In this issue of Circulation, researchers from the Royal Brompton Hospital and University College London have published a long-anticipated report on the ability of magnetic resonance imaging (MRI) to predict cardiac dysfunction in transfusional siderosis.1
Briefly, they report that a cardiac T2* value <10 ms had a sensitivity of 98% and a specificity of 86% for prediction of symptomatic heart failure in 1 year. Risk was graded with respect to T2*, with 47% of patients having T2* <6 ms developing cardiac failure in the same interval. Similar, but less striking, risk stratification was also observed for prospective arrhythmia risk. Metrics of total body iron stores, liver iron concentration, and serum ferritin performed little better than chance in predicting heart failure.
To place these observations in context, it is important to review iron overload and its past and present management. Iron overload is a surprisingly common clinical problem, occurring through increased iron absorption (primary hemochromatosis) or through frequent blood transfusion therapy (secondary hemochromatosis).2 Primary hemochromatosis disorders, such as hfe mutations, are relatively common in white populations. However, variable hfe gene penetrance, increased genetic surveillance, and severity of noncardiac symptoms result in fewer hereditary hemochromatosis patients presenting with iron-mediated cardiac disease. By contrast, iron cardiomyopathy remains a major cause of death in secondary hemochromatosis disorders such as the thalassemia, Blackfan-Diamond anemia, and myelodysplastic syndromes because the iron-loading rates are many-fold greater than for primary hemochromatosis.3 The hemoglobinopathies are the most common genetic disorders in the world, particularly in regions where malaria is or was previously endemic, such as the Mediterranean, northern Africa, the Middle East, and Southeast Asia. Increasing economic and ethnic globalization has increased the importance of these disorders in the United States, and their impact is increasing. Iron overload is also becoming an increasingly critical problem in myelodysplasia syndromes because novel therapeutics have markedly increased life expectancy in low- and intermediate-risk patients.
Before the availability of deferoxamine iron chelation therapy, patients receiving long-term transfusions succumbed to arrhythmias and congestive heart failure once their transfusional burden exceeded approximately 200 U, usually in the second decade of life.4 Cardiac signs and symptoms were a late finding, usually heralding death within 6 months. Birth cohorts born after routine implementation of iron chelation demonstrate steadily improved survival.5 Classically, screening for iron cardiomyopathy consisted of serum ferritin or liver iron measurements combined with assessments of cardiac systolic function. This regimen was successful in reducing cardiac deaths in the second and third decade of life.6,7 Nonetheless, iron cardiomyopathy continues to be the leading cause of death in thalassemia patients, appearing even in patients with apparently good control of their somatic iron stores.8 Some postulated that such patients were succumbing to myocarditis, not iron cardiomyopathy, but autopsies almost inevitably confirmed severe, previously silent, cardiac iron accumulation. Cardiac biopsy was performed in some patients having decreased ventricular function or arrhythmias, but its invasiveness and variability precluded use in routine screening.
