INTRODUCTION
Cardiovascular disease is the leading cause of death in women regardless of race or ethnicity.1 Coronary heart disease (CHD), including myocardial infarction (MI) and acute coronary syndrome (ACS), are associated with adverse outcomes in women. One potential reason women with CHD have such poor outcomes is that women are more likely to experience a myriad of non-chest pain symptoms than are men, contributing to difficulty receiving a correct diagnosis, and delay in receiving optimal treatment.2–5
Most studies on symptoms of CHD formulate a list of the participants’ most frequently reported symptoms.6–8 This descriptive information on women’s most commonly reported prodromal and acute symptoms associated with CHD is especially important since it provides vital information to assist clinicians in diagnosing this often elusive disease in women.5,7 Canto and colleagues9 conducted a thorough review of the literature on women’s symptoms associated with ACS to provide additional guidance on women’s symptom presentation. However, women, as men, seldom experience an individual symptom indicative of CHD but instead present with numerous symptoms.
Researchers in other specialties suggest that clusters of symptoms, as opposed to individual symptoms, are more clinically important.10–13 Leaders in symptom management research14–16 conceptualize symptom clusters as multiple symptoms that are related to each other and are experienced concurrently. Symptom clusters often share a common etiology. Additionally, measurements of frequency and/or severity of symptoms within clusters typically correlate with each other.14
Symptom cluster analysis is especially prominent in the cancer literature and several authors have performed a concept analysis related to symptom clusters.17,18 Others have reported on the usefulness of symptom clusters in developing a plan for symptom management of cancer pain and other related symptoms.11,12, 17 Symptom clusters may also be useful in formulating diagnostic criteria and evaluating patterns of symptoms that typify different diseases and symptom progression.15
Unlike the cancer literature, there are few reported cluster analyses in the field of cardiovascular disease. Several researchers investigated clusters of CHD risk factors, such as in young adults in Ireland,19 patients with type 2 diabetes,20 or with metabolic syndrome.21 Another article based on cluster analysis focused on the knowledge of individuals at risk for developing MI and what symptoms they perceived might be indicative of MI.22 These researchers initially used Q methodology to develop a group of 49 potential MI symptoms. Participants then responded to these statements to identify the symptoms they believed were most and least likely associated with MI. Next they conducted a factor analysis that resulted in 4 clusters of possible symptoms of MI. The 61 subjects in this study had not experienced a MI so their responses were not based on actual symptoms. Although this information is useful for possible development and modification of educational interventions to promote early recognition of MI symptoms in the public, it is not useful in assisting clinicians in recognizing clusters of actual MI symptoms.
A recent study23 described characteristics of older adults (≥ 65) who ranked seven symptoms (chosen a priori by the research team) based on symptoms they experienced 1 week prior to hospitalization after their MI. Participants completed the questionnaire as soon as stable and while still hospitalized. The investigators analyzed symptoms using hierarchical cluster analysis to identify groups of patients who were most similar in the symptoms they experienced related to certain individual characteristics (demographics, comorbidities, cardiac risk factors, quality of life, and mood states). The 247 participants were divided into 3 groups according to reported symptoms that the authors described as clustering into discrete patterns. They labeled these clusters as the classic ACS Symptom Group, Weary Group, and Diffuse Symptom Group. They described the classic ACS group (n= 53) as those with severe chest pain and moderate fatigue with significant differences in patient characteristics of less heart failure; the Weary Group (n= 72) as those reporting severe fatigue, sleep disturbance, shortness of breath and moderate chest pain with characteristics of younger age (mean=74), diabetes, more likely to be treated with bypass surgery, lower quality of life and vitality scores, and higher fatigue, anxiety, depression, and anger scores. Lastly, the Diffuse Symptom Group (n=122) described low symptom intensity, mild shortness of breath and fatigue and had significantly different characteristics of older age and less hypertension. Fatigue emerged as a significant symptom in all three clusters with an overall prevalence of 76.1% while shortness of breath was the second most prevalent symptom (61.5%). Chest pain was reported by only 56% while the four other symptoms were reported by less than 50% of the participants (palpitations, sleep disturbance, nausea and vomiting). They did not conduct analyses related to ethnicity or race since the sample was 92% White but reported no significant differences related to sex. Since the symptom instrument was limited to seven symptoms, a full description of actual symptoms experienced by participants was likely restricted. Additionally, participants could only choose chest pain and not other descriptors of chest discomfort, likely reducing reports of other discomfort sensations more commonly reported by women.2,9
Therefore, the state of the knowledge of women’s CHD symptoms is expanding but remains in an early phase of development. Other studies have described women’s symptoms by race,2,6 but no studies could be located that performed a cluster analysis of CHD symptoms reported by racially diverse women prior to and during MI.
PURPOSE
We performed a secondary analysis to: a) generate naturally occurring symptom clusters based on women’s prodromal and acute MI symptoms separately, b) examine the association between women’s characteristics and symptom clusters, and c) describe the percentage of women who reported experiencing the same symptoms in both the prodromal and acute MI phases.
METHODS
Sample and Description of Database
We performed a secondary analysis of retrospective self-reported data obtained by telephone survey from 1270 women (43% Black, 42% White, 15% Hispanic) with a confirmed MI recruited from 15 geographically diverse sites located in eight states. Human Subject Review Committees approved the study at each of the sites. The purposes of the original studies were to describe and compare the prodromal and acute MI symptoms in White, Black, and Hispanic women. A detailed description of the inclusion criteria, recruitment procedure, and sample description of women in this database is described elsewhere.2,24 Briefly, due to the retrospective nature of the study, all women in the database had to pass (achieve a score of 16 or less) a six-item cognitive screen appropriate for assessing cognition prior to telephone surveys.25 Interviews with Hispanic women were conducted in the language of their choice (Spanish or English). Since Hispanic women were fluent in either Mexican-Spanish or Caribbean-Spanish, research assistants who were fluent in both dialects administered the telephone surveys. Trained registered nurse research assistants, located at one site, conducted telephone surveys 4 months after the MI to allow sufficient time for women to recognize prodromal symptoms. Research assistants entered responses directly into an ACCESS database while conducting the one-time 60 minute interview. Although telephone surveys have both advantages and disadvantages,26 this cost-effective data collection method allowed participation of a large number of racially and ethnically diverse women from a wide geographical area. This was essential for statistical and clinically meaningful comparisons of symptoms and generalizability of findings.
White women were older (67± 12) than minority women (63±13) (p<0.001) but minority women were not significantly different from each other (Hispanics 64±13 and Blacks 63±13) ( p = 0.385). White women were better educated and reported higher incomes than minority women.
