Abstract
Purpose of review
Growth differentiation factor 15 (GDF15) was identified as a hepcidin-suppression factor that is expressed at high levels in patients with ineffective erythropoiesis. This review addresses the regulation, expression and potential functions of GDF15 in the context of erythroid biology.
Recent findings
GDF15 expression during late erythroid differentiation was discovered as part of an erythroblast transcriptome project. Since GDF15 expression is associated with cellular stress or apoptosis, further investigation of the cytokine was focused upon its involvement in ineffective erythropoiesis. Remarkably high serum levels were detected in patients with thalassemia syndromes, congenital dyserythropoiesis and some acquired sideroblastic anemias. Similarly high-level GDF15 expression is not a feature of normal erythropoiesis, or erythroid recovery after bone marrow transplantation. Since GDF15 is a TGF-β superfamily member, it was investigated as an effector of ineffective erythropoiesis that suppresses hepcidin expression despite iron overloading.
Summary
In contrast to the low-levels of GDF15 expressed during normal erythropoiesis, ineffective erythropoiesis causes high-level expression of GDF15. In patients with thalassemia and related anemias, GDF15 expression may contribute to iron overloading or other features of the disease phenotype.
Keywords: GDF15, ineffective erythropoiesis, iron regulation
Introduction
Ineffective erythropoiesis describes the suboptimal production of mature erythrocytes from a proliferating pool of immature erythroblasts. In many patients, secondary hemochromatosis develops with iron loading in which stores are beyond the physiological levels needed to support erythropoiesis. Studies of iron homeostasis in patients with ineffective erythropoiesis led to the notion that erythroblast-expressed factors may cause excess iron loading through suppression of the hormone hepcidin. Recently, transcriptome studies of ex vivo human erythropoiesis identified growth differentiation factor 15 (GDF15) as a candidate molecule in this regard. In this review, erythroblast expression and the potential functions of GDF15 are discussed.
The GDF15 gene and its expression
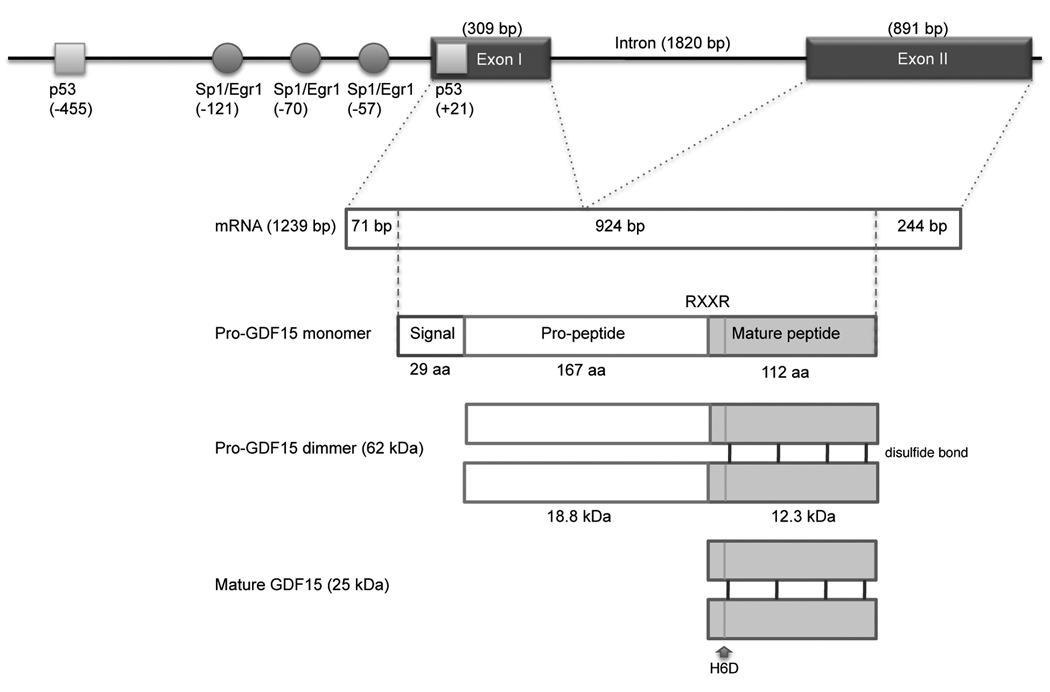
The human GDF15 locus was mapped by fluorescence in situ hybridization (FISH) to chromosome 19p12.1-13.1 [1]. As shown in Figure 1, the gene contains a single 1820 bp intron [1]. The GDF15 protein is encoded by two exons: the 309 bp Exon I contains a 71 bp 5’ untranslated region (UTR) and 238 bp of coding region, and the 647 bp Exon II contains a 3’ UTR. GDF15 is one of the major secreted proteins induced by the tumor suppressor protein p53 [2]. Two p53 binding sites are located within the −500 bp promoter with a site located in the 5’ UTR [3,4]. Several studies suggest that GDF15 induction is associated with cell cycle arrest and apoptosis [5]. Hence, GDF15 may be an excellent in vivo biomarker of the p53 pathway activation [6]. However, p53 is not the only transcription factor regulating GDF15 expression. The GDF15 promoter contains motifs for several additional transcription factors [7]. Sp1 and COUP-TF1 transcriptional factors regulate the basal transcription of GDF15 through the GC box located within −133 bp of the GDF15 promoter [8]. The Egr-1 binding sites in the GDF15 promoter overlap with an Sp1 binding sites. GATA binding motifs are also encoded in the promoter region [3]. Thus, the transcriptional activity of GDF15 likely depends on the balance of transacting factors that may be regulated as part of an apoptotic or stress response as well as tissue differentiation. Since hypoxia or other cellular stresses increase p53, Sp1, and Egr-1 expression, increased serum levels of this cytokine may reflect cellular stress or death [9]. Additional transcription factors may be involved in the GDF15 response to hypoxia [10].
