Abstract
In this article, we discuss the hypothesis that affect is a fundamental, psychologically irreducible property of the human mind. We begin by presenting historical perspectives on the nature of affect. Next, we proceed with a more contemporary discussion of core affect as a basic property of the mind that is realized within a broadly distributed neuronal workspace. We then present the affective circumplex, a mathematical formalization for representing core affective states, and show that this model can be used to represent individual differences in core affective feelings that are linked to meaningful variation in emotional experience. Finally, we conclude by suggesting that core affect has psychological consequences that reach beyond the boundaries of emotion, to influence learning and consciousness.
“… stimuli do something more than arouse sensation; they give rise to processes of a different kind, to “feelings” in a special sense; we do not merely take the impressions as they come, but we are affected by them, we feel them”
Titchener (1909, p. 226)
In English, the word “affect” means “to produce a change.” To be affected by something is to be influenced by it. In science, and particularly in psychology, “affect” refers to a special kind of influence—something’s ability to influence your mind in a way that is linked to your body. Historically, “affect” referred to a simple feeling—to be affected is to feel something. In modern psychological usage, “affect” refers to the mental counterpart of internal bodily representations associated with emotions, actions that involve some degree of motivation, intensity, and force, or even personality dispositions. In the science of emotion, “affect” is a general term that has come to mean anything emotional. A cautious term, it allows reference to something’s effect or someone’s internal state without specifying exactly what kind of an effect or state it is. It allows researchers to talk about emotion in a theory-neutral way.
In this review, we begin with a historical account of the concept of affect in psychology. This sets the stage for discussing the contemporary view of core affect as a basic, universal, and psychologically irreducible property of the mind. We then describe the brain areas that are responsible for realizing core affect, illustrating its central role in mental life. Next, we present the affective circumplex as a mathematical formalization for representing core affective states. We then describe evidence from our own laboratory demonstrating that the circumplex can model and represent individual variation in core affective feelings that are linked to differences in the precision of emotional experience (termed emotional granularity). Finally, we end by describing our most recent research on how affective variation has important psychological consequences that reach beyond the boundaries of emotion. We describe how core affect forms a basis for learning and grounds consciousness for other senses like seeing.
1. Affect in the History of Psychology
WilhemWundt (1998b/1897), along with William James (1890), crafted the first psychological constructionist approaches to psychology (Gendron & Barrett, in press). Constructivist approaches are united in the assumption that the mental phenomena people experience and name (e.g., “thoughts,” “emotions,” “memories,” and “beliefs”) are events that result from the interplay of more basic psychological ingredients that are not themselves specific to any single psychological phenomenon. Whereas James focused on the importance of raw sensory processing of somatic, visceral, vascular, and motor cues from the body as the basic building block of the mind, Wundt focused on the mental counterpart of those internal cues, which he called “affect.”1
Affect, according to Wundt, is a feeling state that is a fundamental ingredient of the human mind. People are, wrote Wundt, likely “never in a state entirely free from feeling” (1897/1998b, p. 92). Wundt argued that affect is a direct (uninterpreted), psychologically primitive (psychologically irreducible) experience. He also argued that internally-generated sensations were as important to mental life as externally-driven sensations, so that affect (what he called “simple feelings”) and sensation were two sides of the same mental coin. Internal and external sensations “do not indicate separate objects,” wrote Wundt, “but different points of view from which we start in the consideration and scientific treatment of a unitary experience” (1897/1998b, p. 2). Wundt referred to simple feelings as the “affective tone of a sensation” (1987/1998b, p. 75).
Wundt described momentary affective states as having three independent qualities—pleasantness/unpleasantness (now called hedonic valence), arousing/subduing (arousal), and strain/relaxation (intensity). According to Wundt, these properties were not ingredients that make an affective response, because affect itself is irreducible and cannot be decomposed into more basic parts. Instead, valence, arousal, and intensity are descriptive features of a unified state. These three properties define the multidimensional affective space that people inhabit, such that a person’s momentary affective state can be described in these terms. Furthermore, Wundt believed that there was great variety in the nature of simple feelings, so that pleasure and displeasure did not refer to uniform states. It is “entirely untenable,” wrote Wundt, that the “unpleasurableness of a toothache, of an intellectual failure, and of a tragic experience are all regarded as identical in their affective contents” (p. 85).
