.
Signal could not be assigned.
Marinopyrrole B (2)
UV/Vis (CH3CN/H2O): λmax = 274, 322, 348 nm; [α]D −72 (c 0.20, MeOH); IR (film): ν̃ = 1622, 1599 cm−1; 1H NMR: see Table 1; 13C NMR: 188.5, 185.3, 162.5, 161.4, 137.3, 136.4, 134.2, 133.7, 130.0, 124.4, 121.9, 121.7, 120.0, 119.2, 118.8, 118.6, 118.5, 118.1, 117.9, 114.7, 104.7 (one quaternary carbon signal was not detected); HRMS (ESI-TOF): m/z (M–H)− calcd for C22H1079Br35Cl4N2O4 584.8578, found 584.8588; m/z (M+Na)+ calcd for C22H1179Br35Cl4N2O4Na 608.8554, found 608.8570.
Marinopyrrole C (3)
UV/Vis (CH3CN/H2O): λmax = 270, 324, 346 nm; [α]D −100 (c 0.040, MeOH); IR (film): ν̃ = 1623, 1597 cm−1; 1H NMR: see Table 1; 13C NMR (HSQC and HMBC): 186.7, 184.5, 162.4, 159.4, 136.1, 135.5, 131.1, 129.6, 128.9, 123.8, 123.8, 120.9, 118.9, 118.9, 118.3; HRMS (ESI-TOF): m/z (M+H)+ calcd for C22H1235Cl5N2O4 542.9240, found 542.9229.
Marinopyrrole D (4)
UV/Vis (CH3CN/H2O): λmax = 270, 320, 352 nm; [α]D −55 (c 0.080, MeOH); IR (film): ν̃ = 1624, 1593 cm−1; 1H NMR: see Table 1; 13C NMR (HSQC and HMBC): 185.3, 185.1, 161.0, 160.7, 136.3, 136.3, 130.3, 129.9, 128.4, 124.9, 123.7, 120.4, 120.1, 118.8, 118.0; HRMS (ESI-TOF): m/z (M+H)+ calcd for C22H1235Cl5N2O4 542.9240, found 542.9217.
Marinopyrrole E (5)
UV/Vis (CH3CN/H2O): λmax = 270, 320, 350 nm; [α]D −100 (c 0.005, MeOH); IR (film): ν̃ = 1623, 1593 cm−1; 1H NMR: see Table 1; 13C NMR (HSQC and HMBC): 185.3, 185.1, 161.2, 161.0, 139.0, 136.0, 133.4, 129.7, 128.4, 124.9, 120.6, 120.4, 120.0, 119.1, 118.4; HRMS (ESI-TOF): m/z (M+H)+ calcd for C22H1279Br35Cl4N2O4 586.8735, found 586.8740.
Marinopyrrole F (6)
UV/Vis (CH3CN/H2O): λmax = 264, 336 nm; [α]D 0 [c 0.057, MeOH:Me2CO (2:1)]; IR (film): ν̃ = 1620, 1601 cm−1; 1H NMR (DMSO-d6): see Table 1; 13C NMR (DMSO-d6): 183.8, 175.8, 158.2, 157.0, 145.8, 135.9, 134.0, 132.7, 130.9, 129.0, 127.7, 125.6, 123.2, 123.1, 121.6, 121.5, 120.9, 120.4, 118.8, 117.0, 105.7, 99.2; HRMS (ESI-TOF): m/z (M+H)+ calcd for C22H1235Cl3N2O4 472.9863, found 472.9858. (±)-Marinopyrrole F (6) was prepared from marinopyrrole A (1): A solution of metabolite 1 (4.5 mg, 0.0088 mmol) in DMA (1.0 mL) was heated at 145 °C for 3 d. The mixture was allowed to cool and concentrated under full vacuum. The product was purified by reversed-phase HPLC (65% CH3CN in H2O, 0.2% TFA, tR = 27 min) to give 2.6 mg (62%) of (±)-6 as a white solid. All analytical and spectroscopic data agreed with the natural product.
4,4′,5,5′-Tetrachloro-1′H-1,3′-bipyrrole-2,2′-diyl)bis((2-acetoxyphenyl)methanone) (11)
To a solution of metabolite 1 (18.0 mg, 0.0353 mmol) and DMAP (1 mg) in pyridine (1.0 mL) was added acetic anhydride (0.20 mL, 2.7 mmol) dropwise via syringe. The mixture was stirred at room temperature for 5 h and concentrated. The product was purified twice by reversed-phase HPLC (70% CH3CN in H2O, 0.2% TFA, tR = 18 min; then 80% MeOH in H2O, 0.2% TFA, tR = 25 min) to yield 12.9 mg (62%) of 11 as a white solid: [α]D +20 (c 0.38, MeOH); IR (film): ν̃ = 1767, 1640 cm−1; 1H NMR: δ 9.66 (br s, 1H), 7.58–7.51 (m, 2H), 7.38 (t, J = 7.8 Hz, 1H), 7.35–30 (m, 2H), 7.18 (d, J = 7.8 Hz, 1H), 7.12–7.08 (m, 2H), 6.52 (s, 1H), 2.20 (s, 3H), 2.19 (s, 3H); 13C NMR (125 MHz): δ 181.4, 180.2, 169.5, 169.3, 148.8, 148.4, 132.4, 132.2, 130.8, 130.1, 129.9, 129.2, 128.8, 125.4, 125.3, 125.3, 124.6, 123.8, 123.4, 123.3, 121.6, 120.4, 112.2, 111.2, 20.9, 20.7; HRMS (ESI-TOF): m/z (M+H)+ calcd for C26H1735Cl4N2O6 592.9841, found 592.9840.
