Abstract
The encoding of reward-predictive stimuli by neurons in the nucleus accumbens (NAcc) depends on integrated synaptic activity from the basolateral amygdala (BLA) and medial prefrontal cortex (mPFC) afferent inputs. In a previous study, we found that single electrical stimulation pulses applied to the BLA facilitate mPFC-evoked spiking in NAcc neurons in a timing-dependent manner, presumably by a fast glutamatergic mechanism. In the present study, the ability of repetitive BLA activation to modulate synaptic inputs to NAcc neurons through dopamine- or N-methyl-d-aspartate (NMDA)-dependent mechanisms is characterized. NAcc neurons receiving excitatory input from both mPFC and BLA were recorded in urethane-anesthetized rats. Train stimulation of the BLA depressed mPFC-evoked spiking in these neurons. This was not attributable to mechanisms involving NMDA or dopamine D1, D2, D3 or D5 receptors, since blockade of these receptors did not affect the BLA-mediated depression. BLA-mediated depression was only evident when the BLA stimulation evoked spikes in the recorded neuron; thus, depolarization of the recorded neuron may be critical for this effect. The ability of the BLA to suppress mPFC-to-NAcc signaling may be a mechanism by which normal or pathologically heightened emotional states disrupt goal-directed behavior in favor of emotionally-driven responses.
Keywords: electrophysiology, in vivo, heterosynaptic, limbic, reward, addiction
The pursuit of natural rewards is essential to survival. The nucleus accumbens (NAcc) and its direct innervation by the medial prefrontal cortex (mPFC) and basolateral amygdala (BLA) form a circuit that supports this ability by encoding information about reward-associated stimuli. In the presence of conditioned cues, normally quiescent BLA neurons exhibit phasic increases in firing rate (Pratt and Mizumori, 1998; Schoenbaum et al., 1999), and the direct excitation of NAcc neurons by the BLA is essential for NAcc neurons to encode reward-predictive cues and for those conditioned cues to guide subsequent actions (Ambroggi et al., 2008). Similarly, mPFC neurons, including those projecting to NAcc (McGinty and Grace, 2008), are also excited by conditioned stimuli (Jodo et al., 2000; Gilmartin and McEchron, 2005; Laviolette et al., 2005), and the mPFC-to-NAcc projection is necessary for cue-related NAcc firing and cued reward seeking behavior (Ishikawa et al., 2008). These findings suggest that NAcc neurons encode cues and influence behavior only if they receive sufficient synaptic activity from BLA and mPFC afferent inputs. In this way, the physiological interaction of BLA and mPFC synaptic activity in single NAcc neurons may be a critical mechanism that supports reward-directed actions.
We have shown previously that activation of BLA and mPFC mutually facilitates spiking in the NAcc neurons on which those inputs converge, and that this facilitation likely relies on fast synaptic glutamatergic transmission (McGinty and Grace, 2009). However, other neurotransmitter systems can also be recruited by activation of BLA-to-NAcc afferents. Although the BLA projection to the NAcc is gluta-matergic, high frequency BLA stimulation has been reported to increase extracellular dopamine (DA) in the NAcc, and induce N-methyl-d-aspartate (NMDA)- and DA D1-dependent plasticity of BLA inputs (Floresco et al., 1998, 2001b). DA modulates corticostriatal transmission through both D1-like and D2-like receptors (West and Grace, 2002; Goto and Grace, 2005). Thus, BLA activation could potentially modulate mPFC inputs onto NAcc neurons through both NMDA-and DA-dependent mechanisms, in addition to the facilitatory, presumably glutamatergic interactions described previously (McGinty and Grace, 2009).
This model was examined by recording from NAcc neurons receiving both BLA and mPFC input, and observing the effects of high frequency BLA stimulation on mPFC-evoked spiking. The evoked responses were depressed both during and after BLA activation. Interestingly, while the post-activation depression of mPFC inputs was dependent on DA D1-like receptors, the depression observed during BLA activation was unchanged by DA receptor or NMDA channel blockade.
EXPERIMENTAL PROCEDURES
Subjects and surgery
All studies were carried out in accordance with the Institutional Animal Care and Use Committee at the University of Pittsburgh, and the Guide for the Care and Use of Laboratory Animals published by the United States Public Health Service. A total of 80 NAcc neurons were recorded from male Sprague–Dawley rats with an average weight of 310 g (range 270–360 g). The rats were anesthetized with a single injection of urethane (1.4–1.5 g/kg in distilled water); this provided stable anesthesia for the duration of the experiment, which did not exceed 8 h. Surgery was initiated when the rat no longer displayed a reflexive withdrawal in response to a foot pinch (20–60 min after injection). For experiments in which antagonist drugs were delivered, the rats were first implanted with a femoral vein catheter. The rats were placed in a stereotaxic apparatus (David Kopf Instruments, Tujunga, CA, USA), and the skull was exposed and cleaned. Burr holes were drilled in the skull overlying the electrode targets, and the overlying dura was carefully resected. The coordinates of the target regions are given relative to the bregma suture: AP for anterior–posterior distance; ML for medial-lateral; DV for dorsal–ventral. The target regions were all within the right hemisphere.
Stimulating electrodes were lowered into the BLA (AP −3.6, ML 4.8, DV −9.0) and the mPFC (AP +3.0, ML 0.8, DV −5.5). The electrodes consisted of Plastics One (Roanoke, VA, USA) model C232G-MS303/2 bipolar electrodes with 0.8 mm of wire exposed at the tip of each pole. The two poles of the BLA electrode were oriented in the same sagittal plane, and the two poles of the mPFC electrode were in approximately the same coronal plane. The stereotaxic coordinates and stimulating electrode locations all refer to the negative pole. A recording electrode was lowered to the dorsal border of the NAcc, near the border of the shell and core subregions (AP + 1.2, ML 0.8–1.0, DV −6.0). The recording electrodes were pulled from 2 mm outside diameter, filamented borosilicate glass (World Precision Instruments, Sarasota, FL, USA). The tips were broken back under microscopic control to achieve an impedance of 8–12 MΩ as measured in situ through the amplifier (Fintronics model WDR-420, Orange, CT, USA), and filled with 2 M NaCl with Pontamine Sky Blue dye.
