Abstract
Patients with sickle cell disease (SCD) appear to be at lower risk of endocrinopathies and cardiac dysfunction than those with thalassemia major (TM). Circulating redox active iron is lower in these patients, possibly due to increased systemic inflammation and circulating cytokines. Hepcidin synthesis is upregulated during chronic inflammation, reducing intestinal iron absorption and promoting retention of iron in the reticuloendothelial cells. Hence, we hypothesized that livers of patients with SCD would exhibit greater iron deposition in sinusoidal spaces relative to hepatocytes and less in portal tracts when compared to patients with TM. To test this hypothesis, iron scoring analysis was performed on 70 clinically indicated liver biopsy specimens from children and young adults with the two syndromes. Sinusoidal scores were lower in around 1 of 4 patients with TM but the relative iron loading in hepatocytes, and portal tracts was identical in both diseases. Sinusoidal iron burdens saturated at low hepatic iron concentration (HIC) while hepatocyte and portal iron depots increased proportionally to HIC. Liver fibrosis was increased in patients with TM regardless of their chronic hepatitis status. Overall, liver iron distribution was relatively insensitive to differences in disease type and to the presence or absence of hepatitis.
Introduction
Patients with β-thalassemia (TM) and chronically transfused patients with sickle cell disease (SCD) develop severe iron overload with iron deposition in liver, heart, spleen, and endocrine organs [1–5]. Although having similar transfusional burdens and somatic iron stores, patients with chronically transfused SCD appear to be at lower risk for endocrinopathy, cardiac dysfunction, iron-mediated oxidative stress, and extrahepatic iron deposition [6–8]. Transferrin saturation and circulating nontransferrin bound iron levels are lower in transfused patients with SCD relative to patients with TM having comparable somatic iron stores, suggesting disease-specific iron handling. It has been postulated that the greater systemic inflammation observed in transfused patients with SCD may limit reticuloendothelial iron export and iron absorption through hepcidin or other iron mediators, but this hypothesis has never been proven [7].
Liver iron distribution offers a potential window into this question through the relative iron accumulation in the sinusoidal, portal, and hepatocyte compartments. Systemic inflammatory states, such as the anemia of chronic disease, favor sinusoidal iron loading [9,10]. Conditions with ineffective erythropoiesis, such as intermittently transfused thalassemia intermedia, are characterized by significant portal (from iron absorption) and hepatocyte iron loading [11,12]. Since patients with chronically transfused SCD have greater systemic inflammation and lower ineffective erythropoiesis than patients with TM [7], we postulated that patients with SCD would have relatively greater sinusoidal and lower portal iron loading than patients with TM.
Results
The demographics and biopsy scoring parameters for the patients with TM and SCD are summarized in Table I; all patients with chronic hepatitis were excluded. The populations were well balanced for age, gender, chelator use, pretransfusion hemoglobin, and hepatic iron concentration (HIC) by liver biopsy. Total iron score (TIS), hepatocyte iron score (HIS), and portal iron score (PIS) were statistically identical in the two groups. Patients with TM exhibited a trend toward lower mean sinusoidal iron score (SIS). Patients with TM also demonstrated greater liver fibrosis.
TABLE I.
Demographics and Iron-Scoring of Hepatis-Negative Patients with SCD and TM
| SCD | TM | P-value | |
|---|---|---|---|
| Age | 14.3 ± 6.5 | 14.9 ± 8.8 | 0.73 |
| Gender | 15 M, 14 F | 17 M, 24 F | 0.47 |
| Chelator | DFO, 23 | DFO, 36 | – |
| None, 5 | None, 4 | ||
| DFP, 1 | |||
| Hemoglobin | 9.3 ± 0.7 | 9.6 ± 0.8 | 0.15 |
| Liver iron by biopsy | 16.2 ± 8.6 | 18.1 ± 15.9 | 0.56 |
| Total score | 29.3 ± 7.5 | 26.5 ± 8.9 | 0.20 |
| Hepatocyte score | 17.1 ± 5.7 | 15.5 ± 6.8 | 0.32 |
| Sinusoidal score | 9.1 ± 1.6 | 7.9 ± 2.5 | 0.036 |
| Portal score | 3.1 ± 2.0 | 3.1 ± 2.0 | 0.92 |
| Fibrosis | 0.6 ± 0.6 | 1.2 ± 0.8 | 0.003* |
| Inflammation | 0.7 ± 0.7 | 0.9 ± 0.7 | 0.17 |
Significant at P < 0.05 after Bonferroni correction.
