Abstract
Introduction
Combined therapy with deferoxamine (DFO) and deferasirox (DFX) may be performed empirically when DFX monotherapy fails. Given the lack of published data on this therapy, the study goal was to assess the safety and efficacy of combined DFO/DFX therapy in a gerbil model.
Methods
Thirty-two female Mongolian gerbils 8–10 weeks old were divided into 4 groups (sham chelated, DFO, DFX, DFO/DFX). Each received 10 weekly injections of 200 mg/kg iron dextran prior to initiation of 12 weeks of chelation. Experimental endpoints were heart and liver weights, iron concentration and histology.
Results
In the heart, there was no significant difference among the treatment groups for wet-to-dry ratio, iron concentration and iron content. DFX-treated animals exhibited lower organ weights relative to sham-chelated animals (less iron-mediated hypertrophy). DFO-treated organs did not differ from sham-chelated organs in any aspects. DFX significantly cleared hepatic iron. No additive effects were observed in the organs of DFO/DFX-treated animals.
Conclusions
Combined DFO/DFX therapy produced no detectable additive effect above DFX monotherapy in either the liver or heart, suggesting competition with spontaneous iron elimination mechanisms for chelatable iron. Combined therapy was well tolerated, but its efficacy could not be proven due to limitations in the animal model.
Keywords: Deferasirox, Deferoxamine, Iron overload
Introduction
Although iron chelation therapy with deferoxamine (DFO) improves life expectancy in thalassemia major, iron cardiomyopathy remains the leading cause of death [1]. Effective chelation therapy entails both prevention and removal of stored cardiac iron. Even though DFO efficiently clears cardiac iron if administered continuously, its cardiac efficacy diminishes significantly when administered intermittently, even if liver iron levels appear sufficiently regulated [2, 3]. Patient tolerance and compliance with this regime often compromise effective treatment and remain the best predictors of successful therapy [4, 5]. Oral chelators such as deferiprone and deferasirox (DFX) were developed to reduce these tolerance and compliance problems. DFX is a novel, tridentate chelator that is well tolerated in thalassemia major patients. It has a half-life of 14–16 h, leading to excellent suppression of the labile iron pool [6, 7]. Its long half-life makes it not only an excellent potential drug for cardiac iron prophylaxis, but also for cardiac iron extraction. Recent studies have shown that DFX can access and remove cardiac iron in cardiomyocytes, animal models and humans [7–9].
Due to its ease of administration, many patients have been switching to DFX since its approval by the Food and Drug Administration. However, while monotherapy at 30 mg/kg places approximately 85% of patients in negative iron balance, some patients remain in positive balance even with doses of 40 mg/kg [10–13]. Twice or thrice weekly DFO, taken in addition to daily DFX, might rectify this problem while still being acceptable to patients. More importantly, combination therapy may have significant advantages with respect to cardiac iron chelation. Prior in vitro and in vivo studies have shown that combined chelation therapy using deferiprone and DFO results in iron shuttling between the 2 compounds. That is, the smaller, lipophilic deferiprone molecule accesses stubborn cardiac iron stores and carries them to cellular locations accessible to DFO [14, 15]. DFO, with its higher affinity for iron, strips iron from deferiprone, which allows the now iron-free deferiprone to access additional cardiac iron. DFX, although not as permeable as deferiprone, readily enters myocytes and combines with iron [7, 16]. Since DFX has a lower iron affinity than DFO, there is a similar potential for a shuttle mechanism. As a result, some physicians are using combined DFO and DFX for patients that remain in positive iron balance or who are responding slowly to DFX monotherapy. However, there are no published animal or human data on sustained dual DFO/DFX therapy. Thus, the goal of this study was to evaluate the safety and efficacy of combined therapy in a gerbil model of iron overload.
Materials and Methods
Animals
Thirty-two female Mongolian gerbils 8–10 weeks old were obtained from Charles River Laboratories and housed in the Children’s Hospital of Los Angeles accredited animal care facility. Iron loading of animals was accomplished by 10 weekly, subcutaneous injections of iron dextran at a dose of 200 mg/kg (Sigma, St. Louis, Mo., USA). Following a 2-week washout period, chelation therapy was initiated in 30 animals (2 animals died unexpectedly during the iron loading).
