SUMMARY
Rapid, experience-dependent plasticity in developing visual cortex is thought to be competitive. After monocular visual deprivation, the reduction in response of binocular neurons to one eye is matched by a corresponding increase to the other. Chronic optical imaging in wild type and mutant mice deficient in TNFα reveals similar initial losses of response to the deprived eye, but the subsequent increase in response to the open eye is absent in the mutant animals. This mutation also blocks homeostatic synaptic scaling of mEPSCs in visual cortex in vitro, without affecting LTP. In monocular cortex, thought not to be subject to competition, response to TNFα mutants are reduced as in the binocular zone. Local pharmacological inhibition of endogenous TNFα in the visual cortex of wild type mice phenocopies the knockout. These findings suggest that experience-dependent competition in developing visual cortex is the outcome of two distinct, non-competitive processes, the first a loss of deprived-eye responses, the second an apparently homeostatic increase in responses dependent on TNFα signaling.
INTRODUCTION
Refinement of neuronal connectivity during development requires activity-dependent synaptic plasticity, which may include both correlation-based (Hebbian) forms and more global, homeostatic forms of plasticity. The former, represented by long-term potentiation and depression (LTP and LTD), rapidly alters connections in a synapse-specific manner, and considerable evidence has accumulated for its role in synaptic refinement during development as well as in learning. The latter adjusts synaptic strengths slowly and globally in response to prolonged changes in activity; such mechanisms can provide homeostatic stability to neuronal networks (Burrone and Murthy, 2003; Davis and Bezprozvanny, 2001; Turrigiano and Nelson, 2004).
The postnatal development of the mammalian visual cortex has been a classic model for the experience-dependent fine-tuning of cortical circuitry. For example, occluding the vision of one eye (monocular deprivation, MD) during a critical period of heightened sensitivity in early postnatal life dramatically alters visual function, producing a loss of visual cortical responsiveness to the deprived eye and decreases in visual acuity (Wiesel, 1982). Such visual cortical plasticity is also competitive in that responses to the open eye grow stronger and more widespread as those to the deprived eye decrease, and such plasticity has not been thought to take place in regions of the cortex receiving input from only one eye (Wiesel, 1982). Other manipulations of visual experience such as alternating monocular occlusion or strabismus provide even stronger evidence for the competitive nature of visual cortical plasticity (Wiesel, 1982).
Although the competitive nature of visual cortical plasticity has been firmly established, the mechanistic basis of this competition is not well understood. Early single unit recordings in kitten striate cortex provided evidence that the decrease in deprived eye responses precedes the increase in open eye responses (Mioche and Singer, 1989; Olson and Freeman, 1975). A recent study using visual evoked potentials in mice has found similar results (Frenkel and Bear, 2004). These observations of a temporal separation in the changes in responses to the two eyes raise the possibility that binocular competition may be mediated by distinct mechanisms, one for the decrease and another for the increase.
Visual cortical plasticity has conventionally been ascribed to Hebbian or correlation-based mechanisms similar to LTP and LTD (Bear and Rittenhouse, 1999; Bienenstock et al., 1982; Crozier et al., 2007; Heynen et al., 2003; Katz and Shatz, 1996; Kirkwood and Bear, 1994; Miller et al., 1989). However, patch recordings in cortical slices from visually-deprived animals have revealed increased mEPSCs in layer 2/3 pyramidal neurons (Desai et al., 2002; Goel and Lee, 2007), suggesting that homeostatic scaling of synaptic strength similar to that observed in vitro may be induced in vivo by sensory manipulations. Recent two-photon observations of calcium signals in deprived visual cortex have shown homeostatic response compensation following MD (Mrsic-Flogel et al., 2007). Since both Hebbian and homeostatic mechanisms may interact to produce a final outcome in vivo following sensory manipulations such as MD, a means to dissociate one mechanism from the other is necessary to reveal the contributions of each.
Previously we demonstrated that synaptic scaling in response to prolonged activity blockade in dissociated and slice cultures of hippocampus was deficient in mice lacking TNFα (Tnf knockout mice: Tnf−/−), although NMDA receptor-dependent LTP and LTD in the hippocampus were normal (Stellwagen and Malenka, 2006). These findings prompted us to search for a role for homeostatic synaptic scaling in cortical development by studying visual cortical plasticity in these mice in vivo.
RESULTS
Impaired homeostatic synaptic scaling in the visual cortex of TNFα-deficient mice
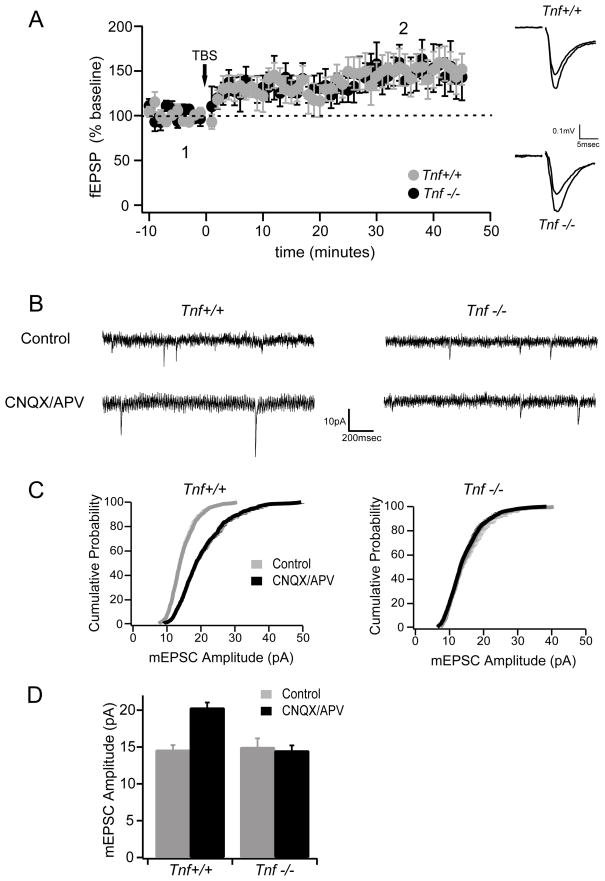
Because other knockout mice have shown differences between hippocampal and cortical plasticity (Frankland et al., 2001), we first sought to determine whether synapse-specific mechanisms of plasticity were normal in Tnf−/− mice by examining in vitro LTP of the layer 4 inputs to layer 2/3 of visual cortex. The magnitude of LTP in slices from Tnf−/− mice at P26-32, the same age we investigated cortical plasticity in vivo (see below), was indistinguishable from that in slices from wild type mice (Fig. 1A), indicating that, as in the hippocampus, Tnf−/− mice have no deficits in cortical LTP. In contrast, synaptic scaling induced by prolonged activity-blockade was deficient in the visual cortical slice cultures. The increase in mEPSC amplitude in slices treated with APV and CNQX for 2 days, prominently observed in wild type neurons, did not occur in cortical neurons from Tnf−/− mice (Fig. 1B–D). Frequency of mEPSCs were not changed significantly by activity blockade in either Tnf+/+ (control vs. blocked: 0.4 +/− 0.1 Hz, 0.8 +/− 0.3 Hz; P>0.10) or Tnf−/− mice (0.8 +/− 0.2 Hz, 1.4 +/− 0.5 Hz; P>0.25). These observations confirm that, similar to hippocampal neurons, visual cortical neurons of Tnf−/− mice lack a homeostatic increase in excitatory synaptic strength in response to long-term decrease of neuronal activity, while Hebbian plasticity remains intact.