(4,4′,5,5′-Tetrachloro-1′-methyl-1′H-1,3′-bipyrrole-2,2′-diyl)bis((2-methoxyphenyl)methanone) (12)
To a solution of metabolite 1 (25.2 mg, 0.0494 mmol) and potassium carbonate (200 mg) in acetone (1.0 mL) was added dimethyl sulfate (0.10 mL, 1.1 mmol) dropwise via syringe. The mixture was heated at 40 °C overnight, allowed to cool, filtered through Celite with acetone washings, and concentrated. The product was purified by reversed-phase HPLC (73% CH3CN in H2O, 0.2% TFA, tR = 23 min) to furnish 27.3 mg (100%) of 12 as a white solid: [α]D −92 (c 0.42, MeOH); IR (film): ν̃ = 1647, 1597 cm−1; 1H NMR: δ 7.40 (t, J = 7.8 Hz, 1H), 7.24–7.18 (m, 3H), 6.98–6.93 (m, 2H), 6.75 (d, J = 8.2 Hz, 1H), 6.67 (t, J = 7.8 Hz, 1H), 6.25 (s, 1H), 4.05 (s, 3H), 3.80 (s, 3H), 3.75 (s, 3H); 13C NMR: δ 184.2, 182.9, 157.3, 157.0, 131.9, 131.9, 130.7, 129.4, 129.1, 127.5, 127.4, 126.4, 125.6, 125.0, 123.6, 121.2, 119.8, 119.6, 111.7, 111.5, 110.5, 109.6, 55.6, 55.5, 34.7; HRMS (ESI-TOF): m/z (M+H)+ calcd for C25H1935Cl4N2O4 551.0099, found 551.0100.
(4,4′,5,5′-Tetrachloro-1′-methyl-1′H-1,3′-bipyrrole-2,2′-diyl)bis((2-hydroxyphenyl)methanone) (13)
To a solution of metabolite 1 (8.7 mg, 0.017 mmol) in ether (2.0 mL) at 0 °C was added an ethereal solution of diazomethane via pipette (torched to remove sharp edges). After one minute, several drops of acetic acid were added (until gas evolution ceased), and the solution was concentrated. The product was purified by reversed-phase HPLC (72% CH3CN in H2O, 0.2% TFA, tR = 33 min) to afford 7.7 mg (86%) of 13 as a pale yellow solid: [α]D −62 (c 0.35, MeOH); IR (film): ν̃ = 1624, 1596 cm−1; 1H NMR: δ 11.17 (s, 1H), 10.94 (s, 1H), 7.64 (d, J = 7.8 Hz, 1H), 7.54 (d, J = 7.8 Hz, 1H), 7.49 (t, J = 7.8 Hz, 1H), 7.34 (t, J = 7.8 Hz, 1H), 7.01 (d, J = 8.2 Hz, 1H), 6.91–6.87 (m, 2H), 6.59 (s, 1H), 6.55 (t, J = 7.8 Hz, 1H), 3.89 (s, 3H); 13C NMR: δ 187.9, 186.7, 162.4, 161.9, 136.4, 136.1, 131.9, 131.6, 129.0, 125.1, 124.6, 122.6, 122.5, 120.3, 119.7, 119.1, 118.9, 118.5, 118.3, 117.6, 112.3, 108.7, 34.3; HRMS (ESI-TOF): m/z (M+H)+ calcd for C23H1535Cl4N2O4 522.9786, found 522.9778.