Chelation Therapy
Animals were divided into 4 treatment groups: control, DFO, DFX and combined DFO/DFX. In order to administer DFO continuously, we used an ex vivo osmotic pump system. A 2-ml AL-ZET osmotic pump was placed in a sealed PBS-filled tube and carried by the gerbils on a rodent jacket (Braintree Scientific, Inc., Braintree, Mass., USA). At room temperature, the pump released 2.5 µ l of DFO per hour for 28 days via a catheter at a dose of 200 mg/kg/day. During the 3 months of chelation therapy, the pumps were replaced twice. To control for effects of the delivery mechanism, 4 sham-chelated animals were harnessed and cannulated with PBS-filled pumps. DFX was mixed in peanut butter and administered orally via syringe at a daily dose of 100 mg/kg. As a control, 3 sham-chelated animals received plain peanut butter.
Histology and Quantitative Iron Determination
At the end of the chelation therapy, animals were euthanized by CO 2 inhalation according to institution guidelines. Hearts and livers of all animals were harvested and processed for histology and iron assessment. The mid-papillary sections of the hearts (40 mg) and small pieces (80 mg) of the livers were sent for quantitative iron determination (Mayo Medical Laboratories, Rochester, Minn., USA). The rest of the organs were immersion fixed in 10% formalin, paraffin embedded and stained with Prussian blue and hematoxylin and eosin (H&E).
Statistical Analysis
All results are expressed as mean values ± standard deviations. One-way analysis of variance was used to determine statistical differences among groups. To correct for unequal variance, tests were performed on a log-transformed scale. The mean of each treatment group was compared with the mean value from the control animals using Dunnett’s test, which corrects for multiple comparisons. p < 0.05 was considered significant.
Results
The ex vivo pump systems were able to expel 92.2 ± 6.6% of the target dose. Some animals were able to temporarily dislodge their cannulae. Cannulation was also temporarily halted if animals appeared ill. Nine out of 30 animals displayed clinical signs of ill health during the whole experiment. Ill health was defined as weight loss, dehydration, hunched back, ruffled fur or lethargy. These findings were observed in 8 animals receiving DFO, either alone or in combination, and in 1 cannulated, sham-chelated animal. Upon diagnosis, backpacks with pumps were removed to allow recovery. On average, DFO gerbils missed 3 days and DFO/DFX gerbils about 5 days of DFO chelation therapy for health concerns. Animals in all 3 treatment groups were slightly lighter than control animals, but this difference reached statistical significance only for DFO-treated animals. Control animals weighed 86.9 ± 7.3 g. The body weights of DFX-, DFO/DFX- and DFO-treated animals were 80.5 ± 8.1, 80.6 ± 4.6 and 79.2 ± 5.8 g (p = 0.05), respectively. Total cannulation efficiency was 94.2 ± 4.2% and was not statistically different among the groups.
Organ weight, wet-to-dry weight ratio, dry weight iron concentration and organ iron content are displayed in table 1. Iron loading produces organ hypertrophy in the gerbil [17, 18]. For reference, normal organ weights in age-matched gerbils are 333 ± 42 mg for the heart and 2.13 ± 0.26 g for the liver; they do not vary significantly with age [19]. DFX treatment partially normalized liver and cardiac weights relative to controls: 344 ± 31 versus 417 ± 60 mg for the heart and 4.38 ± 0.67 versus 5.94 ± 0.88 g for the liver. The wet-to-dry ratio, cardiac iron concentrations and iron content did not statistically differ among all the groups.
Table 1.
