Abstract
Patients with transfusion-dependent anemia develop cardiac and endocrine toxicity from iron overload. Classically, serum ferritin and liver biopsy have been used to monitor patient response to chelation therapy. Recently, magnetic resonance imaging (MRI) has proven effective in detecting and quantifying iron in the heart and liver. Tissue iron is paramagnetic and increases the MRI relaxation rates R2 and R2* in a quantifiable manner. This review outlines the principles and validation of non invasive iron estimation by MRI, as well as discussing some of the technical considerations necessary for accurate measurements. Specifically, the use of R2 or R2* methods, choice of echo times, appropriate model for data fitting, the use of a pixel-wise or region-based measurement, and the choice of field strength are discussed.
Keywords: Iron Overload, Magnetic resonance imaging (MRI), Thalassemia, Sickle Cell Disease, Liver, Heart
INTRODUCTION
Iron overload is a surprisingly common clinical problem, resulting from disorders of iron absorption, such as hereditary hemochromatosis and thalassemia intermedia, defects in heme metabolism, as well as from chronic transfusion therapy (1,2). Iron is toxic to many tissues, particularly the heart and endocrine tissues, and iron overload is universally lethal unless controlled by phlebotomy or iron chelation therapy (3). As a result, methods to estimate total body iron stores are quite important. Trends in serum ferritin serve as a reasonable surrogate marker but can yield inappropriate results in the presence of inflammation or ascorbate deficiency (4–6). Ferritin measurements are also poorly correlated with cardiac iron stores (7,8).
In contrast, liver iron measurement is a better marker of total body iron stores. The liver accounts for more than 70% of somatic iron stores (even more in splenectomized patients). Liver iron concentration tracks net iron balance in both chelated (9) and non chelated patients (10). Elevated liver iron also conveys prospective risk of cardiac complications (9,11). Unfortunately, significant cardiac iron burden and toxicity can occur despite low liver iron concentrations (12).
Classically, liver iron assessment has been performed by needle biopsy. This procedure carries a 0.5% complication risk (13). It is also disliked by patients, making it difficult to perform serially at reasonable intervals. Although the superconducting quantum interference device (SQUID) has been used as a non invasive alternative to liver iron estimation, there are only four functioning devices in the world, critically limiting this technique (14).
Recently, magnetic resonance imaging (MRI) has gained acceptance as a novel tool for iron quantitation in the body. Although the principles were demonstrated nearly 25 years ago (15), it is only recently that MRI has become sufficiently robust for clinical practice. Magnetic resonance imaging has the ability of being non invasive, inexpensive and widely available in developed countries. More importantly, it can also estimate iron concentrations in a wide range of tissues such as the heart (7,8,16,17). Magnetic resonance imaging does not image the iron directly but instead images water protons as they diffuse near iron deposits in the tissue of interest (18,19). The iron acts as little magnets, destroying the homogeneity of the magnetic field in iron laden tissues. The moving water protons each experience significantly different magnetic profiles and become desynchronized from one another. This causes the image to darken at a rate proportional to the iron concentration.
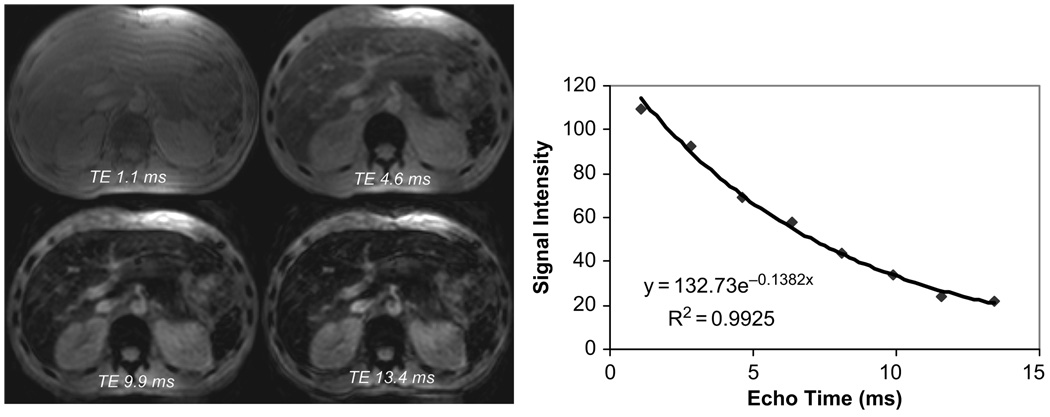
There are two fundamentally different ways to generate MRI images for iron quantitation. To make an image, desynchronized water protons must be refocused to form an echo that the MRI scanner can perceive. This can be achieved using a radio-frequency (rf) pulse, or spin echo, or by applying a strong additional magnetic field known as a gradient (forming a gradient echo). The timing of this echo is controlled by the MRI scanner. Figure 1 illustrates this process. The longer the echo time (or TE), the darker the resultant image. If one looks at the signal intensity within a certain region (Figure 1, right panel), it is clear that the decline in image intensity follows a pattern similar to radioactive decay. Therefore, the iron-mediated darkening can be characterized by a half-life time constant. If a spin echo is used, the half life is known as T2, whereas it is known as T2* if a gradient echo is used. Often, the darkening is described as a rate, R2 or R2*, rather than a time constant. The relaxation rates are just the reciprocal of the time constants, thus: R2 = 1000/T2, R2* = 1000/T2*. The factor of 1000 is included because T2 and T2* are expressed in ms and relaxation rates are expressed in Hertz (or sec−1).