DFO, deferoxamine; DFP, deferiprone.
The demographics and biopsy scoring parameters for all patients having chronic hepatitis are shown in Table II. These patients tended to be older, of either gender, and predominantly had TM (9 out of 10). Liver iron concentration by biopsy was slightly lower than the general patient population but both fibrosis and inflammation scores trended higher in this patient subgroup.
TABLE II.
Demographics and Iron-Scoring of Patients with SCD and TM with Respect to Hepatitis Status
| Positive | Negative | P | |
|---|---|---|---|
| Age | 23.4 ± 8.6* | 14.6 ± 8.6 | 0.011 |
| Disease | 1 SCD, 9 TM | 23 SCD, 32 TM | 0.018 |
| Gender | 5 M, 5 F | 27 M, 33 F | 0.77 |
| Hemoglobin | 9.8 ± 1.1 | 9.4 ± 0.8 | 0.32 |
| Liver iron by biopsy | 12.7 ± 11.0 | 17.2 ± 13.0 | 0.26 |
| Total score | 25.3 ± 11.7 | 27.8 ± 8.3 | 0.53 |
| Hepatocyte score | 15.8 ± 7.3 | 16.2 ± 6.3 | 0.85 |
| Sinusoidal score | 6.2 ± 4.3 | 8.5 ± 2.2 | 0.13 |
| Portal score | 3.4 ± 1.7 | 3.1 ± 2.0 | 0.64 |
| Fibrosis | 1.9 ± 1.1 | 0.9 ± 0.8 | 0.019 |
| Inflammation | 1.5 ± 0.8 | 0.8 ± 0.7 | 0.035 |
Significant at P < 0.05 after Bonferroni correction.
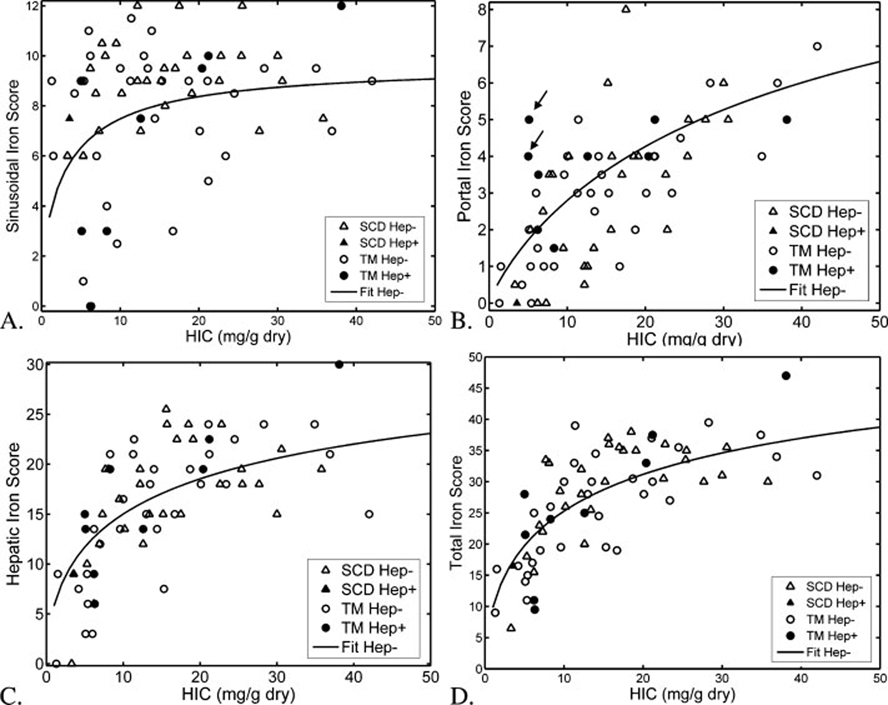
The relationship between histological iron scores and biopsy-proven iron levels in patients with TM and SCD are demonstrated in Fig. 1; corresponding logistic function curve fits are also shown. The iron scores display considerable variability with respect to the underlying HIC, however the logistic equation described central tendencies well. All patient subgroups exhibited a r2 ≥ 0.36, with the exception of SIS (Table III). In patients with SCD, SIS scores were essentially independent of liver iron concentration, with SIS scores > 6 for all but the two lowest liver iron burdens. In patients with TM, there is a bimodal behavior with 3 of 4 patients having a SIS scores distribution similar to the patients with SCD. However, 11 patients with TM had a SIS score < 7, including five patients with a score of 3 or less. These 11 patients exhibited lower liver iron concentration (9.9 ± 6.8 mg/g dry weight versus 20.1 ± 16.4 mg/g dry weight, P = 0.008), and lower hemoglobin (9.1 ± 0.8 mg/dl versus 9.9 ± 0.8 mg/dl, P = 0.0l).