Electrophysiological recordings and neuron selection
Signals from neurons in the NAcc were amplified and band pass filtered at 200–4000 Hz. The amplified signals were passed to an oscilloscope and speaker for real-time monitoring, as well as to a data acquisition board and PC (Microstar Laboratories, Bellevue, WA, USA) for storage and analysis. Off-line analysis was performed using custom software (Neuroscope, Brian Lowry) and scripts for the R programming environment (R Development Core Team, 2005). Electrical stimulation pulses were delivered through the Master 8 Stimulator (AMPI, Jerusalem, Israel) and stimulus isolation units.
The neurons of interest were those NAcc neurons that were excited by stimulation of both ipsilateral mPFC and ipsilateral BLA, indicating converging excitatory inputs from both afferent structures (McGinty and Grace, 2009). These neurons were isolated by slowly advancing the recording electrode through the NAcc while single electrical stimulation pulses were delivered to the mPFC and BLA at an overall rate of 0.5 Hz. NAcc neurons (typically not spontaneously active) were identified by the presence of action potentials following the stimulation pulses (see Fig. 1). In this experiment, NAcc neurons were considered suitable for recording if they met two criteria: they produced at least a 50% evoked spike ratio to 400 µA stimulation of the BLA and 500 µA stimulation of the mPFC; and the evoked spike latency was 20 ms or less, consistent with monosynaptic, orthodromic transmission (McGinty and Grace, 2009). Neurons with sustained spontaneous firing >3 Hz (Wilson et al., 1990) or that produced more than two spikes in response to a single stimulation pulse (Mallet et al., 2005) were considered to be putative interneurons, and were not analyzed.
Fig. 1.
Example of evoked action potentials (spikes) in a recorded neuron and the locations of recorded cells and stimulating electrodes. (A) Traces show the action potentials (small arrows) recorded from a single neuron. Action potentials were evoked by stimulation of the mPFC (left, five traces) or the BLA (right, five traces). The empty arrowheads indicate the stimulation artifacts, which have been cropped for clarity. (B) Two coronal atlas sections show the locations of 76/80 recorded cells in the NAcc. Most neurons were recorded in the shell (sh), and a smaller number were recorded in the core (co). (C) The BLA stimulating electrode tips were placed in the basolateral (BL), lateral (LaV) and basomedial (BM) subnuclei of the amygdala. Placements in BM were acceptable because the exposed lead extended 0.8 mm dorsal of the tip, into the BL subnucleus. (D) The mPFC stimulating electrode tips were placed in the deep layers of infralimbic cortex (IL), with the exposed lead extending into the prelimbic cortex (PL) for placements in dorsal IL. For clarity, only 20 representative placements are shown each in C and D. The numbers indicate stereotaxic coordinates in mm. DP: dorsal peduncular cortex.
BLA train stimulation and drug administration
Once a suitable neuron was found, the effect of BLA train stimulation on the mPFC-evoked spike probability was tested. MPFC stimulation current was adjusted to produce a 40%–60% evoked spike probability when delivered alone; thus, the currents used were different for each neuron. The mPFC was first stimulated for 40 trials at a rate of 0.3 Hz (baseline, 2.2 min). Then, as the mPFC stimulation continued, BLA train stimulation was introduced for 80 trials (4.4 min); in these trials, a single BLA stimulus train (five pulses at 20 Hz) was delivered 2.8 s before the mPFC stimulation pulse. This interval was determined by the overall trial rate of 0.3 Hz (3.3 s interval), and the 0.5 s gap between the mPFC pulse at the end of one trial, and the beginning of the BLA train in the next trial. After these 80 trials, mPFC stimulation continued for as long as the recording exhibited a stable signal-to-noise ratio, low background noise, and consistent action potential shape. Because some recordings became unstable before sufficient post-train responses could be observed, fewer post-train neurons are reported compared to the number reported during BLA trains. Up to two neurons were tested in this way in each rat; the results did not differ between those neurons recorded first and those recorded second (not shown).
In most experiments, the BLA stimulation current was adjusted to threshold intensities, such that the five-pulse BLA stimulation train evoked spikes at about a ~50% spike probability (mean current 330 µA). To test the role of BLA-evoked spiking, in a few experiments (n=7) the BLA current was reduced to produce a near 0% spike probability for the first 40 train stimulation trials (subthreshold, mean 200 µA), and then increased to produce a 50% spike probability in the next 40 train stimulation trials (threshold, mean 320 µA). In 18 additional neurons for which data are not shown, only subthreshold BLA current was applied (mean 170 µA).
The effects of BLA train stimulation were tested after systemic injection of the DA D1/D5 antagonist SCH23390, the DA D2/D3 antagonists eticlopride or raclopride, or the NMDA channel blocker MK-801. All drugs (acquired from Sigma, St. Louis, MO, USA) were dissolved in sterile 0.9% saline. The drug doses were (in mg/kg i.v.): SCH23390 0.5, eticlopride 0.2, raclopride 0.1, MK-801 0.05 or 0.1. These doses have been shown previously to have potent activity at the target receptors and to be effective in relevant behavioral tasks (Caine and Koob, 1994; Nicola et al., 2000; Floresco et al., 2001b; Cervo et al., 2003; Biondo et al., 2005).
The drugs were injected into the femoral vein 5–15 min prior to testing the effects of BLA train stimulation. In these experiments, only a single injection was administered and only one neuron was recorded per rat, with the following exceptions: in seven rats (one SCH23390, six MK-801), the drug was administered, and the BLA train effects were tested on one neuron; then, at least 2 h later, a second dose of the same drug was given and the BLA train effects were tested in a second neuron. In three rats (all SCH23390), two neurons were tested within 2 h of the same injection. These 10 neurons recorded after a second injection or after a delay did not have different responses from the majority of neurons that were recorded immediately after the first injection (not shown). Furthermore, the D1 antagonist effects of SCH23390 were sustained well beyond 2 hours after systemic administration (Schulz et al., 1985; McQuade et al., 1988; Canini and Bourdon, 1998; O’Neill and Shaw, 1999).