FIGURE 1.
Left: gradient echo images of liver at echo times of 1.1, 4.6, 9.9, and 13.4 ms. Liver parenchyma darkens progressively with increasing echo time. Right: signal intensity as a function of time is well described by a monoexponential decay.
MAGNETIC RESONANCE IMAGING VALIDATION
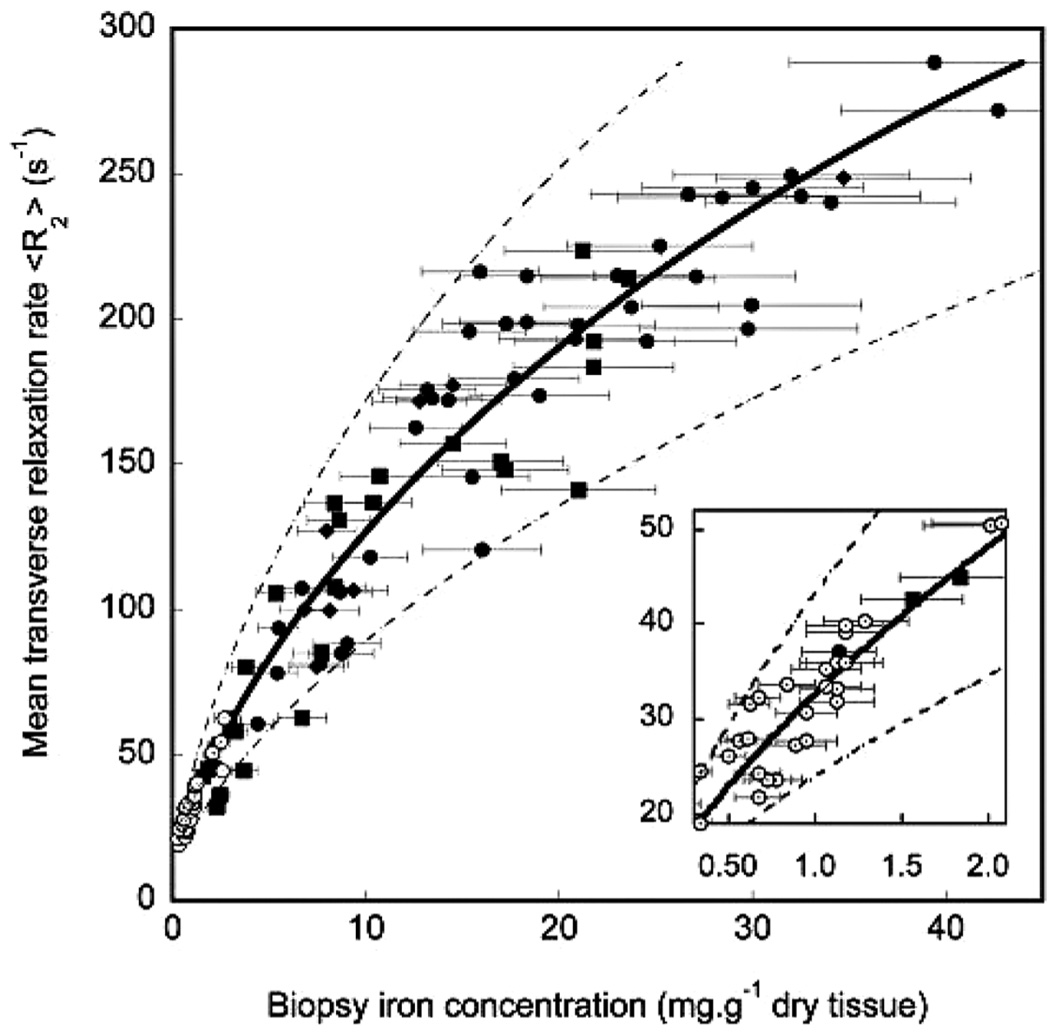
Magnetic resonance imaging assessment of tissue iron was first validated in the liver because biopsies were routinely obtained through clinical care. Although numerous small studies had demonstrated the feasibility of R2 imaging, the studies of St. Pierre et al. (20) and Wood et al. (21) were the largest and most tightly-controlled. St. Pierre et al. (20), measured liver R2 values in over 100 patients with iron overload. Liver R2 demonstrated a curvilinear relationship with iron, having a correlation coefficient of 0.98 (Figure 2). Prediction errors were comparable to those expected from the variability in liver biopsy (22,23). That study also demonstrated inter-exam variability of only 7% and good inter-machine reproducibility. This work ultimately led to Food and Drug Administration (FDA) approval of liver R2 analysis using the FerriScan® technique (http//www.ferriscan.com/) and increasing awareness of MRI’s utility for iron estimation.
FIGURE 2.
Plot of liver R2 vs. biopsied liver iron concentration in 104 iron overloaded patients [reprinted from (20) with permission].