Cardiac and hepatic organ iron contents, dry weight iron concentrations, wet-to-dry weight ratio and organ weights
| Control (n = 7) |
DFO (n = 8) |
DFX (n = 7) |
DFO/DFX (n = 8) |
|
|---|---|---|---|---|
| Heart | ||||
| Organ weight, mg | 417 ± 60 | 408 ± 33 | 344 ± 31* | 409 ± 63 |
| Wet-to-dry ratio | 4.45 ± 0.21 | 4.55 ± 0.31 | 4.53 ± 0.34 | 4.48 ± 0.35 |
| Iron dry weight, mg/g | 2.37 ± 0.41 | 2.09 ± 0.48 | 2.6 ± 0.32 | 1.98 ± 0.39 |
| Organ iron, mg | 0.22 ± 0.04 | 0.19 ± 0.05 | 0.20 ± 0.02 | 0.18 ± 0.01 |
| Liver | ||||
| Organ weight, g | 5.94 ± 0.88 | 5.29 ± 0.57 | 4.38 ± 0.67* | 5.24 ± 0.6 |
| Wet-to-dry ratio | 4.36 ± 0.39 | 4.26 ± 0.27 | 4.25 ± 0.3 | 4.23 ± 0.3 |
| Iron dry weight, mg/g | 30 ± 2.5 | 28.3 ± 4.0 | 19.4 ± 5.2* | 16 ± 4.5* |
| Organ iron, mg | 40.8 ± 7.7 | 35.4 ± 7.2 | 19.3 ± 3.1* | 20.1 ± 7.1* |
Data are means ± SD. Analysis of variance was used to determine statistical differences among groups.
p < 0.05 (Dunnett’s test).
In the liver, there was no statistically significant change among the treatment groups for the wet-to-dry ratio. DFO treatment alone did not lower hepatic iron concentration or content when compared with the control group. DFX removed more than half of the liver iron, comparable with prior studies. However, combined therapy did not produce an additive effect; in fact, organ weight was higher in the combined therapy animals (5.24 ± 0.6 vs. 4.37 ± 0.67 g for DFX therapy alone).
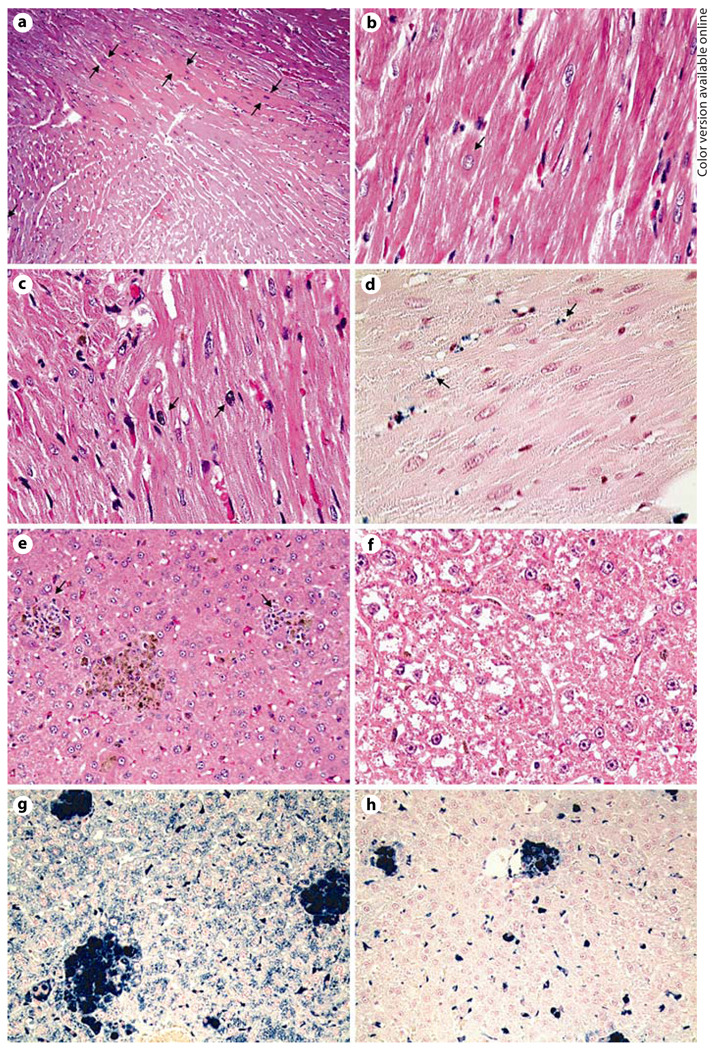
Cardiac histology was relatively similar among the treatment groups. H&E staining revealed mild to moderate myocardial dense fibers and mild signs of nuclear hypertrophy in the hearts among all the treatment groups (fig. 1 a, b). As reported previously, cardiac iron accumulated mainly in the interstitium (fig. 1 d). The only chelator-specific findings were rare misshapen nuclei that were only found in 6 out of 8 animals treated with combined therapy (fig. 1 c). Misshapen nuclei were not associated with necrosis. In addition, 1 DFO/DFX-treated animal displayed a focal area of necrosis and inflammation in the left ventricle wall (data not shown).