Fig. 1.
Normal long-term potentiation (LTP) and deficient synaptic scaling in visual cortical slices prepared from TNFα knockout mice. (A) LTP is normal in Tnf−/− mice (n=6) compared to wild type mice (n=9). The peak amplitudes of field excitatory postsynaptic potentials (fEPSP) recorded in layer 3 in response to layer 4 stimulation are plotted as a function of time. Theta burst stimulation (TBS) was delivered at time 0. Insets on the right show example traces of fEPSP (averaged responses (6 traces = 1 minute) from around 1 and 2 in the graph. The smaller trace is pre-LTP (1) and the larger trace post-LTP (2). (B-D) Homeostatic synaptic scaling assayed in organotypic slice cultures of mouse visual cortex prepared from Tnf−/− and Tnf+/+ mice. The amplitudes of miniature excitatory postsynaptic currents (mEPSC) are increased after 2 days of blockade of glutamatergic excitatory transmission (using CNQX/APV) in Tnf+/+ mice but not in Tnf−/− mice (n = 10 – 13 cells per condition).
Cortical responses to visual stimulation in Tnf−/− mice were similar to those in wild type animals at a global level as measured by intrinsic signal optical imaging (Fig. S1). Magnitudes and areas of responses as well as the quality of the retinotopic maps were statistically indistinguishable between mutant and wild type animals (Fig. S1). In Tnf−/− mice with normal visual experience, visual cortical responses to stimulation of the two eyes (whose relative magnitude is referred to as ocular dominance) were also similar to wild type (see non-deprived (ND) groups in Fig. 2 and pre-MD groups in Fig. 3G). Anatomical experiments demonstrated normal cytoarchitecture in the cerebral cortex and normal segregation of the eye-specific input in the dorsal lateral geniculate nucleus (dLGN) in Tnf−/− mice (Fig. S2). These observations indicate that with normal visual experience, visual cortical function in Tnf−/− mice develops without impairment detectable in any of the measures we evaluated.
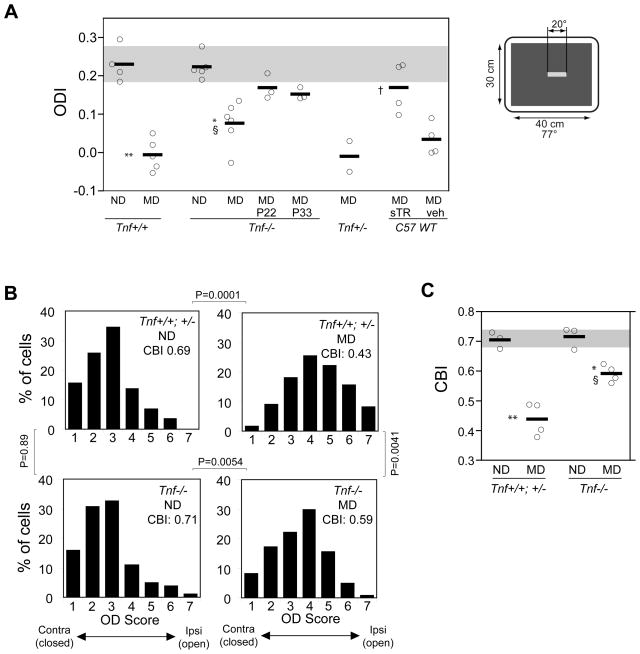
Fig. 2.
Plasticity following monocular visual deprivation is reduced in Tnf−/− mice. (A) Ocular dominance index (ODI) in individual animals (circles) and group average (horizontal line) computed from responses measured by intrinsic signal optical imaging. The gray box represents the mean ± SD of baseline ODI in Tnf+/+ animals with no deprivation. ND: no deprivation, MD: 5 days of monocular deprivation starting at P26-27. Inbred C57/Bl6 wild type animals received cortical infusion of soluble TNF receptor 1 (sTR) or vehicle (veh) during the MD. ** P<0.001 and * P<0.01 vs. corresponding ND group; § P<0.05 vs. Tnf+/+ MD; † P<0.05 vs. vehicle-treated animals. The inset on the right hand illustrates the visual stimuli used to evoke intrinsic signal responses in the binocular zone. (B) Distribution of ocular dominance scores of single units recorded electrophysiologically in Tnf+/+, Tnf+/−, or Tnf−/− mice after 5 days of MD starting at P26-27 and in age-matched animals without visual deprivation (ND). Data from Tnf+/+ and Tnf+/− mice were pooled because no significant difference was detected in ODIs between these two groups. Control (Tnf+/+ and +/−) ND: 3 mice, 78 cells; control MD: 4 mice, 122 cells; Tnf−/− ND: 3 mice, 101 cells; Tnf−/− MD: 4 mice 121 cells. P values in the figure were from Fisher exact test. (C) Contralateral bias index (CBI), which measures relative responses of single neurons to the two eyes, shown for individual animals (circles) and average group values (horizontal lines) computed from single unit data presented in (B). The gray box indicates the mean ± SD of baseline CBI in control animals. **P<0.01 and *P<0.05 vs. corresponding ND group; § P<0.05 vs. MD-Tnf+/+ group. Statistical analyses in A and C were performed using one-way ANOVA with Bonferroni multiple comparisons.