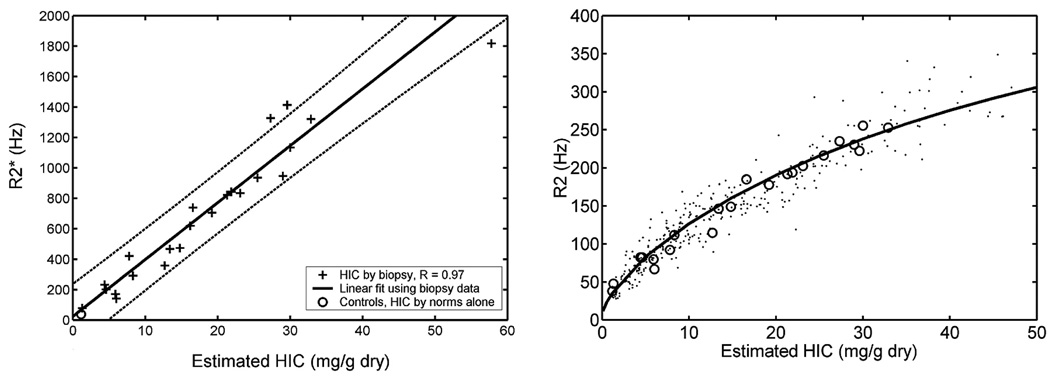
Preliminary liver R2* calibrations were published in 2001 by Anderson et al. (7), but had some technical and patient selection difficulties that limited accuracy. Shortly thereafter, our laboratory published results demonstrating that liver R2* tracked liver iron as R2, rising linearly with iron concentration (Figure 3A) (21). Using this calibration and simultaneous measurements of liver R2 and R2*, we were able to reproduce the R2 calibration curve demonstrated by St. Pierre et al. (20) (Figure 3B). This independent confirmation was convincing evidence that the MRI techniques translate across platforms and processing techniques.
FIGURE 3.
Left: plot of liver R2* vs. biopsied liver iron concentration. Open circle represents value estimated in normal control subjects. Right: plot of liver R2 vs. hepatic iron concentration estimated by liver biopsy (open circles) and by R2* estimate (filled dots). Curve represents the R2 calibration curve derived by St. Pierre et al. (20) [graphs reprinted from (21)].
Choices for Magnetic Resonance Imaging Estimation
Although iron estimation is conceptually straightforward, there are a number of important technical considerations: 1) R2 or R2* method, 2) choice of echo times, 3) model for data fitting, 4) use of a pixel-wise or region-based measurement, and 5) choice of field strength. 6) Target organ. Each point will be discussed in turn.
1. R2 or R2* Methods
As demonstrated in the previous section, either R2 or R2* can yield clinically-relevant assessments of hepatic iron. Table 1 summarizes the advantages and disadvantages of these techniques. R2* methods are significantly faster and easier. Newer techniques can even capture complete R2* information for multiple slices in a single breath-hold (24). Unfortunately, R2* measurements are vulnerable to large-scale distortions in the magnetic field produced by boundaries between materials having different magnetic susceptibility. The largest artifacts are caused by metal implants and air-tissue interfaces. One example is the lung-heart boundary. As a result, the inter-ventricular septum is most often used for cardiac R2* analysis, instead of the posterior wall, because it has blood on both sides of the measurement location. Another example is magnetic distortion introduced from excessive bowel gas which can obscure liver and pancreas imaging. Most of these artifacts are manageable through careful choice of a measurement region.
TABLE 1.
Properties of R2 and R2* Measurements
| R2* | R2 | |
|---|---|---|
| Validation | [++++] | [++++] |
| Speed | [++++] | [++] |
| Breath-hold | Yes | Sometimes |
| Motion sensitivity | [++ ] | [++++] |
| Susceptibility artifacts | [+++ ] | [+] |
R2 imaging is more robust to susceptibility artifacts, but images take longer to create. For exam, the FerriScan technique requires 5 min. per echo time and 25 min. per exam. Obviously, these examinations must be performed with the patient free-breathing and respiratory motion disrupts the image quality. Respiratory motion also compromises T2 measurements in the heart, although clinically diagnostic images can be obtained (25). Some investigators have used a sophisticated diaphragm “tracking” algorithm to reduce respiratory artifact, however these techniques are not universally available and image acquisition is fairly long compared with T2* methods (26).
2. Choice of Echo Times
Good T2 and T2* estimation requires sampling of echo times that “span” the range of expected T2, T2* values. Table 2 demonstrates the complete range of T2 and T2* values in the heart and liver as well as the TEs used for estimation at the Children’s Hospital of Los Angeles, Los Angeles, CA, USA. Ideally, one would like the longest TE to be approximately 2-fold longer than the longest T2 or T2*. However, this is generally not practical because T2* and T2 images degrade at longer echo times from motion artifacts. In fact, the maximum practical gradient echo time is around 18–20 ms, which is only half the T2* in normal hearts; this is one reason why inter-sequence and inter-machine agreement is poor for cardiac T2* longer than 20 ms (24,27,28). Fortunately, the measurements stabilize nicely for iron-loaded tissues and T2* is quite accurate and reproducible in the clinically relevant range (29,30).
TABLE 2.