Analysis and histology
The mPFC-evoked spike probability was measured before, during and after BLA train stimulation, as was the BLA-evoked spike probability during the train. The responses were grouped into bins of 10 trials each and the spike probabilities (spikes/100 stimuli, expressed as a percentage) were compared across stimulation conditions. The differences between treatment conditions were assessed with paired t-tests (within neuron) with Holm’s modified Bonferroni correction for multiple comparisons. All mean values are expressed as “mean±standard error of the mean”; all error bars on graphs show the standard error of the mean. Unless otherwise stated, all P-values described in the text refer to the results of within-neuron paired t-tests, corrected for multiple comparisons if appropriate.
At the end of the experiment, the stimulating electrodes were marked by passing 100 µA of current for 10 s. The final position of the recording electrode was marked by iontophoretic ejection of Pontamine Sky Blue dye (−20 µA for 60 min). The rats were decapitated, and their brains were fixed in 8% paraformaldehyde with 1% potassium ferricyanide. After 48 h, they were transferred to a cryoprotectant solution of 25% sucrose. The brains were sliced on a freezing microtome and mounted onto slides for subsequent Nissl staining using standard histological procedures. The location of the recording and stimulating electrodes was determined using the atlases of (Paxinos and Watson 1998, 2005). Neurons were recorded in both the shell and core subregions of the NAcc. For analysis purposes, only neurons that could be clearly identified to lie within the core or shell were assigned to a subregion; if the actual or reconstructed location of a neuron was within 0.1 mm of the core/shell border, it was not assigned to either subregion.
RESULTS
A total of 80 NAcc neurons that were excited by stimulation of both the mPFC and BLA were recorded (Fig. 1A). The neurons were located in the medial shell or in the medial core near the shell border (Fig. 1B). Because medium spiny neurons compose approximately 95% of striatal neurons (Kemp and Powell, 1971; Jiang and North, 1991) and because putative interneurons were not tested, almost all of the neurons recorded in this study were likely to be the medium spiny subtype. The stimulating electrode tips were placed in the basolateral, lateral and basomedial subnuclei of the ipsilateral BLA (Fig. 1C) and in the deep layers of the infralimbic subregion of the ipsilateral mPFC (Fig. 1D).
BLA train stimulation depressed mPFC-evoked spiking
The probability of mPFC stimulation evoking spike firing in NAcc neurons was measured before, during and after train stimulation of the BLA (Fig. 2A). In seven neurons from four rats, BLA stimulation was tested at both subthreshold (evoking no spikes) and threshold current amplitudes (evoking a ~50% response; Fig. 2B, C). Subthreshold BLA trains did not change mPFC-evoked spike probability (decrease of 2.1%±3.6%); in contrast, mPFC-evoked spike probability was decreased during threshold BLA trains by 19.2%±4.7% compared to the probability before trains, and by 17.0%±3.7% compared to the probability during subthreshold train stimulation (P<0.008 for both comparisons; Fig. 2C).
Fig. 2.
(A) Raster showing mPFC- and BLA-evoked spikes in a single NAcc neuron recorded over 140 trials. Each row shows the stimulation and evoked spikes during one trial. The black dots are spikes; the gray line at t=0 indicates the mPFC stimulation time in each trial; the black and gray stippled lines show BLA stimulation pulses. The mPFC was stimulated alone over trials 1–40. Subthreshold train stimulation of BLA applied 2833 ms before mPFC stimulation (trials 41–80) had no effect on the mPFC-evoked spike probability; however threshold BLA stimulation (trials 81–120, ~50% average BLA-evoked response) reduced mPFC-evoked spiking. (B) In seven NAcc neurons, mPFC-evoked spike probability was reduced during threshold BLA train stimulation. This time series shows the mPFC-evoked spike ratio before BLA stimulation (trials −40 to 0), and during subthreshold (“sub”, 1–40), and threshold (thresh, 41–80) BLA train stimulation. The gray shaded area shows the SEM of the response. The black horizontal lines show the mean and SEM for trials −40 to 0. (C) MPFC-evoked firing decreased only during threshold BLA train stimulation (trials 41–80). The ** symbol indicates significant difference from “Pre” (trials −40 to 0) and “sub” (trials 1–40).
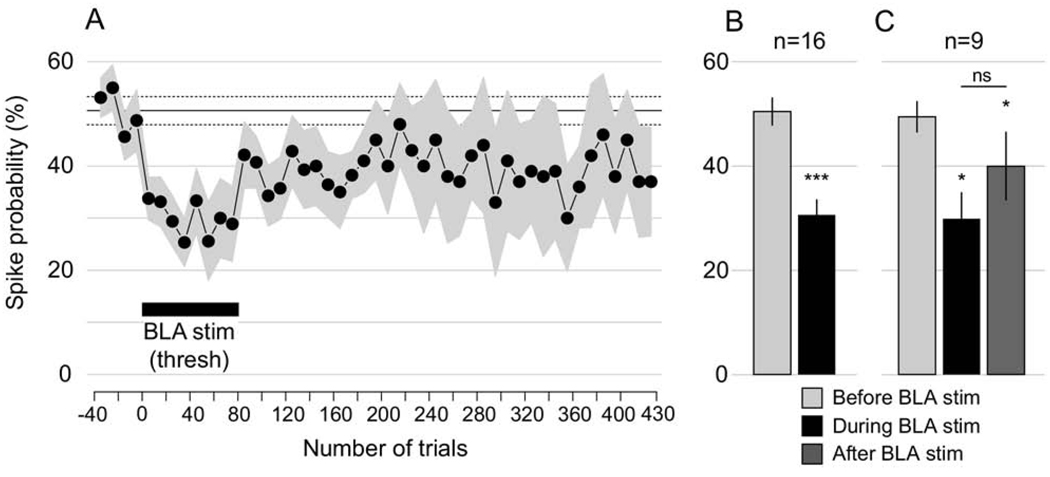
Nine additional neurons were tested with only threshold BLA stimulation (five rats); since the responses were similar, these data were combined with the seven neurons described above. In these 16 neurons, threshold BLA train stimulation decreased the mPFC-evoked spike probability by 19.9%±3.6% (P<0.0004; Fig. 3A, B). In a subset of neurons with stable post-train recording conditions (n=9/16), evoked spiking was recorded for 350 trials after the end of BLA trains. In these neurons the mPFC-evoked spike probability remained depressed by 17.0%±5.1% after BLA stimulation (P<0.022; Fig. 3C). Although threshold stimulation of the BLA appeared to be necessary to decrease mPFC-evoked spiking, there was no correlation between the number of spikes evoked by the BLA stimuli and the resulting decrease in mPFC-evoked spike probability (not shown; r=0.06, P<0.83 for entire BLA train; r=0.02, P<0.94 for the first BLA stimulus). Train stimulation had no effect on the latency of mPFC-evoked spikes (not shown).