(4,4′,5′-Trichloro-5-methoxy-1′H-1,3′-bipyrrole-2,2′-diyl)bis((2-hydroxyphenyl)methanone) (14)
To metabolite 1 (10.0 mg, 0.0196 mmol) and potassium cabonate (100 mg, 0.72 mmol) was added MeOH (2.0 mL). The mixture was heated at 65 °C for 2 d, allowed to cool, diluted with a saturated NH4Cl solution (4 mL), and extracted with EtOAc (2 × 2 mL). The combined extracts were washed with brine (1 mL), dried, filtered, and concentrated. The product was purified by reversed-phase HPLC (65% CH3CN in H2O, 0.2% TFA, tR(14) = 25 min, tR(1) = 30 min) to give 2.1 mg (21%) of 1 and 4.2 mg (42%) of 14 as a yellow residue: [α]D +5 (c 0.22, MeOH); IR (film): ν̃ = 1619, 1592 cm−1; 1H NMR: δ 11.30 (s, 1H), 10.61 (s, 1H), 9.58 (br s, 1H), 7.72 (d, J = 7.8 Hz, 1H), 7.59 (d, J = 7.8 Hz, 1H), 7.48 (t, J = 7.8 Hz, 1H), 7.35 (t, J = 7.8 Hz, 1H), 7.02 (d, J = 8.2 Hz, 1H), 6.95–6.89 (m, 2H), 6.66 (s, 1H), 6.53 (t, J = 7.8 Hz, 1H), 3.79 (s, 3H); 13C NMR: δ 186.3, 186.1, 162.1, 161.1, 146.7, 136.0, 135.5, 131.7, 130.6, 123.3, 122.6, 122.4, 121.4, 119.9, 119.3, 118.9, 118.8, 118.8, 118.2, 117.4, 110.2, 96.5, 60.9; HRMS (ESI-TOF): m/z (M+H)+ calcd for C23H1635Cl3N2O5 505.0125, found 505.0118.
N-(2-(3,4′,5′-Trichloro-2′,5-bis(2-hydroxybenzoyl)-1′H-1,3′-bipyrrol-2-ylthio)ethyl)acetamide (15)
To a solution of metabolite 1 (7.0 mg, 0.014 mmol) and potassium carbonate (100 mg, 0.72 mmol) in DMF (1.0 mL) was added N-acetylcysteamine (20 μL, 0.19 mmol) via syringe. The solution was heated at 80 °C for 20 h, allowed to cool, diluted with a saturated NH4Cl solution (4 mL), and extracted with EtOAc (2 × 2 mL). The combined extracts were washed with brine (1 mL), dried, filtered, and concentrated. The product was purified by reversed-phase HPLC (50% CH3CN in H2O, 0.2% TFA, tR = 24 min) to give 4.5 mg (55%) of 15 as a yellow residue: [α]D +29 (c 0.27, MeOH); IR (film): ν̃ = 1623, 1594 cm−1; 1H NMR: δ 11.35 (s, 1H), 10.58 (br s, 1H), 10.19 (br s, 1H), 7.88 (d, J = 7.8 Hz, 1H), 7.57–7.53 (m, 2H), 7.30 (t, J = 7.8 Hz, 1H), 7.06 (d, J = 8.2 Hz, 1H), 6.97 (t, J = 7.8 Hz, 1H), 6.93 (d, J = 8.2 Hz, 1H), 6.82 (s, 1H), 6.43 (t, J = 7.8 Hz, 1H), 5.70 (br s, 1H), 3.14 (m, 2H), 2.67 (m, 1H), 2.37 (m, 1H), 1.95 (s, 3H); 13C NMR: δ 187.6, 186.1, 170.5, 162.8, 161.1, 136.5, 136.0, 132.2, 131.6, 130.6, 128.5, 124.4, 124.0, 122.2, 119.9, 119.6, 119.2, 119.0, 119.0, 118.7, 118.5, 117.6, 109.8, 38.2, 35.4, 23.1; HRMS (ESI-TOF): m/z (M+H)+ calcd for C26H2135Cl3N3O5S 592.0267, found 592.0274.
(4,4′,5′-Trichloro-5-(dimethylamino)-1′H-1,3′-bipyrrole-2,2′-diyl)bis((2-hydroxyphenyl)methanone) (16)
To metabolite 1 (3.5 mg, 0.0069 mmol) was added a solution of dimethylamine in THF (1.0 mL, 2.0 M) via syringe. The mixture was heated at 65 °C for 4 d, allowed to cool, and concentrated. The product was purified by reversed-phase HPLC (60% CH3CN in H2O for 25 min, 60–100% CH3CN in H2O over 15 min, 0.2% TFA, tR(1) = 35 min, tR(16) = 38 min) to yield 0.7 mg (20%) of 1 and 1.5 mg (42%) of 16 as a yellow residue. Alternatively, to compound 6 (4.1 mg, 0.0087 mmol) was added a solution of dimethylamine in THF (1.0 mL, 2.0 M). The mixture was heated at 55 °C for 2 d, allowed to cool, and concentrated. The product was purified by reversed-phase HPLC (70% CH3CN in H2O, 0.2% TFA, tR = 27 min) to yield 4.9 mg (100%) of 16 as a yellow residue: [α]D +3 (c 0.10, MeOH); IR (film): ν̃ = 1619, 1591 cm−1; 1H NMR (600 MHz): δ 11.41 (br s, 1H), 11.01 (br s, 1H), 10.28 (br s, 1H), 7.92 (d, J = 7.8, 1H), 7.63 (d, J = 7.8 Hz, 1H), 7.51 (t, J = 7.8 Hz, 1H), 7.31 (t, J = 7.8 Hz, 1H), 7.06 (d, J = 8.2 Hz, 1H), 6.96 (t, J = 7.8 Hz, 1H), 6.90 (d, J = 7.8 Hz, 1H), 6.69 (s, 1H), 6.48 (t, J = 7.8 Hz, 1H), 2.30 (s, 6H); 13C NMR: δ 186.6, 186.2, 162.3, 161.6, 143.8, 136.0, 135.5, 132.0, 131.2, 124.0, 123.2, 122.7, 122.0, 119.5, 119.2, 118.9, 118.8, 118.8, 118.2, 117.3, 109.9, 105.8, 41.5; HRMS (ESI-TOF): m/z (M+H)+ calcd for C24H1935Cl3N3O4 518.0441, found 518.0431.