Range of T2, T2* in the Heart and Typical Echo Times
| Heart | TEs | Liver | TEs | |
|---|---|---|---|---|
| T2 | 10–60 ms | 10, 20, 30, 40 ms | 2.5–60 ms | 3, 3.5, 5, 8, 12, 18, 30 ms |
| T2* | 1.5–50 ms | 2.2, 4.4 … 17.6 ms | 0.5–30 ms | 0.9–16 ms, log-spaced |
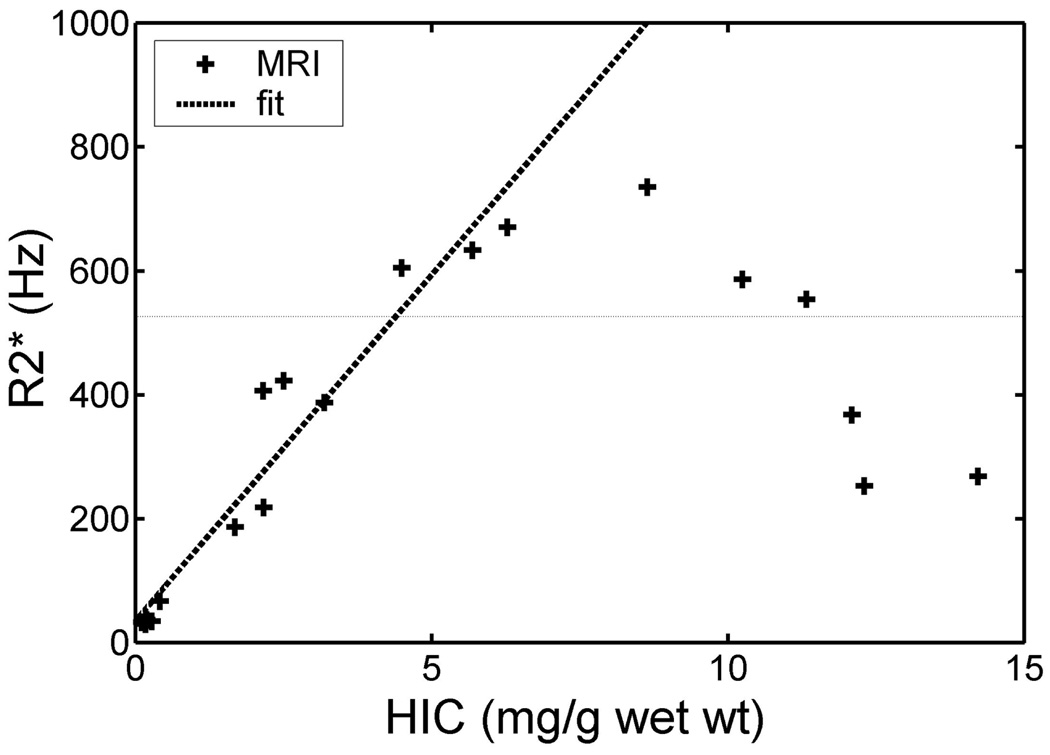
An even more important consideration is the choice of minimum echo time (28). This is usually limited by the hardware and software characteristics of individual MRI scanners. If a T2 or T2* method has too long a minimum echo time, most of the MRI signal will have irreversibly disappeared (28). However, some portions of the tissue will have less iron than others and will still have enough signal to generate an image. This resulting image, though, reflects only the iron concentration from the lightly loaded tissue and will badly underestimate the true tissue iron deposition. This phenomenon is illustrated in Figure 4. These data represent measured R2* values from gerbils that were progressively iron overload. The observed R2* tracks the liver iron concentration up to a R2* of 700 Hz, but falls off precipitously thereafter. A good rule of thumb is that the shortest measurable T2 or T2* is roughly 1.4 times lower than the shortest TE.
FIGURE 4.
Plot of hepatic R2* vs. chemically assayed iron concentration in gerbil liver. There is a linear relationship until R2* surpasses 700 Hz (approximately 1.33/TEmin.) whereupon there is catastrophic breakdown of the relationship [graphs reprinted from (16)].
3. Model for Data Fitting
The parameters T2 and T2* reflect exponential signal losses (image darkening), i.e., the signal may be described as the following:
| (1) |
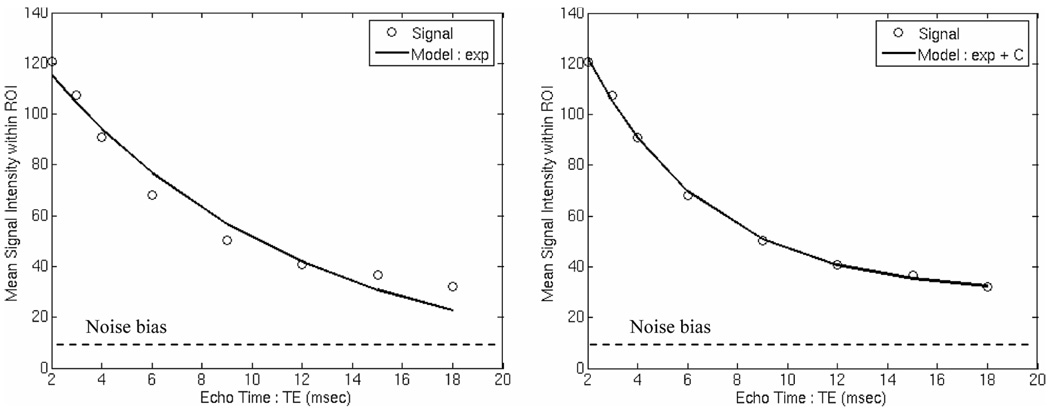
However, not all of the tissue has uniform iron concentration. In fact, each voxel in the image may have iron-dense and iron-sparse regions (such as blood or bile). While the iron-rich areas dominate, the iron-sparse tissues can contribute a small bias or offset in heavily iron loaded tissues. This principle is shown in Figure 5.