Fig. 3.
In 16 neurons, mPFC-evoked spike probability was depressed during and after threshold BLA trains. (A) This time series shows mPFC-evoked spike probability before BLA stimulation (−40 to 0), during threshold BLA trains (“BLAstim (thresh),” 1–80), and up to 350 trials post-BLA stimulation. The gray area shows the SEM, and the black horizontal lines show the mean and SEM over trials −40 to 0. This sample includes the seven neurons shown in Fig. 2; to integrate that data into this figure, the 40 trials of subthreshold train stimulation were omitted from the time series. (B) mPFC-evoked spike probability not only decreased during threshold BLA stimulation (trials 0–80), but remained depressed after BLA trains ended (trials 100–430) in the 9/16 neurons for which data were available (C). The *** and * symbols indicate significant difference from “Pre” (trials −40 to 0). There was no significant difference (ns) between spike probability during and after trains.
Among these 16 neurons, eight were definitively localized to the NAcc shell, and four were localized to the core. BLA train stimulation significantly decreased mPFC-evoked spike probability in both groups of neurons (shell: −21.6%±4.9%, P<0.004; core: −13.4%±2.2%, P<0.001).
To confirm that only threshold intensity BLA stimulation decreases mPFC-evoked firing, 18 additional neurons (12 rats) were tested using only subthreshold BLA trains. The mPFC-evoked spike probability was unchanged during train stimulation in these neurons (not shown; increase of 0.3%±3.7%, P<0.94), and was unchanged within 100 trials following the end of the trains (−3.8%±5.1%, n=16/18, P<0.47). In addition, there was no relationship between the amplitude of the BLA stimulation current and the change in mPFC-evoked spike probability during trains (not shown; r=0.14, P<0.58).
DA or NMDA blockade did not attenuate BLA train effects
To assess the roles of DA and NMDA receptors in the BLA-mediated depression of mPFC-evoked spiking, the effects of BLA train stimulation were measured in the presence of antagonists for DA D1-like receptors (SCH23390), DA D2-like receptors (eticlopride or raclopride), or an NMDA channel blocker (MK-801) (Fig. 4). Typically, only one injection was performed and only one cell recorded per rat (see Experimental Procedures). In 14/14 neurons recorded after systemic injection of MK-801, BLA train stimulation decreased mPFC-evoked spike probability 17.1%±6.3% (P<0.017) during the train (Fig. 4A). Of these 14 neurons, nine were recorded for 350 trials following BLA trains. Although the spike probability both during and after train stimulation appeared to be less than the pre-train baseline in these nine neurons, the differences did not reach statistical significance (during P<0.1, after P<0.07; Fig. 4A). In 17/17 neurons recorded after injection of SCH23390, mPFC-evoked spike probability decreased during train stimulation by 17.6%±5.5% (P<0.006; Fig. 4B). Unlike the other antagonist drugs tested, SCH23390 treatment attenuated the post-train spike probability depression; post-train spiking was only 6.0%±5.4% less than pre-train responses (n=11/17, P<0.30) and was 22.7%±4.7% greater than spiking during the train (n=11/17, P<0.002; Fig. 4B). Finally, in 15/15 neurons recorded after injection of either eticlopride or raclopride (the data are similar and are pooled), mPFC-evoked spike probability was reduced both during (−17.1%±6.3%, P<0.006) and after BLA train stimulation (−13.5%±6.5%, n=11/15, P<0.024; Fig. 4C).
Fig. 4.
The effects of BLA train stimulation were not reversed by NMDA channel or DA receptor blockade. The left two bars in each group show data from all neurons tested; the right three bars in each group show data from the subset of neurons for which sufficient post-train responses were observed. In neurons recorded after systemic injection of MK-801 (A, n=14), SCH23390 (B, n=17), or eticlopride/raclopride (C, n=15), mPFC-evoked spike probability was reduced during threshold BLA trains (left bars). In the presence of MK-801 (A, n=9/14), post-train spiking was not significantly depressed, but this group of nine neurons also exhibited no significant depression during the train (right bars). In neurons recorded with eticlopride or raclopride (C, n=11/15), post-train spike probability was significantly less than pre-train responses and did not differ from spike probability during the train. In contrast, SCH23390 (B, n=11/17) attenuated the post-train depression. The ** and *symbols indicate significant difference from “Before BLA stim” within each set of bars, or between “During” and “After” as indicated. The “ns” symbol indicates no significant difference.
In a subset of neurons, the mPFC-evoked spike probability (with no BLA stimulation) was measured during administration of DA- or NMDA-receptor blocking drugs. Consistent with previous observations (West and Grace, 2002), injection of SCH23390 reduced the mPFC-evoked spike probability by 26.4%±6.5% (not shown, n=8, P<0.003, paired t-test). However, as noted above, this decrease did not occlude spike probability depression during BLA train stimulation. Neither MK-801 (n=8) nor the DA D2 antagonist drugs (n=13) altered mPFC-evoked firing (not shown). The effects of antagonist drugs were similar in NAcc core and shell neurons (not shown).