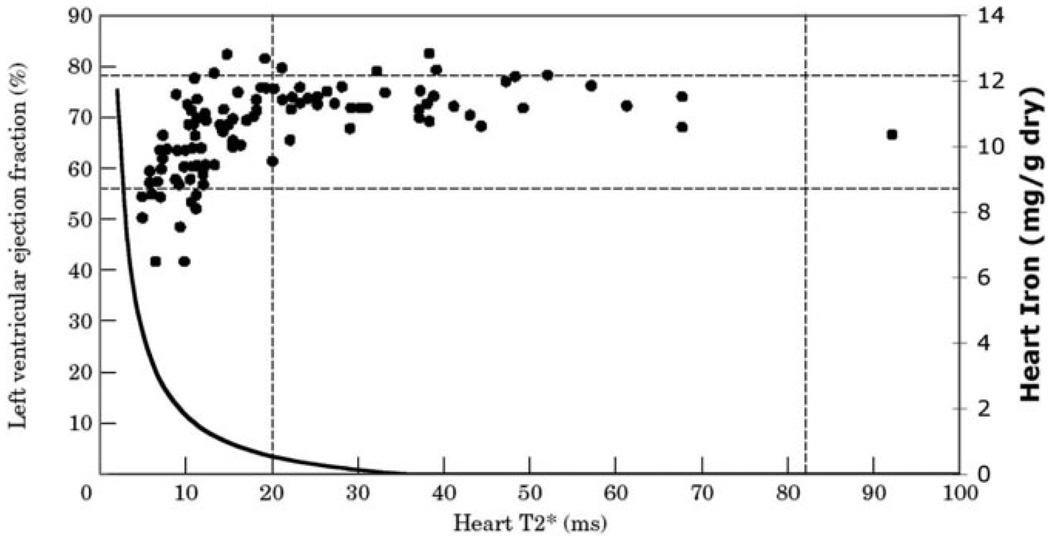
In 2001, the Royal Brompton group published its first report of the use of cardiac T2* to detect preclinical cardiac iron in 103 patients with thalassemia major. Figure 1 demonstrates left ventricular ejection fraction as a function of cardiac T2*.9 The MRI relaxation time, T2*, is typically 37±5 ms in normal subjects but shortens in the presence of iron. A lower cutoff of 20 ms has generally been used to eliminate false-positive diagnosis from motion and magnetic susceptibility artifacts as well as possible contributions from fluctuations in deoxygenated hemoglobin concentration. The authors made 6 key observations in their seminal article: (1) All patients lacking detectable cardiac iron had normal cardiac function. Thus, a T2*>20 ms yields a high negative predictive value. (2) Thalassemia patients lacking cardiac iron appeared to have higher ejection fractions than population norms. This was confirmed in a subsequent study and likely reflects lower cardiac afterload and greater cardiac preload associated with chronic anemia.10 (3) There was a graded negative relationship between cardiac T2* and cardiac ejection fraction as T2* decreased below 20 ms, indicating an association between increased cardiac iron and cardiac dysfunction. (4) Many patients with detectable cardiac iron exhibited normal cardiac function, which suggested that T2* was identifying preclinical iron deposition. (5) Iron appeared to clear more quickly from the liver than from the heart. In a subsequent study, the effective half-life of cardiac iron clearance in response to continuous deferoxamine therapy was found to be 13.5 months compared with 1.4 months for liver iron reduction. 11 (6) Lastly, no statistical association was observed between cardiac and liver iron concentration or serum ferritin.
Figure 1.
Plot of ejection fraction (left axis) versus heart T2* in milliseconds. T2* >20 ms represents patients with no detectable cardiac iron. Horizontal dotted lines represent predicted population norms for ejection fraction in nonthalassemia subjects. Solid line represents calibration curve for cardiac iron (right axis) as a function of cardiac T2*. Predicted cardiac iron for patients having a T2* of 2 to 10 ms is 1.8 to 10 mg/g dry weight, consistent with published autopsy studies. Figure reprinted from Circulation.15
This final observation was particularly challenging to the thalassemia community, and the authors faced severe criticism when they used T2* methods to compare chelator efficacy (see letters to the editor that follow their 2002 case–control study.12 In response to challenges raised, the authors demonstrated that T2* measurements were reproducible over time, across different T2* methods, and across different MRI platforms.13,14 Subsequently, our laboratory demonstrated that cardiac T2* is inversely proportional to cardiac iron in gerbils15 and in humans,16 as predicted by MRI relaxivity relationships. As an indirect validation, we superimposed a crude T2*–cardiac iron calibration curve against the original 2001 data points (Figure 1). Predicted cardiac iron concentration (right axis) rose sharply for T2* values <10 ms, coinciding with declining left ventricular ejection fraction. Furthermore, the predicted cardiac iron concentrations in this range matched directly measured cardiac iron concentrations in autopsy specimens.4 These data, combined with extensive MRI validations in liver, demonstrated that cardiac T2* reflected cardiac iron and was a robust measurement tool.