The original data were collected using the McSweeney Acute and Prodromal Myocardial Infarction Symptom Survey (MAPMISS) that includes intensity and frequency measures of 33 prodromal (early warning intermittent symptoms that change after MI) and intensity of 37 acute MI symptoms (continuously present after onset until time of diagnosis). It also contains comorbidities/risk factors. A complete description of the symptom content of the MAPMISS and definitions of terms is described elsewhere.27 This instrument was developed based on qualitative interviews and incorporates symptoms and their descriptors based on women’s actual accounts. It has been extensively pilot tested with White, Black, and Hispanic women. Although women were offered the opportunity to add additional symptoms or descriptors during data collection, they did not identify any additional items.2
Women were queried about their prodromal symptom experience, including both frequency in days per week (less than monthly (0.167) to daily (7) and severity (0–3, 3=most severe) of symptoms, and about their acute MI symptoms and corresponding severity.2 Since acute symptoms were a one-time event, there was no rating for frequency. Based on women’s responses, we calculated a prodromal and acute symptom score for each woman by summing the individual symptom scores. Scores are calculated by multiplying the intensity by the frequency for each prodromal symptom and is based solely on the intensity for each acute symptom. Women also responded to 10 possible comorbidities/risk factor questions, such as presence of diabetes, hypertension, or hypercholesterimia, and self-reported height and weight.
Statistical Methods
Data management and most analyses were performed using Stata version 10.0 (Stata Corporation, College Station, TX). Recursive partitioning was performed utilizing the algorithm implemented in the Classification Trees for Multiple Binary Responses (CTMBR) program (http://peace.med.yale.edu/pub/mcart).
Cluster analysis was used to group women with MI into similar configurations of prodromal and acute symptoms separately based on frequency and severity of the reported symptoms. Cluster analysis comprises a set of statistical methods, including K Mean and K Median, that seek to divide a population into naturally occurring homogeneous subgroups by minimizing the within-group differences while maximizing between-group differences.28 Thus, our operational definition of clustering is a method of dividing objects into groups based on a measurement of similarity such that the most similar objects are group together while similarities between groups is minimized. In an effort to better describe cluster membership, bivariate analyses by chi-square test were used to examine the association between cluster assignment and women’s characteristics and known risk factors independently, whereas multivariate analysis using multinomial (polytomous) logistic regression was used to examine the association between cluster assignment and characteristic/risk factors (age, race, body mass index [BMI], personal history of heart disease, family history of heart disease, diabetes, smoking, physical activity, hypercholesterimia and hypertension) combined.29
Because of the possibility of complex interplay between patient characteristics, risk factors, and symptom clusters, recursive partitioning was used to explore these non-linear interactions. Recursive partitioning is a multivariable analysis method useful in uncovering hidden patterns and groups within data. It relates an outcome (cluster membership) to a collection of independent predictors (patient characteristics/risk factors). The method partitions the data into homogeneous groups or nodes, while maximizing the differences between groups or nodes. The recursive algorithm used the Gini criterion for splitting nodes and a 10-fold cross-validation for pruning and tree validation. Results from recursive partitioning are routinely presented as a decision tree or a set of classification rules.30
RESULTS
Several clustering algorithms were evaluated after removing infrequently reported prodromal (<20%) and acute symptoms (<15%). Symptoms were removed if rarely reported and not significantly associated with any of the six risk factors. Sixteen of the 33 prodromal and 22 of the 37 acute symptoms were retained for analysis. The K-mean and K-median clustering methods yielded the most informative clusters, both producing similar clustering results. Therefore, only the results from the K-mean method are presented.
Prodromal symptoms
Three clusters of women were identified based on their reported prodromal symptoms and characteristics. Each cluster contained women with increasing frequency and severity of reported prodromal symptoms. Women in the first cluster reported the fewest and least severe prodromal symptoms, whereas women in the third cluster reported having the most prodromal symptoms which were of the greatest severity. The average prodromal score of every prodromal symptom was lowest in Cluster 1 and highest in Cluster 3. For example, the average score (intensity times frequency) of the most frequently reported prodromal symptom, very tired/unusual fatigue, was 4.78 for Cluster 1, 10.26 for Cluster 2, and 12.22 for Cluster 3. The most frequently reported prodromal symptoms by cluster are listed in Table 1.
Table 1.
Prodromal Symptom Clusters: Most Commonly Reported Prodromal Symptoms by Cluster
| Percent of Women Reporting Prodromal Symptoms | ||
|---|---|---|
| Cluster | (40–69.9%) | (70–100%) |
| 1 Older Silent Asymptomatic Group N=552 |
Very tired/unusual fatigue | |
| 2 Diverse Mildly Symptomatic Group N=435 |
Anxious Heart racing Shortness of breath Frequent indigestion Change in thinking/remembering |
Very tired/unusual fatigue Sleep disturbance |
| 3 Younger Minority Multiple Distressing Symptom Group N=283 |
Any chest pain/discomfort Cough Heart racing Difficulty breathing at night Loss of appetite Frequent indigestion Arms ache Numbness or burning in hands/fingers Vision problems Change in thinking/remembering |
Very tired/unusual fatigue Sleep disturbance Anxious Shortness of breath Arms weak/heavy Hand/arms tingling |
The first cluster (Cluster 1) was composed of 552 women. None of the prodromal symptoms was reported by more than 70% of the women in Cluster 1 and only one symptom, very tired, unusual fatigue, was reported by 48.6% of the women. No other symptom was reported by more than 40% of the women. Only two symptoms, very tired, unusual fatigue and sleep disturbance, were reported by more than 70% of the 435 women in Cluster 2. In contrast, over 70% of the 283 women in Cluster 3 reported having the same six prodromal symptoms, and another ten prodromal symptoms were reported by 40% to 69.9 % of them. Unlike women in the other two Clusters, more than 40% of women in the Cluster 3 reported having chest pain/discomfort (59.4%). In summary, women in Cluster 3 reported having the most prodromal symptoms while a lower proportion of women in Clusters 2 and 1 reported having prodromal symptoms.
Next we assessed the association between women’s characteristics and prodromal cluster membership to determine if demographic characteristics or known cardiovascular risk factors could be used to inform cluster membership. Six of ten characteristics were strongly associated with cluster membership: race, age, BMI, personal history of heart disease, smoking status, and diabetes (see Table 2).
Table 2.