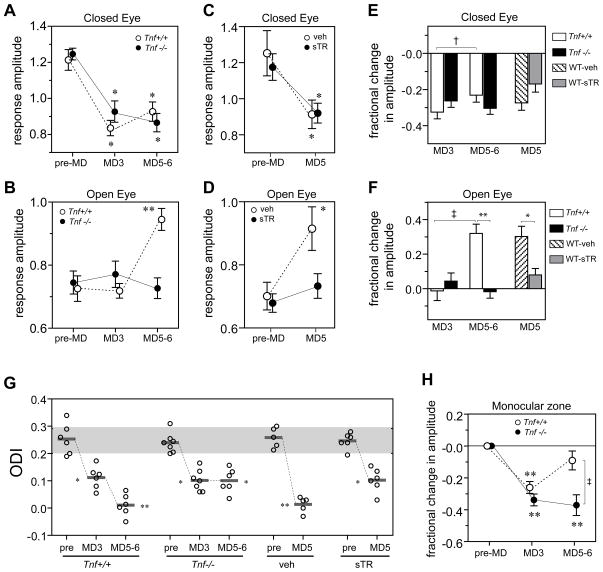
Fig. 3.
Repeated imaging of intrinsic signal reveals that lack of TNFα signaling impairs the delayed component of plasticity induced by MD. (A, B) Average signal amplitude of the deprived-eye response (A) and open-eye response (B) in the binocular visual area, before the lid suture (pre-MD), after 2.5 – 3 days of MD (MD3), and after 5 – 6 days of MD (MD5-6) in Tnf+/+ (n=6) and Tnf−/− (n=7) mice. All data from longitudinal measurements are in the same individual animals. (C, D) Average signal amplitude of the deprived-eye response (C) and open-eye response (D) in the binocular visual area in inbred C57Bl6 wild type mice treated with cortical infusion of soluble TNF receptor1 (sTR) (n=7) or vehicle solution (n=5), before the lid suture and after 5 days of MD. Values in A–D present mean ± S.E.M of the group. **P<0.01, *P<0.05 compared with baseline (pre-MD), repeated measure ANOVA and Bonferroni multiple comparisons. (E, F) Changes in response amplitude from baseline following MD. Fractional changes in average response to deprived eye (E) and open eye (F) were calculated from measurements presented in A–D as (post-MD – pre-MD)/(pre-MD). †P<0.05 and ‡ P<0.01, paired t-test; *P<0.05 and **P<0.01, unpaired t-test. (G) Individual ODIs (circles) and mean group values (horizontal lines). The gray box presents the mean ± SD of baseline (pre-MD) ODI in Tnf+/+ animals. **P<0.01, *P<0.05 vs. corresponding baseline. (H) Response magnitude in the monocular area to the deprived eye in Tnf+/+ animals (open bar, n=6) and Tnf−/− animals (closed bar, n=6). Values present mean changes (±S.E.M.) from the baseline in response magnitude in the monocular area. **P<0.01 compared to baseline (repeated measure ANOVA), ‡ P <0.01 between groups (t-test).
Reduced ocular dominance plasticity in TNFα-deficient mice
To examine the requirement for TNFα in experience-dependent neocortical plasticity in vivo, we assayed ocular dominance plasticity following a short period of monocular deprivation induced by lid suture near the peak of the critical period. We used optical imaging of intrinsic signals to measure cortical responses to right- and left-eye stimulation that was restricted to the binocular portion of the visual field, from which we computed the ocular dominance index (ODI) as described (Cang et al., 2005a) (Fig. S3 for example images). Closing the eye contralateral to the hemisphere under study for 5 days starting at P26-27 produced a robust shift in OD toward the ipsilateral open eye in Tnf+/+ mice (Fig. 2A and Fig. S3). Compared with plasticity in Tnf+/+ animals, the OD shift in Tnf−/− mice was significantly reduced but was not completely eliminated. Thus, the ODI of the Tnf−/− animals with MD fell between the ODIs in animals with normal visual experience (no deprivation: ND groups) and those in Tnf+/+ animals after MD (Fig. 2A).
Recent studies of other transgenic mice have highlighted the possibility that apparent defects in plasticity can arise from changes in the timing of the critical period (Fagiolini and Hensch, 2000; Hanover et al., 1999; Huang et al., 1999). To investigate this possibility, we examined the effect of MD initiated before and after the normal peak of the critical period. MD in Tnf−/− mice started at P22 or P33 failed to induce a larger shift in OD than that at P26-28 (Fig. 2A), indicating that the impaired plasticity in Tnf−/− animals during the critical period cannot be accounted for by a precocious or delayed critical period for OD plasticity.
Although we detected no significant difference in visual responsiveness and LTP induction in the Tnf−/− cortex compared with Tnf+/+ cortex, the lack of TNFα before the critical period could in principle have affected the normal development of cortical circuitry that is necessary for proper OD plasticity. Also, the absence of TNFα in more peripheral stages of the visual pathway, such as the retina or dLGN, might also contribute to reduced OD plasticity because TNFα and its receptors are expressed ubiquitously throughout the CNS (reviewed in (Vitkovic et al., 2000). To address these questions, we sought to perturb TNFα signaling in a temporally and spatially delimited manner, exclusively during the induction of plasticity in the visual cortex of one hemisphere, by studying the effects of MD in wild type animals that received a cortical infusion of soluble TNF receptor 1 (sTNFR1) only during the period of deprivation. After 5 days of infusion, sTNFR1 typically spread within the hemisphere of the infused side but spread very little to the contralateral hemisphere or subcortical areas (Fig. S3). This cortical infusion of sTNFR1 did not affect visual cortical responses at a global level under normal visual experience (Fig. S4). Following MD, the OD in vehicle-treated mice shifted robustly toward the open eyes, whereas sTNFR1-treated wild type animals showed a reduced OD shift (Fig. 2A) like that in the Tnf−/− mice. Thus, interaction of endogenous TNFα with its receptors is required acutely during MD and within the cortex for normal OD plasticity.
Single unit electrophysiological recordings confirmed the observations made using intrinsic signal imaging. After 5-day MD, cortical neurons shifted their responsiveness to favor the open eye in control animals including Tnf+/+ and Tnf+/− (Fisher exact test, P < 0.001 compared to non-deprived mice), whereas such a shift in Tnf−/− cortical cells was significantly reduced (Fisher exact test, P < 0.05 compared either to non-deprived Tnf−/− mice or to deprived control mice) (Fig. 2B). As a result, the mean contralateral bias index (CBI) of Tnf−/− animals lay between those of non-deprived and control MD groups (Fig. 2C).