FIGURE 5.
Left: signal decay curve from the inter ventricular septum of a patient with heavy cardiac iron burden. Signal loss is prominent in early echo times but plateaus during the later echoes. Mono-exponential fit is poor but addition of an offset correction (right) corrects the problem [reprinted from (28)].
If this curve is fitted to a simple exponential function, the “tail” contributed by the low-iron tissues will distort the fit from the iron-rich tissues. Failure to account for this effect will lead to severe systematic overestimation of T2 and T2* values (28). The low-iron tissue distortion may be corrected three ways. Anderson et al. (7) limited the fit to early echo times using a visual assessment of goodness-of-fit. This has the advantage of having only 2 degrees of statistical freedom, but requires some subjective assessment (7). Clark et al. (31), fitted two separate exponentials, i.e.,
| (2) |
The advantage of this approach is that there is information regarding tissue structure in the second long T2 component. However, it has 4 degrees of statistical freedom, making it numerically unstable unless highly mathematically constrained. A compromise approach is used by our laboratory which assumes that contributions from “long” T2 component are essentially constant compared with the decay from the high iron tissue.
| (3) |
This approach is much more mathematically robust than a true biexponential fit, however fitting algorithms still require sensible parameter constraints and intelligent initial starting values. The constant is generally very close to zero (and can be forced to zero if desired) for T2* > around 10 ms. We recommend simplex algorithms (brute-force search methods) for their accuracy and robustness relative to least-squares fitting algorithms.
4. Use of a Pixel-Wise or Region-Based Measurement
Equations (1) through (3) can be applied in two fundamentally different manners. In a region-based fit, an operator draws a region of interest on one of the acquired images and all of the pixels within that region are averaged together. The region of interest is then propagated to the other echo times (either manually or automatically) and average values are calculated from those images as well. A single decay curve then results that generally reflect the average iron concentration in that region. This technique has the advantage that it is relatively quick and easy.
A pixel-wise calculation differs in that equations (1) through (3) are applied to every pixel in the region, yielding a complete T2 or T2* map. This process is computationally more intensive but has the advantage in that it can expose areas of artifact or pathology that might be missed using a region-based approach (20,21,32).
We compared the internal consistency and absolute accuracy of regional and pixel-wise analyses in actual and simulated cardiac T2* images (28). In general, the agreement was quite good, particularly for T2* larger than 5 ms (most of the clinically relevant range). Although we continue to use a pixel-wise approach, some established laboratories do not and the data are not compelling that it is necessary, at least in the heart.
5. Choice of Field Strength
Most clinical imaging is performed at 1.5 Tesla, although some older machines operate at 0.5 and 1.0 Tesla. Recently, 3 Tesla platforms have begun to be installed because of their superiority for certain neurological applications. Both R2 and R2* rise proportionally to field strength (33,34), so calibration curves developed at 1.5 Tesla do not apply to 3 Tesla. Storey et al. (35) recently demonstrated that liver and cardiac R2* images collected at 3 Tesla were almost exactly 2-fold higher than corresponding values measured at 1.5 Tesla.
While 3 Tesla imaging offers twice the signal to noise than 1.5 Tesla, there are two disadvantages for iron estimation. Firstly, susceptibility artifacts are significantly worse than at 1.5 Tesla, requiring good shimming. Secondly, the increasing signal decay cuts the maximum detectable iron by a factor of two. This is rarely problematic in the heart but liver iron levels may easily increase into the undetectable range.
6. Target Organ
Although MRI is most often used to estimate cardiac and hepatic iron, MRI can also been used to characterize tissue iron deposits in the brain, pituitary gland, bone marrow, kidney and pancreas (36–42). These approaches observe the same physical principles as cardiac and hepatic iron imaging but present different imaging challenges. For example, the pituitary is quite small and sits in a magnetically inhomogeneous environment; spin echo (R2) imaging is likely to perform better than gradient echo (R2*) methods. In contrast, splenic iron deposits are quite large and spin-echo (R2) techniques will not yield accurate iron estimates in this organ. The merits and limitations of these other techniques are beyond the scope of this manuscript, but several key references are included for interested readers (36–42).
CONCLUSIONS
Magnetic resonance imaging is a useful and readily available tool for non invasive iron estimation in multiple organs. This review outlines the principles and technical considerations behind robust T2 and T2* measurements, including choice of echo times, fitting techniques, and field strength. Readers are invited to contact the corresponding author with questions.