Association between Participant Characteristics and Prodromal Cluster Membership by Bivariate Analysis
| Prodromal Symptom Clusters | |||||||
|---|---|---|---|---|---|---|---|
| 1a | 2b | 3c | |||||
| Covariate | N | % | N | % | N | % | P-value |
| Race | |||||||
| Black | 198 | 35.87 | 195 | 44.83 | 152 | 53.71 | |
| Hispanic | 83 | 15.04 | 62 | 14.25 | 41 | 14.49 | |
| White | 271 | 49.09 | 178 | 40.92 | 90 | 31.8 | <0.0001 |
| Age>50 | 486 | 88.04 | 367 | 84.37 | 222 | 78.45 | 0.0013 |
| BMI >29 | 215 | 38.95 | 233 | 53.56 | 165 | 58.3 | <0.0001 |
| Personal history heart disease | 311 | 56.34 | 280 | 64.37 | 196 | 69.26 | 0.0006 |
| Smoker | 127 | 23.01 | 115 | 26.44 | 92 | 32.51 | 0.0128 |
| Diabetic | 216 | 39.13 | 196 | 45.06 | 135 | 47.7 | 0.0356 |
Abbreviation: BMI, body mass index.
Older Silent Asymptomatic Group.
Diverse Mildly Symptomatic Group.
Younger Minority Multiple Distressing Symptom Group.
Because of the possibility of complex interactions, we used multinomial (polytomous) logistic regression to examine patient characteristics simultaneously (see Table 3). The women in Cluster 3, who had the most number and greatest severity of prodromal symptoms, were 50% less likely to be White than Black and 33% less likely to be older, after adjusting for the other factors in the model, than women in the other two Clusters. Women in Cluster 3 were also 92% more likely to be obese, 73% more likely to report having a personal history of cardiovascular disease and 71% more likely to be smokers. Diabetes was no longer a significant predictor of cluster membership after accounting for the effect of the other factors in the model. Therefore, based on prodromal symptoms and their descriptors and the characteristics of the women reporting the symptoms in each cluster, we named the clusters as follows: Cluster 1, Older, Silent Asymptomatic Group; Cluster 2, Diverse Mildly Symptomatic Group; and Cluster 3, as Young Minority Multiple Distressing Symptom Group.
Table 3.
Prodromal Symptom Cluster Membership and Participant Characteristics: Adjusted Odds Ratios and 95% Confidence Interval Estimates from Polytomous Logistic Regression
| Covariate | Cluster 2a | Cluster 3a | ||||
|---|---|---|---|---|---|---|
| Diverse Mildly Symptomatic Group | Younger Minority Multiple Distressing Symptom Group | |||||
| OR | 95% CI | P-value | OR | 95% CI | P-value | |
|
Race | ||||||
| Black | Reference | Reference | ||||
| Hispanic | 0.86 | (0.58, 1.29) | 0.4745 | 0.79 | (0.50, 1.24) | 0.3069 |
| White | 0.75 | (0.57, 1.00) | 0.0521 | 0.50 | (0.36, 0.70) | 0.0001 |
| Age>50 | 0.88 | (0.60, 1.30) | 0.5224 | 0.67 | (0.44, 1.01) | 0.0577 |
| BMI >29 | 1.66 | (1.27, 2.18) | 0.0002 | 1.92 | (1.41, 2.62) | <0.0001 |
| Personal history CHD | 1.38 | (1.06, 1.80) | 0.0159 | 1.73 | (1.26, 2.37) | 0.0007 |
| Smoker | 1.24 | (0.91, 1.69) | 0.1746 | 1.71 | (1.20, 2.44) | 0.0030 |
| Diabetic | 1.08 | (0.82, 1.41) | 0.5939 | 1.13 | (0.83, 1.55) | 0.4374 |
Abbreviation: BMI, body mass index; CI, confidence interval; OR, odds ratio.
Women in Cluster 1, Older Silent Asymptomatic Group, were used as reference.
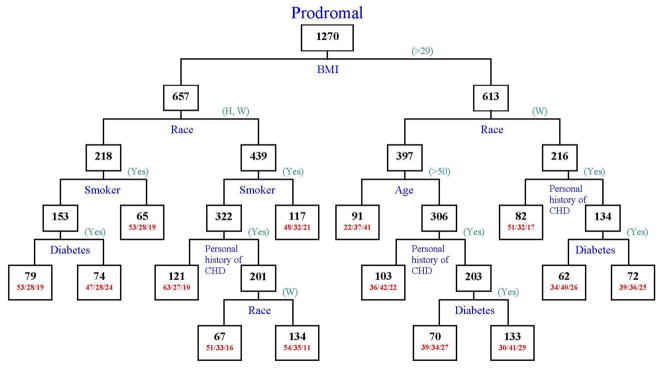
Recursive partitioning identified BMI as the most important factor in classifying prodromal symptoms, followed by race, smoking, age and personal history of CHD (see Figure 1). Approximately 41% of the young (age<50) obese (BMI>29) Black/Hispanic women were classified in Cluster 3, Young Minority Multiple Distressing Symptom Group, whereas 37% were in Cluster 2, Diverse Mildly Symptomatic Group, and 22% of them were classified in Cluster 1, Older, Silent Asymptomatic Group. Only 9.9% of normal weight (BMI<29), non-smoking, minority women without personal history of CHD were in Cluster 3, Young Minority Multiple Distressing Symptom Group; in comparison, 63% of these women were classified in Cluster 1, the Older, Silent Asymptomatic Group.
Figure 1.
Classification Tree from Recursive Partitioning of Prodromal Symptom Clusters.
Numbers inside each node indicate the total number of women in the node. For terminal nodes, the percent of women in Clusters 1, 2 and 3 are indicated as (1/2/3).
Source: Author)
Acute symptoms
Three clusters of women were also identified based on their reported acute symptoms. The most frequently reported acute symptoms by cluster are listed in Table 4. As with the prodromal clusters, each sequential cluster contained women with increasing number of symptoms and a higher of intensity of those symptoms (acute symptom score). Women in Cluster 1 reported having the fewest and least intense acute symptoms, whereas women in the Cluster 3 reported having the most number and intense acute symptoms.
Table 4.