Figure 1. Genomic structure and transcription for protein production of matured GDF15.
The diagram shows GDF15 gene structure with several transcription factors binding sites in the promoter region. GDF15 is synthesized from two exons, and dimerized with disulfide bonds, then cleaved at RXXR site to form C-terminal mature GDF15 protein. H6D denotes a single-nucleotide polymorphism at position 6 of the mature protein resulting in histidine to aspartic acid substitution.
GDF15 is a member of transforming growth factor-β (TGF-β) superfamily that comprises more than 40 members. The TGF-β superfamily is involved in several processes including cell differentiation, development and apoptosis [11]. GDF15 is somewhat unique, in that it shares TGF-β homology according to its cysteine rich domain, but it otherwise shares less than 30% amino acid homology with other TGF-β family members. Among the superfamily, GDF15 is the most divergent member [12]. A single-nucleotide polymorphism at position 6 of the mature protein results in histidine to aspartic acid substitution (H6D, rs1058587) [13]. The H6D variant is associated with functional variation of the protein [14]. GDF15 is synthesized as a precursor protein that undergoes disulfide-linked dimerization like TGF-β. The precursor form mediates binding to theextracellular matrix, creating latent stromal stock of proGDF15. The precursor protein is cleaved at an RXXR furin-like cleavage site to form the mature C-terminal GDF15 peptide, which is subsequently secreted as a 25–30 kDa dimer [15,16]. Mature GDF15 is soluble and easily identified in blood, where it acts as an “extracellular” messenger [17]. Unfortunately, current knowledge regarding specific cellular membrane receptors and signaling cascades (Smad, MAPK, Akt) that transducer GDF15 signals remains superficial to date [18–20].
GDF15 expression in effective and ineffective erythropoiesis
Based upon the Human Genome Project, efforts were made over the last decade to better understand transcriptomes encoded in human erythroblasts. An erythroblast transcriptome project was initiated by first isolating human erythroblasts in real-time as they differentiate in ex vivo cultures [21]. mRNA from those cells was isolated and analyzed to provide a erythroid transcriptome map for further study. Due to the high-levels of erythroblast proliferation followed by incomplete maturation (without enucleation), the transcriptome generated using cultured cells was hypothesized to reflect ineffective erythropoiesis [22]. High-levels of GDF15 gene expression were noted in culture among the more mature erythroblasts, and the cytokine was selected for further study [23].
Along with studies in cultured erythroblasts, clinical studies of GDF15 expression in vivo were begun. A summary of clinical reports is shown in Figure 2. The levels of GDF15 in healthy individuals are generally measured in 200–1,150 pg/ml range by commercial ELISA assays [23,24]. There are no significant differences of GDF15 concentrations among citrated plasma, EDTA-treated plasma, and serum. GDF15 is stable at room temperature in serum, plasma, and whole blood for at least 48 hours. GDF15 is resistant to at least 4 freeze thaw cycles. Anticoagulants, albumin, bilirubin, or hemoglobin do not significantly influence the measurement of GDF15 concentrations [25]. These features suggest GDF15 expression in the serum may eventually be utilized as a clinical marker of cell stress or apoptosis. Additional studies are needed to determine the sensitivity and specificity of GDF15 measurements in a broader range of disease settings.
Figure 2. GDF15 concentrations in human blood from healthy volunteers and several hematological diseases.
The individual data points represent GDF15 concentrations from healthy volunteers (HV; n = 37), sickle cell anemia (SS; n = 13), thalassemia trait (Thal-trait; n = 12), α-thalassemia (α-Thal; n = 20), β-thalassemia (β-Thal; n = 40) [23], and congenital dyserythropoietic anemia type I (CDAI; n = 17) [28]. The lines represent the maximum and minimum concentrations from patients with refractory anemia with ring sideroblasts (RARS, n = 20) [31] and pyruvate kinase deficiency (PKD, n = 22) [34]. Bars show the mean of GDF15 concentrations.