ACKNOWLEDGMENTS
This study was supported by the National Institutes of Health (NIH), Bethesda, MD, USA (RO1 HL075592-01A1), the General Clinical Research Center at the Children’s Hospital of Los Angeles (CHLA), Los Angeles, CA, USA (RR00043-43), Centers for Disease Control, Atlanta, GA, USA (Thalassemia Center Grant U27/CCI922106), Department of Health and Human Services, Bethesda, MD, USA, Novartis Pharma AG, Basel, Switzerland, the Wright Foundation, Los Angeles, CA, USA and the Guenther Foundation, Los Angeles, CA, USA.
Footnotes
Presented at the 16th International Conference on Chelation, Limassol, Cyprus, October 25–31 2006.
REFERENCES
- 1.Gordeuk VR, Bacon BR, Brittenham GM. Iron overload: causes and consequences. Annu Rev Nutr. 1987;7:485–508. doi: 10.1146/annurev.nu.07.070187.002413. [DOI] [PubMed] [Google Scholar]
- 2.McLaren GD, Muir WA, Kellermeyer RW. Iron overload disorders: natural history, pathogenesis, diagnosis, and therapy. Crit Rev Clin Lab Sci. 1983;19(3):205–266. doi: 10.3109/10408368309165764. [DOI] [PubMed] [Google Scholar]
- 3.Hershko C, Link G, Cabantchik I. Pathophysiology of iron overload. Ann NY Acad Sci. 1998;850:191–201. doi: 10.1111/j.1749-6632.1998.tb10475.x. [DOI] [PubMed] [Google Scholar]
- 4.Nielsen P, Gunther U, Durken M, Fischer R, Dullmann J. Serum ferritin iron in iron overload and liver damage: correlation to body iron stores and diagnostic relevance. J Lab Clin Med. 2000;135(5):413–418. doi: 10.1067/mlc.2000.106456. [DOI] [PubMed] [Google Scholar]
- 5.Borgna-Pignatti C, Castriota-Scanderbeg A. Methods for evaluating iron stores and efficacy of chelation in transfusional hemosiderosis. Haematologica. 1991;76(5):409–413. [PubMed] [Google Scholar]
- 6.Chapman RW, Hussain MA, Gorman A, Laulicht M, Politis D, Flynn DM, Sherlock S, Hoffbrand AV. Effect of ascorbic acid deficiency on serum ferritin concentration in patients with β-thalassemia major and iron overload. J Clin Pathol. 1982;35(5):487–491. doi: 10.1136/jcp.35.5.487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Anderson LJ, Holden S, Davis B, Prescott E, Charrier CC, Bunce NH, Firmin DN, Wonke B, Porter J, Walker JM, Pennell DJ. Cardiovascular T2-star (T2*) magnetic resonance for the early diagnosis of myocardial iron overload. Eur Heart J. 2001;22(23):2171–2179. doi: 10.1053/euhj.2001.2822. [DOI] [PubMed] [Google Scholar]
- 8.Wood JC, Tyszka JM, Ghugre N, Carson S, Nelson MD, Coates TD. Myocardial iron loading in transfusion-dependent thalassemia and sickle-cell disease. Blood. 2004;103(5):1934–1936. doi: 10.1182/blood-2003-06-1919. [DOI] [PubMed] [Google Scholar]
- 9.Brittenham GM, Griffith PM, Nienhuis AW, McLaren CE, Young NS, Tucker EE, Allen CJ, Farrell DE, Harris JW. Efficacy of deferoxamine in preventing complications of iron overload in patients with thalassemia major. N Engl J Med. 1994;331(9):567–573. doi: 10.1056/NEJM199409013310902. [DOI] [PubMed] [Google Scholar]
- 10.Angelucci E, Brittenham GM, McLaren CE, Ripalti M, Baronciani D, Giardini C, Galimberti M, Polchi P, Lucarelli G. Hepatic iron concentration and total body iron stores in thalassemia major. N Engl J Med. 2000;343(5):327–331. doi: 10.1056/NEJM200008033430503. [DOI] [PubMed] [Google Scholar]
- 11.Telfer PT, Prestcott E, Holden S, Walker M, Hoffbrand AV, Wonke B. Hepatic iron concentration combined with long-term monitoring of serum ferritin to predict complications of iron overload in thalassaemia major. Br J Haematol. 2000;110(4):971–977. doi: 10.1046/j.1365-2141.2000.02298.x. [DOI] [PubMed] [Google Scholar]
- 12.Anderson LJ, Westwood MA, Prescott E, Walker JM, Pennell DJ, Wonke B. Development of thalassaemic iron overload cardiomyopathy despite low liver iron levels and meticulous compliance to desferrioxamine. Acta Haematol. 2006;115(1–2):106–108. doi: 10.1159/000089475. [DOI] [PubMed] [Google Scholar]
- 13.Angelucci E, Baronciani D, Lucarelli G, Baldassarri M, Galimberti M, Giardini C, Martinelli F, Polchi P, Polizzi V, Ripalti M. Needle liver biopsy in thalassaemia: analyses of diagnostic accuracy and safety in 1184 consecutive biopsies. Br J Haematol. 1995;89(4):757–761. doi: 10.1111/j.1365-2141.1995.tb08412.x. [DOI] [PubMed] [Google Scholar]