Acute Symptom Clusters: Most Commonly Reported Acute Symptoms by Cluster
| Percent of Women Reporting Acute Symptoms | ||
|---|---|---|
| Cluster | (40–69.9%) | (70–100%) |
| 1 Older Silent Asymptomatic Group N=637 |
Any chest pain/discomfort Shortness of breath |
|
| 2 Diverse Mildly Symptomatic Group N=385 |
Any chest pain/discomfort Cold sweat Heart racing Nausea Very tired/unusual fatigue Arms weak/heavy Dizzy or faint Hot, flushed Indigestion Vomiting |
Shortness of breath |
| 3 Younger Minority Multiple Distressing Symptom Group N=248 |
Cold sweat Heart racing Nausea Vision problems Discomfort, Centered high in chest Discomfort, Back, between/under shoulder blades Left arm/shoulder Hand/arms tingling Hot, flushed Indigestion Loss of appetite Headache |
Any chest pain/discomfort Very tired, unusual fatigue Shortness of breath Arms weak/heavy Numbness or burning of arms Numbness in hands/fingers Dizzy or faint |
More women reported having acute symptoms than prodromal symptoms. Any chest pain/discomfort and shortness of breath were the most frequently reported acute symptoms. The average intensity (0=none, 3=severe) of these two symptoms, as with all the acute symptoms, was lowest in Cluster 1 and most severe in Cluster 3. For example, the mean for severity of chest pain/discomfort was 1.23 in Cluster 1, 1.61 for Cluster 2, and 2.01 for Cluster 3. The 635 women with membership in acute symptom Cluster 1 reported only two symptoms more that 40 %: chest pain/discomfort (53.1%) and shortness of breath (44.4%). No other symptom was reported by more than 40% of women in this Cluster. Approximately 75% of 385 women in Cluster 2 reported experiencing shortness of breath with no other symptom being reported by more than 70% of women. In contrast, women in Cluster 3 reported a large number of frequently occurring acute symptoms. Over 91% of women in Cluster 3 (n=248) reported experiencing shortness of breath and over 78% of them reported chest pain/discomfort. Forty to sixty-nine percent of women in Cluster 3 also reported a variety of 12 other symptoms (see Table 4).
Similarly, as for the prodromal symptoms, we assessed the association between women’s characteristics and acute cluster membership to determine if demographic characteristics or known cardiovascular risk factors could be used to inform cluster membership. The same six characteristics found to be associated with prodromal clusters were also found to be associated with acute cluster membership: race, age, BMI, personal history of heart disease, smoking status and diabetes (see Table 5).
Table 5.
Association between Participant Characteristics and Acute Cluster Membership by Bivariate Analysis
| Acute Symptoms Cluster | |||||||
|---|---|---|---|---|---|---|---|
| 1a | 2b | 3c | |||||
| N | % | N | % | N | % | P-value | |
| Race | |||||||
| Black | 254 | 39.87 | 166 | 43.12 | 125 | 50.40 | |
| Hispanic | 88 | 13.81 | 58 | 15.06 | 40 | 16.13 | |
| White | 295 | 46.31 | 161 | 41.82 | 83 | 33.47 | 0.0151 |
| Age>50 | 578 | 90.74 | 308 | 80.00 | 189 | 76.21 | <0.0001 |
| BMI >29 | 274 | 43.01 | 194 | 50.39 | 145 | 58.47 | <0.0001 |
| Personal history heart disease | 369 | 57.93 | 241 | 62.60 | 177 | 71.37 | 0.0010 |
| Smoker | 137 | 21.51 | 116 | 30.13 | 81 | 32.66 | <0.0001 |
| Diabetic | 254 | 39.87 | 175 | 45.45 | 118 | 47.58 | 0.0607 |
Abbreviation: BMI, body mass index.
Older Silent Asymptomatic Group.
Diverse Mildly Symptomatic Group.
Younger Minority Multiple Distressing Symptom Group.
As before, we used multinomial (polytomous) logistic regression to examine patient characteristics simultaneously (see Table 6). Compared to women in Cluster 1, women in Cluster 3 were 60% more likely to be younger, 84% more likely to have a personal history of heart disease and 72% more likely to be smokers. Compared to women in Cluster 1, women in Cluster 2 were also younger and were more often smokers. Therefore, the same names for Prodromal Clusters 1 (Older, Silent Asymptomatic Group), 2 (Diverse Mildly Symptomatic Group), and 3 (Young Minority Multiple Distressing Symptom Group) were retained for the corresponding Acute Clusters.
Table 6.
Acute Symptom Clusters and Participant Membership Characteristics Adjusted Odds Ratios and 95% Confidence Interval Estimates from Polytomous Logistic Regression.
| Cluster 2a | Cluster 3a | |||||
|---|---|---|---|---|---|---|
| Covariate | OR | 95% CI | P-value | OR | 95% CI | P-value |
| Race | ||||||
| Black | Reference | Reference | ||||
| Hispanic | 1.11 | (0.74 1.66) | 0.6072 | 1.18 | (0.75 1.87) | 0.4739 |
| White | 0.93 | (0.70 1.24) | 0.6275 | 0.68 | (0.48 0.96) | 0.0294 |
| Age>50 | 0.47 | (0.32 0.69) | 0.0001 | 0.40 | (0.26 0.62) | <0.0001 |
| BMI >29 | 1.21 | (0.92 1.58) | 0.1700 | 1.59 | (1.15 2.19) | 0.0049 |
| Personal history CHD | 1.20 | (0.92 1.56) | 0.1898 | 1.84 | (1.32 2.57) | 0.0003 |
| Smoker | 1.51 | (1.11 2.05) | 0.0086 | 1.72 | (1.20 2.46) | 0.0030 |
| Diabetic | 1.22 | (0.93 1.60) | 0.1545 | 1.19 | (0.87 1.64) | 0.2805 |
Abbreviation: BMI, body mass index; CI, confidence interval; OR, odds ratio.
Women in Cluster 1 used as reference group.
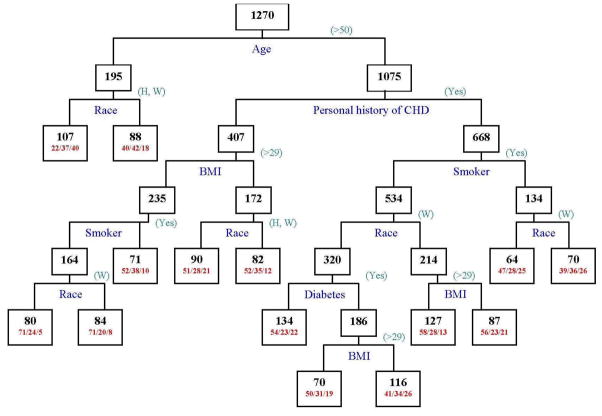
Recursive partitioning yielded age as the most important factor in classifying acute symptoms, followed by race, personal history of CHD, BMI and smoking status (see Figure 2). Approximately 40% of young, Black women were classified in Cluster 3, whereas only 22% of them were in Cluster 1. Over 74% of young Black women reported chest pain/discomfort, whereas less than 50% of normal weight, older, non-smoking, White women, without a personal history of CHD reported chest pain/discomfort. Sixty percent of all other women reported chest pain/discomfort, and 71% of normal weight, older (age>50) women, without a personal history of CHD were in Cluster 1.