Figure 1.
Plots demonstrate sinusoidal (A), portal (B), hepatocyte (C), and total (D) iron scores as a function of hepatic iron concentration in patients with TM (circles) and SCD (triangles). Open symbols represent hepatitis-negative patients; filled symbols reflect hepatitis-positive patients. The curves shown represent logistic functions optimally fitted to the pooled hepatitis-negative data points; logistic function parameters had no significant disease dependence. The logistic function was a poor fit to the sinusoidal iron score-biopsy relationship, but had an r2-value greater than 0.36 for the other three scores.
TABLE III.
Logistic Function Parameters in Hepatitis-Negative Patients with SCD and TM
| Parameter | A | B | r2 |
|---|---|---|---|
| HIS | 5.2 ± 1.6 | 0.57 ± 0.11 | 0.36 |
| SIS | 0.6 ± 1.4 | 0.16 ± 0.15 | 0.02 |
| PIS | 23.8 ± 8.5 | 0.80 ± 0.13 | 0.38 |
| TIS | 5.1 ± 1.1 | 0.57 ± 0.08 | 0.49 |
The values in bold depicts significant regression analysis for A, B, P < 0.0001.
Portal iron rose proportionally to total iron concentration in a similar manner for both patients with TM and SCD. Inappropriately high PIS, for the given liver iron burden, was seen in 2 of 9 patients with TM and chronic hepatitis (see arrows, Fig. 1). HIS and TIS both rose nonlinearly with biopsied liver iron concentration. This relationship was similar in both patients with TM and SCD; no obvious differences were observed in patients with chronic hepatitis.
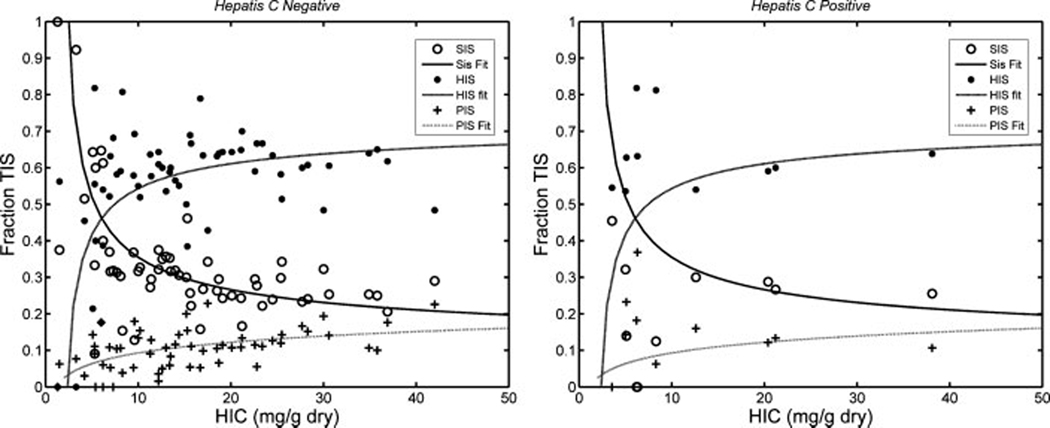
Figure 2 shows iron scores of the three compartments as a percent of TIS for all hepatitis-negative patients (left panel) and hepatitis-positive patients (right panel). Iron loading begins in the Kupffer cells of the sinusoids, but this compartment saturates at an HIC around 8 mg/g. Above this level, hepatocyte iron loading predominates, contributing ~60% of the TIS. The portal iron stores increase gradually with HIC and have a maximum capacity of ~15%.
