Abstract
Rationale
Nicotine serves as a primary reinforcer but also potently enhances responding for non-nicotine stimuli with reinforcing properties. One of the most successful pharmacotherapies for smoking cessation, bupropion, also increases responding for non-drug reinforcers such as food and brain stimulation rewards.
Objective
The present studies investigated whether treatment with bupropion and nicotine had similar effects on responding for a reinforcing visual stimulus (VS). They also investigated whether the effects of bupropion and nicotine depended on common pharmacological substrates.
Results
Nicotine (0.4 mg/kg base) enhanced responding for the VS and this enhancing effect increased across testing sessions, replicating our previous findings. Bupropion (3, 10, and 30 mg/kg salt) dose-dependently increased responding for the VS. Treatment with 10 and 30 mg/kg bupropion resulted in a profile similar to nicotine; operant responding increased over repeated drug treatments. The reinforcement enhancing effect of nicotine, but not bupropion, was blocked by pretreatment with the nicotinic acetylcholine receptor antagonist mecamylamine. In contrast, the reinforcement enhancing effect of bupropion, but not nicotine, was blocked by pretreatment with the alpha noradrenergic antagonist prazosin.
Conclusion
The reinforcement enhancing effects of nicotine and bupropion increased over time and repeated treatments suggesting a shared mechanism of action. However, the reinforcement enhancing effects of nicotine are mediated by nicotinic acetylcholine receptors, whereas the reinforcement enhancing effects of bupropion were mediated by alpha noradrenergic receptors.
INTRODUCTION
Although sustained release bupropion (Zyban SR®) has enjoyed clinical success as a smoking cessation aid (Hurt et al. 1997; Jorenby et al. 1999), the specific reasons for this success remain elusive. Pre-clinical studies have demonstrated that bupropion and nicotine have similar subjective effects (Besheer et al. 2004), both drugs are psychomotor stimulants (Bevins and Palmatier 2003; Wilkinson and Bevins 2007), both serve as primary reinforcers in non-human subjects (Chaudhri et al. 2006a; Tella et al. 1997) and both compounds increase the concentration of extracellular catecholamines in midbrain limbic regions (see Balfour et al. 2000; Dwoskin et al. 2006 for review). Thus, the clinical efficacy of bupropion may depend on its ability to ‘replace’ some of the subjective, psychomotor, and/or reinforcement related effects of nicotine (Warner and Shoaib 2005).
In addition to being primary reinforcers, nicotine and other psychomotor stimulants (i.e., cocaine and amphetamine) have a second spectrum of reinforcement-related effects: they can increase responding for reinforcing non-drug stimuli (Chaudhri et al. 2006b; Palmatier et al. 2007a; Robbins 1976). For example, nicotine (Donny et al. 2003; Palmatier et al. 2007b) cocaine (Chaudhri et al. 2006b) and amphetamine (Glow and Russell 1973; Robbins 1977) can increase responding for visual and auditory stimuli with unconditioned and conditioned reinforcing properties.
Several recent findings suggest that bupropion also increases responding for non-drug reinforcers. Bupropion has been shown to increase responding for food on a progressive ratio schedule of reinforcement (Bruijnzeel and Markou, 2003), lower reward threshold for intracranial self-stimulation (Cryan et al., 2003) and increase cue-induced reinstatement of nicotine-seeking behavior (Liu et al., 2008). In addition, several studies suggest that bupropion can increase responding for nicotine infusions (Rauhut et al. 2003; Shoaib et al. 2003; Stairs and Dworkin 2008), an effect that seems at odds with smoking cessation. Interestingly, in each of these studies, nicotine infusions were delivered in conjunction with a sensory stimulus (infusion/time-out ‘cues’). Hence, the increase in nicotine self-administration may be attributable to the reinforcement-enhancing effects of bupropion on responding for the cues associated with nicotine.
Nicotine and bupropion share two pharmacological actions that may lead to increased responding for other primary rewards. First, both drugs directly affect nicotinic acetylcholine receptors (nAChRs). Although the drugs have opposing effects at these receptors (nicotine serves as an agonist, whereas bupropion is an antagonist, see Fryer and Lukas 1999; Slemmer et al. 2000), nicotine can desensitize nAChRs, and some of the behavioral and physiological effects of nicotine have been attributed to nicotine-induced decreases in nAChR function (Picciotto et al. 2008). Second, nicotine and bupropion also increase the post-synaptic effects of norepinephrine (NE) and dopamine (DA) in midbrain limbic regions (Balfour et al. 2000; Chaudhri et al. 2006b; Dwoskin et al. 2006; Fu et al. 1998; Fu et al. 2003). Increased catecholamine activity in these regions is widely regarded as a necessary component of appetitive and goal-directed behaviors (Robinson and Berridge 1993; Ventura et al. 2003). A shared or comparable ability to enhance responding for non-drug reinforcement may depend on these latter effects of bupropion and nicotine.
The first goal of the present research was to investigate the putative reinforcement enhancing effects of bupropion without the complication of having two or more reinforcers associated with a single operant. A second goal was to compare the reinforcement enhancing effects of bupropion to those of nicotine. Based on previous research we hypothesized that bupropion would enhance responding for a sensory reinforcer in a dose-dependent manner that was comparable to nicotine. A third goal of these studies was to investigate pharmacological substrates of bupropion- and nicotine-enhanced reinforcement. In order to investigate the role of nAChRs, rats were challenged with the non-selective antagonist mecamylamine. Based on previous findings (Palmatier et al. 2007a), we predicted that the reinforcement enhancing effects nicotine, but not bupropion, would be blocked by mecamylamine. We also investigated the role of NE receptors with prazosin and propranolol. Prazosin is a non-selective competitive antagonist at α1-NE receptors, with some affinity for post-synaptic α2-NE receptors (Ordway et al. 1993). Propranolol is a competitive antagonist for β-adrenergic receptors, and non-selective across subtypes (Nahorski 1976). We hypothesized that the reinforcement enhancing effects of bupropion would depend on noradrenergic receptors because the NE system has frequently been implicated in the rewarding and reinforcing effects of psychomotor stimulants (Ventura et al. 2003; Zhang and Kosten 2005) and prazosin can reduce the psychomotor stimulant effects of bupropion in mice (Martin et al. 1990). However, since nicotine self-administration is usually reduced by increasing synaptic NE (Coen et al. 2009; Rauhut et al. 2002), we predicted that blocking post-synaptic NE receptors would not reduce the reinforcement enhancing effect of nicotine.
METHOD
General Method
Subjects
Male Sprague-Dawley rats (Harlan Farms, IN) weighing 174–200 g on arrival were housed individually in hanging wire mesh cages. Rats were housed in a temperature- and humidity-controlled colony room on a reverse 12:12 h light:dark cycle. Unrestricted access to food and water was allowed for 3 days after their arrival. Food access was subsequently restricted to 20 g per day allowing limited growth (approximately 20 g/week) throughout the remainder of the study (Donny et al. 1995).