Fig. 1.
Representative histological examination of the heart and liver following 12 weeks of chelation therapy. Specimens were stained with H&E (a – c, e, f) and Perl’s Prussian blue iron stain (d, g, h). Increased appearance of dense fibers (a) and mild levels of nuclear hypertrophy (b) were observed in all cardiac samples. Misshapen nuclei were present in some of the combined therapy-treated specimens (c). Cardiac iron was mainly found in the interstitium (d). Accumulation and distribution of iron in the heart was very similar for all samples. Signs of focal area of inflammation were present in particular in DFO and control livers (e). Pallor was increasingly observed in ICL and combined treated livers (f). Whereas DFO did not significantly remove hepatic iron (g), ICL and combined treatment cleared hepatocytes from iron (h). ×100 (a), ×200 (e, g, h), ×400 (b–d, f).
Liver histology is highlighted in figure 1 e–h. DFO-treated and sham-chelated liver histology was similar. In general, there were focal glomular inflammatory infiltrates in areas with masses of hemosiderin (fig. 1 e). Prussian blue staining showed increased iron accumulation in hepatocytes, Kupffer cells and infiltrating monocytes (fig. 1 g). Heavy iron deposits were observed, in particular around the central veins. DFX and combined treatment caused clear hepatocyte foci devoid of iron (fig. 1 h). There were no or little signs of hepatic inflammation. However, cytoplasmic pallor along central veins was prominent in some of the DFX- and DFX/DFO-treated animals (fig. 1 f). This phenomenon was also present in 2 control livers.
Discussion
Combination therapy with DFX and DFO is increasingly being used in human studies. The goal of this study was to evaluate the safety and efficacy of this combination in a gerbil model of iron overload.
Efficacy
Our ability to evaluate the efficacy of combined therapy was prevented by the inability of DFO to produce iron chelation detectable beyond the spontaneous iron elimination rate. Gerbils can spontaneously excrete as much as 25% of their stored liver iron in a 4-week period, with detectable iron in both the urine and stool [20]. Large spontaneous iron loss has also been observed in other rodent models of iron overload [21]. If the DFO-chelatable iron is accessible to these vigorous excretion mechanisms, competition may limit the total iron removed. However, the smaller and more lipophilic oral chelators, DFX and deferiprone, likely have greater access to intracellular stores that have rate-limited access to spontaneous elimination mechanisms. The effect of these chelators is more readily detectable above the background iron losses [18].
If this hypothesis is true, why did DFO produce detectable chelation in several prior studies? One key difference is that iron loading and iron chelation were performed simultaneously rather than sequentially as in the present model. If iron dextran and DFO are coadministered in the subcutaneous space, the chelation kinetics will be quite favorable. Some portion of the extravascular iron dextran will be scavenged by DFO, preventing its macrophage uptake and mobilization to the free iron pool; in effect, it is like administering less iron dextran. In contrast, sequential iron loading and chelation produces ample time for the iron dextran to be mobilized from the subcutaneous space before DFO is administered. As a result, DFO must either extract intracellular iron or compete with spontaneous loss mechanisms for circulating non-transferrin-bound iron (NTBI). In addition, other reports of DFO efficacy have used hypertransfused rats where liver iron stores are minimally elevated. Spontaneous iron losses are insignificant at low somatic iron burdens such that DFO competes more favorably for the labile iron pool [22].
The apparent inability of DFO to remove intracellular iron stores, despite being administered continuously, does not imply that DFO does not penetrate these tissues. It simply means that the rate of this process is slower than the spontaneous iron mobilization rate, making it impossible to observe the effect above background losses. This is an unfortunate artifact of rodent models and one that has been underappreciated.
In this trial, cardiac iron reduction with once-daily DFX treatment did not reach statistical significance, comparable with a prior comparison with twice daily dosing, but in contrast to our initial work [18, 23]. Pooling the data from all 3 studies (all had identical loading/unloading protocols) yields a 16% reduction in iron content (p < 0.001). Thus, the negative observation in this trial simply represents a modest effect size and inadequate statistical power for the group size used.