DISCUSSION
The integration of BLA and mPFC synaptic inputs onto single NAcc neurons may be a critical mechanism by which the NAcc supports reward-directed actions. We have previously shown that single-pulse stimulation of BLA and mPFC afferent inputs mutually facilitates the firing of NAcc neurons (McGinty and Grace, 2009). In the present study, repetitive activation of the BLA was found to depress mPFC-to-NAcc input, representing a second mechanism of input integration at the single cell level. In neurons receiving excitatory input from both mPFC and BLA, the probability of evoking a spike by mPFC stimulation was reduced when the BLA was repetitively stimulated at 20 Hz; this reduction persisted for more than 300 trials (15 min) after the BLA train stimulation ended. BLA stimulation was only effective when the BLA current was sufficient to evoke spikes (~50% probability) in the NAcc neuron being recorded. Subthreshold BLA stimulation had no effect on mPFC-evoked responses on average, and there was no relationship between the subthreshold current amplitude and the change in mPFC-evoked firing. Thus, the effects of high frequency BLA stimulation appear to depend on BLA-evoked spiking activity in the recorded neuron, rather than the absolute intensity of BLA stimulation.
Mechanisms for amygdala-mediated response depression
BLA train stimulation was predicted to activate NMDA and DA receptors (Floresco et al., 2001a,b), which we proposed could contribute to the robust depression of mPFC inputs during BLA trains. However this inhibitory action was not reversed by the NMDA channel blocker MK-801, the DA D1/D5 receptor antagonist SCH23390, or the D2/D3 antagonists eticlopride and raclopride. Although these DA antagonist drugs have low affinity for DA D4 receptors (Tang et al., 1994; Vallone et al., 2000), D4 receptors may not be the primary subtype that mediates the effects of NAcc DA: they are relatively sparse in the rat NAcc (Tarazi et al., 1997, 1998), and selective block of NAcc D4 receptors does not reduce the reinforcing effects of cocaine (Bari and Pierce, 2005; Anderson et al., 2006).
BLA stimulation was only effective when it evoked spikes in the recorded neuron, which is strong evidence that sufficient post-synaptic depolarization is necessary for depression of evoked spiking. Because BLA stimulation increases glutamate concentrations outside of the synapse (Jackson and Moghaddam, 2001), volume transmission to elements other than the recorded neuron (e.g. presynaptic terminals of mPFC-to-NAcc inputs, nearby inhibitory interneurons) could also contribute to the activity-dependent depression. One consequence of repetitive BLA-evoked spiking may be increased calcium concentration within the recorded neuron (Kerr and Plenz, 2002; Wolf et al., 2005). This supports a potential mechanism in which calcium influx could reduce synaptic excitability through calcium-activated second messengers. Activity-dependent depression may also be mediated by the activation of depolarization-dependent potassium conductances (Surmeier et al., 1989; Nisenbaum et al., 1994; Mahon et al., 2000) that could increase membrane conductance, reduce excitability and attenuate spiking evoked by mPFC afferent synaptic activation (but see Mahon et al. (2000)). Although in vitro recordings have demonstrated response depression during afferent train stimulation (Pennartz and Lopes da Silva, 1994; Lape and Dani, 2004), these effects are likely mediated by homosynaptic vesicle depletion in the stimulated inputs (Lape and Dani, 2004). Intracellular recording will be necessary to determine whether intracellular calcium or active conductances contribute to the activity-dependent depression of mPFC inputs.
Interestingly, whereas BLA-evoked spiking in the recorded neuron was necessary for the depression of mPFC inputs, the amount of spiking elicited by the BLA trains did not predict the magnitude of the depression. Thus, an activity-dependent mechanism may be necessary to initiate input depression, while other mechanisms may determine its magnitude. Apart from DA, the NAcc is innervated by local and exogenous peptides, local interneurons and other neuromodulatory inputs that could be recruited during BLA stimulation (Delfs et al., 1998; Meredith, 1999; Tepper and Bolam, 2004).
mPFC input interactions with DA
High frequency stimulation of afferents to the NAcc (including BLA) can increase extracellular DA (Floresco et al., 1998; Jackson and Moghaddam, 2001; Howland et al., 2002) and induce DA- and NMDA-dependent synaptic plasticity in the NAcc (Floresco et al., 2001a). Therefore, BLA-mediated depression of mPFC inputs could also be mediated by these receptor systems. However, systemic blockade of DA or NMDA receptors did not attenuate these effects. Interestingly, blockade of D1-like receptors with SCH23390 did attenuate the response depression observed in the minutes after train stimulation ended. This is consistent with previous studies of the hippocampus-to-NAcc pathway, where a DA D1-dependent depression of BLA inputs was measured minutes after 20 Hz hippocam-pal train stimulation (Floresco et al., 2001b). Thus, our results suggest that the afferent input arising from the BLA may also mediate a long-duration, DA D1-mediated depression of NAcc responses, and that this may represent a common consequence of intense afferent activation in this region. While no effect of NMDA channel block on posttrain depression was observed, those data may lack sufficient statistical power due to the variability in the response.
Several studies have reported that both DA D1 and D2 receptors modulate cortically evoked responses in the NAcc and striatum (West and Grace, 2002; Goto and Grace, 2005); however, only D1 receptor blockade was found to alter the evoked responses. This discrepancy may be due to the different ways in which evoked responses were measured or the different locations of the recorded responses: subthreshold EPSPs in dorsal striatum (West and Grace, 2002) and field potentials in NAcc core (Goto and Grace, 2005) compared to extracellular spike activity in NAcc shell and core in the current study. Brady and O’Donnell (2004) found that D2 receptor antagonists facilitated mPFC-evoked EPSPs in NAcc core and shell neurons, but only relative to responses evoked during the up-state, and only when NAcc DA was increased by ventral tegmental area stimulation. They reported no effect of D2 antagonists on responses evoked by mPFC stimulation alone (their Fig. 7C, 7D). Thus, the effects of D2 antagonists may depend on the method of measuring cortically evoked responses, the specific recording site, and the amount of synaptically released DA.