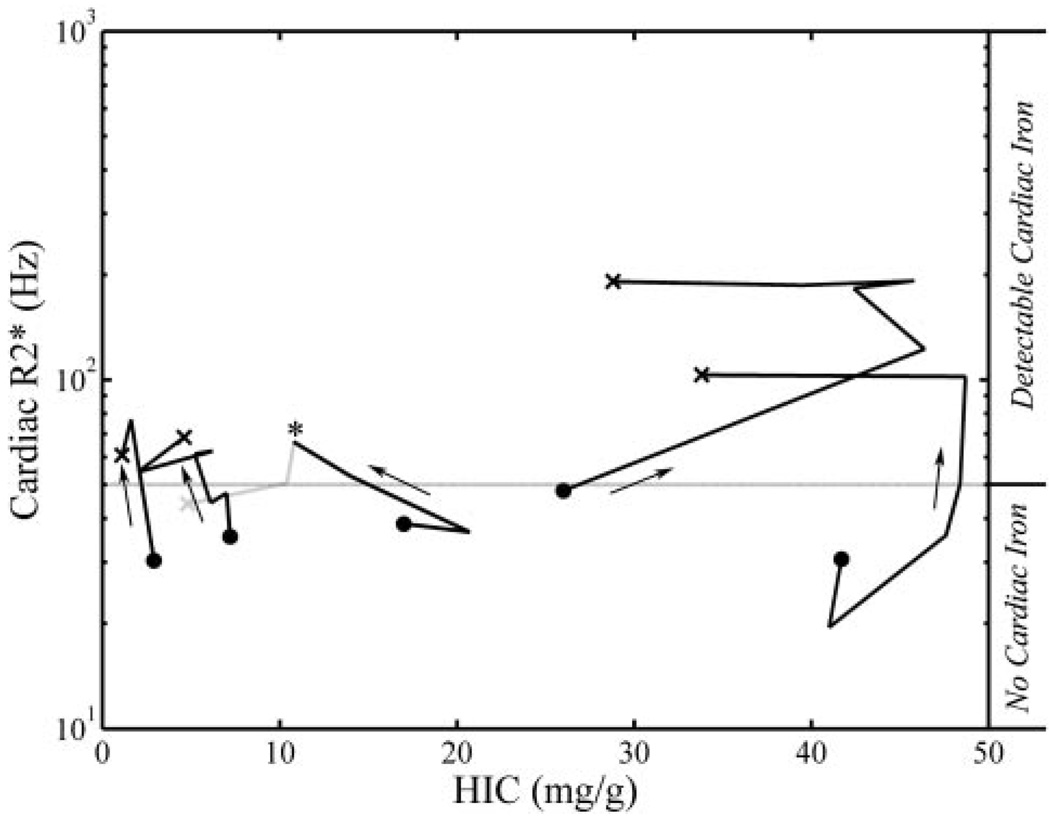
However, several puzzles remained relative to the new method. Why did cardiac and liver iron levels appear to be unrelated, when clinical studies suggested prospective cardiac risk for increased liver iron and serum ferritin? Why did some patients have high cardiac iron but low liver iron? Longitudinal comparisons of heart and liver iron are beginning to clarify these observations.17 One explanation is slow kinetics of cardiac iron clearance.11,17 Intensive chelation can rapidly and completely remove liver iron in 6 months, but clearance of cardiac iron may take several years, leaving patients with high cardiac iron and low liver iron. The converse situation is also common; the heart is initially spared in patients as their liver iron rises. Over time, however, patients with high liver iron concentrations are at risk for precipitous cardiac iron loading. Figure 2 demonstrates the temporal evolution of de novo cardiac siderosis in 5 patients; cardiac R2*, the reciprocal of T2*, is a linear surrogate for cardiac iron.15,16 Each line represents 3 to 7 consecutive heart and liver iron measurements collected 6 to 18 months apart. Two patients with severe hepatic siderosis rapidly developed profound cardiac siderosis, as might be expected from survival data.6,7 Although high liver iron predisposes to cardiac iron accumulation, two patients prospectively developed cardiac siderosis despite excellent control of liver iron. Cardiac and endocrine tissues are known to have different iron uptake mechanisms and kinetics compared with the liver. Extrahepatic organs develop iron overload only when circulating transferrin becomes saturated and labile-free iron species appear in the blood. This represents a steeply thresholded process and is modulated by transfusion rate, liver iron concentration, and systemic inflammation, as well as the type and pattern of chelator usage. As a result, one can deliver iron chelation therapy sufficient to maintain overall iron balance but inadequate to suppress circulating labile iron, leading to slowly progressive cardiac iron deposition. Such patients, who accumulate cardiac iron despite neutral or negative liver iron balance, require either higher drug dosages, longer drug exposure, or addition of a second chelating agent. Thus, while controlling total body iron stores remains an important goal of iron chelation therapy, MRI surveillance of extrahepatic tissues remains essential to exclude maldistribution of iron to sensitive target organs.