Figure 2.
Classification Tree from Recursive Partitioning of Acute Symptom Clusters.
Numbers inside each node indicate the total number of women in the node. For terminal nodes, the percent of women in Clusters 1, 2 and 3 are indicated as (1/2/3).
(Source: Author)
Women reporting the same symptoms in both prodromal and acute MI phases
Finally, we calculated the percentage of women who reported experiencing the same symptoms in both the prodromal and acute MI phases regardless of cluster membership. Of the16 prodromal and 22 acute symptoms, 11 symptoms were assessed in both the prodromal and acute phases and only these were retained for analysis. Of these 11 symptoms, 8 of them were reported in both the prodromal and acute phases by over 50% of women. Two symptoms, any chest pain/discomfort and shortness of breath, had over 80% agreement while arms weak and heavy had over 65% agreement. Although chest pain/discomfort was not a frequently reported prodromal symptom for women in any cluster group, at least 80% of the women who experienced it prodromally also reported it in the acute phase (regardless of cluster membership).
DISCUSSION
Clinicians use clinical reasoning to develop diagnostic hypotheses about patient symptoms, selecting the most specific and critical findings that support a hypothesis. It is in this way that cluster analysis becomes most clinically relevant as it organizes the most associated symptoms and correlates them with readily identifiable characteristics of those patients most likely to experience the symptom cluster. For example, younger Black women in this study who had a high BMI, smoked, and had personal history of CHD tended to present with a variety of vague prodromal symptoms, such as unusual fatigue, sleep disturbance, anxiety, shortness of breath, and uncomfortable arm sensations (weakness, heaviness, tingling). These symptoms, if viewed individually are often unrecognized as potentially related to early CHD and most difficult to diagnose. However, this cluster analysis provides an organization to “sort” symptoms into more manageable groups to provide valuable clues as to which women may require cardiovascular evaluation during the prodromal period.
During the prodromal phase, women in the Older Silent Asymptomatic Group, who had the fewest and least severe symptoms, were more likely to be White, elderly, normal weight, without personal history of CHD, and nonsmokers. Unlike the other cluster groups, no symptoms were reported by more than 70% of women in this cluster and only one symptom, very tired/unusual fatigue, was reported by 48.6% of the women making this group perhaps the most difficult to diagnose during the prodromal phase. The symptom very tired/unusual fatigue was also an important symptom for women in Clusters 2 and 3 as over 70% reported this symptom. Other researchers have identified unusual and profound fatigue as an early symptom of impending MI. 7,23,31–34 However, fatigue as a single symptom, is included in many differential diagnoses such as disorders of mood, infections, metabolic disorders, anemia, and rheumatologic disorders 35 and not very helpful in isolation for diagnosing CHD conditions. In order to increase the usefulness of fatigue in developing the differential diagnosis of CHD for women, especially those in the Older, Silent Asymptomatic Group (Cluster 1), we recommend clinicians thoroughly assess the impact of unusual fatigue on women’s ability to complete their activities of daily living since women have previously reported this fatigue as profound and interfering with completing usual activities.32
Prodromally, women in both the Diverse Mildly Symptomatic Group and the Younger Minority Multiple Distressing Symptom Group with the most CHD risk factors and a multitude of prodromal symptoms, present a more typical picture of CHD. When fatigue is viewed within a cluster of symptoms (heart racing, shortness of breath, anxiety, and frequent indigestion), such as reported by women in these clusters, clinicians may be more likely to recognize CHD as a differential diagnosis. Other researchers have also identified these various symptoms as common CHD symptoms. 23,27,36–38 However, if women and clinicians view each symptom individually, it is easier to attribute these symptoms to other problems such as gastrointestinal distress, anxiety, or stress. Clustering these symptoms provides clinicians with a more complete picture of the pattern of CHD presentation in these women.
For acute symptoms, women in the Older Silent Asymptomatic Group tended to be White, less likely to smoke, have a history of CVD, or to be obese. Proportionately, this Cluster contained the largest number of Hispanic women (88 of 186 Hispanic women). In comparison, over 67% of the women in the Younger Minority Multiple Distressing Symptom Group were minorities, primarily Black. They were also the youngest group, and had the greatest number of risk factors, including smoking, obesity, personal history of CVD, and diabetes. Women in the Diverse Mildly Symptomatic Group (Cluster 2) were also primarily minority women and had more risk factors than those in the Silent Asymptomatic Group (Cluster 1), but less than those in the Younger Minority Multiple Distressing Symptom Group (Cluster 3). It is interesting to note that when viewing characteristics of the women in each cluster, the prevalence of risk factors was least in the Older Silent Asymptomatic Group compared to those in the Younger Minority Multiple Distressing Symptom Group, similarly to the intensity and frequency of symptoms, which were least in the Older Silent Asymptomatic Group and greatest in Younger Minority Multiple Distressing Symptom Group. Unexpectedly, diabetes mellitus was not a significant predictor of cluster membership.
Chest pain/discomfort was reported by 40–69.9% of the 283 women in Cluster 3, the Younger Minority Multiple Distressing Symptom Group, but not reported by women in Cluster 1 or 2 as a common symptom during the prodromal period, and thus was not identified as a primary prodromal symptom. During the acute MI, chest pain/discomfort was identified by 70–100% of women in Cluster 3 and by 40–69.9% of the 1022 women in Clusters 1 and 2. Other researchers have similarly reported chest pain as the most frequent acute symptom, regardless of sex,3,9 but little is known about racially diverse women’s symptoms.39 Interestingly, over 25% of these diverse women did not report any chest pain/discomfort during their acute MI. Brieger et al.3 reported that 8.4% of 20,881 patients admitted for ACS presented without chest pain, 23.8% of whom were misdiagnosed as something other than ACS. Furthermore, they reported that patients without chest pain were significantly older, more likely women, and non-smokers, similar to the women in the Older Silent Asymptomatic Group (Cluster 1) in this analysis. Other researchers report that lack of chest pain in women often leads to misdiagnosis.9,40–42 The only other acute symptom that was reported with chest pain/discomfort by 40% or more women in each cluster was shortness of breath, and was reported by more than 70% of women in Clusters 2 and 3. Shortness of breath was also a frequently reported prodromal symptom by women in these Clusters. Lindren et al. recently performed a cluster analysis of N=247 elderly male and female CHD patients and identified fatigue and shortness of breath as the most frequently reported individual symptoms 1 week prior to MI.23
Younger Black women had the highest number of risk factors and also the highest frequency and intensity of both prodromal and acute symptoms. It is well-known that Black women also have the highest morbidity and mortality associated with CHD and MI as compared to other women.1 It is possible these women may be difficult to diagnose due to sheer number and variety of symptoms reported. Cluster analysis may be more clinically relevant in interpreting their symptom pattern for diagnosis than for the other groups with fewer symptoms.