Consistent with the high levels of GDF15 expressed ex vivo in the culture model described above, serum GDF15 levels are highly-elevated in patients with ineffective erythropoiesis or other hematopoietic disorders (Figure 2). The thalassemia syndromes (α-thalassemia and β-thalassemia) represent the most common causes of ineffective erythropoiesis. In the thalassemia syndromes, imbalances in the production of α- and β-globin chains result in increased apoptosis during erythroblast maturation [26]. Serum GDF15 levels in patients with thalassemia were dramatically elevated compared with healthy volunteers or patients with the thalassemia trait. The median in beta-thalassemia patients (48,000 pg/ml) was particularly high with a detected range of 5,000–250,000 pg/ml [23]. A second group of disorders associated with ineffective erythropoiesis and iron overloading is called dyserythropoietic anemias. Congenital dyserythropoietic anemia type I (CDAI) is caused by CDANI gene deficiency [27]. Like α- and β-thalassemia, very high serum levels of GDF15 are expressed in patients with CDAI [28]. In CDAI patients, serum GDF15 levels were correlated with the level of ineffective erythropoiesis, hepcidin-25, and ferritin. A third group of patients with ineffective erythropoiesis possess an erythroid defect that is characterized by accumulation of iron in mitochondria that “ring” the erythroblast nucleus during terminal maturation [29]. Intramedullary apoptosis is a feature of acquired anemia with ringed sideroblasts [30]. Significant elevations in GDF15 expression were also reported in this group (3,254 ± 1,400 pg/ml vs. 451 ± 87 pg/ml in healthy volunteers) [31]. Finally, significant ineffective erythropoiesis has been reported among some patients with pyruvate kinase deficiency, but the erythroid phenotype is variable [32,33]. GDF15 expression in patients with pyruvate kinase deficiency is elevated, but the magnitude of elevation is considerably lower than measured in patients with thalassemia [34].
Serum GDF15 levels from patients with other erythroid disorders have also been reported. Like thalassemia syndromes, sickle cell syndromes are characterized by increased levels of erythropoiesis, but the primary defect in sickle cell involves destruction of mature erythrocytes. In severe cases of sickle cell disease, some ineffective erythropoiesis may be found [35]. Interestingly, the elevation in the serum level of GDF15 is mild in sickle cell disease compared to thalassemia [23]. Studies of patients with effective erythropoiesis after bone marrow transplantation [36] or after injection of erythropoietin [37] failed to demonstrate major increases in serum GDF15. Indeed, preliminary studies at the National Institutes of Health suggest GDF15 expression is barely detectable in normal bone marrow, compared with the distinct staining in a subset of erythroid precursors in thalassemia bone marrow (Figure 3). Since GDF15 is not expressed specifically in erythroblasts, elevations of this cytokine in patients with cancers and inflammatory disease should not be attributed to erythroblast expression. This point may be particularly important when considering the high-levels of GDF15 reported in patients with anemia of chronic disease [38]. When combined, these data suggest GDF15 measurements may be helpful for predicting ineffective or apoptotic erythropoiesis.
Figure 3. GDF15 expression in bone marrow from a patient with thalassemia.
Brown coloration marks GDF15 present in late-stage erythroblasts in thalassemia bone marrow. No similar staining was noted in bone marrow from a healthy volunteer.
Hepcidin, erythroblast iron loading, and GDF15
Dietary, tissue-stored, and erythrocyte-recycled iron molecules exit cells via a membrane channel named ferroportin. Ferroportin expression on the cell membrane is regulated by a protein named hepcidin [39–41]. Hepcidin is a small peptide that is produced in the liver that is most likely the major regulator of iron [42]. It acts by causing the endocytosis and degradation of ferroportin [43]. Based upon the importance of iron, multiple mechanisms exist for the regulation of hepcidin. Iron levels, inflammation, erythropoiesis and the combined effects of several proteins expressed on hepatocyte membranes are involved [44]. There exists a relatively broad range and diurnal variation in serum hepcidin levels among healthy volunteers. Hence, the interpretation of serum hepcidin levels in disease states may be complex. As an example, it is now recognized that simple iron deficiency causes serum hepcidin to be reduced to low or undetectable levels, and inflammatory disease causes increased serum levels [45]. However, the combination of iron deficiency with inflammation cause a balance of hepcidin-regulating mechanisms that results in serum levels within the normal range.
Hepcidin expression and iron overloading in patients with ineffective erythropoiesis may similarly be explained by a combination of regulatory mechanisms. Erythropoiesis in all forms requires high levels of iron for the production of hemoglobin. The robust demand for iron is supplied by transferrin-bound iron in the plasma [46]. Therefore, erythropoiesis causes the necessary suppression of hepcidin required to maintain adequate transport of iron from macrophage and other cells to support the large demand of iron for hemoglobin production. The mechanism by which normal erythropoiesis regulates the suppression of hepcidin is still not known. Based upon the relative lack of GDF15 regulation by EPO or during marrow recovery, GDF15 is not likely involved in the physiological suppression of hepcidin for normal erythropoiesis [36,37]. Importantly, ineffective erythropoiesis is unique among anemias in that it is associated with iron overloading of the host, which far exceeds the iron loading required for erythropoiesis. [47]. It is proposed here that ineffective erythropoiesis causes iron overloading by not permitting the appropriate rise in hepcidin expression to occur once the erythroid demand is met. The low or low-normal serum hepcidin levels in thalassemia support this hypothesis [48].
Since a pathological signal could explain the inappropriately normal serum hepcidin levels in iron-overloaded patients with ineffective erythropoiesis, studies were focused upon determining whether GDF15 modifies or is modified by iron homeostasis [23,49]. In cultured human hepatocytes, serum GDF15 in thalassemia patients, as well as recombinant GDF15 protein, suppressed hepcidin expression. However, significant suppression of hepcidin required high levels (>5,000 pg/ml) of GDF15, and the suppression was incomplete even after the addition of the highest dose of 100,000 pg/ml GDF15. In addition, serum of thalassemia patients suppressed hepcidin in the cultured cells even after GDF15 was immunoprecipitated [23]. As a result, it was concluded that GDF15 expression at the high levels achieved in the setting of ineffective erythropoiesis contribute to pathological iron overloading through a mechanism of incomplete hepcidin suppression. Others have suggested that in addition to the regulation of hepcidin, iron depletion or chelation in the host may regulate GDF15 expression [50].