Implications for normal and pathological accumbens function
In light of our previous study in this circuit (McGinty and Grace, 2009), the current findings suggest that the BLA can facilitate or depress the mPFC drive of the NAcc depending on the timing and intensity of BLA afferent activity. This dual-nature interaction between the BLA and mPFC inputs has implications for the transition from healthy cognitive function to pathological mental states. The NAcc has been posited as a structure in which emotional and goal-related motor information directly interface: the NAcc integrates signals from the BLA, mPFC and other inputs, activating an appropriate motor plan (Nicola, 2007). We have proposed that in healthy individuals, no single afferent system dominates NAcc activity, and behavior reflects the balanced influences of affective, goal-oriented, and contextual constraints (Grace, 2000). In several psychiatric disorders, the amygdala is persistently active or hyper-excitable. In individuals with schizophrenia, amygdala activation is greater than in healthy subjects in response to both neutral and fearful faces (Hall et al., 2008), and similar high levels of amygdala activation occur in both addicted individuals (Kilts et al., 2001) and sufferers of posttraumatic stress (Shin et al., 2005). The hyper-responsivity of the amygdala in these disorders may lead to an unusually elevated BLA drive of the NAcc and thus a decreased responsivity of NAcc neurons to prefrontal cortical input. Unbalanced BLA/mPFC afferent interactions in the NAcc may lead to impaired motivation and cognitive function, as prefrontal signals relating to learned safety (Milad and Quirk, 2002) or non-drug goals (Kaufman et al., 2003) become less effective in driving NAcc neurons and guiding behavior. Such a reduction in corticostriatal throughput could be a particularly devastating insult in schizophrenia or drug addiction, where the capability of prefrontal cortex itself is diminished during the course of the disease (Lewis et al., 2001; Kalivas and Volkow, 2005). Although the mechanism underlying the amygdala-mediated depression of cortical inputs is unknown, this phenomenon has the potential to be a novel target for pharmacological intervention in the treatment of these disorders.
Acknowledgments
We wish to thank Nicole MacMurdo, Christy Smolak and Emily Mahar for technical assistance, and Brian Lowry for development of the Neuroscope software. Clinton McCracken and Daniel Lodge provided useful discussions and comments on the manuscript.
This work was supported by USPS MH57440 (A.A.G.) and an Andrew Mellon Fellowship (V.B.M.).
Abbreviations
- AP
anterior–posterior
- BLA
basolateral amygdala
- DA
dopamine
- DV
dorsal–ventral
- ML
medial-lateral
- mPFC
medial prefrontal cortex
- NAcc
nucleus accumbens
- NMDA
N-methyl-d-aspartate.
REFERENCES
- Ambroggi F, Ishikawa A, Fields HL, Nicola SM. Basolateral amygdala neurons facilitate reward-seeking behavior by exciting nucleus accumbens neurons. Neuron. 2008;59:648–661. doi: 10.1016/j.neuron.2008.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson SM, Schmidt HD, Pierce RC. Administration of the D2 dopamine receptor antagonist sulpiride into the shell, but not the core, of the nucleus accumbens attenuates cocaine priming-induced reinstatement of drug seeking. Neuropsychopharmacology. 2006;31:1452–1461. doi: 10.1038/sj.npp.1300922. [DOI] [PubMed] [Google Scholar]
- Bari AA, Pierce RC. D1-like and D2 dopamine receptor antagonists administered into the shell subregion of the rat nucleus accumbens decrease cocaine, but not food, reinforcement. Neuroscience. 2005;135:959–968. doi: 10.1016/j.neuroscience.2005.06.048. [DOI] [PubMed] [Google Scholar]
- Biondo A-M, Clements R, Hayes D, Eshpeter B, Greenshaw A. NMDA or AMPA/kainate receptor blockade prevents acquisition of conditioned place preference induced by D2/3 dopamine receptor stimulation in rats. Psychopharmacology. 2005;179:189–197. doi: 10.1007/s00213-005-2201-y. [DOI] [PubMed] [Google Scholar]
- Brady AM, O’Donnell P. Dopaminergic modulation of prefrontal cortical input to nucleus accumbens neurons in vivo. J Neurosci. 2004;24:1040–1049. doi: 10.1523/JNEUROSCI.4178-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caine SB, Koob GF. Effects of dopamine D-1 and D-2 antagonists on cocaine self-administration under different schedules of reinforcement in the rat. J Pharmacol Exp Ther. 1994;270:209–218. [PubMed] [Google Scholar]
- Canini F, Bourdon L. Dopamine involvement in thermoregulatory responses to heat in rats. Neurosci Lett. 1998;241:91–94. doi: 10.1016/s0304-3940(97)00958-0. [DOI] [PubMed] [Google Scholar]
- Cervo L, Carnovali F, Stark JA, Mennini T. Cocaine-seeking behavior in response to drug-associated stimuli in rats: involvement of D3 and D2 dopamine receptors. Neuropsychopharmacology. 2003;28:1150–1159. doi: 10.1038/sj.npp.1300169. [DOI] [PubMed] [Google Scholar]
- Delfs JM, Zhu Y, Druhan JP, Aston-Jones GS. Origin of noradrenergic afferents to the shell subregion of the nucleus accumbens: anterograde and retrograde tract-tracing studies in the rat. Brain Res. 1998;806:127–140. doi: 10.1016/s0006-8993(98)00672-6. [DOI] [PubMed] [Google Scholar]
- Floresco SB, Blaha CD, Yang CR, Phillips AG. Dopamine D1 and NMDA receptors mediate potentiation of basolateral amygdala-evoked firing of nucleus accumbens neurons. J Neurosci. 2001a;21:6370–6376. doi: 10.1523/JNEUROSCI.21-16-06370.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Floresco SB, Blaha CD, Yang CR, Phillips AG. Modulation of hippocampal and amygdalar-evoked activity of nucleus accum-bens neurons by dopamine: cellular mechanisms of input selection. J Neurosci. 2001b;21:2851–2860. doi: 10.1523/JNEUROSCI.21-08-02851.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Floresco SB, Yang CR, Phillips AG, Blaha CD. Basolateral amygdala stimulation evokes glutamate receptor-dependent dopamine efflux in the nucleus accumbens of the anaesthetized rat. Eur J Neurosci. 1998;10:1241–1251. doi: 10.1046/j.1460-9568.1998.00133.x. [DOI] [PubMed] [Google Scholar]