(S,E)-Methyl 2-(benzyloxycarbonylamino)-6-((2-hydroxyphenyl)(4,4′,5,5′-tetrachloro-2′-(2-hydroxybenzoyl)-1′H-1,3′-bipyrrol-2-yl)methyleneamino)hexanoate (17)
To metabolite 1 (8.6 mg, 0.017 mmol) and methyl 6-amino-2-(benzyloxycarbonylamino)hexanoate hydrochloride (Cbz-NH-Lys-OMe•HCl, 40.6 mg, 0.123 mmol) was added pyridine (0.5 mL). The mixture was heated at 80 °C for 2 d, allowed to cool, diluted with a saturated NaHCO3 solution (2 mL) and water (2 mL), and extracted with EtOAc (2 × 3 mL). The combined extracts were washed with brine (1 mL), dried, filtered, and concentrated. The product was purified by reversed-phase HPLC (65% CH3CN in H2O, 0.2% TFA, tR = 21 min) to give 4.9 mg (37%) of 17 as a 1:1 mixture of diastereomers: 1H NMR (600 MHz, CD3CN): δ 7.75 (d, J = 7.8 Hz, 1H), 7.53 (t, J = 7.8 Hz, 1H), 7.49 (t, J = 7.8 Hz, 1H), 7.40 (d, J = 7.8 Hz, 1H), 7.38–7.26 (m, 11H), 7.16 (t, J = 7.8 Hz, 1H), 7.09 (d, J = 7.8 Hz, 1H), 7.01–6.89 (m, 6H), 6.79–6.73 (m, 2H), 6.66 (s, 1H), 6.53 (s, 1H), 6.47 (t, J = 7.8 Hz, 1H), 5.98 (m, 2H), 5.08–5.00 (m, 4H), 4.06 (m, 2H), 3.62 (s, 3H), 3.61 (s, 3H), 3.32 (m, 1H), 3.17 (m, 2H), 3.02 (m, 1H), 1.68–1.10 (m, 12H); 13C NMR (HSQC and HMBC): 187.1/185.9 (C-6′), 173.5 (C-19), 161.9/161.5 (C-6), 156.9 (C-22), 52.4 (C-20); HRMS (ESI-TOF): m/z (M+H)+ calcd for C37H3335Cl4N4O7 785.1103, found 785.1098.
(3-Bromo-4,5-dichloro-1H-pyrrol-2-yl)(2-hydroxyphenyl)methanone (19)
To acetate 21 (115 mg, 0.386 mmol) and N-bromosuccinimide (77.0 mg, 0.433 mmol) was added DCE (8.0 mL). The mixture was heated at 90 °C for 4.5 h, allowed to cool, poured into EtOAc (100 mL), and washed with a saturated NaHCO3 solution (20 mL) and brine (20 mL). The organic layer was dried, filtered, and concentrated. The product was purified by column chromatography (10% EtOAc then 15% EtOAc in hexanes) to afford 83.6 mg (57%) of acetylated bromide 22 as a foamy oil: IR (film): ν̃ = 1769, 1745, 1624 cm−1; 1H NMR: δ 9.77 (br s, 1H), 7.56 (t, J = 8.0 Hz, 1H), 7.51 (d, J = 7.8 Hz, 1H), 7.33 (t, J = 7.8 Hz, 1H), 7.24 (d, J = 8.0 Hz, 1H), 2.18 (s, 3H); 13C NMR: δ 181.8, 169.2, 148.1, 132.4, 130.1, 129.8, 126.9, 125.8, 123.1, 120.9, 114.9, 106.2, 20.8; HRMS (ESI-TOF): m/z (M+Na)+ calcd for C13H879Br35Cl2NO3Na 397.8962, found 397.8957. To a solution of 22 (9.8 mg, 0.026 mmol) in MeOH (2.0 mL) was added 5 drops of conc. HCl. After 4 h at room temperature, the mixture was concentrated. The product was purified by reversed-phase HPLC (50% CH3CN in H2O, 0.2% TFA) to furnish 8.2 mg (94%) of 19 as a yellowish solid: IR (film): ν̃ = 1620, 1585 cm−1; 1H NMR: δ 10.83 (s, 1H), 9.33 (br s, 1H), 7.81 (d, J = 7.8 Hz, 1H), 7.53 (t, J = 7.8 Hz, 1H), 7.05 (d, J = 8.0 Hz, 1H), 6.95 (t, J = 7.8 Hz, 1H); 13C NMR: δ 186.9, 161.8, 136.7, 133.3, 126.7, 119.7, 119.0, 118.3, 118.1, 115.0, 104.8; HRMS (ESI-TOF): m/z (M+H)+ calcd for C11H779Br35Cl2NO2 333.9037, found 333.9020.