Apparatus
Experimental sessions were conducted in 30 operant conditioning chambers (BRS/LVE Model RTC-020, MD; see Donny et al. 1995 for further details) measuring 25 × 31 × 28 (w × l × h) cm. One wall of each chamber was equipped with two retractable levers and stimulus lights located above each lever. A pellet dispenser and food trough were located between the two levers, approximately 2 cm from the floor of the chamber. A houselight fixture was located above the food trough, approximately 1 cm from the top of the chamber. A white house light was illuminated at the beginning and extinguished at the end of each session. The sensory reinforcer (visual stimulus or VS) consisted of 1 s illumination of the stimulus light located directly above the active lever followed by 1 min extinction of the houselight. We have previously established that the VS has moderate reinforcing properties which are potently enhanced by pre-session nicotine administration (Palmatier et al. 2007c)
Drugs
Nicotine hydrogen tartrate salt, bupropion hydrochloride, and (±)-propranolol hydrochloride (Sigma, St. Louis, MO) were dissolved in 0.9% saline. Prazosin hydrochloride (Sigma, St. Louis, MO) was dissolved in distilled water. Subcutaneous (sc) nicotine injections and intraperitoneal (ip) bupropion and propranolol injections were delivered at a volume of 1 ml/kg. Prazosin was injected at a volume of 2 ml/kg, sc. Nicotine solution pH was adjusted to 7.0 (±0.2) with dilute NaOH and dose (0.4 mg/kg) was calculated from the base form. All other drug doses were calculated from the salt form. Mecamylamine doses were derived from our previous studies investigating the reinforcement enhancing effects of nicotine (Liu et al. 2007; Palmatier et al. 2007a). Higher doses were not tested due to the potential for non-cholinergic action of mecamylamine at micromolar brain concentrations (O’Dell and Christensen 1988). Doses of prazosin and propranolol were derived from previous studies investigating the effects of these antagonists on the behavioral effects of nicotine (Hahn and Stolerman 2005; Villegier et al. 2007), cocaine (Zhang and Kosten 2005) and amphetamine (Dickinson et al. 1988; Yokel and Wise 1976).
Procedure
A schematic representation of experimental procedures, dose conditions, and the number of subjects in each condition are presented in Table 1.
Table 1.
Schematic representation of groups, number of subjects, and treatments from each experiment.
| Group | n | Experiment 1 | Experiment 2 | ||
|---|---|---|---|---|---|
| Acquisition (Sessions 1–23) | Drug Pretreatment (Sessions 24–30) | nAChR (Sessions 31–35) | NE-R (Sessions 41–52) | ||
| SAL | 8 | Active Lever: VS (FR1, FR2, FR5) | SAL (IP/SC) | 0, 0.05, 0.1 mg/kg mecamylamine | 0, 0.1, 0.3, 1 mg/kg prazosin |
| 0.4 NIC | 8 | 0.4 NIC | |||
| 3 BUP | 9 | 3 BUP | 0, 2.5, 5, 10 mg/kg propranolol | ||
| 10 BUP | 9 | 10 BUP | |||
| 30 BUP | 8 | 30 BUP | |||
Phase 1: Reinforcement Enhancing Effects of Bupropion & Nicotine
Food Shaping & Acquisition of Stimulus Reinforcement
The first 5 sessions were food auto-shaping and preference-testing sessions, the procedures have been described in detail elsewhere (Palmatier et al. 2006). Briefly, rats were shaped to associate individual lever-extensions with a food reward. After rats met a minimum criterion of 25 total reinforced lever-press responses an extinction test examined unconditional side preferences. Lever (active vs. inactive) was then randomly assigned to each subject for the subsequent stimulus reinforcement sessions, with the constraint that side preference was balanced in each drug condition. Stimulus reinforcement tests were carried out in 60-min sessions over 23 consecutive days. Meeting the fixed ratio (FR) schedule on the active lever resulted in presentation of the moderately reinforcing VS. The fixed ratio schedule was increased from 1 to 2 and subsequently 5 responses across test sessions when rats met a stability criterion under each ratio (≤10% variation in response rate and reinforcers earned for 3 consecutive sessions). As a result, a fixed ratio 1 (FR1) schedule was in force from sessions 1–5, an FR2 was in force from sessions 6–12, and an FR5 was in force from sessions 13–23.
Bupropion & Nicotine Pretreatment Tests
After stable responding was established under the FR5 schedule, rats were randomly assigned to one of five groups according to the bupropion dose (0, 3, 10, or 30 mg/kg BUP) or nicotine comparison condition (0.4 mg/kg NIC). BUP rats were injected ip with the appropriate solution 30 min before each subsequent test session, NIC rats were injected sc 5-min before testing. The nicotine dose was selected based on previous studies from our laboratory suggesting that this dose/route combination produces robust enhancing effects (Palmatier et al. 2007c). Bupropion doses were selected based on previous studies examining the effects of bupropion on operant behavior (Bruijnzeel and Markou 2003; Paterson et al. 2008). Testing occurred over seven consecutive days in order to provide acute (Day 1) and chronic (Day 7) measures of reinforcement enhancing effects. Previous studies suggest that the reinforcement enhancing effects of sc nicotine become asymptotic over this test period, an effect reminiscent of psychomotor stimulant sensitization (Palmatier et al. 2007c).
Phase 2: Pharmacological Substrates of Enhanced Reinforcement
nACh Receptor Antagonist Tests
All rats/groups from Experiment 1 were pretreated with mecamylamine (0, 0.5, or 1 mg/kg) to determine if non-specific blockade of nAChRs altered the reinforcement enhancing effects of bupropion and to confirm our previous finding that pretreatment with mecamylamine (1 mg/kg) abolishes the reinforcement enhancing effect of nicotine (Palmatier et al. 2007a). Testing took place over 5 consecutive sessions in which mecamylamine or vehicle was injected 45-min before testing; the appropriate dose of BUP or NIC was injected 15 or 40 min after mecamylamine pretreatment, respectively. Sessions 31, 33, and 35 were vehicle tests, responses and reinforcers earned were monitored for any long-term or progressive effects of mecamylamine. No progressive effects of mecamylamine treatment were observed (ps≥0.37), so data from these tests were averaged for analyses (0 mg/kg mecamylamine). Sessions 32 and 34 were mecamylamine tests, dose (0.5 or 1 mg/kg) was randomized and balanced within each group.
NE Receptor Antagonist Tests
To further characterize the reinforcement enhancing effects of bupropion and nicotine, additional pretreatment tests included prazosin (0.1, 0.3, or 1 mg/kg) and propranolol (2.5, 5, or 10 mg/kg). Before NE antagonist testing began, all rats received 5 sessions (36–40) with only pre-session injections of their assigned BUP or NIC test solution in order to re-establish the 3-day performance criteria (≤10% variation in responses/reinforcers earned) and prevent any carry-over effect of mecamylamine treatment. NE receptor antagonist tests were conducted over 12 consecutive sessions. Prazosin or propranolol was administered on ‘even’ test days (Sessions 42, 44, 46, etc.) 45 min before testing sessions; compound and dose were assigned by Latin Square. Intervening saline tests (Sessions 41, 43, 45, etc.) were monitored for carryover or progressive effects of the antagonists. Since no carryover or progressive effect of antagonist treatment was found on the vehicle test sessions (ps≥0.46), the dependent measures were averaged across sessions and served as the 0 mg/kg control for both compounds. Doses of each drug were chosen from previous studies and were not expected to have non-specific effects on motor activity or operant performance (e.g., Zhang and Kosten 2005).