Edward Titchener (Wundt’s student) largely agreed with Wundt, save two modifications (Titchener, 1909). First, Titchener believed that affect had only one property—hedonic valence—on the somewhat flawed reasoning that pleasure and displeasure were clearly accessible to introspection. Second, Titchener, more so than Wundt, believed that the content of feelings revealed their process (i.e., those feelings of pleasure and displeasure reveal the process of evaluation). This latter assumption has caused a great degree of confusion in scientific discussions about the basic dimensions of affect, as we discuss later.
Like most “dimensional” approaches, Wundt and Titchener did not argue that mental states are reduced to only affective feelings. Instead, they argued that affect is a mental element that can become an emotion when combined with other mental elements. This assumption inspired many similar models of emotion during the first half of the twentieth century (e.g., Beebe-Center, 1932; Duffy, 1934; Gemelli, 1949a,b; Hunt, 1941; Ruckmick, 1936; Young, 1943) and defined a theoretical tradition that was carried forward by Schachter and Singer (1962), Mandler (1975), Russell (2003), and Barrett (2006b). Wundt, in particular, emphasized that emotions are not static things or entities, but instead are “psychical compounds” or composites that are constituted out of “psychical elements,” like affect, that are simple and irreducible in a psychological sense (1897/1998b, p. 101). He proposed that the additional element in emotion was “ideas,” which he described as “revival of previous experiences” (1894/1998, p. 452).2 For our purposes, the important point is that most theorists who are labeled as having a “dimensional” perspective on emotion, including Wundt and Titchener, did not argue that affect was sufficient to explain mental states. They only proposed that it was necessary.
Wundt and Titchener inspired several decades of debate about affect during the first decades of the twentieth century. First, there was debate over whether affect was more like a sensation (i.e., a sixth sense to vision, taste, etc.) or like a mental feeling. Most writers favored the latter conclusion. For example, Alechsieff (1907; cited in Arnold, 1960) argued that affect is not a sensation on the grounds that it cannot be parsed and analyzed as distinct modalities like vision, audition, and touch. Koch (1913; cited in Arnold, 1960) added that affect is not a distinct sensory modality because it is derived from “diffuse organic sensations,” in effect arguing that affect can be distinguished from sensations that derive from the external sensory world, but not from those sensations that derive from the internal sensory world (i.e., the body). In modern terms, Koch’s proposal would be that affect is, essentially, a redescription of internal sensation in personally relevant terms. In contrast, Arnold herself argued that affect (as feeling) is completely separate from all sensations and always occurs in reaction to them. Importantly, Arnold’s writing forms the basis of most modern appraisal views of emotion.
A second debate inspired by Wundt and Titchener dealt with the question of whether affect is distinct from emotion. Most writers assumed that the answer was yes, but for different reasons. Some argued that feelings of pleasantness and unpleasantness are something more akin to an attitude or an action tendency derived from the feeling of wanting to approach or avoid an object (e.g., Carr, 1925; Hunt, 1939; Peters, 1935; Young, 1943). These feelings could then be shaped into emotion via additional processes. In these models, which have a largely constructionist flavor that is similar to Wundt and Titchener, emotion is just one class of affective feeling. Arnold (1960), on the other hand, used the word “affect” to refer to “feelings” as categorically separate from “emotions” which she described in more behaviorally mechanistic terms (i.e., a tendency to move towards or away from an object during basic emotions). For Arnold, affect is a state of mind that occurs in response to emotion—it is unpleasant to be angry or sad or afraid and pleasant to be excited or happy or tranquil. According to Arnold, both sensations and emotions inspire affective feeling (that are pleasant or unpleasant) by virtue of their influence.
Amidst these debates, the last century has seen a steady accumulating of evidence that Wundt’s initial proposals about affect were largely correct. In the next section, we discuss how a person’s momentary mental state (however it is categorized) can be described as pleasant or unpleasant with some degree of arousal. Together valence and arousal describe something psychologically primitive—a basic or “core” ingredient common to all psychological states. In the section following that, we describe the neuroanatomical evidence that a core affective state is, at once, tied to a person’s interoceptive sensations from the body and exteroceptive sensations from the world.