- 14.Fischer R, Longo F, Nielsen P, Engelhardt R, Hider RC, Piga A. Monitoring long-term efficacy of iron chelation therapy by deferiprone and desferrioxamine in patients with β-thalassaemia major: application of SQUID biomagnetic liver susceptometry. Br J Haematol. 2003;121(6):938–948. doi: 10.1046/j.1365-2141.2003.04297.x. [DOI] [PubMed] [Google Scholar]
- 15.Stark DD, Bass NM, Moss AA, Bacon BR, McKerrow JH, Cann CE, Brito A, Goldberg HI. Nuclear magnetic resonance imaging of experimentally induced liver disease. Radiology. 1983;148(3):743–751. doi: 10.1148/radiology.148.3.6192464. [DOI] [PubMed] [Google Scholar]
- 16.Wood JC, Otto-Duessel M, Aguilar M, Nick H, Nelson MD, Coates TD, Pollack H, Moats R. Cardiac iron determines cardiac T2*, T2, and T1 in the gerbil model of iron cardiomyopathy. Circulation. 2005;112(4):535–543. doi: 10.1161/CIRCULATIONAHA.104.504415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Anderson LJ, Westwood MA, Holden S, Davis B, Prescott E, Wonke B, Porter JB, Walker JM, Pennell DJ. Myocardial iron clearance during reversal of siderotic cardiomyopathy with intravenous desferrioxamine: a prospective study using T2* cardiovascular magnetic resonance. Br J Haematol. 2004;127(3):348–355. doi: 10.1111/j.1365-2141.2004.05202.x. [DOI] [PubMed] [Google Scholar]
- 18.Ghugre NR, Coates TD, Nelson MD, Wood JC. Mechanisms of tissue-iron relaxivity: nuclear magnetic resonance studies of human liver biopsy specimens. Magn Reson Med. 2005;54(5):1185–1193. doi: 10.1002/mrm.20697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wood JC, Fassler J, Meade T. Mimicking liver iron overload using liposomal ferritin preparations. Magn Reson Med. 2004;51(3):607–611. doi: 10.1002/mrm.10735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.St. Pierre TG, Clark PR, Chua-anusorn W, Fleming AJ, Jeffrey GP, Olynyk JK, Pootrakul P, Robins E, Lindeman R. Noninvasive measurement and imaging of liver iron concentrations using proton magnetic resonance. Blood. 2005;105(2):855–861. doi: 10.1182/blood-2004-01-0177. [DOI] [PubMed] [Google Scholar]
- 21.Wood JC, Enriquez C, Ghugre N, Tyzka JM, Carson S, Nelson MD, Coates TD. MRI R2 and R2* mapping accurately estimates hepatic iron concentration in transfusion-dependent thalassemia and sickle cell disease patients. Blood. 2005;106(4):1460–1465. doi: 10.1182/blood-2004-10-3982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Villeneuve JP, Bilodeau M, Lepage R, Cote J, Lefebvre M. Variability in hepatic iron concentration measurement from needle-biopsy specimens. J Hepatol. 1996;25(2):172–177. doi: 10.1016/s0168-8278(96)80070-5. [DOI] [PubMed] [Google Scholar]
- 23.Ambu R, Crisponi G, Sciot R, Van Eyken P, Parodo G, Iannelli S, Marongiu F, Silvagni R, Nurchi V, Costa V. Uneven hepatic iron and phosphorus distribution in β-thalassemia. J Hepatol. 1995;23(5):544–549. doi: 10.1016/0168-8278(95)80060-3. [DOI] [PubMed] [Google Scholar]
- 24.Westwood M, Anderson LJ, Firmin DN, Gatehouse PD, Charrier CC, Wonke B, Pennell DJ. A single breath-hold multiecho T2* cardiovascular magnetic resonance technique for diagnosis of myocardial iron overload. J Magn Reson Imaging. 2003;18(1):33–39. doi: 10.1002/jmri.10332. [DOI] [PubMed] [Google Scholar]
- 25.Mavrogeni SI, Maris T, Gouliamos A, Vlahos L, Kremastinos DT. Myocardial iron deposition in β-thalassemia studied by magnetic resonance imaging. Int J Cardiovasc Imaging. 1998;14(2):117–122. doi: 10.1023/a:1005922016048. [DOI] [PubMed] [Google Scholar]
- 26.Voskaridou E, Douskou M, Terpos E, Papassotiriou I, Stamoulakatou A, Ourailidis A, Loutradi A, Loukopoulos D. Magnetic resonance imaging in the evaluation of iron overload in patients with β thalassaemia and sickle cell disease. Br J Haematol. 2004;126(5):736–742. doi: 10.1111/j.1365-2141.2004.05104.x. [DOI] [PubMed] [Google Scholar]
- 27.Westwood MA, Anderson LJ, Firmin DN, Gatehouse PD, Lorenz CH, Wonke B, Pennell DJ. Inter-scanner reproducibility of cardiovascular magnetic resonance T2* measurements of tissue iron in thalassemia. J Magn Reson Imaging. 2003;18(5):616–620. doi: 10.1002/jmri.10396. [DOI] [PubMed] [Google Scholar]