Data Analyses
Responding on the active lever served as the main dependent variable. Where appropriate, inactive lever responses were included or analyzed separately. Analyses of variance (ANOVAs) were the primary significance tests. During Phase 1, mixed-factors ANOVAs included the linear effect of Session and Lever as within subject factors and Group (NIC, 0, 3, 10, and 30 BUP) as the between subjects factor. Significant effects involving more than two Groups were explored using pairwise comparisons. For Phase 1, pairwise ANOVAs were used to assess main effects of Group, the linear effect of Session, and Group by Session interactions. Additional t tests (with Bonferroni correction) were conducted to specify the group differences at the start and end of the study (i.e., Days 1 and 7 of treatment), as illustrated graphically in Figure 2c. . During Phase 2, mixed factors ANOVAs included Dose (antagonist treatment dose) as a within subjects factor, Group was the between subjects factor. For Phase 2, which did not assess change in responding over time, pairwise comparisons were made with t-tests using Bonferroni’s correction. All t tests are reported as ts(df) and include corrected p-values (calculated p multiplied by the number of comparisons).
Figure 2.
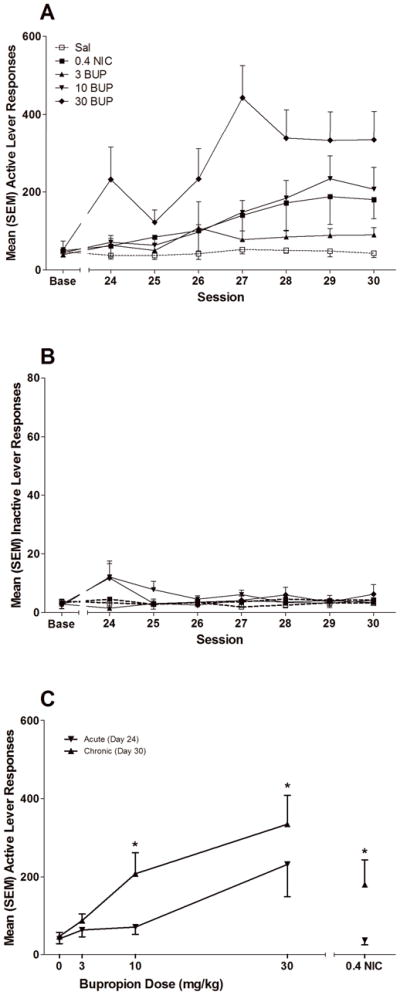
illustrates operant responding during the Bupropion and Nicotine Pretreatment Phase. Panel A illustrates active lever responses over testing sessions for rats in the five treatment groups. Panel B illustrates inactive lever responses over testing sessions for each group. Panel C illustrates the effects of acute (first drug-pretreatment test) and chronic (seventh drug pretreatment test) drug administration on active lever responding in each drug/dose condition. * indicates that responding in chronic test differs significantly from responding on the acute test, p<0.05.
RESULTS
Phase 1: Reinforcement Enhancing Effects of Bupropion & Nicotine
Acquisition of Stimulus Reinforcement (Sessions 1–23)
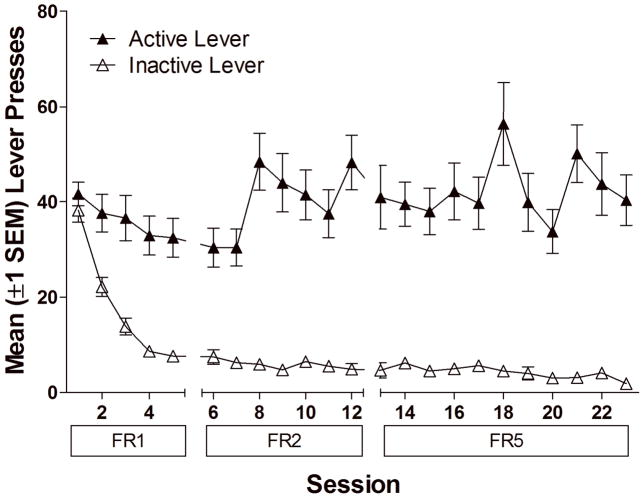
Despite equal training on both levers, responding on the active lever was higher than responding on the inactive lever across the acquisition phase (main effect of Lever for FR1, FR2 and FR5 schedules, p<0.01), demonstrating that the VS served as a primary reinforcer (Figure 1).
Figure 1.
illustrates operant responding over testing sessions during the Stimulus Reinforcement Phase. Closed symbols represent active lever responses (responses leading to the visual stimulus or VS), open symbols represent responses on the inactive lever (responses with no programmed consequences). As illustrated in the figure, responses producing the VS remained high, while inactive lever presses decreased over testing sessions.
Bupropion & Nicotine Pretreatment Tests (Sessions 24–30)
Both nicotine and bupropion increased responding at the active lever preferentially and the enhancing effect of bupropion was systematically related to bupropion dose. The two-way ANOVA confirmed that active lever responding was high than inactive lever responding during this phase (p<0.01), but the rate of active lever responding increased linearly with Session (p<0.01) and depended on Group (p<0.01); there was also a significant interaction between Group and the linear change over Sessions (p<0.05, Figure 2a–b). For inactive lever responding, the effects of Session (p=0.275, Group (p=0.367), and the Group × Session interaction (p=0.379) all failed to reach significance.
T tests were used to illustrate the effects of nicotine and bupropion relative to saline on active lever responding on sessions 24 and 30 (Day 1 and Day 7 of drug pretreatment) and additional pairwise ANOVAs with linear contrasts were conducted to assess the pattern of change in active lever responding over sessions relative to saline. Active lever responding in both the NIC and BUP groups failed to significantly differ from saline on Session 24, although BUP pretreatment revealed a non-significant trend for increased responding (Fig. 2a). However, group differences emerged over repeated sessions. With the exception of 3 mg/kg BUP, there was a significant linear increase in the active lever drug conditions (0.4 mg/kg NIC, p<0.01; 10 mg/kg BUP, p<0.01; 30 mg/kg BUP, p<0.01). This increase contributed to significant differences in active lever responding between the saline group and the 0.4 mg/kg NIC [t(13)=2.843, p=0.014], 3 mg/kg BUP [t(10.913)=2.297, p=0.042], 10 mg/kg BUP [t(7.477)=2.786, p=0.025] and 30 mg/kg BUP [t(13)=5.159, p<0.001] groups on Day 30 as illustrated by a leftward/upward shift in the dose-response curve in Figure 2c. These findings indicate that the acute effects of nicotine and bupropion were marginal, but a significant reinforcement-enhancing effect of both drugs emerged after repeated testing.