Although our study is the first report of chronic combined DFX/DFO therapy, Hershko et al. [22] reported single-dose combination therapy in hypertransfused rats. Combined therapy produced additive effects for DFX doses of 25–50 mg/kg [22]. However, DFX dosing at 100–200 mg/kg/day produced identical iron chelation with or without subcutaneous DFO at 200 mg/kg/day, suggesting competition for chelatable iron. The 100 mg/kg/day DFX dose chosen for this study was based on an earlier dose-response study, where DFX doses of 25–50 mg/kg/day were not distinguishable above the background iron elimination rate [20]. However, 100 mg/kg/day appears to maximize cardiac and hepatic iron clearance. In gerbils, cardiac iron clearance was about 5% per month, which is consistent with human data where the maximal clearance rate is approximately 3–5% per month [24, 25].
Safety
In general, combined chelation therapy was well tolerated. Although chelation regimes were very aggressive, no animal died during iron chelation therapy. Single chelation therapy seemed to be better tolerated overall. Six out of 8 combined treated animals got sick during the experiment, compared with 2 DFO animals and 1 sham-chelated animal. Combined therapy caused nuclear irregularity in a few cardiomyocytes; however, the physiological consequences are not known. Similar nuclear changes have been observed in long-term hepatocyte cultures exposed to iron [26]. In the prior study, nuclear morphologic changes were associated with oxidative damage from increased levels of NTBI. In the present work, NTBI levels are likely to be decreased in combined therapy-treated cardiomyocytes; however, it is possible that an increased unbound chelator could have induced oxidative stress [26]. No nuclear changes were seen in our liver samples, but DFX- and DFX/DFO-treated animals showed increased central pallor. The etiology and consequences of this observation are also unknown; they were not associated with necrosis, hepatocellular atrophy, increased tissue water content, or sinusoidal fibrosis.
Limitations
Osmotic pumps offer the benefit of continuous chelator administration; however, there are some limitations associated with their use. Drug delivery via pump is less precise than subcutaneous bolus injections. Since the pump size was too big for subcutaneous insertion, we had to create an ex vivo pump and harness delivery system. Animals did decannulate themselves for an average of 3.8 ± 3.5% of the time for the DFO-treated animals and for 5.6 ± 2.3% of the time for the DFO/DFX-treated animals. The decannulation time is still favorable compared with DFO dosing in humans. The gerbil DFX and DFO doses used in this study corresponded to 67% of the standard human dose when normalized to body surface area; this dose is appropriate given the lack of ongoing transfusional iron accumulation.
There is no perfect animal model to simulate iron overload in humans. Although the gerbil model of iron overload is well established for evaluating chelation efficiency, its limitations need to be recognized. The distribution patterns and kinetics of heart and liver iron deposition and removal are somewhat different from those found in humans.
In summary, DFX monotherapy at 100 mg/kg/day produced comparable hepatic and cardiac iron chelation as in prior studies. DFO was ineffective either alone or in combination with DFX, presumably because of unfavorable competition with spontaneous iron loss mechanisms. Unfortunately, these findings reflect a fundamental inadequacy of the animal model and cannot be extrapolated to human subjects. Better characterization of spontaneous iron loss mechanisms in rodents will be necessary to adequately conduct these studies in the future.
Acknowledgements
This work was supported by Novartis Pharma AG and the National Institute of Health (1RO1 HL075592-01A1).