- 28.Ghugre NR, Enriquez CM, Coates TD, Nelson MD, Jr, Wood JC. Improved R2* measurements in myocardial iron overload. J Magn Reson Imaging. 2006;23(1):9–16. doi: 10.1002/jmri.20467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Westwood MA, Firmin DN, Gildo M, Renzo G, Stathis G, Markissia K, Vasili B, Pennell DJ. Intercentre reproducibility of magnetic resonance T2* measurements of myocardial iron in thalassaemia. Int J Cardiovasc Imaging. 2005;21(5):531–538. doi: 10.1007/s10554-005-0651-2. [DOI] [PubMed] [Google Scholar]
- 30.Tanner MA, He T, Westwood MA, Firmin DN, Pennell DJ. Multi-center validation of the transferability of the magnetic resonance T2* technique for the quantification of tissue iron. Haematologica. 2006;91(10):1388–1391. [PubMed] [Google Scholar]
- 31.Clark PR, Chua-anusorn W, St. Pierre TG. Bi-exponential proton transverse relaxation rate (R2) image analysis using RF field intensity-weighted spin density projection: potential for R2 measurement of iron-loaded liver. Magn Reson Imaging. 2003;21(5):519–530. doi: 10.1016/s0730-725x(03)00080-8. [DOI] [PubMed] [Google Scholar]
- 32.Clark PR, St. Pierre TG. Quantitative mapping of transverse relaxivity (1/T(2)) in hepatic iron overload: a single spin-echo imaging methodology. Magn Reson Imaging. 2000;18(4):431–438. doi: 10.1016/s0730-725x(00)00118-1. [DOI] [PubMed] [Google Scholar]
- 33.Bulte JW, Miller GF, Vymazal J, Brooks RA, Frank JA. Hepatic hemosiderosis in non-human primates: quantification of liver iron using different field strengths. Magn Reson Med. 1997;37(4):530–536. doi: 10.1002/mrm.1910370409. [DOI] [PubMed] [Google Scholar]
- 34.Yablonskiy DA, Haacke EM. Theory of NMR signal behavior in magnetically inhomogeneous tissues: the static dephasing regime. Magn Reson Med. 1994;32(6):749–763. doi: 10.1002/mrm.1910320610. [DOI] [PubMed] [Google Scholar]
- 35.Storey P, Thompson AA, Carqueville CL, Wood JC, de Freitas RA, Rigsby CK. R2* imaging of transfusional iron burden at 3T and comparison with 1.5T. J Magn Reson Imaging. 2007;25(3):540–547. doi: 10.1002/jmri.20816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Argyropoulou MI, Metafratzi Z, Kiortsis DN, Bitsis S, Tsatsoulis A, Efremidis S. T2 relaxation rate as an index of pituitary iron overload in patients with β-thalassemia major. Am J Roentgenol. 2000;175(6):1567–1569. doi: 10.2214/ajr.175.6.1751567. [DOI] [PubMed] [Google Scholar]
- 37.Sparacia G, Banco A, Midiri M, Iaia A. MR imaging technique for the diagnosis of pituitary iron overload in patients with transfusion-dependent β-thalassemia major. Am J Neuroradiol. 1998;19(10):1905–1907. [PMC free article] [PubMed] [Google Scholar]
- 38.Miszkiel KA, Paley MN, Wilkinson ID, Hall-Craggs MA, Ordidge R, Kendall BE, Miller RF, Harrison MJ. The measurement of R2, R2* and R2’ in HIV-infected patients using the prime sequence as a measure of brain iron deposition. Magn Reson Imaging. 1997;15(10):1113–1119. doi: 10.1016/s0730-725x(97)00089-1. [DOI] [PubMed] [Google Scholar]
- 39.Graham JM, Paley MN, Grunewald RA, Hoggard N, Griffiths PD. Brain iron deposition in Parkinson’s disease imaged using the PRIME magnetic resonance sequence. Brain. 2000;123(Pt 12):2423–2431. doi: 10.1093/brain/123.12.2423. [DOI] [PubMed] [Google Scholar]
- 40.Isokawa M, Kimura F, Matsuki T, Omoto E, Otsuka K, Kurokawa H, Togami I, Hiraki Y, Kimura I, Harada M. Evaluation of bone marrow iron by magnetic resonance imaging. Ann Hematol. 1997;74(6):269–274. doi: 10.1007/s002770050298. [DOI] [PubMed] [Google Scholar]
- 41.Drakonaki E, Papakonstantinou O, Maris T, Vasiliadou A, Papadakis A, Gourtsoyiannis N. Adrenal glands in β-thalassemia major: magnetic resonance (MR) imaging features and correlation with iron stores. Eur Radiol. 2005;15(12):2462–2468. doi: 10.1007/s00330-005-2855-1. [DOI] [PubMed] [Google Scholar]
- 42.Ooi GC, Khong PL, Chan GC, Chan KN, Chan KL, Lam W, Ng I, Ha SY. Magnetic resonance screening of iron status in transfusion-dependent β-thalassaemia patients. Br J Haematol. 2004;124(3):385–390. doi: 10.1046/j.1365-2141.2003.04772.x. [DOI] [PubMed] [Google Scholar]