Phase 2: Pharmacological Substrates of Enhanced Reinforcement
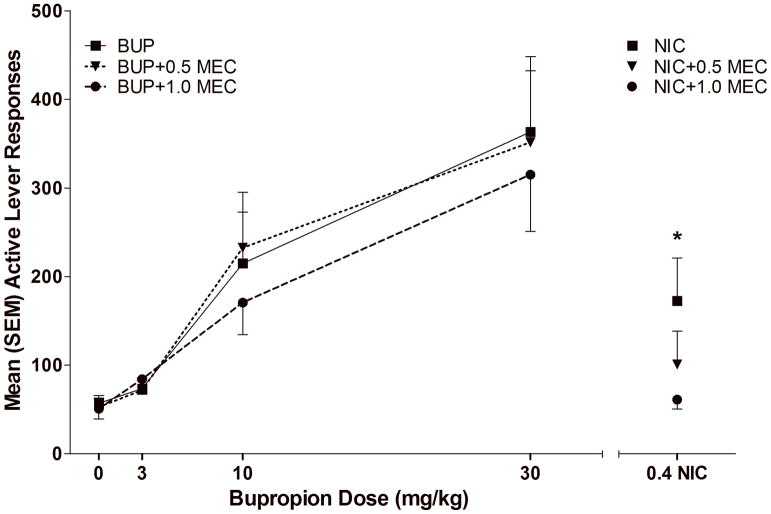
nACh Receptor Antagonist Challenge (Sessions 31–35)
Mecamylamine (0.5 and 1.0 mg/kg) reduced the reinforcement enhancing effects of nicotine but did not alter the reinforcement enhancing effects of bupropion. The two-way ANOVA revealed that active lever responding decreased as a function of Group (p<0.001); there was also a Group × Dose interaction (p<0.001). Paired t-tests investigating the interaction revealed a significant decrease in active lever responding when rats in the NIC group were pretreated with both 0.5 [t(6)=2.537, p<0.05] and 1.0 mg/kg MEC [t(6)=3.255, p<0.05, (Figure 3)]. There were no significant effects of MEC on active lever responding for the 0, 3, 10 or 30 mg/kg BUP groups (ps≥0.45). Furthermore, in each case, the decrease in VS responding associated with MEC (0.5 or 1.0 mg/kg) was greater in NIC than BUP pre-treated animals (ps<.05).
Figure 3.
illustrates active lever responding in each drug/dose condition during the nACh Receptor Antagonist Challenge Tests. * indicates that 0.5 and 1 mg/kg mecamylamine pretreatment significantly reduced responding for the NIC group, ps<0.05.
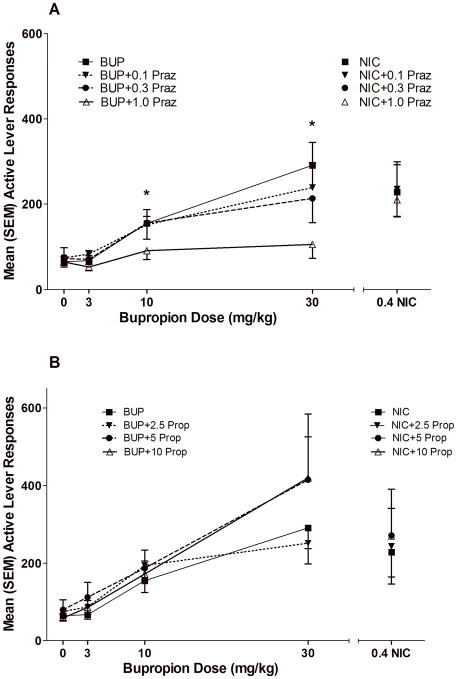
NE Receptor Antagonist Challenge (Sessions 41–52)
Prazosin (PRAZ; 0.1, 0.3 and 1.0 mg/kg) dose-dependently decreased the reinforcement enhancing effects of bupropion, but did not alter the reinforcement enhancing effects of nicotine. The two-way ANOVA revealed a main effect of main effect of Dose (p<0.001) on active lever responding, but this effect was highly dependent on Group (Dose × Group interaction, p<0.001). Pairwise comparisons revealed a significant decrease in active lever responding in the 10 [t(7)=3.613, p<0.01] and 30 mg/kg [t(6)=5.693, p=0.001] BUP groups for the highest dose of PRAZ (1.0 mg/kg). There were no significant effects of PRAZ on active lever responding for the 0.4 mg/kg NIC or 0 or 3 mg/kg BUP groups. Direct comparison of the NIC and the 30 mg/kg BUP group confirmed the greater PRAZ-induced decrease in VS responding in the BUP compared to NIC group (Figure 4a). There were no detectable effects on active lever responding across all groups that were pretreated with propanolol (Figure 4b).
Figure 4.
illustrates active lever responding in each drug/dose condition during the NE Receptor Antagonist Challenge Tests. * indicates that 1 mg/kg prazosin pretreatment (Panel A) reduced responding for the 10 and 30 BUP groups. Panel B illustrates active lever responses from the propranolol pretreatment tests.
DISCUSSION
The present studies confirmed previous findings that bupropion enhances responding for a non-drug reinforcer (Bruijnzeel and Markou 2003; Liu et al. 2008; Paterson et al. 2008). We also extended these findings by demonstrating that the reinforcement enhancing effects of bupropion (10 and 30 mg/kg) were comparable in profile to those of nicotine (increasing over time/repeated treatments). The subjects’ history of reinforcement on both levers during shaping suggests that the sensory reinforcement that we observed (Figure 1) was not the result of habitual performance. Despite a history in which both levers had previously been reinforced, the effect of bupropion was specific to lever response producing the VS, suggesting that it was not the result of simple motor activation. The initial trend for increased responses on the inactive lever decreased over the first 3 testing sessions (Figure 2a). Finally, the present studies demonstrate an apparent contrast between the reinforcement enhancing effects of bupropion and nicotine. The enhancing effects of nicotine, but not bupropion, depended exclusively on nAChRs. Instead, the enhancing effects of bupropion were completely abolished by prazosin pretreatment, suggesting that they are critically dependent on α-NE receptors. Notably, the blockade of β-NE receptors did not alter the enhancing effects of either drug.
Several of the present findings bear further discussion. First, the parallel observations of increased responding over repeated treatments for both bupropion and nicotine suggests that convergent pharmacological systems mediate the reinforcement enhancing effects of both drugs. The dependence on catecholaminergic receptors for bupropion is also consistent with the enhancing effects of other psychomotor stimulants such as cocaine and amphetamine (Chu and Kelley 1992; Ranaldi and Beninger 1993; Wolterink et al. 1993). Although bupropion is an antagonist at nAChRs (Slemmer et al. 2000), we did not find evidence for an additive effect between bupropion and mecamylamine over the dose-rang tested. In addition, a previous study from our laboratory found that repeated daily treatment with mecamylamine alone does not enhance responding for the VS (Palmatier et al. 2007a). Combined, these findings suggest that reduced activity of nAChRs does not produce enhanced reinforcement.