It is well recognized that women delay seeking treatment when experiencing acute MI symptoms. 43,44 One reason women delay seeking treatment may pertain to symptoms experienced during the prodromal phase. Women describe that prodromal symptoms are intermittent and resolve spontaneously after rest with little if any, lasting effects.7 This study indicates a high percent-recurrence between prodromal and acute symptoms; that is, symptoms that presented prodromally, are likely to reoccur acutely. Similarly, other researchers reported that 57% of 356 patients (44% women) admitted with ACS described their ACS symptoms as similar to symptoms they had experienced in the past.8 Even though prodromal chest pain/discomfort was not a commonly reported prodromal symptom for women in this study, for those women who experienced it prodromally, 82% also reported it with their acute MI. The other prodromal symptom with the highest percent recurrence (83%) was shortness of breath and 6 other symptoms were over 50% likely to reoccur. Due to this high rate of agreement between prodromal and acute symptoms, we suggest that women may delay seeking treatment for acute symptoms because they expect that acute MI symptoms will resolve spontaneously, as they had in the past since prodromal symptoms previously resolved without intervention. Once symptoms do not resolve, women may rest, try home remedies or continue to wait, contributing to delay in treatment-seeking and poor outcomes.
Results from this study provide useful information to assist women to recognize potential CHD symptom clusters, especially in the presence of known CHD risk factors, and this knowledge may prompt them to seek treatment while still in the prodromal phase, perhaps delaying or preventing MI. We need future research to replicate our findings and to fully describe symptom clusters and the temporal process of these symptoms for maximum diagnostic usefulness.
This study offers the most complete picture to-date of how racially diverse women’s prodromal and acute MI symptoms naturally cluster and describes the associated characteristics of the women who comprise each cluster. This study also found that women’s symptoms reported in the prodromal symptom phase were likely to reoccur with the acute event, offering information about symptom patterns and how women’s symptoms may evolve over time. Identifying CHD symptoms clusters and characteristics of women who experience specific symptom clusters offers insight into multi-symptom complaints that are challenging for patients and clinicians to identify and diagnose.
LIMITATIONS
Only women with a diagnosis of CHD and MI were included in the database selected for this secondary analysis. Having a control group would have strengthened this analysis. Further, clustering, like modeling in general, fits the data on which they are developed better than an arbitrary data set. However, because we used various clustering methods that yielded similar results, confidence in the conclusions is enhanced. Additionally, original data were self-reported data based on participant’s recall that may have been inaccurate. To minimize this limitation, all participants passed a cognitive screen prior to answering questions. Because MI is a life-altering experience, women reported no difficulty differentiating between intermittent prodromal and unrelenting acute symptoms. Others have reported that persons have accurate recall of experiences surrounding profound life events.45,46
SUMMARY
Since CHD is a disabling and deadly disease in women, prompt diagnosis and efficacious treatment are essential in improving women’s CHD outcomes. Identifying naturally occurring clusters of CHD symptoms in women and characteristics of women who experience specific symptom clusters could potentially assist women to recognize these symptom clusters as significant and promptly seek medical attention and could increase clinicians’ suspicion of CHD when symptom clusters are present, thus promoting prompt and appropriate diagnostic testing and treatment. Additionally describing women’s acute MI symptom clusters should assist health personnel in emergency departments in making informed and appropriate triaging decisions for women who present with a variety of non-chest pain symptoms but who may be experiencing a MI or acute coronary syndrome.
Summary & Implications for Practice
Older, White women without a history of diabetes or smoking were less likely to experience clusters of prodromal symptoms but rather a single symptom of unusual profound fatigue.
Younger (<50 years), Black women with multiple risk factors as compared to other women were more likely to complain of a cluster of symptoms composed of multiple distressing prodromal symptoms.
During the prodromal period, chest pain/discomfort was notably lacking in all three symptom clusters.
Smoking, younger, obese diabetic Black women with a personal history of CHD reported the largest cluster of acute MI symptoms.
Older, normal weight, White women without diabetes who were not smokers reported the smallest cluster of acute MI symptoms.
Women were likely to experience the same prodromal (early warning) symptoms and acute MI symptoms regardless of cluster membership.
Clusters of women’s CHD and MI symptoms provide useful diagnostic clues to inform clinicians, facilitate timely diagnoses and early treatment, and potentially improve CHD outcomes.
MI symptom clusters could assist ED clinicians in making appropriate triaging decisions for women who present with a variety of non-chest pain symptoms but who may be experiencing a MI or ACS episode.
What’s New.
Younger (<50 years), Black women with multiple risk factors as compared to other women were more likely to complain of a cluster of symptoms composed of multiple distressing prodromal symptoms.
During the prodromal period, chest pain/discomfort was notably lacking in all three
Older, normal weight, White women without diabetes who were not smokers reported the smallest cluster of acute MI symptoms.
Table 7.
Percent of Women Reporting a Symptom in the Prodromal Phase Reporting the Same Symptom During the Acute MI
| Symptom | Number of Women Reporting Symptom Prodromally | Number of Women Reporting Recurrence of Symptom at Acute Stage | % Recurrence |
|---|---|---|---|
| Shortness of breath | 565 (44.5%) | 469 | 83.01 |
| Any chest pain/discomfort | 458 (36.1%) | 374 | 81.66 |
| Arms weak/heavy | 336 (26.5%) | 221 | 65.77 |
| Arms ache | 273 (21.5%) | 161 | 58.97 |
| Frequent indigestion | 494 (38.9%) | 279 | 56.48 |
| Very tired/unusual fatigue | 930 (73.2%) | 505 | 54.30 |
| Loss of appetite | 357 (28.1%) | 185 | 51.82 |
| Heart racing | 454 (35.7%) | 233 | 51.32 |
| Hand/arms tingling | 344 (27.1%) | 170 | 49.42 |
| New vision problems | 392 (30.9%) | 172 | 43.88 |
| Cough | 304 (23.9%) | 113 | 37.17 |
Acknowledgments
Acknowledgement of Financial Support:
The National Institute of Nursing Research supported this work with two grants, 1 RO1 NR04908 and 1 R01 NR05265.
Contributor Information
Jean C. McSweeney, University of Arkansas for Medical Sciences, Little Rock, AR.