(4,5-Dichloro-3-iodo-1H-pyrrol-2-yl)(2-hydroxyphenyl)methanone (20)
To acetate 21 (24.0 mg, 0.0805 mmol) and N-iodosuccinimide (26.0 mg, 0.116 mmol) was added DCE (2.0 mL). The mixture was heated at 90 °C for 6 h, allowed to cool, poured into EtOAc (50 mL), and washed with a saturated NaHCO3 solution (10 mL) and brine (10 mL). The organic layer was dried, filtered, and concentrated. The product was purified by column chromatography (20% EtOAc in hexanes) to afford 22.3 mg (65%) of acetylated iodide 23 as a foamy oil: IR (film): ν̃ = 1765, 1745, 1623 cm−1; 1H NMR: δ 7.57 (t, J = 7.8 Hz, 1H), 7.48 (d, J = 7.8 Hz, 1H), 7.34 (t, J = 7.8 Hz, 1H), 7.25 (d, J = 8.0 Hz, 1H), 2.16 (s, 3H); 13C NMR: δ 182.5, 169.3, 148.2, 132.4, 130.1, 129.9, 129.8, 126.0, 123.2, 120.7, 119.3, 76.2, 20.8; HRMS (ESI-TOF): m/z (M+Na)+ calcd for C13H835Cl2INO3Na 445.8824, found 445.8818. To a solution of 23 (16.8 mg, 0.0396 mmol) in MeOH (2.0 mL) was added 5 drops of conc. HCl. After 18 h at room temperature, the mixture was concentrated. The product was purified by reversed-phase HPLC (55% CH3CN in H2O, 0.2% TFA) to furnish 14.2 mg (94%) of 20 as a yellowish solid: IR (film): ν̃ = 1621, 1588 cm−1; 1H NMR: δ 10.82 (s, 1H), 9.28 (br s, 1H), 7.78 (d, J = 7.8 Hz, 1H), 7.54 (t, J = 7.8 Hz, 1H), 7.06 (d, J = 8.0 Hz, 1H), 6.96 (t, J = 7.8 Hz, 1H); 13C NMR: δ 187.5, 161.7, 136.7, 133.6, 129.6, 119.3, 119.2, 119.0, 118.3, 118.1, 75.2; HRMS (ESI-TOF): m/z (M+H)+ calcd for C11H735Cl2INO2 381.8899, found 381.8897.
2-(1,3-Dibromo-4,5-dichloro-1H-pyrrole-2-carbonyl)phenyl acetate (24)
To a solution of acetate 21 (14.8 mg, 0.0763 mmol) in DCE (1.0 mL) was added N-bromosuccinimide (30 mg, 0.17 mmol). The mixture was heated to 90 °C for 3 h, allowed to cool, and concentrated. The product was purified by column chromatography (10% EtOAc in hexanes) to give 22.0 mg (82%) of 24 as a white solid: IR (film): ν̃ = 1771, 1725 cm−1; 1H NMR: δ 7.69 (t, J = 7.8 Hz, 1H), 7.60 (d, J = 7.8 Hz, 1H), 7.39 (t, J = 7.8 Hz, 1H), 7.25 (d, J = 8.0 Hz, 1H), 2.20 (s, 3H); 13C NMR: δ 168.5, 162.7, 150.4, 135.6, 132.2, 126.5, 125.6, 124.2, 115.6, 114.6, 105.1, 101.3, 20.5; HRMS (ESI-TOF): m/z (M+Na)+ calcd for C13H779Br235Cl2NO3Na 475.8067, found 475.8078.