Figure 2.
Time course of cardiac R2* (as a surrogate for heart iron) versus hepatic iron concentration (HIC); each line represents 3 to 6 sequential measurements. Cardiac R2* is plotted on a log scale. Solid points represent first examinations, crosses represent final measurements, and arrows reinforce the temporal direction; each examination was separated by 6–18 months. As a time course crosses the gray horizontal line (lower limit of detection for cardiac iron) it represents de novo accumulation of cardiac iron. Figure adapted from Wood JC, Enriquez C, Ghugre N, Tyzka JM, Carson S, Nelson MD, Coates TD. MRI R2 and R2* mapping accurately estimates hepatic iron concentration in transfusion-dependent thalassemia and sickle cell disease patients. Blood. 2005;106:1460–1465.
A second puzzle was the observation that some patients with low T2* were asymptomatic whereas others were in heart failure. The key was recognizing which iron species MRI detected. We demonstrated that dispersed iron-loaded ferritin molecules produce little MRI relaxation18; free inorganic iron molecules have even lower influence. However, attaching the same amount of ferritin to 800-nm synthetic liposomes increased the relativity (lowered the T2*) 6-fold.18 Thus, MRI T2 and T2* measurements primarily detect lysosomally stored iron in the form of hemosiderin, not ferritin or free iron. Cardiac arrhythmias and dysfunction are caused by labile inorganic iron, not ferritin or hemosiderin. As a result, some patients with heavy cardiac iron burden may be initially asymptomatic. However, labile iron stores are in equilibrium with short-term (ferritin) and long-term (hemosiderin) buffering mechanisms. Consequently, cardiac T2* conveys high relative risk for prospective development of iron cardiomyopathy.
The third, and related, puzzle was how a clinician should respond to T2* measurements that were highly abnormal (<10 ms) when patients were asymptomatic and had normal cardiac testing. Did low T2* convey prospective risk of heart failure? Should one stay the course unless cardiac dysfunction appeared? Intuitively, this latter approach was unappealing. Although cardiac dysfunction may be entirely reversible when detected on routine screening, rescue chelation therapy requires 2 to 5 years of strict and arduous compliance with continuous deferoxamine infusion.19 Mortality was universal in patients unable or unwilling to maintain intensified chelation (21% of all patients with asymptomatic dysfunction).19 The present work strongly suggests that patients with cardiac T2* <10 ms should preemptively undergo chelation intensification regardless of ejection fraction. Although controlled studies are lacking, early recognition of cardiac iron appears to be translating into improved survival.20
Taken together, all evidence indicates that cardiac T2* measurements are reproducible, transferable, and strong predictors of clinical outcome. They represent robust and sensitive metrics for clinical trials of iron chelation therapy and should become the standard of care for all patients receiving long-term transfusions. Unfortunately, technical barriers to widespread implementation still exist. Although all major MRI vendors have pulse sequences capable of generating T2* images on their newest software releases, the cost is prohibitive for many centers, and the software is incompatible with older hardware. Commercial postprocessing software approved by the US Food and Drug Administration is available (CMR Tools, London, UK), but annual licensing fees may be prohibitive for some institutions. Offline analysis packages are also offered by some vendors, but these have not been extensively validated to date. Nonetheless, increasing awareness of the prevalence and clinical consequences of iron overload in hemoglobinopathies, myelodysplasia, cancer, and hemochromatosis has increased market pressure for “push-button” T2* measurements. As these techniques permeate regional hospitals over the next 5 years we will witness the translation of a novel research idea to the clinical standard of care.