Figure 2.
Plot demonstrates hepatocyte, sinusoid, and portal iron scores as a percent of TIS. Sinusoidal and hepatocyte loading exhibit a reciprocal relationship with HIC, with iron deposition beginning in the sinusoids and propagates into the hepatocyte and portal areas as sinusoids are saturated (HIC between 5 and 10 mg/g). Left hand panel represents hepatitis-negative patients and their corresponding curve fit. Right hand panel depicts hepatitis-positive patients compared with the curve fit’s derived from the hepatitis-negative patients.
Interobserver iron score correlations ranged from r2 of 0.44 for SIS to 0.78 for TIS. The slope of these linear relationships was not statistically different from unity and confidence intervals for the intercepts always contained the origin. Bland Altman analysis demonstrated no significant absolute or relative bias between the two observers.
Discussion
Relative hepatic iron compartmentalization is governed by the balance of systemic inflammation and ineffective erythropoiesis. This balance appears to be regulated primarily by the iron hormone, hepcidin, through its modulation of ferroportin in the gut and reticuloendothelial system. In this study, most patients with TM had iron distribution similar to patients with SCD. However, 25% of patients exhibited SIS less than 7; these patients had lower liver iron stores and hemoglobin levels, concordant with known hepcidin modulators [12]. In contrast, portal iron deposition was similar in patients with TM and SCD and did not suggest relative differences in gastrointestinal iron absorption.
Despite comparable liver iron levels in both groups, patients with TM exhibited greater liver fibrosis, regardless of their hepatitis status. Hepatocyte fibrosis is thought to result from oxidative stress induced by the chronic exposure to increased labile plasma iron (LPI). Hepatic LPI uptake is unregulated and thought to be more toxic than transferrin-mediated iron loading [13]. Patients with SCD generally have a shorter duration of transfusional exposure [14,15] in addition to having lower LPI levels for comparable iron burdens [7,16]. Thus, the difference in hepatic toxicity parallels previously described differences in the heart and endocrine organs [6,8,17,18].
Patients with TM and active hepatitis exhibited a trend toward greater liver inflammation and fibrosis; all patients with grade II liver fibrosis or higher had chronic, active hepatitis. With respect to hepcidin secretion, systemic and hepatic inflammation act in diametric opposition. Patients with chronic hepatitis have lower hepcidin RNA levels (relative to their iron stores), greater intestinal iron absorption, and increased labile iron export from the reticuloendothelial system [19–21]. Although the sinusoid-to-portal iron ratio was abnormal in a few patients with chronic hepatitis, our sample size was too small to power a formal comparison. In addition, there was an uncorrectable age bias between patients with and without hepatitis C. Only one patient with SCD had hepatitis, making it impossible to make any inference with respect to hepatitis in this disease.
It is well known that transfusional iron overload beings in the liver sinusoids and then progresses into once sinusoidal capacity is saturated [3,22]. However, the effects of chronic chelation therapy on this relationship are unknown. Relative chelation efficiency of the reticuloendothelial and hepatocyte compartments appears to be drug specific [23,24]. In this study, we observed iron distributions comparable to those described in unchelated patients, suggesting that iron chelation (primarily deferoxamine) was relatively “balanced” between the two compartments. There was a clear “break-point” near an HIC of 8 mg/g where the reticuloendothelial system appeared to saturate and hepatocyte iron loading rose dramatically.