References
- 1.Borgna-Pignatti C, Rugolotto S, De Stefano P, Piga A, Di Gregorio F, Gamberini MR, Sabato V, Melevendi C, Cappellini MD, Verlato G. Survival and disease complications in thalassemia major. Ann NY Acad Sci. 1998;850:227–231. doi: 10.1111/j.1749-6632.1998.tb10479.x. [DOI] [PubMed] [Google Scholar]
- 2.Lerner N, Blei F, Bierman F, Johnson L, Piomelli S. Chelation therapy and cardiac status in older patients with thalassemia major. Am J Pediatr Hematol Oncol. 1990;12:56–60. doi: 10.1097/00043426-199021000-00010. [DOI] [PubMed] [Google Scholar]
- 3.Aldouri MA, Wonke B, Hoffbrand AV, Flynn DM, Ward SE, Agnew JE, Hilson AJ. High incidence of cardiomyopathy in beta-thalassaemia patients receiving regular transfusion and iron chelation: reversal by intensified chelation. Acta Haematol. 1990;84:113–117. doi: 10.1159/000205046. [DOI] [PubMed] [Google Scholar]
- 4.Modell B, Khan M, Darlison M. Survival in beta-thalassaemia major in the UK: data from the UK Thalassaemia Register. Lancet. 2000;355:2051–2052. doi: 10.1016/S0140-6736(00)02357-6. [DOI] [PubMed] [Google Scholar]
- 5.Schrier S, Angelucci E. New strategies in the treatment of the thalassemias. Annu Rev Med. 2005;56:157–171. doi: 10.1146/annurev.med.56.082103.104718. [DOI] [PubMed] [Google Scholar]
- 6.Daar S, Taher A, Pathare A. Plasma LPI in beta-thalassemia patients before and after treatment with deferasirox (Exjade ®, ICL670) Blood. 2005;106:758a. [Google Scholar]
- 7.Glickstein H, El RB, Shvartsman M, Cabantchik ZI. Intracellular labile iron pools as direct targets of iron chelators: a fluorescence study of chelator action in living cells. Blood. 2005;106:3242–3250. doi: 10.1182/blood-2005-02-0460. [DOI] [PubMed] [Google Scholar]
- 8.Anderson LJ, Westwood MA, Prescott E, Walker JM, Pennell DJ, Wonke B. Development of thalassaemic iron overload cardiomyopathy despite low liver iron levels and meticulous compliance to desferrioxamine. Acta Haematol. 2006;115:106–108. doi: 10.1159/000089475. [DOI] [PubMed] [Google Scholar]
- 9.Otto-Duessel M, Aguilar M, Moats R, Wood JC. Antioxidant-mediated effects in a gerbil model of iron overload. Acta Haematol. 2007;118:193–199. doi: 10.1159/000109879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cohen AR, Glimm E, Porter JB. Effect of transfusional iron intake on response to chelation therapy in beta-thalassemia major. Blood. 2008;111:583–587. doi: 10.1182/blood-2007-08-109306. [DOI] [PubMed] [Google Scholar]
- 11.Nisbet-Brown E, Olivieri NF, Giardina PJ, Grady RW, Neufeld EJ, Sechaud R, Krebs-Brown AJ, Anderson JR, Alberti D, Sizer KC, Nathan DG. Effectiveness and safety of ICL670 in iron-loaded patients with thalassaemia: a randomised, double-blind, placebo-controlled, dose-escalation trial. Lancet. 2003;361:1597–1602. doi: 10.1016/S0140-6736(03)13309-0. [DOI] [PubMed] [Google Scholar]
- 12.Porter J, Galanello R, Saglio G, Neufeld EJ, Vichinsky E, Cappellini MD, Olivieri N, Piga A, Cunningham MJ, Soulières D, Gattermann N, Tchernia G, Maertens J, Giardina P, Kwiatkowski J, Quarta G, Jeng M, Forni GL, Stadler M, Cario H, Debusscher L, Della Porta M, Cazzola M, Greenberg P, Alimena G, Rabault B, Gathmann I, Ford JM, Alberti D, Rose C. Relative response of patients with myelodysplastic syndromes and other transfusion-dependent anaemias to deferasirox (ICL670): a 1-year prospective study. Eur J Haematol. 2007;80:168–176. doi: 10.1111/j.1600-0609.2007.00985.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cappellini MD, Bejaoui M, Perrotta S, Agaoglu L, Kattamis A, Giardina PJ, Janka-Schaub G, Opitz H, Ressyare-Djaffer C, Alberti D. A phase 3 study of deferasirox (ICL670), a once-daily oral iron chelator, in patients with beta-thalassemia. Blood. 2006;107:3455–3462. doi: 10.1182/blood-2005-08-3430. [DOI] [PubMed] [Google Scholar]