In summary, the authors should be commended not only for an excellent article but for changing the management and outlook of an important international disease. The present article culminates almost a decade of creative and systematic exploration of the role of cardiovascular MRI in the management of transfusional siderosis.
Footnotes
Disclosures
Dr Wood’s work is supported by the General Clinical Research Center at CHLA/USC (NIH #RR00043–43), NHLBI (1 RO1 HL075592–01A1), and the Centers for Disease Control (Thalassemia Center Grant U27/CCU922106). Dr Wood receives research funding from Novartis.
References
- 1.Kirk P, Roughton M, Porter JB, Walker JM, Tanner MA, Patel J, Wu D, Taylor J, Westwood MA, Anderson LJ, Pennell DJ. Cardiac T2* magnetic resonance for prediction of cardiac complications in thalassemia major. Circulation. 2009;120:1961–1968. doi: 10.1161/CIRCULATIONAHA.109.874487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gordeuk VR, Bacon BR, Brittenham GM. Iron overload: causes and consequences. Annu Rev Nutr. 1987;7:485–508. doi: 10.1146/annurev.nu.07.070187.002413. [DOI] [PubMed] [Google Scholar]
- 3.Porter JB. Concepts and goals in the management of transfusional iron overload. Am J Hematol. 2007;82:1136–1139. doi: 10.1002/ajh.21100. [DOI] [PubMed] [Google Scholar]
- 4.Buja LM, Roberts WC. Iron in the heart: etiology and clinical significance. Am J Med. 1971;51:209–221. doi: 10.1016/0002-9343(71)90240-3. [DOI] [PubMed] [Google Scholar]
- 5.Borgna-Pignatti C, Rugolotto S, De Stefano P, Piga A, Di Gregorio F, Gamberini MR, Sabato V, Melevendi C, Cappellini MD, Verlato G. Survival and disease complications in thalassemia major. Ann N Y Acad Sci. 1998;850:227–231. doi: 10.1111/j.1749-6632.1998.tb10479.x. [DOI] [PubMed] [Google Scholar]
- 6.Brittenham GM, Griffith PM, Nienhuis AW, McLaren CE, Young NS, Tucker EE, Allen CJ, Farrell DE, Harris JW. Efficacy of deferoxamine in preventing complications of iron overload in patients with thalassemia major. N Engl J Med. 1994;331:567–573. doi: 10.1056/NEJM199409013310902. [DOI] [PubMed] [Google Scholar]
- 7.Olivieri NF, Nathan DG, MacMillan JH, Wayne AS, Liu PP, McGee A, Martin M, Koren G, Cohen AR. Survival in medically treated patients with homozygous beta-thalassemia. N Engl J Med. 1994;331:574–578. doi: 10.1056/NEJM199409013310903. [DOI] [PubMed] [Google Scholar]
- 8.Modell B, Khan M, Darlison M. Survival in beta-thalassaemia major in the UK: data from the UK Thalassaemia Register. Lancet. 2000;355:2051–2052. doi: 10.1016/S0140-6736(00)02357-6. [DOI] [PubMed] [Google Scholar]
- 9.Anderson LJ, Holden S, Davis B, Prescott E, Charrier CC, Bunce NH, Firmin DN, Wonke B, Porter J, Walker JM, Pennell DJ. Cardiovascular T2-star (T2*) magnetic resonance for the early diagnosis of myocardial iron overload. Eur Heart J. 2001;22:2171–2179. doi: 10.1053/euhj.2001.2822. [DOI] [PubMed] [Google Scholar]