This study had a number of important limitations. Because of its retrospective nature, we did not have data on LPI, systemic inflammation, or hepcidin levels/mRNA, forcing us to rely on the results from the literature. The retrospective design also prevents us from capturing the morphodynamics of the storage process. Our sample size was too small and poorly age-matched to adequately assess the consequences of hepatitis and its potential interaction with the underlying disease state. Imperfect interobserver iron-scoring hindered our ability to detect the underlying iron distribution relationships. Nonetheless, our observations offer important insights into the pathophysiologic disparities between SCD and TM and may be useful for hypothesis-generation in prospective studies.
Methods
Iron storage pattern was reviewed in 70 liver biopsies obtained from patients with TM (N = 41) and SCD (N = 29) between 09/1996 and 04/ 2005. A subset of the patients participated in additional research trials that have been reported elsewhere; informed consent was obtained from those patients and/or their guardians [25]. This study represented a retrospective review of the biopsy specimens and clinical data and waiver of consent for the remaining patients was approved by the institutional review board at Children’s Hospital Los Angeles and Children’s Hospital & Research Center at Oakland. All histologic sections were evaluated by one experienced pathologist at each institution (IGG, RW). Each specimen was graded for the relative iron distribution in the three zones of Rappaport, acinus in the hepatocytes and Kupffer cells (sinusoids) and different structures of portals tracts (connective tissue, biliary ducts, and vascular walls), based on scoring developed by Deugnier et al. [26]. Hepatocytes and sinusoids were graded between a score of 0–36 (0–12 per zone) and 0–12 (0–4 per zone), respectively, while portal structures between 0 and 12 (0–4 per structure); higher score corresponded to higher iron burden. Finally, TIS for each specimen was calculated as the sum of the scores of the individual compartments (maximum of 60). Iron scores were compared between hepatitis-negative patients with TM and SCD using unpaired T-test with Bonferroni correction. A similar comparison was performed between patients with TM with and without chronic hepatitis. To control for possible systematic differences among the two reviewers, 20 specimens were jointly read and scores compared to one another using correlation and Bland-Altman analysis.
Quantitative iron analysis was performed using inductively coupled mass spectrometry (Metals Laboratory, Mayo Clinic, Rochester, MN). To compare the compartmental iron scores between patients with TM and SCD, it was necessary to normalize them to the biopsied liver iron level. To accomplish this, we use a logistic or sigmoid function to describe the relationship between iron score and HIC. A logistic function is popularly used to describe biological population growth and can be represented as,
| (1) |
where x and y are the independent (input) and the dependent (output: population) variables, respectively, C is the maximum value that the function, y, can take, A reflects the ratio of the C and the initial population and B represents how quickly y attains C. In our context, y corresponded to the observed iron scores and x equals log (HIC). We found that log-transforming HIC significantly improved curve fitting; HIC were converted to units of mg/g dry weight of liver before log transformation. Since C represents the maximum observable score (the denominator of eq. 1 is always ≥1), we set C to 36, 12, 12, and 60 (maximum scores possible) for hepatocyte, sinusoidal, portal, and total scores, respectively. Equation 1 can be recast as a linear equation by isolating the Ae−Bx term, followed by log transformation. We then used standard linear least-squares algorithms to calculate the A and B terms and their confidence intervals. JMP™ statistical discovery software (SAS Institute, Cary, NC) was used to identify outliers using jackknifed Mahalanobis outlier distance algorithm and these data points were excluded during curve fitting.
Differences in iron distribution between sickle cell and thalassemia were assessed by comparing the 95% confidence intervals for the A and B coefficient values for both diseases. As the confidence intervals were heavily overlapping for patients with TM and SCD, data from both groups were pooled together. Hepatic C-positive patients were excluded from the logistic function calculations. Calculated logistic functions for HIS, PIS, and SIS were subsequently normalized to TIS to represent a percent of TIS.
Biopsy specimens were also graded for fibrosis and inflammation using a 0–4 grading scale. Scores were compared among disease and hepatitis states by Student’s T-test with Bonferroni correction.