2. A Modern Wundtian View: Core Affect
Core affect is a state of pleasure or displeasure with some degree of arousal (Barrett, 2006b,c; Russell, 2003; Russell & Barrett, 1999). Together, valence and arousal form a unified state, so although it is possible to focus on one property or the other, people cannot feel pleasant or unpleasant in a way that is isolated from their degree of arousal.3 This kind of affect is referred to as “core” for a number of reasons.
Barring injury, core affect is grounded in the somatovisceral, kinesthetic, proprioceptive, and neurochemical fluctuations that take place within the core of body (Barrett, 2006a; Nauta, 1971). As we will see in the next section, core affect is realized by integrating incoming sensory information from the external world with homeostatic and interoceptive information from the body. The result is a mental state that can be used to safely navigate the world by predicting reward and threat, friend and foe.
Affect is a central feature in many psychological phenomena, including emotion (Barrett, 2006a,b; Diener, 1999; Russell, 2003), attitudes (e.g., Cacioppo & Berntson, 1994; Eagly & Chaiken, 1998; Ito & Cacioppo, 2001), stereotyping and prejudice (e.g., Cacioppo & Berntson, 2001; Forgas & Fiedler, 1996; Mackie & Hamilton, 1993; Moreno & Bodenhausen, 2001), verbal communication and negotiation strategies (e.g., Forgas, 1998, 1999a,b), judgment and decision-making (e.g., Forgas, 1995; Haidt, 2002; Slovic et al., 2002), predicting the future (e.g., Gilbert & Ebert, 2002; Gilbert et al., 1998), work motivation (e.g., Seo et al., 2004), psychopathology (e.g., Davidson, 2000; Davidson et al., 2002), well-being, (e.g., Davidson, 2004), health (Gallo et al., 2005), and personality (e.g., Revelle, 1995; Watson, 2000; Yik et al., 2002). Core affect provides a common metric (or what neuroeconomists call a “common currency”) for comparing qualitatively different events (Cabanac, 2002), and can serve as the basis for moral judgments of right and wrong (Greene et al., 2001; Haidt, 2001). It also serves as a basic of language comprehension. A speaker’s tone of voice (speaking rate, tone of voice, and intonation) as well as acoustical cues to the identity of a speaker routinely impacts the affective state of the listener (Nygaard & Lunders 2002; Owren & Rendell, 1997) and these cues influence lexical processing (Schirmer & Kotz, 2003; Wurm et al., 2001). Affective tone even influences the perception of spoken words, making it easier to recognize some words and harder to recognize others (Nygaard & Queen, 2008). In the final section of the paper, we discuss how core affect is important in normal object perception (see Barrett & Bar, in press). People see with feeling. We “gaze,” “behold,” “stare,” “gape,” and “glare.” Without affect, there is visual sensation, but no sight.
Core affect also represents a basic kind of psychological meaning. The basic acoustical properties of animal calls (and human voices) directly act on the nervous system of the perceiving animal to change its affective state and in so doing conveys the meaning of the sound (Owren & Rendall, 1997, 2001).4 All words (regardless of language) have an affective dimension of meaning (Osgood et al., 1957), so that people cannot communicate without also (often inadvertently) communicating something about their affective state. Learning a new language fluently does not merely require making a link between the phonological forms of words and their denotation, but a connection to affective changes must also be forged.
Finally, as we discuss in the final section of the paper, affective changes are “core” because they are crucial to the conscious experience of the world around us (for a discussion, see Duncan & Barrett, 2007). Affective changes are often experienced as a property of an object, in much the same way as color (people say “The sky is blue” rather than “I experience the sky as blue” or “Light from the sky at 500 nm is striking my retina which I experience as blue”). Indeed, objects in the world are said to be “positive” or “negative” by virtue of their capacity to influence a person’s core affective state. For example, if the perception of a snake involves unpleasant, high arousal affect, then the snake is said to be negative and arousing.