- Gilmartin MR, McEchron MD. Single neurons in the medial prefrontal cortex of the rat exhibit tonic and phasic coding during trace fear conditioning. Behav Neurosci. 2005;119:1496–1510. doi: 10.1037/0735-7044.119.6.1496. [DOI] [PubMed] [Google Scholar]
- Goto Y, Grace AA. Dopaminergic modulation of limbic and cortical drive of nucleus accumbens in goal-directed behavior. Nat Neurosci. 2005;8:805–812. doi: 10.1038/nn1471. [DOI] [PubMed] [Google Scholar]
- Grace AA. Gating of information flow within the limbic system and the pathophysiology of schizophrenia. Brain Res Brain Res Rev. 2000;31:330–341. doi: 10.1016/s0165-0173(99)00049-1. [DOI] [PubMed] [Google Scholar]
- Hall J, Whalley HC, McKirdy JW, Romaniuk L, McGonigle D, McIntosh AM, Baig BJ, Gountouna VE, Job DE, Donaldson DI, Sprengelmeyer R, Young AW, Johnstone EC, Lawrie SM. Overactivation of fear systems to neutral faces in schizophrenia. Biol Psychiatry. 2008;54:70–73. doi: 10.1016/j.biopsych.2007.12.014. [DOI] [PubMed] [Google Scholar]
- Howland JG, Taepavarapruk P, Phillips AG. Glutamate receptor-dependent modulation of dopamine efflux in the nucleus ac-cumbens by basolateral, but not central, nucleus of the amygdala in rats. J Neurosci. 2002;22:1137–1145. doi: 10.1523/JNEUROSCI.22-03-01137.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishikawa A, Ambroggi F, Nicola SM, Fields HL. Dorsomedial prefrontal cortex contribution to behavioral and nucleus accumbens neuronal responses to incentive cues. J Neurosci. 2008;28:5088–5098. doi: 10.1523/JNEUROSCI.0253-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson ME, Moghaddam B. Amygdala regulation of nucleus accumbens dopamine output is governed by the prefrontal cortex. J Neurosci. 2001;21:676–681. doi: 10.1523/JNEUROSCI.21-02-00676.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang ZG, North RA. Membrane properties and synaptic responses of rat striatal neurones in vitro. J Physiol. 1991;443:533–553. doi: 10.1113/jphysiol.1991.sp018850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jodo E, Suzuki Y, Kayama Y. Selective responsiveness of medial prefrontal cortex neurons to the meaningful stimulus with a low probability of occurrence in rats. Brain Res. 2000;856:68–74. doi: 10.1016/s0006-8993(99)02386-0. [DOI] [PubMed] [Google Scholar]
- Kalivas PW, Volkow ND. The neural basis of addiction: a pathology of motivation and choice. Am J Psychiatry. 2005;162:1403–1413. doi: 10.1176/appi.ajp.162.8.1403. [DOI] [PubMed] [Google Scholar]
- Kaufman JN, Ross TJ, Stein EA, Garavan H. Cingulate hypoactivity in cocaine users during a GO-NOGO task as revealed by event-related functional magnetic resonance imaging. J Neurosci. 2003;23:7839–7843. doi: 10.1523/JNEUROSCI.23-21-07839.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kemp JM, Powell TP. The structure of the caudate nucleus of the cat: light and electron microscopy. Philos Trans R Soc Lond B Biol Sci. 1971;262:383–401. doi: 10.1098/rstb.1971.0102. [DOI] [PubMed] [Google Scholar]
- Kerr JN, Plenz D. Dendritic calcium encodes striatal neuron output during up-states. J Neurosci. 2002;22:1499–1512. doi: 10.1523/JNEUROSCI.22-05-01499.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilts CD, Schweitzer JB, Quinn CK, Gross RE, Faber TL, Muhammad F, Ely TD, Hoffman JM, Drexler KP. Neural activity related to drug craving in cocaine addiction. Arch Gen Psychiatry. 2001;58:334–341. doi: 10.1001/archpsyc.58.4.334. [DOI] [PubMed] [Google Scholar]
- Lape R, Dani JA. Complex response to afferent excitatory bursts by nucleus accumbens medium spiny projection neurons. J Neurophysiol. 2004;92:1276–1284. doi: 10.1152/jn.00066.2004. [DOI] [PubMed] [Google Scholar]
- Laviolette SR, Lipski WJ, Grace AA. A subpopulation of neurons in the medial prefrontal cortex encodes emotional learning with burst and frequency codes through a dopamine D4 receptor-dependent basolateral amygdala input. J Neurosci. 2005;25:6066–6075. doi: 10.1523/JNEUROSCI.1168-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis DA, Cruz DA, Melchitzky DS, Pierri JN. Lamina-specific deficits in parvalbumin-immunoreactive varicosities in the prefrontal cortex of subjects with schizophrenia: evidence for fewer projections from the thalamus. Am J Psychiatry. 2001;158:1411–1422. doi: 10.1176/appi.ajp.158.9.1411. [DOI] [PubMed] [Google Scholar]
- Mahon S, Delord B, Deniau JM, Charpier S. Intrinsic properties of rat striatal output neurones and time-dependent facilitation of cortical inputs in vivo. J Physiol. 2000;527(2):345–354. doi: 10.1111/j.1469-7793.2000.t01-1-00345.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mallet N, Le Moine C, Charpier S, Gonon F. Feedforward inhibition of projection neurons by fast-spiking GABA interneurons in the rat striatum in vivo. J Neurosci. 2005;25:3857–3869. doi: 10.1523/JNEUROSCI.5027-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGinty VB, Grace AA. Selective activation of medial prefrontal-to-accumbens projection neurons by amygdala stimulation and Pavlovian conditioned stimuli. Cereb Cortex. 2008;18:1961–1972. doi: 10.1093/cercor/bhm223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGinty VB, Grace AA. Timing-dependent regulation of evoked spiking in nucleus accumbens neurons by integration of limbic and prefrontal cortical inputs. J Neurophysiol. 2009;101:1823–1835. doi: 10.1152/jn.91162.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McQuade RD, Chipkin R, Amlaiky N, Caron M, Iorio L, Barnett A. Characterization of the radioiodinated analogue of SCH 23390: in vitro and in vivo D-1 dopamine receptor binding studies. Life Sci. 1988;43:1151–1160. doi: 10.1016/0024-3205(88)90474-2. [DOI] [PubMed] [Google Scholar]