Acknowledgments
Contract grant sponsor: National Institutes of Health (General Clinical Research Center); Contract grant number: RR00043-43. Contract grant sponsor: Pediatric Clinical Research Center at the Children’s Hospital & Research Center, Oakland; Contract grant number: M01 RR01271. Contract grant sponsor: Center for Disease Control (Thalassemia Center); Contract grant number: U27/CCU922106. Contract grant sponsor: Department of Pediatrics at Children’s Hospital Los Angeles (CHLA), National Heart Lung and Blood Institute of the National Institutes of Health; Contract grant number: 1 R01 HL75592-01A1. Contract grant sponsor: National Institute for Diabetes, Digestion, and Kidney Disease; Contract grant number: R01 DK057778. Contract grant sponsor: Saban Research Institute at CHLA.
Footnotes
Conflict of Interest: Dr. Wood, Dr. Coates, and Dr. Harmatz have research funding from Novartis and had received speakers honoraria from Novartis and Apotex.
References
- 1.Darbari DS, Kple-Faget P, Kwagyan J, et al. Circumstances of death in adult sickle cell disease patients. Am J Hematol. 2006;81:858–863. doi: 10.1002/ajh.20685. [DOI] [PubMed] [Google Scholar]
- 2.Sonakul D, Pacharee P, Thakerngpol K. Pathologic findings in 76 autopsy cases of thalassemia. Birth Defects Orig Artic Ser. 1988;23:157–176. [PubMed] [Google Scholar]
- 3.Olivieri NF. Progression of iron overload in sickle cell disease. Semin Hematol. 2001;38:57–62. doi: 10.1016/s0037-1963(01)90060-5. [DOI] [PubMed] [Google Scholar]
- 4.Anderson GJ. Mechanisms of iron loading and toxicity. Am J Hematol. 2007;82:1128–1131. doi: 10.1002/ajh.21075. [DOI] [PubMed] [Google Scholar]
- 5.Porter JB. Concepts and goals in the management of transfusional iron overload. Am J Hematol. 2007;82:1136–1139. doi: 10.1002/ajh.21100. [DOI] [PubMed] [Google Scholar]
- 6.Vichinsky E, Butensky E, Fung E, et al. Comparison of organ dysfunction in transfused patients with SCD or beta thalassemia. Am J Hematol. 2005;80:70–74. doi: 10.1002/ajh.20402. [DOI] [PubMed] [Google Scholar]
- 7.Walter PB, Fung EB, Killilea DW, et al. Oxidative stress and inflammation in iron-overloaded patients with beta-thalassaemia or sickle cell disease. Br J Haematol. 2006;135:254–263. doi: 10.1111/j.1365-2141.2006.06277.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fung EB, Harmatz PR, Lee PD, et al. Increased prevalence of iron-overload associated endocrinopathy in thalassaemia versus sickle-cell disease. Br J Haematol. 2006;135:574–582. doi: 10.1111/j.1365-2141.2006.06332.x. [DOI] [PubMed] [Google Scholar]
- 9.Ganz T. Molecular pathogenesis of anemia of chronic disease. Pediatr Blood Cancer. 2006;46:554–557. doi: 10.1002/pbc.20656. [DOI] [PubMed] [Google Scholar]
- 10.Sohn YS, Breuer W, Munnich A, et al. Redistribution of accumulated cell iron: A modality of chelation with therapeutic implications. Blood. 2008;111:1690–1699. doi: 10.1182/blood-2007-07-102335. [DOI] [PubMed] [Google Scholar]
- 11.Kattamis A, Papassotiriou I, Palaiologou D, et al. The effects of erythropoetic activity and iron burden on hepcidin expression in patients with thalassemia major. Haematologica. 2006;91:809–812. [PubMed] [Google Scholar]
- 12.Kearney SL, Nemeth E, Neufeld EJ, et al. Urinary hepcidin in congenital chronic anemias. Pediatr Blood Cancer. 2007;48:57–63. doi: 10.1002/pbc.20616. [DOI] [PubMed] [Google Scholar]