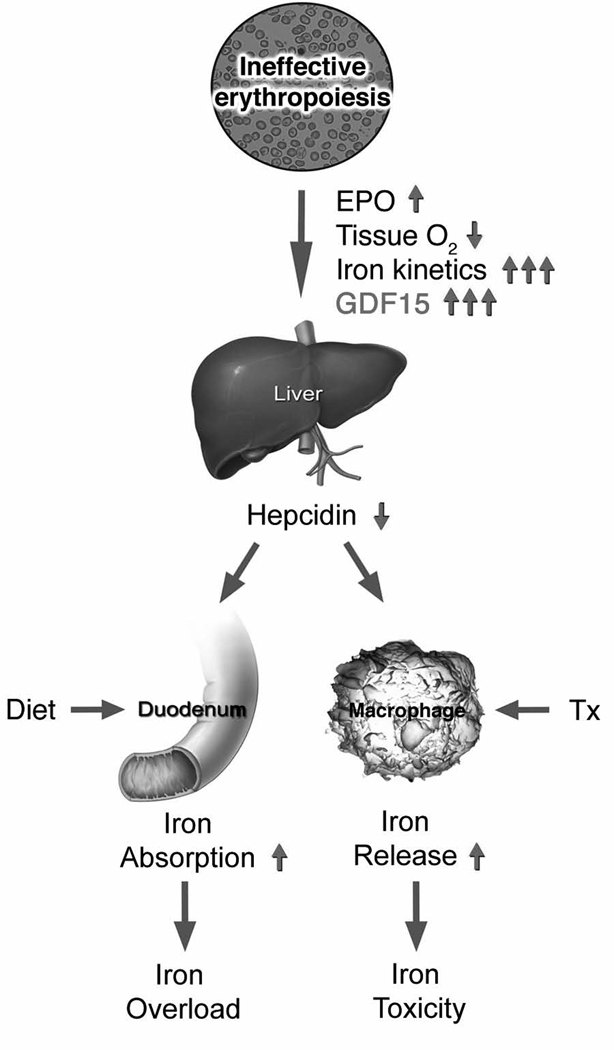
A summary model of iron homeostasis in patients with ineffective erythropoiesis is shown in Figure 4. Based upon the vast expansion of medullary and extramedullary erythropoiesis, the erythroblast demand for iron is enormous. There is a clear increase in iron kinetics, tissue hypoxia, and erythropoietin in these patients that act together with other physiological mechanisms to suppress hepcidin and meet the erythroblast demand for iron. Once the host tissues load enough iron to meet the erythroblast demand, hepcidin expression should increase to levels sufficient for prevention of tissue iron overloading. Instead, hepcidin expression remains within the normal range, partially due to over-expression of GDF15. Among patients who are transfused, the effects of iron loading are made worse by the host’s inability to express sufficient hepcidin to maintain the sequestration of erythrocyte-recycled iron safely inside macrophages.
Figure 4. Model of iron homeostasis in patients with ineffective erythropoiesis.
With permission from reference 23
The curiosity of GDF15 function
GDF15 expression is not erythroid-specific. Human GDF15 is highly expressed in the placenta during pregnancy in the second and third trimester [51,52]. Increased serum GDF15 has also been described in several disease states including acute injury, inflammation, and cancer [53–55]. In contrast, murine GDF15 is not expressed at high levels in the placenta [56]. Instead, murine GDF15 is found in the CNS, its site of highest expression being the choroid plexus [57]. GDF15 mRNA is prominently upregulated in neurons [58]. Recently, Strelau et al. demonstrated that GDF15 is a genuine novel trophic factor for motor and sensory neurons using GDF15 deficient mice [59]. GDF15 shows remarkably low homology conservation between human and murine species. While mature forms of human and murine TGF-β1 and BMP-2 show 99% and 100% sequence similarity between the murine and human species, GDF15 shares only 66% amino acid sequence similarity [60,61]. Promoter homology between human and mouse is even less, with only 39% homology [8]. GDF15 null mice show few obvious abnormalities [56]. Overexpression of GDF15 in mice demonstrated a reduction in body weight [62]. GDF15 also suppresses intestinal and gastrointestinal tumorigenesis in the transgenic mice, suggesting that GDF15 may act as a tumor suppressor gene in those animals [19, 63]. Despite these interesting findings, the interpretation for clinical application is blurred by major differences in homology and tissue expression between mice and men.
Beyond having a potential role in iron regulation, additional functions for human GDF15 have been proposed. Human GDF15-encoded protein has many alternative names [placental bone morphogenetic protein (PLAB), placental transforming growth factor-β (PTGF-β), macrophage inhibitory cytokine-1 (MIC-1), prostate derived factor (PDF), and nonsteroidal anti-inflammatory drug activated gene (NAG-1)]. In tumor cells, GDF15 can exhibit tumorigenic and antitumorigenic activity in different circumstances. GDF15 may also be protective in heart failure [64]. Due to elevated levels of GDF15 observed in patients with rheumatoid arthritis, it has been proposed to participate in inflammatory responses [65]. Johnen et al. described GDF15 as an appetite suppressor at high concentrations, promoting cachexia in patients with advanced cancers [62]. GDF15 also induces osteoclast formation in vitro, and osteoclast formation in vivo [66]. The effects upon appetite and bone metabolism may be worthy of additional studies in patients with thalassemia or related anemias. Overall, GDF15 expression seems to be that of a stress effector with subtle and protean functions that facilitate the host’s response to or recovery from cellular damage or death. In cases of ineffective erythropoiesis, the incomplete suppression of hepcidin by GDF15 may occur when the host response to erythroblast death becomes exaggerated by the shear magnitude and chronicity of the disease.