- 10.Westwood MA, Anderson LJ, Maceira AM, Shah FT, Prescott E, Porter JB, Wonke B, Walker JM, Pennell DJ. Normalized left ventricular volumes and function in thalassemia major patients with normal myocardial iron. J Magn Reson Imaging. 2007;25:1147–1151. doi: 10.1002/jmri.20915. [DOI] [PubMed] [Google Scholar]
- 11.Anderson LJ, Westwood MA, Holden S, Davis B, Prescott E, Wonke B, Porter JB, Malcolm Walker J, Pennell DJ. Myocardial iron clearance during reversal of siderotic cardiomyopathy with intravenous desferrioxamine: a prospective study using T2* cardiovascular magnetic resonance. Br J Haematol. 2004;127:348–355. doi: 10.1111/j.1365-2141.2004.05202.x. [DOI] [PubMed] [Google Scholar]
- 12.Anderson LJ, Wonke B, Prescott E, Holden S, Walker JM, Pennell DJ. Comparison of effects of oral deferiprone and subcutaneous desferrioxamine on myocardial iron concentrations and ventricular function in beta-thalassaemia. Lancet. 2002;360:516–520. doi: 10.1016/s0140-6736(02)09740-4. [DOI] [PubMed] [Google Scholar]
- 13.Tanner MA, He T, Westwood MA, Firmin DN, Pennell DJ. Multi-center validation of the transferability of the magnetic resonance T2* technique for the quantification of tissue iron. Haematologica. 2006;91:1388–1391. [PubMed] [Google Scholar]
- 14.Westwood M, Anderson LJ, Firmin DN, Gatehouse PD, Charrier CC, Wonke B, Pennell DJ. A single breath-hold multiecho T2* cardiovascular magnetic resonance technique for diagnosis of myocardial iron overload. J Magn Reson Imaging. 2003;18:33–39. doi: 10.1002/jmri.10332. [DOI] [PubMed] [Google Scholar]
- 15.Wood JC, Otto-Duessel M, Aguilar M, Nick H, Nelson MD, Coates TD, Pollack H, Moats R. Cardiac iron determines cardiac T2*, T2, and T1 in the gerbil model of iron cardiomyopathy. Circulation. 2005;112:535–543. doi: 10.1161/CIRCULATIONAHA.104.504415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ghugre NR, Enriquez CM, Gonzalez I, Nelson MD, Jr, Coates TD, Wood JC. MRI detects myocardial iron in the human heart. Magn Reson Med. 2006;56:681–686. doi: 10.1002/mrm.20981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Noetzli LJ, Carson SM, Nord AS, Coates TD, Wood JC. Longitudinal analysis of heart and liver iron in thalassemia major. Blood. 2008;112:2973–2978. doi: 10.1182/blood-2008-04-148767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wood JC, Fassler J, Meade T. Mimicking liver iron overload using liposomal ferritin preparations. Mag Res Med. 2004;51:607–611. doi: 10.1002/mrm.10735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Davis BA, O’Sullivan C, Jarritt PH, Porter JB. Value of sequential monitoring of left ventricular ejection fraction in the management of thalassemia major. Blood. 2004;104:263–269. doi: 10.1182/blood-2003-08-2841. [DOI] [PubMed] [Google Scholar]
- 20.Modell B, Khan M, Darlison M, Westwood MA, Ingram D, Pennell DJ. Improved survival of thalassaemia major in the UK and relation to T2* cardiovascular magnetic resonance. J Cardiovasc Magn Reson. 2008;10:42. doi: 10.1186/1532-429X-10-42. [DOI] [PMC free article] [PubMed] [Google Scholar]