People are often aware of their core affective state, although they need not be. The capacity to have core affective states is psychologically universal and biologically basic, although people largely learn which sensory patterns predict threat and reward through experience. Infants (Lewis, 2000) and people in all cultures around the world have core affective experiences (Mesquita, 2003). Scientists can clearly measure core affect in the face (for reviews, see Cacioppo et al., 2000), in the voice (for reviews, see Bachorwoski, 1999; Russell et al., 2003), and in the peripheral nervous system (for reviews, see Bradley & Lang, 2000; Cacioppo et al., 2000). As a consequence, core affect can be thought of as a neurophysiologic barometer of the individual’s relationship to an environment at a given point in time, with self-reported feelings as the barometer readings.
3. The Neural Reference Space for Core Affect
With several decades of modern neuroscience evidence to draw from, it is now possible to see that Wundt was probably right about the relation between affect and external sensations. Both neuroanatomical and neuroimaging evidence suggests that people don’t evaluate an object for its personal significance once they already know what it is. Their affective reaction to the external sensory array helps the brain to make external sensations meaningful, aiding perception in a very basic way.
The distributed circuitry for core affect can be found in every mammalian brain and is particularly elaborated in the human brain (Fig. 4.1). These areas represent crucial components of a network that bind sensory stimulation from inside the body to that coming from outside the body, and in so doing each gives the other informational value. Some parts of affective circuitry are strongly interconnected with sensory cortical areas, whereas others are strongly interconnected with areas that direct the autonomic and hormonal responses to regulate the homeostatic state of the body (Barrett & Bar, in press). The strongly re-entrant nature of neural activity makes it difficult to derive simple cause and effect relationships between the brain and the body, or between sensory and affective processing.
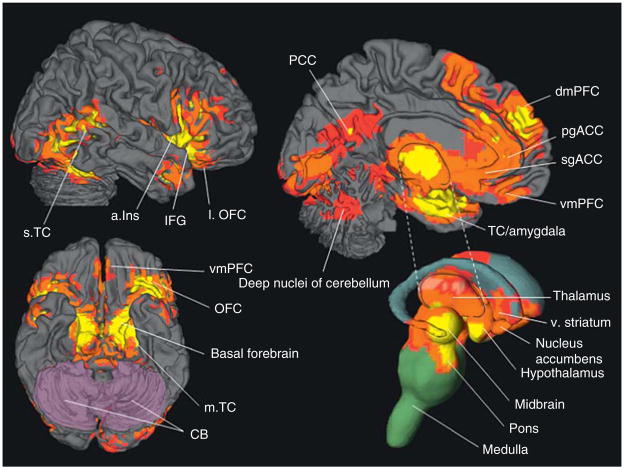
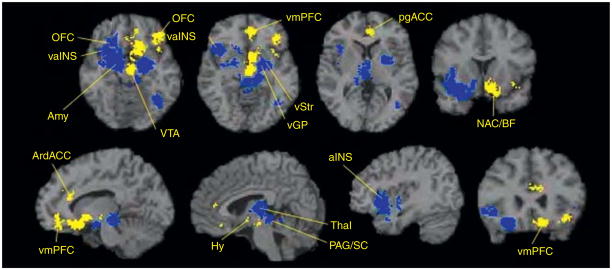
Figure 4.1.
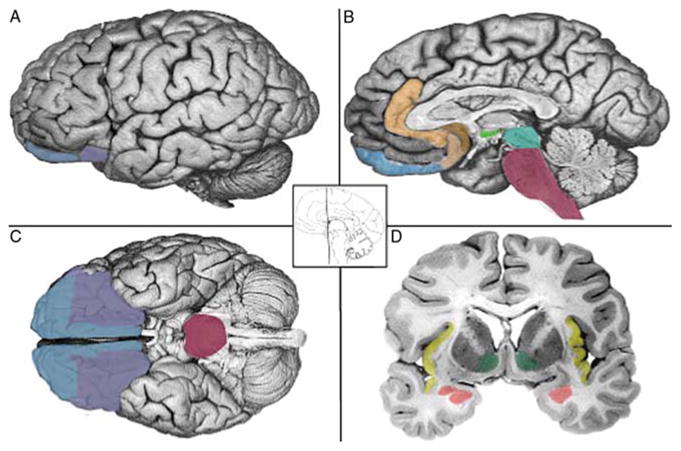
The hypothesized neural reference space for core affect. Brain areas that realize core affect include the visceromotor and sensory integration networks in the OFC (A–C, blue, and purple, respectively), the anterior insula (D, yellow), the amygdala (D, rose), subgenual and pregenual parts of the ACC (B, copper, tan), the hypothalamus (B, light green), and the ventral striatum (D, dark green). Also included are the midbrain (B, turquoise) and brainstem (B, C, dark pink). Adapted from Barrett et al. (2007). Refer online version of the chapter for color figure.