Conclusion
GDF15 expression is usually associated with cellular stress or apoptosis. In patients with thalassemia and other diseases manifested by ineffective erythropoiesis, particularly high levels of GDF15 are present in the serum. It is proposed that the elevated levels of GDF15 contributes to the suppression of hepcidin and subsequent tissue iron overloading in those patients. A more complete understanding of GDF15 signal transduction and functions in health and disease are topics for future research.
Acknowledgment
We thank Colleen Byrnes and Y. Terry Lee for help in preparing this manuscript. This research was supported by the Intramural Research Program of the NIH, NIDDK.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References and recommended reading
Papers of particular interest, published with the annual period of review, have been highlighted as:
* Of special interest
** Of outstanding interest
- 1.Lawton LN, Bonaldo MF, Jelenc PC, et al. Identification of a novel member of the TGF-beta superfamily highly expressed in human placenta. Gene. 1997;203:17–26. doi: 10.1016/s0378-1119(97)00485-x. [DOI] [PubMed] [Google Scholar]
- 2.Tan M, Wang Y, Guan K, et al. PTGF-beta, a type beta transforming growth factor (TGF-beta) superfamily member, is a p53 target gene that inhibits tumor cell growth via TGF-beta signaling pathway. Proc Natl Acad Sci U S A. 2000;97:109–114. doi: 10.1073/pnas.97.1.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Li PX, Wong J, Ayed A, et al. Placental transforming growth factor-beta is a downstream mediator of the growth arrest and apoptotic response of tumor cells to DNA damage and p53 overexpression. J Biol Chem. 2000;275:20127–20135. doi: 10.1074/jbc.M909580199. [DOI] [PubMed] [Google Scholar]
- 4.Lim JH, Park JW, Min DS, et al. NAG-1 up-regulation mediated by EGR-1 and p53 is critical for quercetin-induced apoptosis in HCT116 colon carcinoma cells. Apoptosis. 2007;12:411–421. doi: 10.1007/s10495-006-0576-9. [DOI] [PubMed] [Google Scholar]
- 5.Bauskin AR, Brown DA, Kuffner T, et al. Role of macrophage inhibitory cytokine-1 in tumorigenesis and diagnosis of cancer. Cancer Res. 2006;66:4983–4986. doi: 10.1158/0008-5472.CAN-05-4067. [DOI] [PubMed] [Google Scholar]
- 6.Yang H, Filipovic Z, Brown D, et al. Macrophage inhibitory cytokine-1: a novel biomarker for p53 pathway activation. Mol Cancer Ther. 2003;2:1023–1029. [PubMed] [Google Scholar]
- 7.Baek SJ, Kim JS, Moore SM, et al. Cyclooxygenase inhibitors induce the expression of the tumor suppressor gene EGR-1, which results in the up-regulation of NAG-1, an antitumorigenic protein. Mol Pharmacol. 2005;67:356–364. doi: 10.1124/mol.104.005108. [DOI] [PubMed] [Google Scholar]
- 8.Baek SJ, Horowitz JM, Eling TE. Molecular cloning and characterization of human nonsteroidal anti-inflammatory drug-activated gene promoter. Basa transcription is mediated by Sp1 and Sp3. J Biol Chem. 2001;276:33384–33392. doi: 10.1074/jbc.M101814200. [DOI] [PubMed] [Google Scholar]
- 9.Cummins EP, Taylor CT. Hypoxia-responsive transcription factors. Pflugers Arch. 2005;450:363–371. doi: 10.1007/s00424-005-1413-7. PMID: 16007431. [DOI] [PubMed] [Google Scholar]
- 10. Krieg AJ, Rankin EB, Chan D, et al. Regulation of the Histone Demethylase JMJD1A by HIF-1α Enhances Hypoxic Gene Expression and Tumor Growth. Mol Cell Biol. 2009 Oct 26; doi: 10.1128/MCB.00444-09. * Hypoxia stress induces GDF15 expression in cancer.