- 13.Mcnamara L, Macphail AP, Mandishona E, et al. Non-transferrin-bound iron and hepatic dysfunction in African dietary iron overload. J Gastroenterol Hepatol. 1999;14:126–132. doi: 10.1046/j.1440-1746.1999.01830.x. [DOI] [PubMed] [Google Scholar]
- 14.Harmatz P, Butensky E, Quirolo K, et al. Severity of iron overload in patients with sickle cell disease receiving chronic red blood cell transfusion therapy. Blood. 2000;96:76–79. [PubMed] [Google Scholar]
- 15.Fung EB, Harmatz PR, Milet M, et al. Disparity in the management of iron overload between patients with sickle cell disease and thalassemia who received transfusions. Transfusion. 2008;48:1971–1980. doi: 10.1111/j.1537-2995.2008.01775.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schein A, Enriquez C, Coates TD, et al. Magnetic resonance detection of kidney iron deposition in sickle cell disease: A marker of chronic hemolysis. J Magn Reson Imaging. 2008;28:698–704. doi: 10.1002/jmri.21490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fung EB, Harmatz P, Milet M, et al. Morbidity and mortality in chronically transfused subjects with thalassemia and sickle cell disease: A report from the multi-center study of iron overload. Am J Hematol. 2007;82:255–265. doi: 10.1002/ajh.20809. [DOI] [PubMed] [Google Scholar]
- 18.Wood JC, Tyszka JM, Ghugre N, et al. Myocardial iron loading in transfusion-dependent thalassemia and sickle-cell disease. Blood. 2004;103:1934–1936. doi: 10.1182/blood-2003-06-1919. [DOI] [PubMed] [Google Scholar]
- 19.Nishina S, Hino K, Korenaga M, et al. Hepatitis C virus-induced reactive oxygen species raise hepatic iron level in mice by reducing hepcidin transcription. Gastroenterology. 2008;134:226–238. doi: 10.1053/j.gastro.2007.10.011. [DOI] [PubMed] [Google Scholar]
- 20.Takeo M, Kobayashi Y, Fujita N, et al. Upregulation of transferrin receptor 2 and ferroportin 1 mRNA in the liver of patients with chronic hepatitis C. J Gastroenterol Hepatol. 2005;20:562–569. doi: 10.1111/j.1440-1746.2005.03770.x. [DOI] [PubMed] [Google Scholar]
- 21.Fujita N, Sugimoto R, Takeo M, et al. Hepcidin expression in the liver: Relatively low level in patients with chronic hepatitis C. Mol Med. 2007;13:97–104. doi: 10.2119/2006-00057.Fujita. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gabutti V, Borgna-Pignatti C. Clinical manifestations and therapy of transfusional haemosiderosis. Baillieres Clin Haematol. 1994;7:919–940. doi: 10.1016/s0950-3536(05)80131-3. [DOI] [PubMed] [Google Scholar]
- 23.Wood J, Aguilar M, Otto-Duessel M, et al. Influence of iron chelation therapy on R1 and R2 calibration curves in gerbil liver and heart. Magn Reson Med. 2008;60:82–89. doi: 10.1002/mrm.21660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wood JC, Otto-Duessel M, Gonzalez I, et al. Deferasirox and deferiprone remove cardiac iron in the iron-overloaded gerbil. Transl Res. 2006;148:272–280. doi: 10.1016/j.trsl.2006.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Butensky E, Fischer R, Hudes M, et al. Variability in hepatic iron concentration in percutaneous needle biopsy specimens from patients with transfusional hemosiderosis. Am J Clin Pathol. 2005;123:146–152. doi: 10.1309/puuxegxdlh26nxa2. [DOI] [PubMed] [Google Scholar]
- 26.Deugnier YM, Loreal O, Turlin B, et al. Liver pathology in genetic hemochromatosis: A review of 135 homozygous cases and their bioclinical correlations. Gastroenterology. 1992;102:2050–2059. doi: 10.1016/0016-5085(92)90331-r. [DOI] [PubMed] [Google Scholar]