- Meredith GE. The synaptic framework for chemical signaling in nucleus accumbens. Ann N Y Acad Sci. 1999;877:140–156. doi: 10.1111/j.1749-6632.1999.tb09266.x. [DOI] [PubMed] [Google Scholar]
- Milad MR, Quirk GJ. Neurons in medial prefrontal cortex signal memory for fear extinction. Nature. 2002;420:70–74. doi: 10.1038/nature01138. [DOI] [PubMed] [Google Scholar]
- Nicola SM. The nucleus accumbens as part of a basal ganglia action selection circuit. Psychopharmacology (Berl) 2007;191:521–550. doi: 10.1007/s00213-006-0510-4. [DOI] [PubMed] [Google Scholar]
- Nicola SM, Surmeier J, Malenka RC. Dopaminergic modulation of neuronal excitability in the striatum and nucleus accumbens. Annu Rev Neurosci. 2000;23:185–215. doi: 10.1146/annurev.neuro.23.1.185. [DOI] [PubMed] [Google Scholar]
- Nisenbaum ES, Xu ZC, Wilson CJ. Contribution of a slowly inactivating potassium current to the transition to firing of neostriatal spiny projection neurons. J Neurophysiol. 1994;71:1174–1189. doi: 10.1152/jn.1994.71.3.1174. [DOI] [PubMed] [Google Scholar]
- O’Neill MF, Shaw G. Comparison of dopamine receptor antagonists on hyperlocomotion induced by cocaine, amphetamine, MK-801 and the dopamine D1 agonist C-APB in mice. Psychopharmacology (Berl) 1999;145:237–250. doi: 10.1007/s002130051055. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The rat brain in stereotaxic coordinates. 4th ed. Academic Press; 1998. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The rat brain in stereotaxic coordinates. 5th ed. San Diego, CA: Elsevier Academic Press; 2005. [Google Scholar]
- Pennartz CM, Lopes da Silva FH. Muscarinic modulation of synaptic transmission in slices of the rat ventral striatum is dependent on the frequency of afferent stimulation. Brain Res. 1994;645:231–239. doi: 10.1016/0006-8993(94)91656-x. [DOI] [PubMed] [Google Scholar]
- Pratt WE, Mizumori SJ. Characteristics of basolateral amygdala neuronal firing on a spatial memory task involving differential reward. Behav Neurosci. 1998;112:554–570. doi: 10.1037//0735-7044.112.3.554. [DOI] [PubMed] [Google Scholar]
- R Development Core Team. R: a language and environment for statistical computing. Vienna, Austria: R. Foundation for Statistical Computing; 2005. [Google Scholar]
- Schoenbaum G, Chiba AA, Gallagher M. Neural encoding in orbitofrontal cortex and basolateral amygdala during olfactory discrimination learning. J Neurosci. 1999;19:1876–1884. doi: 10.1523/JNEUROSCI.19-05-01876.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulz D, Staples L, Mailman R. SCH23390 causes persistent antidopaminergic effects in vivo: evidence for longterm occupation of receptors. Life Sci. 1985;36:1941–1948. doi: 10.1016/0024-3205(85)90443-6. [DOI] [PubMed] [Google Scholar]
- Shin LM, Wright CI, Cannistraro PA, Wedig MM, McMullin K, Martis B, Macklin ML, Lasko NB, Cavanagh SR, Krangel TS, Orr SP, Pitman RK, Whalen PJ, Rauch SL. A functional magnetic resonance imaging study of amygdala and medial prefrontal cortex responses to overtly presented fearful faces in posttraumatic stress disorder. Arch Gen Psychiatry. 2005;62:273–281. doi: 10.1001/archpsyc.62.3.273. [DOI] [PubMed] [Google Scholar]
- Surmeier DJ, Bargas J, Kitai ST. Two types of A-current differing in voltage-dependence are expressed by neurons of the rat neostriatum. Neurosci Lett. 1989;103:331–337. doi: 10.1016/0304-3940(89)90122-5. [DOI] [PubMed] [Google Scholar]
- Tang L, Todd RD, Heller A, O’Malley KL. Pharmacological and functional characterization of D2, D3 and D4 dopamine receptors in fibroblast and dopaminergic cell lines. J Pharmacol Exp Ther. 1994;268:495–502. [PubMed] [Google Scholar]
- Tarazi FI, Campbell A, Yeghiayan SK, Baldessarini RJ. Localization of dopamine receptor subtypes in corpus striatum and nucleus accumbens septi of rat brain: comparison of D1-, D2-, and D4-like receptors. Neuroscience. 1998;83:169–176. doi: 10.1016/s0306-4522(97)00386-2. [DOI] [PubMed] [Google Scholar]
- Tarazi FI, Kula NS, Baldessarini RJ. Regional distribution of dopamine D4 receptors in rat forebrain. Neuroreport. 1997;8:3423–3426. doi: 10.1097/00001756-199711100-00001. [DOI] [PubMed] [Google Scholar]
- Tepper JM, Bolam JP. Functional diversity and specificity of neostriatal interneurons. Curr Opin Neurobiol. 2004;14:685–692. doi: 10.1016/j.conb.2004.10.003. [DOI] [PubMed] [Google Scholar]
- Vallone D, Picetti R, Borrelli E. Structure and function of dopa-mine receptors. Neurosci Biobehav Rev. 2000;24:125–132. doi: 10.1016/s0149-7634(99)00063-9. [DOI] [PubMed] [Google Scholar]
- West AR, Grace AA. Opposite influences of endogenous dopamine D1 and D2 receptor activation on activity states and electro-physiological properties of striatal neurons: studies combining in vivo intracellular recordings and reverse microdialysis. J Neurosci. 2002;22:294–304. doi: 10.1523/JNEUROSCI.22-01-00294.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson CJ, Chang HT, Kitai ST. Firing patterns and synaptic potentials of identified giant aspiny interneurons in the rat neostri-atum. J Neurosci. 1990;10:508–519. doi: 10.1523/JNEUROSCI.10-02-00508.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf JA, Moyer JT, Lazarewicz MT, Contreras D, Benoit-Marand M, O’Donnell P, Finkel LH. NMDA/AMPA ratio impacts state transitions and entrainment to oscillations in a computational model of the nucleus accumbens medium spiny projection neuron. J Neurosci. 2005;25:9080–9095. doi: 10.1523/JNEUROSCI.2220-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]