- 11.Whitman M. Smads and early developmental signaling by the TGFbeta superfamily. Genes Dev. 1998;12:2445–2462. doi: 10.1101/gad.12.16.2445. [DOI] [PubMed] [Google Scholar]
- 12.Bootcov MR, Bauskin AR, Valenzuela SM, et al. MIC-1, a novel macrophage inhibitory cytokine, is a divergent member of the TGF-beta superfamily. Proc Natl Acad Sci U S A. 1997;94:11514–11519. doi: 10.1073/pnas.94.21.11514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Brown DA, Stephan C, Ward RL, et al. Measurement of serum levels of macrophage inhibitory cytokine 1 combined with prostate-specific antigen improves prostate cancer diagnosis. Clin Cancer Res. 2006;12:89–96. doi: 10.1158/1078-0432.CCR-05-1331. [DOI] [PubMed] [Google Scholar]
- 14.Lindmark F, Zheng SL, Wiklund F, et al. H6D polymorphism in macrophage-inhibitory cytokine-1 gene associated with prostate cancer. J Natl Cancer Inst. 2004;96:1248–1254. doi: 10.1093/jnci/djh227. [DOI] [PubMed] [Google Scholar]
- 15.Fairlie WD, Zhang H, Brown PK, et al. Expression of a TGF-beta superfamily protein, macrophage inhibitory cytokine-1, in the yeast Pichia pastoris. Gene. 2000;254:67–76. doi: 10.1016/s0378-1119(00)00295-x. [DOI] [PubMed] [Google Scholar]
- 16.Bauskin AR, Zhang HP, Fairlie WD, et al. The propeptide of macrophage inhibitory cytokine (MIC-1), a TGF-beta superfamily member, acts as a quality control determinant for correctly folded MIC-1. EMBO J. 2000;19:2212–2220. doi: 10.1093/emboj/19.10.2212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bauskin AR, Brown DA, Junankar S, et al. The propeptide mediates formation of stromal stores of PROMIC-1: role in determining prostate cancer outcome. Cancer Res. 2005;65:2330–2336. doi: 10.1158/0008-5472.CAN-04-3827. [DOI] [PubMed] [Google Scholar]
- 18.Vanhara P, Lincová E, Kozubík A, et al. Growth/differentiation factor-15 inhibits differentiation into osteoclasts--a novel factor involved in control of osteoclast differentiation. Differentiation. 2009;78:213–222. doi: 10.1016/j.diff.2009.07.008. [DOI] [PubMed] [Google Scholar]
- 19.Cekanova M, Lee SH, Donnell RL, et al. Nonsteroidal anti-inflammatory drug-activated gene-1 expression inhibits urethane-induced pulmonary tumorigenesis in transgenic mice. Cancer Prev Res (Phila Pa) 2009;2:450–458. doi: 10.1158/1940-6207.CAPR-09-0057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kim KK, Lee JJ, Yang Y, et al. Macrophage inhibitory cytokine-1 activates AKT and ERK-1/2 via the transactivation of ErbB2 in human breast and gastric cancer cells. Carcinogenesis. 2008;29:704–712. doi: 10.1093/carcin/bgn031. [DOI] [PubMed] [Google Scholar]
- 21.Miller JL, Njoroge JM, Gubin AN, et al. Prospective identification of erythroid elements in cultured peripheral blood. Exp Hematol. 1999;27:624–629. doi: 10.1016/s0301-472x(98)00086-1. [DOI] [PubMed] [Google Scholar]
- 22.Wojda U, Noel P, Miller JL. Fetal and adult hemoglobin production during adult erythropoiesis: coordinate expression correlates with cell proliferation. Blood. 2002;99:3005–3013. [PubMed] [Google Scholar]
- 23.Tanno T, Bhanu NV, Oneal PA, et al. High levels of GDF15 in thalassemia suppress expression of the iron regulatory protein hepcidin. Nat Med. 2007;13:1096–1101. doi: 10.1038/nm1629. [DOI] [PubMed] [Google Scholar]
- 24.Brown DA, Ward RL, Buckhaults P, et al. MIC-1 serum level and genotype: associations with progress and prognosis of colorectal carcinoma. Clin Cancer Res. 2003;9:2642–2650. [PubMed] [Google Scholar]
- 25.Peter T S, et al. Prognostic utility of growth differentiation factor-15 in patients with chronic heart failure. J Am Coll Cardiol. 2007;50:1054–1060. doi: 10.1016/j.jacc.2007.04.091. [DOI] [PubMed] [Google Scholar]
- 26.Schrier SL. Pathophysiology of thalassemia. Curr Opin Hematol. 2002;9:123–126. doi: 10.1097/00062752-200203000-00007. [DOI] [PubMed] [Google Scholar]
- 27.Heimpel H, Schwarz K, Ebnöther M, et al. Congenital dyserythropoietic anemia type I (CDA I): molecular genetics, clinical appearance, and prognosis based on long-term observation. Blood. 2006;107:334–340. doi: 10.1182/blood-2005-01-0421. [DOI] [PubMed] [Google Scholar]
- 28. Tamary H, Shalev H, Perez-Avraham G, Zoldan M, Levi I, Swinkels DW, Tanno T, Miller JL. Elevated growth differentiation factor 15 expression in patients with congenital dyserythropoietic anemia type I. Blood. 2008;112:5241–5244. doi: 10.1182/blood-2008-06-165738. * Serum GDF15 levels in congenital dyserythropoietic anemia type I.
- 29.Bowman WD., Jr Abnormal ("ringed") sideroblasts in various hematologic and non-hematologic disorders. Blood. 1961;18:662–671. [PubMed] [Google Scholar]
- 30.Merchant SH, Gonchoroff NJ, Hutchison RE. Apoptotic index by Annexin V flow cytometry: adjunct to morphologic and cytogenetic diagnosis of myelodysplastic syndromes. Cytometry. 2001;46:28–32. doi: 10.1002/1097-0320(20010215)46:1<28::aid-cyto1034>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- 31. Ramirez JM, Schaad O, Durual S, et al. Growth differentiation factor 15 production is necessary for normal erythroid differentiation and is increased in refractory anemia with ring-sideroblasts. Br J Haematol. 2009;144:251–262. doi: 10.1111/j.1365-2141.2008.07441.x. * Serum GDF15 levels in refractory anemia with ring-sideroblasts.