Mechanism underlying the reduced ODP in TNFα-deficient mice
The OD shift produced by MD during the critical period is the result of two distinct changes in visual cortical responsiveness: a decrease in deprived-eye responses and an absolute increase in responses to the open eye (Wiesel, 1982). A decreased OD shift could result from a lack of one of these changes or else from partial impairment of both. We sought to determine which of these components were responsible for reduced plasticity observed in the absence of TNFα. Although the intrinsic signal imaging experiments described above give a reliable measure of ocular dominance from the difference in responses to the two eyes, which is constant over a large range of response magnitudes, the acute nature of the experimental setting made it difficult to be confident of the absolute magnitudes of cortical responses, or to infer changes in the magnitudes of cortical responses from comparisons among groups of different animals. To address this issue, we measured response magnitudes repeatedly in the same individuals before, during, and immediately after MD, by imaging transcranially under isoflurane anesthesia (similar to (Hofer et al., 2006). The validity of this method was confirmed by the similarity of its OD measurements to those of the established, acute procedure using optical imaging or single-unit recordings (compare Figs 2A, C with 3G). With this method, response magnitudes within individuals were stable across experimental days and the within-animal variability was significantly smaller than the variability among animals (Fig. S5).
Chronic imaging revealed a clear temporal separation between the effects of MD on responses to the two eyes, consistent with a previous report of visually evoked potential recording (Frenkel and Bear, 2004). In Tnf+/+ mice, MD produced an initial rapid and profound decrease in deprived-eye responses after 2.5 – 3 days (MD3) (Fig. 3A, E, Fig. S6). This decrease was followed over the subsequent 2 – 3 days by a second phase of ocular dominance plasticity, in which there is a prominent increase in open-eye responses (Fig. 3B, F, Fig. S7). Responses to the deprived-eye also showed a small but significant increase during this same period (P<0.05, compare MD3 to MD5-6).
In Tnf−/− mice, this second phase of ocular dominance plasticity was absent. Most prominently, open-eye responses showed no significant change significantly during MD (P = 0.734, pre vs. MD3; and P = 0.657, pre vs. MD5-6, paired t-test) (Fig. 3B, F). While the initial decrease in deprived-eye responses in Tnf−/− mice was similar to that in Tnf+/+ mice, no subsequent increase occurred (Fig. 3A, E). The effects of the knockout on plasticity were largely phenocopied in wild type animals that received cortical infusion of sTNFR1: increases in open-eye responses were significantly smaller than those in vehicle-treated control animals, while decreases in deprived-eye responses were similar to control (Fig. 3C, D).
These changes in response amplitude led to changes in ODIs (Fig. 3G) that reproduced the results obtained in acute imaging experiments. Notably, Tnf−/− animals showed a change in mean ODI to the same degree as Tnf+/+ mice at MD3, but unlike wild type animals, their OD did not shift further at MD5-6. The data from these chronic imaging experiments revealed that impaired TNFα signaling selectively blocked the delayed changes produced by MD, but not the early decrease in deprived-eye responses.
Plasticity in the monocular zone induced by deprivation
The monocular zone of the visual cortex, which represents the region of the visual field seen only by the contralateral eye, provides a further test of our dissection of competition into mechanistically distinct phases, the second of which appears homoeostatic and involves TNFα signaling. This region of visual cortex has appeared to lack rapid, activity-dependent plasticity (Pham et al., 2004; Tohmi et al., 2006), which in the binocular zone is thought to result from competition between inputs serving the two eyes (Wiesel and Hubel, 1965). Chronic imaging in wild type mice revealed that responses in the monocular zone to the deprived eye initially decreased to levels comparable to those in the binocular zone at MD3, but they were restored to the baseline level by MD5-6. Tnf−/− mice showed a similar decrease at MD3, and this decrease persisted at MD5-6 (Fig. 3H). This observation suggests that the monocular zone undergoes an initial loss of deprived-eye responses followed by a homeostatic form of plasticity that restores the global activity level, a process in which TNFα plays a critical role. Consistent with this interpretation are the observations in the rat of a significant decrease in deprived-eye visual evoked potentials in the monocular zone after 24 hr of MD (Heynen et al., 2003) together with the increase in mEPSCs seen in pyramidal neurons of monocular visual cortex after 2 days of monocular application of tetrodotoxin (Desai et al., 2002).
DISCUSSION
The present findings reveal that distinct mechanisms mediate two phases of the competitive, activity-dependent neural plasticity induced in the binocular zone of developing visual cortex by monocular visual deprivation: first, the rapid depression of deprived-eye responses, and second, the delayed potentiation of open-eye (and to a lesser extent deprived-eye) responses, where only the second process requires TNFα signaling. Supporting this dissection of activity-dependent competition into mechanistically distinct processes are our findings in the monocular zone of cortex, where MD had been thought not to cause rapid plasticity because of the lack of a competing input. In the monocular zone we found transient reductions in visual responses in wild type animals subjected to MD, followed by a restoration of responses to their initial levels, but lasting reductions in TNFα knockout animals, which were of similar magnitude to those in the binocular zone. Competition is thus not a unitary phenomenon, but instead is the result of at least two cellular processes that are temporally and mechanistically distinct. The evidence for two temporally distinct phases of plasticity induced by MD was first shown in cat striate cortex, most clearly by chronic extracellular recordings from single sites (Mioche and Singer, 1989), and more recently in mouse visual cortex using evoked potentials (Frenkel and Bear, 2004). Our findings now provide evidence that the two phases are mechanistically distinct.