Mario A. Cleves, University of Arkansas for Medical Sciences, Little Rock, AR.
Weizhi Zhao, University of Arkansas for Medical Sciences, Little Rock, AR.
Leanne L. Lefler, University of Arkansas for Medical Sciences, Little Rock, AR.
Shengping Yang, St. Jude Children’s Research Hospital, Memphis, TN.
References
- 1.American Heart Association. Heart Disease and Stroke Statistics - 2009 Update. Dallas, Tx: American Heart Association; 2009. [Google Scholar]
- 2.McSweeney J. Racial differences in women’s prodromal and acute myocardial infarction symptoms. Am J Crit Care. 2009 doi: 10.4037/ajcc2010372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brieger D, Eagle KA, Goodman SG, et al. Acute coronary syndromes without chest pain, an underdiagnosed and undertreated high-risk group: insights from the Global Registry of Acute Coronary Events. Chest. 2004;126(2):461–469. doi: 10.1378/chest.126.2.461. [DOI] [PubMed] [Google Scholar]
- 4.Moser DK, McKinley S, Dracup K, Chung ML. Gender differences in reasons patients delay in seeking treatment for acute myocardial infarction symptoms. Patient Educ Couns. 2005;56(1):45–54. doi: 10.1016/j.pec.2003.11.011. [DOI] [PubMed] [Google Scholar]
- 5.Dracup K. The challenge of women and heart disease. Arch Intern Med. 2007;167(22):2396. doi: 10.1001/archinte.167.22.2396. [DOI] [PubMed] [Google Scholar]
- 6.Arslanian-Engoren C. Black, Hispanic, and White women’s knowledge of the symptoms of acute myocardial infarction. J Obstet Gynecol Neonatal Nurs. 2005;34(4):505–511. doi: 10.1177/0884217505278222. [DOI] [PubMed] [Google Scholar]
- 7.McSweeney JC, Cody M, O’Sullivan P, Elberson K, Moser DK, Garvin BJ. Women’s early warning symptoms of acute myocardial infarction. Circulation. 2003;108(21):2619–2623. doi: 10.1161/01.CIR.0000097116.29625.7C. [DOI] [PubMed] [Google Scholar]
- 8.DeVon HA, Ryan CJ, Ochs AL, Shapiro M. Symptoms across the continuum of acute coronary syndromes: Differences between women and men. Am J Crit Care. 2008;17(1):14–24. [PMC free article] [PubMed] [Google Scholar]
- 9.Canto JG, Goldberg RJ, Hand MM, et al. Symptom presentation of women with acute coronary syndromes: myth vs reality. Arch Intern Med. 2007;167(22):2405–2413. doi: 10.1001/archinte.167.22.2405. [DOI] [PubMed] [Google Scholar]
- 10.Sakimura JN, Dang MT, Ballard KB, Hansen RL. Cognitive and temperament clusters in 3- to 5-year-old children with aggressive behavior. J Sch Health. 2008;78(1):38–45. doi: 10.1111/j.1746-1561.2007.00264.x. [DOI] [PubMed] [Google Scholar]
- 11.Hadi S, Fan G, Hird AE, Kirou-Mauro A, Filipczak LA, Chow E. Symptom clusters in patients with cancer with metastatic bone pain. J Palliat Med. 2008;11(4):591–600. doi: 10.1089/jpm.2007.0145. [DOI] [PubMed] [Google Scholar]
- 12.Dodd MJ, Miaskowski C, Paul SM. Symptom clusters and their effect on the functional status of patients with cancer. Oncol Nurs Forum. 2001;28(3):465–470. [PubMed] [Google Scholar]
- 13.Gift AG, Jablonski A, Stommel M, Given CW. Symptom clusters in elderly patients with lung cancer. Oncol Nurs Forum. 2004;31(2):202–212. doi: 10.1188/04.ONF.202-212. [DOI] [PubMed] [Google Scholar]
- 14.Miaskowski C, Dodd M, Lee K. Symptom clusters: the new frontier in symptom management research. J Natl Cancer Inst Monogr. 2004;(32):17–21. doi: 10.1093/jncimonographs/lgh023. [DOI] [PubMed] [Google Scholar]
- 15.Barsevick AM, Whitmer K, Nail LM, Beck SL, Dudley WN. Symptom cluster research: conceptual, design, measurement, and analysis issues. J Pain Symptom Manage. 2006;31(1):85–95. doi: 10.1016/j.jpainsymman.2005.05.015. [DOI] [PubMed] [Google Scholar]
- 16.Dodd M, Janson S, Facione N, et al. Advancing the science of symptom management. J Adv Nurs. 2001;33(5):668–676. doi: 10.1046/j.1365-2648.2001.01697.x. [DOI] [PubMed] [Google Scholar]
- 17.Barsevick AM. The elusive concept of the symptom cluster. Oncol Nurs Forum. 2007;34(5):971–980. doi: 10.1188/07.ONF.971-980. [DOI] [PubMed] [Google Scholar]
- 18.Kim HJ, McGuire DB, Tulman L, Barsevick AM. Symptom clusters: concept analysis and clinical implications for cancer nursing. Cancer Nurs. 2005;28(4):270–282. doi: 10.1097/00002820-200507000-00005. [DOI] [PubMed] [Google Scholar]
- 19.Andersen LB, Boreham CA, Young IS, et al. Insulin sensitivity and clustering of coronary heart disease risk factors in young adults. The Northern Ireland Young Hearts Study. Prev Med. 2006;42(1):73–77. doi: 10.1016/j.ypmed.2005.10.009. [DOI] [PubMed] [Google Scholar]
- 20.Sajjadi F, Mohammadifard N, Kelishadi R, Ghaderian N, Alikhasi H, Maghrun M. Clustering of coronary artery disease risk factors in patients with type 2 diabetes and impaired glucose tolerance. East Mediterr Health J. 2008;14(5):1080–1089. [PubMed] [Google Scholar]
- 21.Saylor J. Risk factor clusters for metabolic syndrome in coronary heart disease: state of the science. Dimens Crit Care Nurs. 2005;24(2):64–69. doi: 10.1097/00003465-200503000-00005. [DOI] [PubMed] [Google Scholar]
- 22.Ryan CJ, Zerwic JJ. Knowledge of symptom clusters among adults at risk for acute myocardial infarction. Nurs Res. 2004;53(6):363–369. doi: 10.1097/00006199-200411000-00004. [DOI] [PubMed] [Google Scholar]