Core affective circuitry includes brain areas that are traditionally considered to be “emotional,” such as the amygdala and ventral striatum. The amygdala’s role in affective circuitry is not to code for fear, or threat, or anything negative per se. Instead, the amygdala’s function is to direct the various sources of attention (Holland & Gallagher, 1999) towards a source of sensory stimulation (such as an object) when the predictive value of that stimulation is unknown or uncertain (cf. Barrett et al., 2007). As a consequence, the brain can orchestrate physiology and physical actions that allow it to learn more about the object to better predict its value on future encounters. The amygdala’s work is complete once an object’s value is known for that particular context and in that particular instance. When the threat or rewarding value again becomes uncertain the amygdala is once again engaged (e.g., Barad et al., 2006; Herry et al., 2007). This interpretation is not only consistent with the neuroscience research showing that rats freeze during aversive classical conditioning (in our view mistakenly called “fear” conditioning), but it is also consistent with the research showing that the amygdala is selectively engaged by novelty (e.g., Dubois et al., 1999; Schwartz et al., 2003; Wilson & Rolls, 1990; Wright et al., 2003, 2006, 2007, 2008) and ambiguity (Hsu et al., 2005), and quickly habituates to stimuli as they become familiar (Breiter et al., 1996; Wedig et al., 2005; Wright et al., 2001, 2003).5 Furthermore, amygdala lesions disrupt normal responses to novelty in primates (e.g. Prather et al., 2001). For a related view, see Whalen (1998).
The ventral striatum (and the larger mesolimbic dopamine system of which it is a part) does not to code for reward or positivity per se, but instead gates attention to novel, salient, or unexpected environmental events that require an effortful (usually behavioral) response, regardless of whether they are positive or negative (e.g., Berridge & Robinson, 1998; Horvitz, 2000, 2002; Salamone et al., 2005, 2007; Schultz et al., 1993). Consistent with this view, both approach and withdrawal behaviors in rats are facilitated via electrical stimulation of the rostral and caudal shells of the nucleus accumbens (which is part of the ventral striatum; Reynolds & Berridge, 2001, 2002, 2003) and approach behaviors become dopamine independent with overtraining (Choi et al., 2005). Dopamine neurons within the ventral striatum increase their firing rates when surprising or unexpected appetitive events are presented (McCullough & Salamone, 1992), but firing rates do not increase when appetitive events are predictable (Mirenowicz & Schultz, 1994). New evidence in rats demonstrates a context dependent functional remapping of cells in the nucleus accumbens; the same cells code for reward or threat depending on the context in which the rat is placed (Reynolds & Berridge, 2008).
Core affective circuitry also includes paralimbic portions of prefrontal cortex that until recently have been considered “cognitive” (cf. Duncan & Barrett, 2007). These areas include the lateral portions of the orbitofrontal cortex (OFC) extending back to the agranular insula and laterally to the ventrolateral prefrontal cortex (vlPFC), as well as the medial portions of the OFC (sometimes included in the ventromedial prefrontal cortex or vmPFC) extending back to the subgenual and pregenual portions of the anterior cingulate cortex (ACC) on the medial wall. The OFC is a hetero-modal association area that integrates sensory inputs from the external world and from the internal body to create a multimodal representation of the world at a particular moment in time (Mesulam, 2000). It plays a role in representing reward and threat (e.g., Kringelbach & Rolls, 2004) as well as in hedonic experience (Kringelbach, 2005; Wager et al., 2008).