- 14.Link G, Konijn AM, Breuer W, Cabantchik ZI, Hershko C. Exploring the ‘iron shuttle’ hypothesis in chelation therapy: effects of combined deferoxamine and deferiprone treatment in hypertransfused rats with labeled iron stores and in iron-loaded rat heart cells in culture. J Lab Clin Med. 2001;138:130–138. doi: 10.1067/mlc.2001.116487. [DOI] [PubMed] [Google Scholar]
- 15.Hershko C, Abrahamov A, Konijn AM, Breuer W, Cabantchik IZ, Pootrakul P, Link G. Objectives and methods of iron chelation therapy. Bioinorg Chem Appl. 2003:151–168. doi: 10.1155/S1565363303000128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Glickstein H, El RB, Link G, Breuer W, Konijn AM, Hershko C, Nick H, Cabantchik ZI. Action of chelators in iron-loaded cardiac cells: accessibility to intracellular labile iron and functional consequences. Blood. 2006;108:3195–3203. doi: 10.1182/blood-2006-05-020867. [DOI] [PubMed] [Google Scholar]
- 17.Yang T, Brittenham GM, Dong WQ, Levy MN, Obejero-Paz CA, Kuryshev YA, Brown AM. Deferoxamine prevents cardiac hypertrophy and failure in the gerbil model of iron-induced cardiomyopathy. J Lab Clin Med. 2003;142:332–340. doi: 10.1016/S0022-2143(03)00135-5. [DOI] [PubMed] [Google Scholar]
- 18.Wood J, Otto-Duessel M, Aguilar M, Nick H, Moats R. Deferasirox and deferiprone remove cardiac iron in the iron-overloaded gerbil. Transl Res. 2006;148:272–280. doi: 10.1016/j.trsl.2006.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wood JC, Otto-Duessel M, Aguilar M, Nick H, Nelson MD, Coates TD, Pollack H, Moats R. Cardiac iron determines cardiac T2 *, T2, and T1 in the gerbil model of iron cardiomyopathy. Circulation. 2005;112:535–543. doi: 10.1161/CIRCULATIONAHA.104.504415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wood JC, Otto-Duessel M, Aguilar M, Nick H, Coates TD, Moats R. Dose response of deferoxamine, deferiprone, and ICL670 chelation therapy in a gerbil model of iron overload. Blood. 2004;104:985a. [Google Scholar]
- 21.Oates PS, Jeffrey GP, Basclain KA, Thomas C, Morgan EH. Iron excretion in iron-overloaded rats following the change from an iron-loaded to an iron-deficient diet. J Gastroenterol Hepatol. 2000;15:665–674. doi: 10.1046/j.1440-1746.2000.02210.x. [DOI] [PubMed] [Google Scholar]
- 22.Hershko C, Konijn AM, Nick HP, Breuer W, Cabantchik ZI, Link G. ICL670A: a new synthetic oral chelator: evaluation in hypertransfused rats with selective radioiron probes of hepatocellular and reticuloendothelial iron stores and in iron-loaded rat heart cells in culture. Blood. 2001;97:1115–1122. doi: 10.1182/blood.v97.4.1115. [DOI] [PubMed] [Google Scholar]
- 23.Otto-Duessel M, Aguilar M, Nick H, Moats R, Wood J. Comparison of BID versus QD deferasirox dosing in a gerbil model of iron cardiomyopathy. Exp Hematol. 2007;35:1069–1073. doi: 10.1016/j.exphem.2007.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Anderson LJ, Westwood MA, Holden S, Davis B, Prescott E, Wonke B, Porter JB, Walker JM, Pennell DJ. Myocardial iron clearance during reversal of siderotic cardiomyopathy with intravenous desferrioxamine: a prospective study using T2 * cardiovascular magnetic resonance. Br J Haematol. 2004;127:348–355. doi: 10.1111/j.1365-2141.2004.05202.x. [DOI] [PubMed] [Google Scholar]
- 25.Tanner MA, Galanello R, Dessi C, Smith GC, Westwood M, Agus A, Roughton M, Assomull R, Nair SV, Walker JM, Pennell DJ. A randomized, placebo-controlled, doubleblind trial of the effect of combined therapy with deferoxamine and deferiprone on myocardial iron in thalassemia major using cardiovascular magnetic resonance. Circulation. 2007;115:1876–1884. doi: 10.1161/CIRCULATIONAHA.106.648790. [DOI] [PubMed] [Google Scholar]
- 26.Cable EE, Connor JR, Isom HC. Accumulation of iron by primary rat hepatocytes in long-term culture. Am J Pathol. 1998;152:781–792. [PMC free article] [PubMed] [Google Scholar]