- 32.Aizawa S, Kohdera U, Hiramoto M, et al. Ineffective erythropoiesis in the spleen of a patient with pyruvate kinase deficiency. Am J Hematol. 2003;74:68–72. doi: 10.1002/ajh.10380. [DOI] [PubMed] [Google Scholar]
- 33.Zanella A, Berzuini A, Colombo MB, et al. Iron status in red cell pyruvate kinase deficiency: study of Italian cases. Br J Haematol. 1993;83:485–490. doi: 10.1111/j.1365-2141.1993.tb04675.x. [DOI] [PubMed] [Google Scholar]
- 34. Finkenstedt A, Bianchi P, Theurl I, et al. Regulation of iron metabolism through GDF15 and hepcidin in pyruvate kinase deficiency. Br J Haematol. 2009;144:789–793. doi: 10.1111/j.1365-2141.2008.07535.x. * Serum GDF15 levels in pyruvate kinase deficiency.
- 35.Wu CJ, Krishnamurti L, Kutok JL, et al. Evidence for ineffective erythropoiesis in severe sickle cell disease. Blood. 2005;106:3639–3645. doi: 10.1182/blood-2005-04-1376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kanda J, Mizumoto C, Kawabata H, et al. Serum hepcidin level and erythropoietic activity after hematopoietic stem cell transplantation. Haematologica. 2008;93:1550–1554. doi: 10.3324/haematol.12399. [DOI] [PubMed] [Google Scholar]
- 37.Ashby DR, Gale DP, Busbridge M, et al. Erythropoietin administration in humans causes a marked and prolonged reduction in circulating hepcidin. Haematologica. 2009 Oct 14; doi: 10.3324/haematol.2009.013136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Theurl I, Finkenstedt A, Schroll A, et al. Growth differentiation factor 15 in anemia of chronic disease, iron deficiency anemia and mixed type anemia. Br J Haematol. 2009 Oct 15; doi: 10.1111/j.1365-2141.2009.07961.x. ** GDF15 levels in iron deficiency anemia and anemia chronic disease. An excellent demonstration of the complexities of GDF15 expression and hepcidin regulation among anemia patients.
- 39.Park CH, Valore EV, Waring AJ, et al. Hepcidin, a urinary antimicrobial peptide synthesized in the liver. J Biol Chem. 2001;276:7806–7810. doi: 10.1074/jbc.M008922200. [DOI] [PubMed] [Google Scholar]
- 40.Nemeth E, Tuttle MS, Powelson J, et al. Hepcidin regulates cellular iron efflux by binding to ferroportin and inducing its internalization. Science. 2004;306:2090–2093. doi: 10.1126/science.1104742. [DOI] [PubMed] [Google Scholar]
- 41.De Domenico I, Lo E, Ward DM, et al. Hepcidin-induced internalization of ferroportin requires binding and cooperative interaction with Jak2. Proc Natl Acad Sci U S A. 2009;106:3800–3805. doi: 10.1073/pnas.0900453106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wrighting DM, Andrews NC. Iron homeostasis and erythropoiesis. Curr Top Dev Biol. 2008;82:141–167. doi: 10.1016/S0070-2153(07)00006-3. [DOI] [PubMed] [Google Scholar]
- 43.Ganz T. Hepcidin--a regulator of intestinal iron absorption and iron recycling by macrophages. Best Pract Res Clin Haematol. 2005;18:171–182. doi: 10.1016/j.beha.2004.08.020. [DOI] [PubMed] [Google Scholar]
- 44. Nemeth E, Ganz T. The role of hepcidin in iron metabolism. Acta Haematol. 2009;122:78–86. doi: 10.1159/000243791. ** Current review of the fast-paced field of hepcidin biology.
- 45. Ganz T, Nemeth E. Iron sequestration and anemia of inflammation. Semin Hematol. 2009;46:387–393. doi: 10.1053/j.seminhematol.2009.06.001. * Good review for hepcidin regulation under inflammation.
- 46.Goodnough LT, Skikne B, Brugnara C. Erythropoietin, iron, and erythropoiesis. Blood. 2000;96:823–833. [PubMed] [Google Scholar]
- 47.Pippard MJ, Callender ST, Warner GT, et al. Iron absorption and loading in beta-thalassaemia intermedia. Lancet. 1979;2:819–821. doi: 10.1016/s0140-6736(79)92175-5. [DOI] [PubMed] [Google Scholar]
- 48.Kemna EH, Kartikasari AE, van Tits LJ, et al. Regulation of hepcidin: insights from biochemical analyses on human serum samples. Blood Cells Mol Dis. 2008;40:339–346. doi: 10.1016/j.bcmd.2007.10.002. [DOI] [PubMed] [Google Scholar]
- 49.Zimmermann MB, Fucharoen S, Winichagoon P, et al. Iron metabolism in heterozygotes for hemoglobin E (HbE), alpha-thalassemia 1, or beta-thalassemia and in compound heterozygotes for HbE/beta-thalassemia. Am J Clin Nutr. 2008;88:1026–1031. doi: 10.1093/ajcn/88.4.1026. [DOI] [PubMed] [Google Scholar]
- 50. Lakhal S, Talbot NP, Crosby A, et al. Regulation of growth differentiation factor 15 expression by intracellular iron. Blood. 2009;113:1555–1563. doi: 10.1182/blood-2008-07-170431. * An initial report suggesting that sever iron deficiency may regulate GDF15 expression in vivo and in vitro.