- 23.Lindgren TG, Fukuoka Y, Rankin SH, Cooper BA, Carroll D, Munn YL. Cluster analysis of elderly cardiac patients’ prehospital symptomatology. Nurs Res. 2008;57(1):14–23. doi: 10.1097/01.NNR.0000280654.50642.1a. [DOI] [PubMed] [Google Scholar]
- 24.McSweeney JC, Lefler LL, Fischer EP, Naylor AJ, Jr, Evans LK. Women’s prehospital delay associated with myocardial infarction: does race really matter? J Cardiovasc Nurs. 2007;22(4):279–285. doi: 10.1097/01.JCN.0000278958.98124.6e. [DOI] [PubMed] [Google Scholar]
- 25.Katzman R, Brown T, Fuld P, Peck A, Schechter R, Schimmel H. Validation of a short orientation-memory-concentration test of cognitive impairment. Am J Psychiatry. 1983;140:734–739. doi: 10.1176/ajp.140.6.734. [DOI] [PubMed] [Google Scholar]
- 26.Musselwhite K, Cuff L, McGregor L, King KM. The telephone interview is an effective method of data collection in clinical nursing research: a discussion paper. Int J Nurs Stud. 2007;44(6):1064–1070. doi: 10.1016/j.ijnurstu.2006.05.014. [DOI] [PubMed] [Google Scholar]
- 27.McSweeney JC, O’Sullivan P, Cody M, Crane PB. Development of the McSweeney Acute and Prodromal Myocardial Infarction Symptom Survey. J Cardiovasc Nurs. 2003;19(1):58–67. doi: 10.1097/00005082-200401000-00010. [DOI] [PubMed] [Google Scholar]
- 28.Everitt B. Cluster analysis. 3. London: Edward Arnold; 1993. [Google Scholar]
- 29.Hosmer D, Lemeshow S. Applied logistic regression. 2. New York: John Wiley & Sons; 2000. [Google Scholar]
- 30.Zhang H, Singer B. Recursive partitioning in the health sciences. New York: Springer; 1999. [Google Scholar]
- 31.Lee H, Bahler R, Park OJ, Kim CJ, Lee HY, Kim YJ. Typical and atypical symptoms of myocardial infarction among African-Americans, whites, and Koreans. Crit Care Nurs Clin North Am. 2001;13(4):531–539. [PubMed] [Google Scholar]
- 32.McSweeney JC, Crane PB. Challenging the rules: Women’s prodromal and acute symptoms of myocardial infarction. Res Nurs Health. 2000;23(2):135–146. doi: 10.1002/(sici)1098-240x(200004)23:2<135::aid-nur6>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- 33.Hofgren C, Karlson B, Herlitz J. Prodromal symptoms in subsets of patients hospitalized for suspected acute myocardial infarction. Heart Lung. 1995;24(1):3–10. doi: 10.1016/s0147-9563(05)80089-5. [DOI] [PubMed] [Google Scholar]
- 34.Schuitemaker GE, Dinant GJ, van der Pol GA, Appels A. Assessment of vital exhaustion and identification of subjects at increased risk of myocardial infarction in general practice. Psychosomatics. 2004;45(5):414–418. doi: 10.1176/appi.psy.45.5.414. [DOI] [PubMed] [Google Scholar]
- 35.LeBlond RF, DeGowin RL, Brown DD. DeGowin’s Diagnostic Examination. 9. New York: McGraw Hill; 2009. [Google Scholar]
- 36.Goldberg R, Goff D, Cooper L, et al. Age and sex differences in presentation of symptoms among patients with acute coronary disease: The REACT trial. Coron Artery Dis. 2000;11(5):399–407. doi: 10.1097/00019501-200007000-00004. [DOI] [PubMed] [Google Scholar]
- 37.Ottolini F, Modena MG, Rigatelli M. Prodromal symptoms in myocardial infarction. Psychother Psychosom. 2005;74(5):323–327. doi: 10.1159/000086324. [DOI] [PubMed] [Google Scholar]
- 38.Chen W, Woods SL, Wilkie DJ, Puntillo KA. Gender differences in symptom experiences of patients with acute coronary syndromes. J Pain Symptom Manage. 2005;30(6):553–562. doi: 10.1016/j.jpainsymman.2005.06.004. [DOI] [PubMed] [Google Scholar]
- 39.Fang J, Keenan N, Dai S, Denny C. Disparities in adult awareness of heart attack warning signs and symptoms--14 states, 2005. MMWR. 2008;57(7):175–179. [PubMed] [Google Scholar]
- 40.de Torbal A, Boersma E, Kors JA, et al. Incidence of recognized and unrecognized myocardial infarction in men and women aged 55 and older: the Rotterdam Study. Eur Heart J. 2006;27(6):729–736. doi: 10.1093/eurheartj/ehi707. [DOI] [PubMed] [Google Scholar]
- 41.Sheifer SE, Manolio TA, Gersh BJ. Unrecognized myocardial infarction. Ann Intern Med. 2001;135(9):801–811. doi: 10.7326/0003-4819-135-9-200111060-00010. [DOI] [PubMed] [Google Scholar]
- 42.Canto JG, Shlipak MG, Rogers WJ, et al. Prevalence, clinical characteristics, and mortality among patients with myocardial infarction presenting without chest pain. J Am Med Assoc. 2000;283(24):3223–3229. doi: 10.1001/jama.283.24.3223. [DOI] [PubMed] [Google Scholar]
- 43.Lefler LL, Bondy KN. Women’s delay in seeking treatment with myocardial infarction: A meta-synthesis. J Cardiovasc Nurs. 2004;19(4):251–268. doi: 10.1097/00005082-200407000-00005. [DOI] [PubMed] [Google Scholar]
- 44.Moser DK, Kimble LP, Alberts MJ, et al. Reducing delay in seeking treatment by patients with acute coronary syndrome and stroke. A scientific statement from the American Heart Association Council on Cardiovascular Nursing and Stroke Council. Circulation. 2006;114(2):168–182. doi: 10.1161/CIRCULATIONAHA.106.176040. [DOI] [PubMed] [Google Scholar]
- 45.Green A. An exploratory study of patient’s memory recall of their stay in an adult intensive therapy unit. Intensive & Critical Care Nursing. 1996;12(3):131–137. doi: 10.1016/s0964-3397(96)80435-6. [DOI] [PubMed] [Google Scholar]
- 46.Githens PB, Glass CA, Sloan FA, Entman SS. Maternal recall and medical records: An examination of events during pregnancy, childbirth, and early infancy. Birth. 1993;20(3):136–141. doi: 10.1111/j.1523-536x.1993.tb00438.x. [DOI] [PubMed] [Google Scholar]