2,3-Dichlorochromeno[3,2-b]pyrrol-9(1H)-one (25)
To bromide 19 (22.2 mg, 0.0663 mmol), copper(I) iodide (8.0 mg, 0.042 mmol), and potassium carbonate (40 mg, 0.29 mmol) was added DMF (2.0 mL). The mixture was heated at 110 °C for 2 d, allowed to cool, diluted with a saturated NH4Cl solution (8 mL), and extracted with EtOAc (3 × 5 mL). The combined extracts were washed with brine (4 mL), dried, filtered, and concentrated. The product was purified by reversed-phase HPLC (70% MeOH in H2O, 0.2% TFA, tR = 25 min) to yield 6.9 mg (41%) of 25 as a white solid: IR (film): ν̃ = 1646 cm−1; 1H NMR (DMSO-d6): δ 13.82 (br s, 1H), 8.22 (d, J = 7.8 Hz, 1H), 7.81 (t, J = 7.8 Hz, 1H), 7.75 (d, J = 7.8 Hz, 1H), 7.50 (t, J = 7.8 Hz, 1H); 13C NMR (DMSO-d6): δ 165.3, 154.9, 145.5, 133.4, 125.5, 124.3, 123.0, 122.8, 118.0, 115.9, 95.0; HRMS (ESI-TOF): m/z (M+H)+ calcd for C11H635Cl2NO2 253.9776, found 253.9770.
Diethyl 1′H-1,3′-bipyrrole-2,2′-dicarboxylate (30)
To a solution of ethyl 3-amino-1H-pyrrole-2-carboxylate (29) (160 mg, 1.04 mmol) and crude ethyl 2,5-dioxopentanoate (247 mg, 1.56 mmol) in dry THF (5.0 mL) was added p-toluenesulfonic acid (PTSA, 2 mg). The mixture was heated at 60 °C overnight, allowed to cool, and concentrated. The product was purified by column chromatography (30% EtOAc in hexanes) to furnish 66.7 mg (23%) of bipyrrole 30 as a colorless oil. Analytically pure 30 was obtained using reversed-phase HPLC (52% CH3CN in H2O, tR = 16 min): IR (film): ν̃ = 1696 cm−1; 1H NMR (600 MHz): δ 9.42 (br s, 1H), 7.06 (m, 1H), 6.89 (m, 1H), 6.88 (m, 1H), 6.29 (m, 1H), 6.25 (m, 1H), 4.16 (q, J = 7.2 Hz, 2H), 4.12 (q, J = 7.2 Hz, 2H), 1.22 (t, J = 7.2 Hz, 3H), 1.10 (t, J = 7.2 Hz, 3H); 13C NMR: δ 160.6, 160.0, 130.0, 129.7, 124.6, 120.4, 117.8, 117.5, 109.9, 108.5, 60.3, 59.6, 14.2, 13.9; HRMS (ESI-TOF): m/z (M+H)+ calcd for C14H17N2O4 277.1188, found 277.1183.
Diethyl 4,4′,5,5′-tetrabromo-1′H-1,3′-bipyrrole-2,2′-dicarboxylate (31)
To bipyrrole 30 (20.0 mg, 0.0724 mmol) and N-bromosuccinimide (50 mg, 0.28 mmol) was added DCE (2.0 mL). The mixture was heated at 80 °C for 2 h, allowed to cool and concentrated. The product was purified by reversed-phase HPLC (65% CH3CN in H2O, tR = 25 min) to furnish 36.7 mg (86%) of tetrabromide 31 as a white solid: IR (film): ν̃ = 1699 cm−1; 1H NMR (600 MHz): δ; 10.45 (br s, 1H), 7.17 (s, 1H), 4.20 (q, J = 7.2 Hz, 2H), 4.13 (m, 2H), 1.26 (t, J = 7.2 Hz, 3H), 1.08 (t, J = 7.2 Hz, 3H); 13C NMR: δ 158.6, 158.2, 126.8, 125.9, 120.5, 120.0, 114.3, 106.0, 102.2, 100.5, 61.3, 60.7, 14.1, 13.8; HRMS (ESI-TOF): m/z (M+H)+ calcd for C14H1379Br4N2O4 588.7609, found 588.7607.
Supplementary Material
Acknowledgments
This work was supported by the National Institutes of Health, National Cancer Institute under grants CA44848 and CA052955. We thank Drs. Arnold L. Rheingold and Antonio G. D’Pasquale (UCSD) for providing X-ray diffraction data and Sara Kelly (SIO) for performing the MRSA and HCT-116 bioassays.
Footnotes
Supporting Information Available: Selected 1H, 13C, and 2D NMR spectra and HRMS data for compounds 1–6, 11–17, 19, 20, 22–25, 30, 31. CD spectra for 1–6. Crystallographic data for 6 (CCDC 764039), 19 (CCDC 764038), and 31 (CCDC 764040) in CIF format. This material is available free of charge via the Internet at http://pubs.acs.org.