We suggest that the second process is a form of homeostatic synaptic scaling. What is the evidence for this suggestion? The phenomenon of homeostatic synaptic scaling has been demonstrated repeatedly in many systems (Burrone and Murthy, 2003; Davis and Bezprozvanny, 2001; Turrigiano and Nelson, 2004), but unlike the synapse-specific forms of plasticity such as LTP and LTD, little of the cell biology underlying homeostatic synaptic scaling has been worked out. The dependence of homeostatic synaptic scaling on TNFα signaling, both in hippocampus (Stellwagen and Malenka, 2006) and visual cortex (present study), provides a molecular handle on the phenomenon. Our observation that impairment of TNFα signaling blocks the second phase of ocular dominance plasticity therefore provides evidence of a role for synaptic scaling in ocular dominance plasticity in vivo. Second, in both the binocular zone and the monocular zone, the second phase of plasticity, like homeostatic synaptic scaling, lacks input specificity, in that responses to the deprived eye increase rather than decrease despite continued visual deprivation when TNFα signaling is intact. Third, dark-rearing or visual deprivation has been shown to increase the amplitudes of mEPSCs subsequently recorded in vitro in developing visual cortex, consistent with the operation of synaptic scaling (Desai et al., 2002; Goel and Lee, 2007). Fourth, homeostatic synaptic scaling can also explain observations made using two-photon calcium imaging that responses in a population of neurons dominated by the deprived eye become stronger rather than weaker as a result of MD (Mrsic-Flogel et al., 2007). Together, these observations make it reasonable to conclude that the second phase of MD-induced plasticity is a result of homeostatic synaptic scaling.
Correlation-based synaptic plasticity has been the prevailing explanation for the changes in the visual cortical responsiveness induced by visual deprivation (Bear and Rittenhouse, 1999; Bienenstock et al., 1982; Katz and Shatz, 1996; Kirkwood and Bear, 1994; Miller et al., 1989), and particularly a role for LTD in the decrease in deprived-eye responses (Crozier et al., 2007; Heynen et al., 2003). While the relationship between ocular dominance plasticity in visual cortex vivo and LTP/LTD in vitro is not straightforward (Hensch, 2005), they share a number of common features, including dependence on NMDA receptors (Fagiolini et al., 1994) and its downstream pathways including calcium calmodulin kinase autophosphorylation (Taha et al., 2002). The present findings are perfectly consistent with a role for Hebbian mechanisms in contributing to the plasticity induced by MD. In particular, we found that the lack of TNFα did not alter the time course or magnitude of depression of the closed eye responses in binocular region, suggesting that a weakening of synaptic strength subserving the deprived eye reminiscent of LTD is indeed induced, which leads to a decrease in overall activity in the visual cortex. This decrease, and not just reduced afferent excitatory inputs, could trigger or drive the homeostatic change. We do not know what signaling pathways other than those requiring TNFα are involved specifically in the second phase of competitive ocular dominance plasticity because manipulations that block the first phase, the reduction in deprived-eye responses, could remove the stimulus for the second phase. We suggest that competitive, activity dependent plasticity during the critical period in developing visual cortex involves a coordinated interplay between Hebbian and homeostatic forms of plasticity, which together determine final consequences of visual deprivation.
The phenomenon of ocular dominance plasticity during the critical period includes multiple changes in responses and connections that take place in different cells and different times (Trachtenberg and Stryker, 2001; Trachtenberg et al., 2000). The capacity for homeostatic plasticity itself differs among neurons in different cortical layers and at different times (Desai et al., 2002; Goel and Lee, 2007; Maffei et al., 2006; Maffei et al., 2004). In layer 4, visual deprivation induces mEPSC scaling that disappears by P21 (Desai et al., 2002) while it potentiates inhibition in deprived eye pathway at later ages (Maffei et al., 2006). In layer 2/3, such mEPSC scaling starts later than layer 4 (Desai et al., 2002) and persists into adulthood (Goel and Lee, 2007). The intrinsic optical signal used in the present experiments reflects mainly the activity in layer 2/3 with a significant contribution from layer 4 (Trachtenberg et al., 2000), and its findings were consistent with those of our single unit recordings, which were mainly from the superficial layers, 4 and above. Heterogeneity of cells and mechanisms did not obscure the overall changes that we measured.
If homeostatic synaptic scaling plays an important role in the development of neural circuitry, it may be thought surprising that the visual cortex in Tnf−/− animals appears to develop normally with normal visual experience. In vitro measurements revealed no differences in mEPSC amplitude between wild type and knockout animals, and response magnitude and retinotopic organization examined at population level with intrinsic signal optical imaging were also similar in the two genotypes. The normality of the mutant animals is likely to be due to other, more slowly acting mechanisms for the homeostatic regulation of excitability, which compensate for the lack of TNFα during development. For example, BDNF-TrkB signaling (Rutherford et al., 1998) and Arc (Shepherd et al., 2006) have each been shown to play a role in synaptic scaling. A lack of effect on normal development is indeed common to perturbations of many molecules and signaling pathways involved in neural plasticity. For example, response properties of visual cortical neurons of knockout animals for alpha-calcium/calmodulin-dependent protein kinase (Gordon et al., 1996), RII-beta isoform of PKA (Fischer et al., 2004), RII-alpha isoform of cAMP-dependent protein kinase (Rao et al., 2004), paired-immunoglobulin-like receptor B (Syken et al., 2006) or tissue-type plasminogen activator (Mataga et al., 2002) are apparently normal in normally reared animals, and where abnormalities are found, as in GAD-65 knockout mice (Hensch et al., 1998), they are commonly slight. It is only the manipulation of neural activity that reveals important roles of these molecules in cortical plasticity.
For many decades, the effect of MD on visual cortical circuitry has served as the exemplar of experience-dependent plasticity in the mammalian brain. The disruption of TNFα signaling may allow many forms of experience dependent changes in brain and behavior, including those involved in learning and memory, to be dissected into their synapse-specific and homeostatic components.
EXPERIMENTAL PROCEDURES
Animals
Tnf−/− mice were obtained from Jackson Laboratories, bred homozygously, and compared to wild type mice of matched genetic background also obtained from Jackson Laboratories (#101045 B6129SF2/J )
Acute Slice Electrophysiology
Acute coronal slices of visual cortex (400 μm) were prepared from P26-32 mice. Slices were perfused with external medium containing (in mM): 119 NaCl, 2.5 KCl, 4 CaCl2, 4 MgSO4, 1 NaH2PO4, 26.2 NaHCO3, 11 glucose and 0.05 picrotoxin (PTX), saturated with 95% O2/5% CO2, and maintained at 28–30°C. EPSPs were evoked by stimulating layer IV at a frequency of 0.1 or 0.05 Hz with a glass pipette, field potentials recorded in layers II/III, and LTP was induced using 4 trains of theta burst stimulation (TBS), consisting of 5 pulses delivered at 100Hz repeated 10 times at 5Hz. Field EPSP amplitude was obtained using a 2msec window before the stimulus (baseline) and a 1msec window around the peak of the EPSP after TBS.