The pharmacological dissociation between the reinforcement enhancing effects of nicotine and bupropion are probably best explained by different primary targets of the drugs with comparable or convergent downstream effects. For example, nicotine is relatively selective for nAChRs, both increasing and decreasing their activity (Picciotto et al. 2008; Wooltorton et al. 2003); but downstream effects include increased release of DA (Benwell and Balfour 1992), NE (Fu et al. 1998; Fu et al. 2003), and endogenous opioids (Dhatt et al. 1995; Wewers et al. 1999) in brain regions that may be critical to appetitive or goal-directed operant behaviors. These effects of nicotine are normally attributed to ascending projections from the ventral tegmental area (VTA) to the nucleus accumbens (NAc; Balfour et al. 2000; Dani 2003; Dani et al. 2001; Picciotto et al. 2008), but may also depend on descending projections to the pontine tegmentum (Laviolette and van der Kooy 2004), local cholinergic signaling in the NAc (McGehee 2006), or descending projections from the prefrontal cortex (PFC; McGehee 2007).
Bupropion affects multiple neurotransmitter systems within the central nervous system, including, but not limited to, antagonism of nAChRs (Fryer and Lukas 1999), reduction of NE, DA, and serotonin (5-HT) reuptake, and increasing the activity of vesicular monoamine transporters (see Dwoskin et al. 2006 for review). The reinforcement enhancing effects of bupropion in this study may be dependent on the drugs ability to directly reduce DA uptake in the NAc (Paterson et al. 2007). However, this does not explain the effect of prazosin in the present studies and suggests that the reinforcement enhancing effect of bupropion may be depend, at least partially, on an interaction between noradrenergic function in the PFC and dopaminergic function in the NAc. For example, both amphetamine and bupropion increase extracellular concentrations of DA and NE in the nucleus accumbens and PFC of rats (Li et al. 2002; Rothman et al. 2001). Prazosin administration in the PFC can block amphetamine-induced hyperlocomotion (Blanc et al. 1994) and also attenuates NAc dopamine release induced by systemic amphetamine injections (Darracq et al. 1998). In fact, these latter studies and others (Nichols and Selken 2007; Tassin et al. 2006) implicating the α-NE system in the motivational and psychomotor stimulant effects of amphetamines converge with our present findings with bupropion. Further studies are required to determine whether bupropion also increases NE release in the PFC, and the role of this putative effect in enhanced reinforcement; however, bupropion and amphetamine have similar chemical structures (Mehta 1983), act on similar targets (Li et al. 2002; Rothman et al. 2001), and share an ability to increase responding for sensory reinforcers (present studies; Glow and Russell 1973).
Although we have previously hypothesized that the DA system may be critical to the reinforcement enhancing effects of nicotine (Chaudhri et al. 2006b) and by extension bupropion, we chose not to investigate that hypothesis in the present studies. Midbrain DA function is critical to the primary reinforcing effects of food (Thanos et al. 2008), abused drugs (Yokel and Wise 1976), and sensory stimuli (Liu et al. 2006). Accordingly, antagonizing this system might directly reduce the reinforcement enhancing effects of bupropion and nicotine, but could also indirectly affect drug-enhanced responding by changing the status of (or motivation to obtain) the primary reinforcer. To understand the precise role of DA in enhanced reinforcement it may necessary to investigate the points of convergence and divergence with other neurological systems (e.g., Burns et al. 1994; Phillips et al. 1994) or to dissociate the roles of tonic and phasic dopamine responses (Exley et al. 2008; Rice and Cragg 2004).
The final question prompted by the present findings, as well as previous studies (Bruijnzeel and Markou 2003; Liu et al. 2008; Paterson et al. 2008) is whether the reinforcement enhancing effects of bupropion have clinical relevance in smoking cessation. One recent study in human smokers demonstrated that bupropion and amphetamine increased ad-lib smoking (Cousins et al. 2001). Both drugs also increased measures of positive mood and euphoria, replicating previous findings for amphetamine (Henningfield and Griffiths 1981; Schuster et al. 1979). Although they seem counterintuitive, the results are easily explained by reinforcement enhancing effects of bupropion and amphetamine. As reinforcement enhancers, bupropion and amphetamine could increase the valence of smoking-related conditioned reinforcers (Rose et al. 2003), increasing the number of cigarettes smoked and the satisfaction derived from smoking.
An important facet of bupropion- potentiated smoking in humans was that the participants were smokers who were not intending to quit (Cousins et al. 2001). For individuals attempting to quit smoking, the role that reinforcement enhancing effects of bupropion might play in smoking cessation is less clear. One hypothesis is that the enhancing effects of bupropion functionally replace the enhancing effects of nicotine, allowing smokers to retain the degree of reinforcement normally experienced in the presence of nicotine (Warner and Shoaib 2005). This replacement hypothesis prompts further questions about bupropion and nicotine self-administration in humans and non-human animals. For example, if nicotine self-administration was not confounded by the co-delivery of non-drug reinforcers (Palmatier et al. 2006), would bupropion treatment increase or decrease responding for nicotine? However, metabolism of bupropion produces several active metabolites in humans (i.e., hydroxybupropion) but not in rodent subjects (Borges et al. 2004). Thus, the role of bupropion in human smoking may depend on these metabolites, rather than a reinforcement enhancing effect. These possibilities pose exciting questions for future research on the primary reinforcing and reinforcement enhancing effects of nicotine and bupropion and may inform the development of therapeutic agents for smoking cessation.