References
- 1.(a) von Nussbaum F, Brands M, Hinzen B, Weigand S, Haebich D. Angew Chem, Int Ed. 2006;45:5072–5129. doi: 10.1002/anie.200600350. [DOI] [PubMed] [Google Scholar]; (b) Walsh C. Antibiotics: Actions, Origins, Resistance. ASM Press; Washington, D.C.: 2003. [Google Scholar]; (c) Schito GC. Clin Microbiol Infect. 2006;12:3–8. doi: 10.1111/j.1469-0691.2006.01343.x. [DOI] [PubMed] [Google Scholar]; (d) Palavecino E. In: Methicillin-resistant Staphylococcus Aureus (MRSA) Protocols. Ji Y, editor. Humana Press; Totowa: 2007. pp. 1–19. [Google Scholar]
- 2.Smith TL, Pearson ML, Wilcox KR, Cruz C, Lancaster MV, Robinson-Dunn B, Tenover FC, Zervos MJ, Band JD, White E, Jarvis WR. N Engl J Med. 1999;340:493–501. doi: 10.1056/NEJM199902183400701. [DOI] [PubMed] [Google Scholar]
- 3.Maldonado LA, Fenical W, Jensen PR, Kauffman CA, Mincer TJ, Ward AC, Bull AT, Goodfellow M. Int J Syst Evol Microbiol. 2005;55:1759–1766. doi: 10.1099/ijs.0.63625-0. [DOI] [PubMed] [Google Scholar]
- 4.(a) Fenical W, Jensen PR. Nat Chem Biol. 2006;2:666–673. doi: 10.1038/nchembio841. [DOI] [PubMed] [Google Scholar]; (b) Hughes CC, MacMillan JB, Gaudêncio SP, Jensen PR, Fenical W. Angew Chem, Int Ed. 2009;48:725–727. doi: 10.1002/anie.200804890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hughes CC, Prieto-Davo A, Jensen PR, Fenical W. Org Lett. 2008;10:629–631. doi: 10.1021/ol702952n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.(a) Chang C, Adams R. J Am Chem Soc. 1931;53:2353–2357. [Google Scholar]; (b) Webb JLA. J Org Chem. 1953;18:1423–1427. [Google Scholar]; (c) Dey S, Pal C, Nandi D, Giri VS, Zaidlewicz M, Krzeminski M, Smentek L, Hess BA, Gawronski J, Kwit M, Babu NJ, Nangia A, Jaisankar P. Org Lett. 2008;10:1373–1376. doi: 10.1021/ol800115p. [DOI] [PubMed] [Google Scholar]
- 7.Wu J, Vetter W, Gribble GW, Schneekloth JS, Jr, Blank DH, Gorls H. Angew Chem, Int Ed. 2002;41:1740–1743. doi: 10.1002/1521-3773(20020517)41:10<1740::aid-anie1740>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- 8.(a) Mingoia F. Tetrahedron. 2001;57:10147–10153. [Google Scholar]; (b) Rochais C, Lisowski V, Dallemagne P, Rault S. Tetrahedron Lett. 2004;45:6353–6355. [Google Scholar]; (c) Santo RD, Massa S, Costi R, Simonetti G, Retico A. Il Farmaco. 1994;49:229–236. [PubMed] [Google Scholar]
- 9.(a) Weisburg WG, Barns SM, Pelletier DA, Lane DJ. J Bacteriol. 1991;173:697–703. doi: 10.1128/jb.173.2.697-703.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Fox GE, Wisotzkey JD, Jurtshuk P., Jr Int J Syst Bacteriol. 1992;42:166–170. doi: 10.1099/00207713-42-1-166. [DOI] [PubMed] [Google Scholar]; (c) Stackebrandt E, Goebel BM. Int J Syst Bacteriol. 1994;44:846–849. [Google Scholar]; (d) Drancourt M, Bollet C, Carlioz A, Martelin R, Gayral JP, Raoult D. J Clin Microbiol. 2000;38:3623–3630. doi: 10.1128/jcm.38.10.3623-3630.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]; (e) Stach JEM, Maldonado LA, Masson DG, Ward AC, Goodfellow M, Bull AT. Appl Environ Microbiol. 2003;69:6189–6200. doi: 10.1128/AEM.69.10.6189-6200.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.In general, compounds that have few protons have proven to be resistant to confident structure assignment by NMR methods. When H/C ratios reach 0.5 and below, and the number of heteroatoms increases, the use of 2D NMR experiments such as COSY, TOCSY, HMQC and HMBC are rendered ineffective. Marinopyrrole A has a H/C ratio (non-exchangeable H) of 0.41. This limitation was first described by Professor Phillip Crews, UC Santa Cruz, 7th US-Japan Conference on Marine Natural Products Chemistry (June 2007).
- 11.The placement of the halogen atom in 3–5, para to the hydroxyl group, agrees with a biosynthetic mechanism involving an electrophilic halogenating species. See: van Pée KH. Annu Rev Microbiol. 1996;50:375–399. doi: 10.1146/annurev.micro.50.1.375.