Slice Culture Electrophysiology
Organotypic slice cultures were prepared from coronal slices of visual cortex of P4-5 mice essentially as described (Schlueter, et al., 2006). Slice cultures were recorded at DIV 6–12, following 48hr treatment of 20μM CNQX and 50μM APV. Whole-cell voltage-clamp recordings were made from the superficial layers of primary visual cortex using an Axopatch 1D amplifier (Axon Instruments). The whole-cell pipette solution contained (in mM): 117 Cs-MeSO4, 20 HEPES, 5 TEA, 0.4 EGTA, 3 NaCl, 2.5 ATP, and 0.3 GTP (pH=7.3). Miniature EPSCs were recorded from cells perfused with room temperature external medium (as above) with 2.5mM CaCl2, 1.3mM MgSO4, 400 nM TTX, and 50 μM PTX. Analysis of mEPSCs was performed using Mini Analysis 5 software (Synaptosoft). All mEPSCs above a threshold value (6 pA) were included in the data analysis and each mEPSC was verified visually. Group results were compared using Student’s t-test, and mEPSC cumulative distributions were compared using a Kolmogorov-Smirnov two sample test.
Monocular deprivation (MD)
MD was performed according to Gordon and Stryker (1996), except 2–3% isofluorane in oxygen was used for anesthesia. The lids of the right eye were sutured at P26-27, the peak of the critical period (Gordon and Stryker, 1996), except for 6 Tnf−/− mice that received lid suture at P22 (n = 3) and P33 (n = 3). Animals were checked daily to ensure that the eyes remained closed during the MD period. In acute experiments including single unit recording, the MD period lasted for approximately 5 days. In chronic imaging experiments, lids were sutured immediately following the first (pre-MD) imaging session; a subset of animals in each genotype underwent the second imaging after the first half (2.5 – 3 days) of MD, followed by another 2.5 – 3 days of MD before the third and final examination. In these animals, an extra care was taken to minimize the exposure of the deprived eye to the light during the second imaging session. The other subset of animals was examined only before and after 5 days of MD. In chronic experiments with cortical infusions, the cannula was implanted right after the pre-MD imaging, and the animals were allowed to recover for 2 days before the lid suture.
Optical imaging of intrinsic signals
(a) Acute preparation
Intrinsic signal optical imaging and quantification of ocular dominance were performed as described (Cang et al., 2005a; Kalatsky and Stryker, 2003). Briefly, under anesthesia with Nembutal (50 mg/kg; Abbott, North Chicago, IL) and chlorprothixene (0.2 mg), mice were placed in a stereotaxic apparatus and a craniotomy was made over the visual cortex of the left hemisphere, over which low-melting point agarose (3% in saline) and a glass coverslip were placed. Intrinsic signal images were obtained with Dalsa 1M30 CCD camera (Dalsa, Waterloo, Canada) with 135×50 mm tandem lens (Nikon Inc., Melville, NY) and red interference filter (610 ± 10 nm). Frames were acquired at the rate of 30 fps, temporally binned by 4 frames, and stored as 512×512 pixel images after binning the 1024×1024 camera pixels by 2×2 pixels spatially. A high refresh rate monitor was placed in front of the animal to display visual stimuli. The monitor was 25 cm away from the mouse, with its center aligned to the animal’s midline. The visual stimulus for recording the binocular zone consisted of 2°-wide bars, which were presented between −5° and 15° on the stimulus monitor (0° = center of the monitor) and moving continuously and periodically upward or downward at a speed of 10°/sec. The phase and amplitude of cortical responses at the stimulus frequency were extracted by Fourier analysis as described (Kalatsky and Stryker, 2003). The map of response magnitude was obtained by averaging the response amplitudes of individual pixels in maps to upward and downward moving bars. We chose the binocularly responsive region of interest (ROI) based on the ipsilateral eye response map after smoothing by low-pass filtering using a uniform kernel of 5×5 pixels and thresholding at 40 % of peak response amplitude. We then computed OD score, (C−I)/(C+I), for every pixel in this ROI, where C and I represent the magnitude of response to contralateral and ipsilateral eye stimulation, followed by calculation of the ocular dominance index (ODI) as the average of OD score for all responsive pixels (Cang et al., 2005a).
For assessment of retinotopy and response magnitude as shown in Fig. S1, intrinsic signal imaging was performed in animals at P32-37 using full-screen bar stimulus. Response area was calculated by selecting pixels that had response magnitude larger than 30% of maximum value. Inverse magnification and map scatter were computed as described (Cang et al., 2005b).
(b) Chronic preparation
For repeated intrinsic signal recording, we modified the acute preparation as follows. First, we used isoflurane 0.8% in oxygen applied via a home-made nose mask during the imaging session, supplemented with a single intramuscular injection of 20–25 μg chlorprothixene. Second, we recorded transcranially without craniotomy; the scalp was suture closed at the end of the session and re-opened at the same location in the consequent session(s). Third, we shortened the duration of each run by a quarter, reducing the length of each imaging session to minimize the effects of anesthesia on OD plasticity and overall physical condition. Typically, preparations took about 30–40 min and animals stayed in a relatively stable anesthetic level for 2–3 hours with constant isoflurane inhalation. After discontinuation of isoflurane, righting reflex started within 30 min and normal behavior such as walking, eating, and grooming returned in 2–3 hours.
Single unit recording
Following a brief session of optical imaging of intrinsic signals to locate the binocular visual cortical area contralateral to the closed eye, electrophysiological single unit recordings were performed according to Gordon and Stryker (Gordon and Stryker, 1996). In each mouse, single units were isolated at intervals of 60 μm or more, using a standard tungsten electrode (FHC, Inc., Bowdoinham, ME). A hand-held lamp was used to project moving bars onto a tangent screen to drive neuronal responses. Optimal stimuli were presented to either eye alternately, and the relative strength of the response was determined. Each unit was scored on the 1-to-7 scale (Hubel and Wiesel, 1962), where 1 and 7 indicate exclusive response to the contralateral and ipsilateral eyes, respectively. A score of 4 was assigned to units responding equally well to either eye, and scores 2 or 3 and 5 or 6 were to those responding better or dominated by the contralateral and ipsilateral eyes, respectively. For each mouse, a contralateral bias index (CBI) was calculated according to the formula:
where N = total number of cells and nx = number of cells with OD scores equal to x.