References
- Balfour DJ, Wright AE, Benwell ME, Birrell CE. The putative role of extra-synaptic mesolimbic dopamine in the neurobiology of nicotine dependence. Behav Brain Res. 2000;113:73–83. doi: 10.1016/s0166-4328(00)00202-3. [DOI] [PubMed] [Google Scholar]
- Benwell ME, Balfour DJ. The effects of acute and repeated nicotine treatment on nucleus accumbens dopamine and locomotor activity. Br J Pharmacol. 1992;105:849–56. doi: 10.1111/j.1476-5381.1992.tb09067.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Besheer J, Palmatier MI, Metschke DM, Bevins RA. Nicotine as a signal for the presence or absence of sucrose reward: a Pavlovian drug appetitive conditioning preparation in rats. Psychopharmacology. 2004;172:108–17. doi: 10.1007/s00213-003-1621-9. [DOI] [PubMed] [Google Scholar]
- Bevins RA, Palmatier MI. Nicotine-conditioned locomotor sensitization in rats: assessment of the US-preexposure effect. Behav Brain Res. 2003;143:65–74. doi: 10.1016/s0166-4328(03)00009-3. [DOI] [PubMed] [Google Scholar]
- Blanc G, Trovero F, Vezina P, Herve D, Godeheu AM, Glowinski J, Tassin JP. Blockade of prefronto-cortical alpha 1-adrenergic receptors prevents locomotor hyperactivity induced by subcortical D-amphetamine injection. Eur J Neurosci. 1994;6:293–8. doi: 10.1111/j.1460-9568.1994.tb00272.x. [DOI] [PubMed] [Google Scholar]
- Borges V, Yang E, Dunn J, Henion J. High-throughput liquid chromatography-tandem mass spectrometry determination of bupropion and its metabolites in human, mouse and rat plasma using a monolithic column. Journal of chromatography B, Analytical technologies in the biomedical and life sciences. 2004;804:277–87. doi: 10.1016/j.jchromb.2004.01.024. [DOI] [PubMed] [Google Scholar]
- Bruijnzeel AW, Markou A. Characterization of the effects of bupropion on the reinforcing properties of nicotine and food in rats. Synapse. 2003;50:20–8. doi: 10.1002/syn.10242. [DOI] [PubMed] [Google Scholar]
- Burns LH, Everitt BJ, Kelley AE, Robbins TW. Glutamate-dopamine interactions in the ventral striatum: role in locomotor activity and responding with conditioned reinforcement. Psychopharmacology (Berl) 1994;115:516–28. doi: 10.1007/BF02245576. [DOI] [PubMed] [Google Scholar]
- Chaudhri N, Caggiula AR, Donny EC, Booth S, Gharib M, Craven L, Palmatier MI, Liu X, Sved AF. Operant responding for conditioned and unconditioned reinforcers in rats is differentially enhanced by the primary reinforcing and reinforcement-enhancing effects of nicotine. Psychopharmacology. 2006a;189:27–36. doi: 10.1007/s00213-006-0522-0. [DOI] [PubMed] [Google Scholar]
- Chaudhri N, Caggiula AR, Donny EC, Palmatier MI, Liu X, Sved AF. Complex interactions between nicotine and nonpharmacological stimuli reveal multiple roles for nicotine in reinforcement. Psychopharmacology. 2006b;184:353–66. doi: 10.1007/s00213-005-0178-1. [DOI] [PubMed] [Google Scholar]
- Chu B, Kelley AE. Potentiation of reward-related responding by psychostimulant infusion into nucleus accumbens: Role of dopamine receptor subtypes. Psychobiology. 1992;20:153–162. [Google Scholar]
- Coen K, Adamson KL, Corrigall W. Medication-related pharmacological manipulations of nicotine self-administration in the rat maintained on fixed- and progressive-ratio schedules of reinforcement. Psychopharmacology. 2009;201:557–68. doi: 10.1007/s00213-008-1321-6. [DOI] [PubMed] [Google Scholar]
- Cousins MS, Stamat HM, de Wit H. Acute doses of d-amphetamine and bupropion increase cigarette smoking. Psychopharmacology. 2001;157:243–53. doi: 10.1007/s002130100802. [DOI] [PubMed] [Google Scholar]
- Dani JA. Roles of dopamine signaling in nicotine addiction. Mol Psychiatry. 2003;8:255–6. doi: 10.1038/sj.mp.4001284. [DOI] [PubMed] [Google Scholar]
- Dani JA, Ji D, Zhou FM. Synaptic plasticity and nicotine addiction. Neuron. 2001;31:349–52. doi: 10.1016/s0896-6273(01)00379-8. [DOI] [PubMed] [Google Scholar]
- Darracq L, Blanc G, Glowinski J, Tassin JP. Importance of the noradrenaline-dopamine coupling in the locomotor activating effects of D-amphetamine. J Neurosci. 1998;18:2729–39. doi: 10.1523/JNEUROSCI.18-07-02729.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhatt RK, Gudehithlu KP, Wemlinger TA, Tejwani GA, Neff NH, Hadjiconstantinou M. Preproenkephalin mRNA and methionine-enkephalin content are increased in mouse striatum after treatment with nicotine. J Neurochem. 1995;64:1878–83. doi: 10.1046/j.1471-4159.1995.64041878.x. [DOI] [PubMed] [Google Scholar]
- Dickinson SL, Gadie B, Tulloch IF. Alpha 1- and alpha 2-adrenoreceptor antagonists differentially influence locomotor and stereotyped behaviour induced by d-amphetamine and apomorphine in the rat. Psychopharmacology. 1988;96:521–7. doi: 10.1007/BF02180034. [DOI] [PubMed] [Google Scholar]
- Donny EC, Caggiula AR, Knopf S, Brown C. Nicotine self-administration in rats. Psychopharmacology. 1995;122:390–94. doi: 10.1007/BF02246272. [DOI] [PubMed] [Google Scholar]
- Donny EC, Chaudhri N, Caggiula AR, Evans-Martin FF, Booth S, Gharib MA, Clements LA, Sved AF. Operant responding for a visual reinforcer in rats is enhanced by noncontingent nicotine: implications for nicotine self-administration and reinforcement. Psychopharmacology. 2003;169:68–76. doi: 10.1007/s00213-003-1473-3. [DOI] [PubMed] [Google Scholar]
- Dwoskin LP, Rauhut AS, King-Pospisil KA, Bardo MT. Review of the pharmacology and clinical profile of bupropion, an antidepressant and tobacco use cessation agent. CNS Drug Rev. 2006;12:178–207. doi: 10.1111/j.1527-3458.2006.00178.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Exley R, Clements MA, Hartung H, McIntosh JM, Cragg SJ. Alpha6-containing nicotinic acetylcholine receptors dominate the nicotine control of dopamine neurotransmission in nucleus accumbens. Neuropsychopharmacology. 2008;33:2158–66. doi: 10.1038/sj.npp.1301617. [DOI] [PubMed] [Google Scholar]
- Fryer JD, Lukas RJ. Noncompetitive functional inhibition at diverse, human nicotinic acetylcholine receptor subtypes by bupropion, phencyclidine, and ibogaine. J Pharmacol Exp Ther. 1999;288:88–92. [PubMed] [Google Scholar]
- Fu Y, Matta SG, James TJ, Sharp BM. Nicotine-induced norepinephrine release in the rat amygdala and hippocampus is mediated through brainstem nicotinic cholinergic receptors. J Pharmacol Exp Ther. 1998;284:1188–96. [PubMed] [Google Scholar]
- Fu Y, Matta SG, Kane VB, Sharp BM. Norepinephrine release in amygdala of rats during chronic nicotine self-administration: an in vivo microdialysis study. Neuropharmacology. 2003;45:514–23. doi: 10.1016/s0028-3908(03)00201-6. [DOI] [PubMed] [Google Scholar]