- 51.Marjono AB, Brown DA, Horton KE, et al. Macrophage inhibitory cytokine-1 in gestational tissues and maternal serum in normal and pre-eclamptic pregnancy. Placenta. 2003;24:100–106. doi: 10.1053/plac.2002.0881. [DOI] [PubMed] [Google Scholar]
- 52.Moore AG, Brown DA, Fairlie WD, et al. The transforming growth factor-β superfamily cytokine macrophage inhibitory cytokine-1 is present in high concentrations in the serum of pregnant women. J Clin Endocrinol Metab. 2000;85:4781–4788. doi: 10.1210/jcem.85.12.7007. [DOI] [PubMed] [Google Scholar]
- 53.Fairlie WD, Moore AG, Bauskin AR, et al. MIC-1 is a novel TGF-beta superfamily cytokine associated with macrophage activation. J Leukoc Biol. 1999;65:2–5. doi: 10.1002/jlb.65.1.2. [DOI] [PubMed] [Google Scholar]
- 54.Welsh JB, Sapinoso LM, Kern SG, et al. Large-scale delineation of secreted protein biomarkers overexpressed in cancer tissue and serum. Proc Natl Acad Sci U S A. 2003;100:3410–3415. doi: 10.1073/pnas.0530278100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Koopmann J, Buckhaults P, Brown DA, et al. Serum macrophage inhibitory cytokine 1 as a marker of pancreatic and other periampullary cancers. Clin Cancer Res. 2004;10:2386–2392. doi: 10.1158/1078-0432.ccr-03-0165. [DOI] [PubMed] [Google Scholar]
- 56.Hsiao EC, Koniaris LG, Zimmers-Koniaris T, et al. Characterization of growth-differentiation factor 15, a transforming growth factor beta superfamily member induced following liver injury. Mol Cell Biol. 2000;20:3742–3751. doi: 10.1128/mcb.20.10.3742-3751.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Strelau J, Sullivan A, Böttner M, et al. Growth/differentiation factor-15/macrophage inhibitory cytokine-1 is a novel trophic factor for midbrain dopaminergic neurons in vivo. J Neurosci. 2000;20:8597–8603. doi: 10.1523/JNEUROSCI.20-23-08597.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Schober A, Böttner M, Strelau J, et al. Expression of growth differentiation factor-15/ macrophage inhibitory cytokine-1 (GDF-15/MIC-1) in the perinatal, adult, and injured rat brain. J Comp Neurol. 2001;439:32–45. doi: 10.1002/cne.1333. [DOI] [PubMed] [Google Scholar]
- 59. Strelau J, Strzelczyk A, Rusu P, et al. Progressive postnatal motoneuron loss in mice lacking GDF-15. J Neurosci. 2009;29:13640–13648. doi: 10.1523/JNEUROSCI.1133-09.2009. * Knockout study for GDF15 function suggesting a neurological defect consistent with brain expression of the cytokine in mice.
- 60.Bootcov MR, Bauskin AR, Valenzuela SM, et al. MIC-1, a novel macrophage inhibitory cytokine, is a divergent member of the TGF-beta superfamily. Proc Natl Acad Sci U S A. 1997;94:11514–11519. doi: 10.1073/pnas.94.21.11514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Böttner M, Laaff M, Schechinger B, et al. Characterization of the rat, mouse, and human genes of growth/differentiation factor-15/macrophage inhibiting cytokine-1 (GDF-15/MIC-1) Gene. 1999;237:105–111. doi: 10.1016/s0378-1119(99)00309-1. [DOI] [PubMed] [Google Scholar]
- 62.Johnen H, Lin S, Kuffner T, et al. Tumor-induced anorexia and weight loss are mediated by the TGF-beta superfamily cytokine MIC-1. Nat Med. 2007;13:1333–1340. doi: 10.1038/nm1677. [DOI] [PubMed] [Google Scholar]
- 63.Baek SJ, Okazaki R, Lee SH, et al. Nonsteroidal anti-inflammatory drug-activated gene-1 over expression in transgenic mice suppresses intestinal neoplasia. Gastroenterology. 2006;131:1553–1560. doi: 10.1053/j.gastro.2006.09.015. [DOI] [PubMed] [Google Scholar]
- 64.Xu J, Kimball TR, Lorenz JN, et al. GDF15/MIC-1 functions as a protective and antihypertrophic factor released from the myocardium in association with SMAD protein activation. Circ Res. 2006;98:342–350. doi: 10.1161/01.RES.0000202804.84885.d0. [DOI] [PubMed] [Google Scholar]
- 65.Brown DA, Moore J, Johnen H, et al. Serum macrophage inhibitory cytokine 1 in rheumatoid arthritis: a potential marker of erosive joint destruction. Arthritis Rheum. 2007;56:753–764. doi: 10.1002/art.22410. [DOI] [PubMed] [Google Scholar]
- 66.Wakchoure S, Swain TM, Hentunen TA, et al. Expression of macrophage inhibitory cytokine-1 in prostate cancer bone metastases induces osteoclast activation and weight loss. Prostate. 2009;69:652–661. doi: 10.1002/pros.20913. [DOI] [PubMed] [Google Scholar]