Intracortical infusion
Implantation of a cortical cannula was performed as described (Taha and Stryker, 2002) 2 days prior to MD. The cannulae were connected with Alzet osmotic minipumps filled either with vehicle solution (PBS containing 0.1% bovine serum albumin as a carrier) or 35 μg/ml of soluble TNF receptor-1 (sTNFR1, R&D systems, Inc. Minneapolis, MN). The distribution of sTNFR1 after infusion was examined in a separate group of age-matched animals. Briefly, after 5 days of infusion, the animal was deeply anesthetized with Nembutal and the brain was dissected and fixed in 4% paraformaldehyde overnight. The brain was then sliced in a vibratome and stained immunohistochemically using an anti-TNFR1 antibody (H-271, Santa Cruz Biotechnology, Santa Cruz, CA).
Supplementary Material
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Bear MF, Rittenhouse CD. Molecular basis for induction of ocular dominance plasticity. J Neurobiol. 1999;41:83–91. doi: 10.1002/(sici)1097-4695(199910)41:1<83::aid-neu11>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- Bienenstock EL, Cooper LN, Munro PW. Theory for the development of neuron selectivity: orientation specificity and binocular interaction in visual cortex. J Neurosci. 1982;2:32–48. doi: 10.1523/JNEUROSCI.02-01-00032.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burrone J, Murthy VN. Synaptic gain control and homeostasis. Curr Opin Neurobiol. 2003;13:560–567. doi: 10.1016/j.conb.2003.09.007. [DOI] [PubMed] [Google Scholar]
- Cang J, Kalatsky VA, Lowel S, Stryker MP. Optical imaging of the intrinsic signal as a measure of cortical plasticity in the mouse. Vis Neurosci. 2005a;22:685–691. doi: 10.1017/S0952523805225178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cang J, Kaneko M, Yamada J, Woods G, Stryker MP, Feldheim DA. Ephrin-as guide the formation of functional maps in the visual cortex. Neuron. 2005b;48:577–589. doi: 10.1016/j.neuron.2005.10.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crozier RA, Wang Y, Liu CH, Bear MF. Deprivation-induced synaptic depression by distinct mechanisms in different layers of mouse visual cortex. Proc Natl Acad Sci U S A. 2007;104:1383–1388. doi: 10.1073/pnas.0609596104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis GW, Bezprozvanny I. Maintaining the stability of neural function: a homeostatic hypothesis. Annu Rev Physiol. 2001;63:847–869. doi: 10.1146/annurev.physiol.63.1.847. [DOI] [PubMed] [Google Scholar]
- Desai NS, Cudmore RH, Nelson SB, Turrigiano GG. Critical periods for experience-dependent synaptic scaling in visual cortex. Nat Neurosci. 2002;5:783–789. doi: 10.1038/nn878. [DOI] [PubMed] [Google Scholar]
- Fagiolini M, Hensch TK. Inhibitory threshold for critical-period activation in primary visual cortex. Nature. 2000;404:183–186. doi: 10.1038/35004582. [DOI] [PubMed] [Google Scholar]
- Fagiolini M, Pizzorusso T, Berardi N, Domenici L, Maffei L. Functional postnatal development of the rat primary visual cortex and the role of visual experience: dark rearing and monocular deprivation. Vision Res. 1994;34:709–720. doi: 10.1016/0042-6989(94)90210-0. [DOI] [PubMed] [Google Scholar]
- Fischer QS, Beaver CJ, Yang Y, Rao Y, Jakobsdottir KB, Storm DR, McKnight GS, Daw NW. Requirement for the RIIbeta isoform of PKA, but not calcium-stimulated adenylyl cyclase, in visual cortical plasticity. J Neurosci. 2004;24:9049–9058. doi: 10.1523/JNEUROSCI.2409-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frankland PW, O’Brien C, Ohno M, Kirkwood A, Silva AJ. Alpha-CaMKII-dependent plasticity in the cortex is required for permanent memory. Nature. 2001;411:309–313. doi: 10.1038/35077089. [DOI] [PubMed] [Google Scholar]
- Frenkel MY, Bear MF. How monocular deprivation shifts ocular dominance in visual cortex of young mice. Neuron. 2004;44:917–923. doi: 10.1016/j.neuron.2004.12.003. [DOI] [PubMed] [Google Scholar]
- Goel A, Lee HK. Persistence of experience-induced homeostatic synaptic plasticity through adulthood in superficial layers of mouse visual cortex. J Neurosci. 2007;27:6692–6700. doi: 10.1523/JNEUROSCI.5038-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon JA, Cioffi D, Silva AJ, Stryker MP. Deficient plasticity in the primary visual cortex of alpha-calcium/calmodulin-dependent protein kinase II mutant mice. Neuron. 1996;17:491–499. doi: 10.1016/s0896-6273(00)80181-6. [DOI] [PubMed] [Google Scholar]
- Gordon JA, Stryker MP. Experience-dependent plasticity of binocular responses in the primary visual cortex of the mouse. J Neurosci. 1996;16:3274–3286. doi: 10.1523/JNEUROSCI.16-10-03274.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanover JL, Huang ZJ, Tonegawa S, Stryker MP. Brain-derived neurotrophic factor overexpression induces precocious critical period in mouse visual cortex. J Neurosci. 1999;19:RC40. doi: 10.1523/JNEUROSCI.19-22-j0003.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hensch TK. Critical period plasticity in local cortical circuits. Nat Rev Neurosci. 2005;6:877–888. doi: 10.1038/nrn1787. [DOI] [PubMed] [Google Scholar]
- Hensch TK, Fagiolini M, Mataga N, Stryker MP, Baekkeskov S, Kash SF. Local GABA circuit control of experience-dependent plasticity in developing visual cortex. Science. 1998;282:1504–1508. doi: 10.1126/science.282.5393.1504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heynen AJ, Yoon BJ, Liu CH, Chung HJ, Huganir RL, Bear MF. Molecular mechanism for loss of visual cortical responsiveness following brief monocular deprivation. Nat Neurosci. 2003;6:854–862. doi: 10.1038/nn1100. [DOI] [PubMed] [Google Scholar]
- Hofer SB, Mrsic-Flogel TD, Bonhoeffer T, Hubener M. Prior experience enhances plasticity in adult visual cortex. Nat Neurosci. 2006;9:127–132. doi: 10.1038/nn1610. [DOI] [PubMed] [Google Scholar]