- Glow PH, Russell A. Effects of dexamphetamine, amylobarbitone sodium and their mixture on sensory contingent bar pressing behaviour in the rat. Psychopharmacologia. 1973;31:239–251. doi: 10.1007/BF00422514. [DOI] [PubMed] [Google Scholar]
- Hahn B, Stolerman IP. Modulation of nicotine-induced attentional enhancement in rats by adrenoceptor antagonists. Psychopharmacology. 2005;177:438–47. doi: 10.1007/s00213-004-1969-5. [DOI] [PubMed] [Google Scholar]
- Henningfield JE, Griffiths RR. Cigarette smoking and subjective response: effects of d-amphetamine. Clin Pharmacol Ther. 1981;30:497–505. doi: 10.1038/clpt.1981.194. [DOI] [PubMed] [Google Scholar]
- Hurt RD, Sachs DP, Glover ED, Offord KP, Johnston JA, Dale LC, Khayrallah MA, Schroeder DR, Glover PN, Sullivan CR, Croghan IT, Sullivan PM. A comparison of sustained-release bupropion and placebo for smoking cessation [see comment] New England Journal of Medicine. 1997;337:1195–202. doi: 10.1056/NEJM199710233371703. [DOI] [PubMed] [Google Scholar]
- Jorenby DE, Leischow SJ, Nides MA, Rennard SI, Johnston JA, Hughes AR, Smith SS, Muramoto ML, Daughton DM, Doan K, Fiore MC, Baker TB. A controlled trial of sustained-release bupropion, a nicotine patch, or both for smoking cessation. The New England Journal Of Medicine. 1999;340:685–691. doi: 10.1056/NEJM199903043400903. [DOI] [PubMed] [Google Scholar]
- Laviolette SR, van der Kooy D. The neurobiology of nicotine addiction: bridging the gap from molecules to behaviour. Nat Rev Neurosci. 2004;5:55–65. doi: 10.1038/nrn1298. [DOI] [PubMed] [Google Scholar]
- Li SX, Perry KW, Wong DT. Influence of fluoxetine on the ability of bupropion to modulate extracellular dopamine and norepinephrine concentrations in three mesocorticolimbic areas of rats. Neuropharmacology. 2002;42:181–90. doi: 10.1016/s0028-3908(01)00160-5. [DOI] [PubMed] [Google Scholar]
- Liu X, Caggiula AR, Palmatier MI, Booth S, Gharib M, Craven L, Donny EC, Sved AF. Reinforcement enhancing effect of nicotine and its attenuation by nicotinic and dopaminergic antagonists. Proceedings of the Society for Research on Nicotine and Tobacco.2006. [Google Scholar]
- Liu X, Caggiula AR, Palmatier MI, Donny EC, Sved AF. Cue-induced reinstatement of nicotine-seeking behavior in rats: effect of bupropion, persistence over repeated tests, and its dependence on training dose. Psychopharmacology. 2008;196:365–75. doi: 10.1007/s00213-007-0967-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X, Palmatier MI, Caggiula AR, Donny EC, Booth S, Gharib M, Craven L, Sved AF. Cholinergic substrates of the reinforcement enhancing effects of nicotine. Psychopharmacology. 2007 doi: 10.1007/s00213-005-0183-4. Submitted. [DOI] [PubMed] [Google Scholar]
- Martin P, Massol J, Colin JN, Lacomblez L, Puech AJ. Antidepressant profile of bupropion and three metabolites in mice. Pharmacopsychiatry. 1990;23:187–94. doi: 10.1055/s-2007-1014505. [DOI] [PubMed] [Google Scholar]
- McGehee DS. Nicotinic and opioid receptor interactions in nicotine addiction. Mol Interv. 2006;6:311–4. doi: 10.1124/mi.6.6.4. [DOI] [PubMed] [Google Scholar]
- McGehee DS. Nicotine and synaptic plasticity in prefrontal cortex. Sci STKE. 2007;2007:pe44. doi: 10.1126/stke.3992007pe44. [DOI] [PubMed] [Google Scholar]
- Mehta NB. The chemistry of bupropion. J Clin Psychiatry. 1983;44:56–9. [PubMed] [Google Scholar]
- Nahorski SR. Association of high affinity stereospecific binding of 3H-propranolol to cerebral membranes with beta adrenoceptors. Nature. 1976;259:488–9. doi: 10.1038/259488a0. [DOI] [PubMed] [Google Scholar]
- Nichols D, Selken J. Alpha1-adrenergic receptors mediate the locomotor response to systemic administration of ()-3,4-methylenedioxymethamphetamine (MDMA) in rats. Pharmacology, biochemistry and behavior. 2007;86:622–30. doi: 10.1016/j.pbb.2007.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Dell TJ, Christensen BN. Mecamylamine is a selective non-competitive antagonist of N-methyl-D-aspartate- and aspartate-induced currents in horizontal cells dissociated from the catfish retina. Neurosci Lett. 1988;94:93–8. doi: 10.1016/0304-3940(88)90276-5. [DOI] [PubMed] [Google Scholar]
- Ordway GA, Jaconetta SM, Halaris AE. Characterization of subtypes of alpha-2 adrenoceptors in the human brain. J Pharmacol Exp Ther. 1993;264:967–76. [PubMed] [Google Scholar]
- Palmatier MI, Evans-Martin FF, Hoffman A, Caggiula AR, Chaudhri N, Donny EC, Liu X, Booth S, Gharib M, Craven L, Sved AF. Dissociating the primary reinforcing and reinforcement-enhancing effects of nicotine using a rat self-administration paradigm with concurrently available drug and environmental reinforcers. Psychopharmacology. 2006;184:391–400. doi: 10.1007/s00213-005-0183-4. [DOI] [PubMed] [Google Scholar]
- Palmatier MI, Liu X, Caggiula AR, Donny EC, Sved AF. The role of nicotinic acetylcholine receptors in the primary reinforcing and reinforcement-enhancing effects of nicotine. Neuropsychopharmacology. 2007a;32:1098–108. doi: 10.1038/sj.npp.1301228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmatier MI, Liu X, Matteson GL, Donny EC, Caggiula AR, Sved AF. Conditioned reinforcement in rats established with self-administered nicotine and enhanced by noncontingent nicotine. Psychopharmacology. 2007b;195:235–243. doi: 10.1007/s00213-007-0897-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmatier MI, Matteson GL, Black JJ, Liu X, Caggiula AR, Craven L, Donny EC, Sved AF. The reinforcement enhancing effects of nicotine depend on the incentive value of non-drug reinforcers and increase with repeated drug injections. Drug Alcohol Depend. 2007c doi: 10.1016/j.drugalcdep.2006.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paterson N, Balfour D, Markou A. Chronic bupropion attenuated the anhedonic component of nicotine withdrawal in rats via inhibition of dopamine reuptake in the nucleus accumbens shell. The European journal of neuroscience. 2007;25:3099–108. doi: 10.1111/j.1460-9568.2007.05546.x. [DOI] [PubMed] [Google Scholar]
- Paterson NE, Balfour DJ, Markou A. Chronic bupropion differentially alters the reinforcing, reward-enhancing and conditioned motivational properties of nicotine in rats. Nicotine Tob Res. 2008;10:995–1008. doi: 10.1080/14622200802097571. [DOI] [PubMed] [Google Scholar]