- 12.Hall DM, Harris MM. J Chem Soc. 1960:490–494. [Google Scholar]
- 13.(a) Durham DG, Hughes CG, Rees AH. Can J Chem. 1972;50:3223–3228. [Google Scholar]; (b) Petruso S, Bonanno S, Caronna S, Ciofalo M, Maggio B, Schillaci D. J Heterocyclic Chem. 1994;31:941–945. [Google Scholar]
- 14.(a) Takeda RJ. J Am Chem Soc. 1958;80:4749–4750. [Google Scholar]; (b) Takeda RJ. Bull Agr Chem Soc Japan. 1959;23:126–130. [Google Scholar]; (c) Birch AJ, Hodge P, Rickards RW, Takeda R, Watson TR. J Chem Soc. 1964:2641–2644. [Google Scholar]; (d) Kaneda M, Nakamura S, Ezaki N, Iitaka Y. J Antibiotics. 1981;34:1366–1368. doi: 10.7164/antibiotics.34.1366. [DOI] [PubMed] [Google Scholar]; (e) Ezaki N, Koyama M, Shamura T, Tsuruoka T, Inouye S. J Antibiotics. 1983;36:1263–1267. doi: 10.7164/antibiotics.36.1263. [DOI] [PubMed] [Google Scholar]; (f) Ezaki N, Koyama M, Kodama Y, Shomura T, Tashiro K, Tsuruoka T, Inouye S. J Antibiotics. 1983;36:1431–1438. doi: 10.7164/antibiotics.36.1431. [DOI] [PubMed] [Google Scholar]
- 15.Vlasov VM. Russ Chem Rev. 2003;72:681–703. [Google Scholar]
- 16.The imine was resistant to in situ reduction with sodium cyanoborohydride.
- 17.The atropo-purity of 17 degraded slightly, though not enough to account for the 1:1 mixture of diastereomers. The natural product, liberated during prolonged storage of the imine, was isolated in 74% ee.
- 18.Both H-12 resonances were not clearly resolved from the other aromatic signals.
- 19.Hughes CC, Yang YL, Liu WT, Dorrestein PC, La Clair JJ, Fenical W. J Am Chem Soc. 2009;131:12094–12096. doi: 10.1021/ja903149u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.(a) Kunz K, Scholz U, Ganzer D. Synlett. 2003:2428–2439. [Google Scholar]; (b) Antilla JC, Baskin JM, Barder TE, Buchwald SL. J Org Chem. 2004;69:5578–5587. doi: 10.1021/jo049658b. [DOI] [PubMed] [Google Scholar]; (c) Cristau HJ, Cellier PP, Spindler JF, Taillefer M. Chem Eur J. 2004;10:5607–5622. doi: 10.1002/chem.200400582. [DOI] [PubMed] [Google Scholar]; (d) Cai Q, Zhu W, Zhang H, Zhang Y, Ma D. Synthesis. 2005:496–499. [Google Scholar]; (e) Taillefer M, Xia N, Ouali A. Angew Chem, Int Ed. 2007;46:934–936. doi: 10.1002/anie.200603173. [DOI] [PubMed] [Google Scholar]; (f) Correa A, Bolm C. Angew Chem, Int Ed. 2007;46:8862–8865. doi: 10.1002/anie.200703299. [DOI] [PubMed] [Google Scholar]; (g) Mino T, Harada Y, Shindo H, Sakamoto M, Fujita T. Synlett. 2008:614–620. [Google Scholar]
- 21.For a review of the synthesis of axially chiral biaryl compounds, see: Bringmann G, Mortimer AJP, Keller PA, Gresser MJ, Garner J, Breuning M. Angew Chem, Int Ed. 2005;44:5384–5427. doi: 10.1002/anie.200462661.
- 22.Hasegawa H, Shiori N, Mogi K, Asaoka T, Ono J, Kuraishi T, Katori T. Patent no. JP 63183562. Jpn Kokai Tokkyo Koho. 1988
- 23.For other examples of pyrrole N-halogenation, see: De Rosa M. J Org Chem. 1982;47:1008–1010.De Leon CY, Ganem B. Tetrahedron. 1997;53:7731–7752.
- 24.Paparao C, Rao KV, Sundaramurthy V. Synthesis. 1981:234–236. [Google Scholar]
- 25.Macritchie JA, Silcock A, Willis CL. Tetrahedron Asym. 1997;8:3895–3902. [Google Scholar]
- 26.Furneaux RH, Tyler PC. J Org Chem. 1999;64:8411–8412. doi: 10.1021/jo990903e. [DOI] [PubMed] [Google Scholar]
- 27.Microdilution broth method: (a) Clinical and Laboratory Standards Institute 2009, M7-A8, M100-S19, CLSI, Wayne, Pa. (b) ref. 1d, pp. 29–49.
- 28.MTT method: Mosmann T. J Immunol Methods. 1983;65:55–63. doi: 10.1016/0022-1759(83)90303-4.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.