- Huang ZJ, Kirkwood A, Pizzorusso T, Porciatti V, Morales B, Bear MF, Maffei L, Tonegawa S. BDNF regulates the maturation of inhibition and the critical period of plasticity in mouse visual cortex. Cell. 1999;98:739–755. doi: 10.1016/s0092-8674(00)81509-3. [DOI] [PubMed] [Google Scholar]
- Hubel DH, Wiesel TN. Receptive fields, binocular interaction and functional architecture in the cat’s visual cortex. J Physiol. 1962;160:106–154. doi: 10.1113/jphysiol.1962.sp006837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalatsky VA, Stryker MP. New paradigm for optical imaging: temporally encoded maps of intrinsic signal. Neuron. 2003;38:529–545. doi: 10.1016/s0896-6273(03)00286-1. [DOI] [PubMed] [Google Scholar]
- Katz LC, Shatz CJ. Synaptic activity and the construction of cortical circuits. Science. 1996;274:1133–1138. doi: 10.1126/science.274.5290.1133. [DOI] [PubMed] [Google Scholar]
- Kirkwood A, Bear MF. Hebbian synapses in visual cortex. J Neurosci. 1994;14:1634–1645. doi: 10.1523/JNEUROSCI.14-03-01634.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mataga N, Nagai N, Hensch TK. Permissive proteolytic activity for visual cortical plasticity. Proc Natl Acad Sci U S A. 2002;99:7717–7721. doi: 10.1073/pnas.102088899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller KD, Keller JB, Stryker MP. Ocular dominance column development: analysis and simulation. Science. 1989;245:605–615. doi: 10.1126/science.2762813. [DOI] [PubMed] [Google Scholar]
- Mioche L, Singer W. Chronic recordings from single sites of kitten striate cortex during experience-dependent modifications of receptive-field properties. J Neurophysiol. 1989;62:185–197. doi: 10.1152/jn.1989.62.1.185. [DOI] [PubMed] [Google Scholar]
- Mrsic-Flogel TD, Hofer SB, Ohki K, Reid RC, Bonhoeffer T, Hubener M. Homeostatic regulation of eye-specific responses in visual cortex during ocular dominance plasticity. Neuron. 2007;54:961–972. doi: 10.1016/j.neuron.2007.05.028. [DOI] [PubMed] [Google Scholar]
- Olson CR, Freeman RD. Progressive changes in kitten striate cortex during monocular vision. J Neurophysiol. 1975;38:26–32. doi: 10.1152/jn.1975.38.1.26. [DOI] [PubMed] [Google Scholar]
- Pham TA, Graham SJ, Suzuki S, Barco A, Kandel ER, Gordon B, Lickey ME. A semi-persistent adult ocular dominance plasticity in visual cortex is stabilized by activated CREB. Learn Mem. 2004;11:738–747. doi: 10.1101/lm.75304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao Y, Fischer QS, Yang Y, McKnight GS, LaRue A, Daw NW. Reduced ocular dominance plasticity and long-term potentiation in the developing visual cortex of protein kinase A RII alpha mutant mice. Eur J Neurosci. 2004;20:837–842. doi: 10.1111/j.1460-9568.2004.03499.x. [DOI] [PubMed] [Google Scholar]
- Rutherford LC, Nelson SB, Turrigiano GG. BDNF has opposite effects on the quantal amplitude of pyramidal neuron and interneuron excitatory synapses. Neuron. 1998;21:521–530. doi: 10.1016/s0896-6273(00)80563-2. [DOI] [PubMed] [Google Scholar]
- Shepherd JD, Rumbaugh G, Wu J, Chowdhury S, Plath N, Kuhl D, Huganir RL, Worley PF. Arc/Arg3.1 mediates homeostatic synaptic scaling of AMPA receptors. Neuron. 2006;52:475–484. doi: 10.1016/j.neuron.2006.08.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stellwagen D, Malenka RC. Synaptic scaling mediated by glial TNF-alpha. Nature. 2006;440:1054–1059. doi: 10.1038/nature04671. [DOI] [PubMed] [Google Scholar]
- Syken J, Grandpre T, Kanold PO, Shatz CJ. PirB restricts ocular-dominance plasticity in visual cortex. Science. 2006;313:1795–1800. doi: 10.1126/science.1128232. [DOI] [PubMed] [Google Scholar]
- Taha S, Hanover JL, Silva AJ, Stryker MP. Autophosphorylation of alphaCaMKII is required for ocular dominance plasticity. Neuron. 2002;36:483–491. doi: 10.1016/s0896-6273(02)00966-2. [DOI] [PubMed] [Google Scholar]
- Taha S, Stryker MP. Rapid ocular dominance plasticity requires cortical but not geniculate protein synthesis. Neuron. 2002;34:425–436. doi: 10.1016/s0896-6273(02)00673-6. [DOI] [PubMed] [Google Scholar]
- Tohmi M, Kitaura H, Komagata S, Kudoh M, Shibuki K. Enduring critical period plasticity visualized by transcranial flavoprotein imaging in mouse primary visual cortex. J Neurosci. 2006;26:11775–11785. doi: 10.1523/JNEUROSCI.1643-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trachtenberg JT, Stryker MP. Rapid anatomical plasticity of horizontal connections in the developing visual cortex. J Neurosci. 2001;21:3476–3482. doi: 10.1523/JNEUROSCI.21-10-03476.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trachtenberg JT, Trepel C, Stryker MP. Rapid extragranular plasticity in the absence of thalamocortical plasticity in the developing primary visual cortex. Science. 2000;287:2029–2032. doi: 10.1126/science.287.5460.2029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turrigiano GG, Nelson SB. Homeostatic plasticity in the developing nervous system. Nat Rev Neurosci. 2004;5:97–107. doi: 10.1038/nrn1327. [DOI] [PubMed] [Google Scholar]
- Vitkovic L, Bockaert J, Jacque C. “Inflammatory” cytokines: neuromodulators in normal brain? J Neurochem. 2000;74:457–471. doi: 10.1046/j.1471-4159.2000.740457.x. [DOI] [PubMed] [Google Scholar]
- Wiesel TN. Postnatal development of the visual cortex and the influence of environment. Nature. 1982;299:583–591. doi: 10.1038/299583a0. [DOI] [PubMed] [Google Scholar]
- Wiesel TN, Hubel DH. Comparison of the effects of unilateral and bilateral eye closure on cortical unit responses in kittens. J Neurophysiol. 1965;28:1029–1040. doi: 10.1152/jn.1965.28.6.1029. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.