- Phillips GD, Robbins TW, Everitt BJ. Mesoaccumbens dopamine-opiate interactions in the control over behaviour by a conditioned reinforcer. Psychopharmacology (Berl) 1994;114:345–59. doi: 10.1007/BF02244858. [DOI] [PubMed] [Google Scholar]
- Picciotto MR, Addy NA, Mineur YS, Brunzell DH. It is not “either/or”: activation and desensitization of nicotinic acetylcholine receptors both contribute to behaviors related to nicotine addiction and mood. Prog Neurobiol. 2008;84:329–42. doi: 10.1016/j.pneurobio.2007.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ranaldi R, Beninger RJ. Dopamine D1 and D2 antagonists attenuate amphetamine-produced enhancement of responding for conditioned reward in rats. Psychopharmacology (Berl) 1993;113:110–8. doi: 10.1007/BF02244342. [DOI] [PubMed] [Google Scholar]
- Rauhut AS, Mullins SN, Dwoskin LP, Bardo MT. Reboxetine: attenuation of intravenous nicotine self-administration in rats. J Pharmacol Exp Ther. 2002;303:664–72. doi: 10.1124/jpet.303.2.664. [DOI] [PubMed] [Google Scholar]
- Rauhut AS, Neugebauer N, Dwoskin LP, Bardo MT. Effect of bupropion on nicotine self-administration in rats. Psychopharmacology. 2003;169:1–9. doi: 10.1007/s00213-003-1450-x. [DOI] [PubMed] [Google Scholar]
- Rice ME, Cragg SJ. Nicotine amplifies reward-related dopamine signals in striatum. Nat Neurosci. 2004;7:583–4. doi: 10.1038/nn1244. [DOI] [PubMed] [Google Scholar]
- Robbins TW. Relationship between reward-enhancing and stereotypical effects of psychomotor stimulant drugs. Nature. 1976;264:57–9. doi: 10.1038/264057a0. [DOI] [PubMed] [Google Scholar]
- Robbins TW. Reward enhancement by psychomotor stimulant drugs [proceedings] Neuropharmacology. 1977;16:529–30. doi: 10.1016/0028-3908(77)90015-6. [DOI] [PubMed] [Google Scholar]
- Robinson TE, Berridge KC. The neural basis of drug craving: an incentive-sensitization theory of addiction. Brain Res Brain Res Rev. 1993;18:247–91. doi: 10.1016/0165-0173(93)90013-p. [DOI] [PubMed] [Google Scholar]
- Rose JE, Behm FM, Westman EC, Bates JE, Salley A. Pharmacologic and sensorimotor components of satiation in cigarette smoking. Pharmacol Biochem Behav. 2003;76:243–50. doi: 10.1016/j.pbb.2003.07.002. [DOI] [PubMed] [Google Scholar]
- Rothman RB, Baumann MH, Dersch CM, Romero DV, Rice KC, Carroll FI, Partilla JS. Amphetamine-type central nervous system stimulants release norepinephrine more potently than they release dopamine and serotonin. Synapse. 2001;39:32–41. doi: 10.1002/1098-2396(20010101)39:1<32::AID-SYN5>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- Schuster CR, Lucchesi BR, Emley GS. The effects of d-amphetamine, meprobamate, and lobeline on the cigarette smoking behavior of normal human subjects. NIDA Res Monogr. 1979:91–9. [PubMed] [Google Scholar]
- Shoaib M, Sidhpura N, Shafait S. Investigating the actions of bupropion on dependence-related effects of nicotine in rats. Psychopharmacology. 2003;165:405–12. doi: 10.1007/s00213-002-1277-x. [DOI] [PubMed] [Google Scholar]
- Slemmer JE, Martin BR, Damaj MI. Bupropion is a nicotinic antagonist. Journal of Pharmacology & Experimental Therapeutics. 2000;295:321–7. [PubMed] [Google Scholar]
- Stairs DJ, Dworkin SI. Rate-dependent effects of bupropion on nicotine self-administration and food-maintained responding in rats. Pharmacol Biochem Behav. 2008 doi: 10.1016/j.pbb.2008.05.014. [DOI] [PubMed] [Google Scholar]
- Tassin J-P, Glowinski J, Lanteri C, Salomon L. Behavioral sensitization to amphetamine results from an uncoupling between noradrenergic and serotonergic neurons. Proceedings of the National Academy of Sciences of the United States of America. 2006;103:7476–81. doi: 10.1073/pnas.0600839103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tella SR, Ladenheim B, Cadet JL. Differential regulation of dopamine transporter after chronic self-administration of bupropion and nomifensine. J Pharmacol Exp Ther. 1997;281:508–13. [PubMed] [Google Scholar]
- Thanos PK, Michaelides M, Ho CW, Wang GJ, Newman AH, Heidbreder CA, Ashby CR, Jr, Gardner EL, Volkow ND. The effects of two highly selective dopamine D3 receptor antagonists (SB-277011A and NGB-2904) on food self-administration in a rodent model of obesity. Pharmacol Biochem Behav. 2008;89:499–507. doi: 10.1016/j.pbb.2008.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ventura R, Cabib S, Alcaro A, Orsini C, Puglisi-Allegra S. Norepinephrine in the prefrontal cortex is critical for amphetamine-induced reward and mesoaccumbens dopamine release. J Neurosci. 2003;23:1879–85. doi: 10.1523/JNEUROSCI.23-05-01879.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villegier AS, Lotfipour S, Belluzzi JD, Leslie FM. Involvement of alpha1-adrenergic receptors in tranylcypromine enhancement of nicotine self-administration in rat. Psychopharmacology. 2007;193:457–65. doi: 10.1007/s00213-007-0799-7. [DOI] [PubMed] [Google Scholar]
- Warner C, Shoaib M. How does bupropion work as a smoking cessation aid? Addict Biol. 2005;10:219–31. doi: 10.1080/13556210500222670. [DOI] [PubMed] [Google Scholar]
- Wewers ME, Dhatt RK, Snively TA, Tejwani GA. The effect of chronic administration of nicotine on antinociception, opioid receptor binding and met-enkelphalin levels in rats. Brain Res. 1999;822:107–13. doi: 10.1016/s0006-8993(99)01095-1. [DOI] [PubMed] [Google Scholar]
- Wilkinson JL, Bevins RA. Bupropion hydrochloride produces conditioned hyperactivity in rats. Physiol Behav. 2007;90:790–6. doi: 10.1016/j.physbeh.2007.01.003. [DOI] [PubMed] [Google Scholar]
- Wolterink G, Phillips G, Cador M, Donselaar-Wolterink I, Robbins TW, Everitt BJ. Relative roles of ventral striatal D1 and D2 dopamine receptors in responding with conditioned reinforcement. Psychopharmacology. 1993;110:355–64. doi: 10.1007/BF02251293. [DOI] [PubMed] [Google Scholar]
- Wooltorton JR, Pidoplichko VI, Broide RS, Dani JA. Differential desensitization and distribution of nicotinic acetylcholine receptor subtypes in midbrain dopamine areas. J Neurosci. 2003;23:3176–85. doi: 10.1523/JNEUROSCI.23-08-03176.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yokel RA, Wise RA. Attenuation of intravenous amphetamine reinforcement by central dopamine blockade in rats. Psychopharmacology. 1976;48:311–8. doi: 10.1007/BF00496868. [DOI] [PubMed] [Google Scholar]
- Zhang XY, Kosten TA. Prazosin, an alpha-1 adrenergic antagonist, reduces cocaine-induced reinstatement of drug-seeking. Biol Psychiatry. 2005;57:1202–4. doi: 10.1016/j.biopsych.2005.02.003. [DOI] [PubMed] [Google Scholar]