Figure 4.1 demonstrates how the amygdala, ventral striatum, and OFC (including the vmPFC), along with the ACC, insula, thalamus, hypothalamus, and autonomic control centers in the midbrain brainstem, constitute a large-scale neural reference space that realizes neural representations of sensory information from the world as well as its somatovisceral impact (Barbas, 2007; Ghashghaei & Barbas, 2002; Ongur et al., 2003; reviewed in Duncan & Barrett, 2007). This description of affective circuitry is meant to be nonspecific without sounding vague, in that a “neural reference space” (according to neuroscientist Gerald Edelman) refers to a neuronal work-space that implements the brain states that correspond to mental states. Different brain states are implemented as flexible neuronal assemblies, so that a given neuron need not participate in every brain state within a class (such as reward or hedonic pleasure), or even in the exact same mental state at two different points in time. The assembly of neurons involved in realizing the constantly changing flow of affective states shifts from moment to moment, so that particular neurons are selective for affect but may not be specific to affect in any way.6 Furthermore, this circuitry, although not specific to emotion, is nicely illustrated within a meta-analysis summarizing functional magnetic resonance imaging (fMRI) and positron emission tomography (PET) studies of emotion and affect published between 1990 and 2005 (see Fig. 4.2; Wager et al., 2008).
Figure 4.2.
The observed neural reference space for core affect. 165 neuroimaging studies of emotion (58 using PET and 107 using fMRI) published from 1990 to 2005 were summarized in a multilevel meta-analysis to produce the observed neural reference space for emotion (Wager et al., 2008). These areas include (from top left, clockwise) anterior insula (aIns), lateral OFC (lOFC), pregenual cingulate cortex (pgACC), subgenual cingulate cortex (sgACC), ventral medial prefrontal cortex (vmPFC), temporal cortex/amygdala (TC/Amygdala), thalamus, ventral striatrum (v Striatum), nucleus accumbens, hypothalamus, midbrain, pons, medulla, OFC, and basal forebrain. Other areas shown in this figure (e.g., inferior frontal gyrus (IFG), superior temporal cortex (sTC), dorsal medial prefrontal cortex (dmPFC), posterior cingulate cortex (PCC), medial temporal cortex (mTC), and cerebellum (CB)) relate to other psychological processes involved with emotion perception and experience. (See online version of the chapter for color figure).
Although the details are continually being researched, the available evidence suggests that this larger neutral reference space for core affect can be subdivided into two related functional networks (for reviews, see Barbas & Pandya, 1989; Carmichael & Price, 1996; Hurliman et al., 2005; Ongur & Price, 2000; Ongur et al., 2003). The first functional network is a sensory integration network. This network establishes an experience-dependent, value-based representation of an object that includes both external sensory features of an object along with its impact on the homeostatic state of the body. It includes the cortical aspects of the amygdala (specifically, the baso-lateral complex (BL)), the central and lateral portions of OFC, as well as most of the adjacent agranular insular areas. The sensory integration network has robust connections with unimodal association areas of many sensory modalities (Barbas, 1993, 2000; Carmichael & Price, 1995; Cavada et al., 2000; Ghashghaei & Barbas, 2002; McDonald, 1998), including the anterior insula that represents interoceptive sensations (Craig, 2002).
The second functional network is a visceromotor network and is part of a functional circuit that guides autonomic, endocrine, and behavioral responses to an object. It includes the medial portions of the OFC (extending into what is sometimes called the vmPFC), as well as subgenual and pregenual areas of the ACC, with robust reciprocal connections to all limbic areas (including many nuclei within the amygdala, and the ventral striatum), as well as to the hypothalamus, midbrain, brainstem, and spinal cord areas that are involved in internal-state regulation (Barbas & De Olmos, 1990; Barbas et al., 2003; Carmichael & Price, 1995, 1996; Ghashghaei & Barbas, 2002; Ongur et al., 1998; Price, 2007; Rempel-Clower & Barbas, 1998). These areas modulate changes in the viscera associated with the autonomic nervous system (including tissues and organs made of smooth muscle, such as the heart and lungs) and neuroendocrine changes that affect the same organs by way of the chemicals released into the bloodstream via hypothalamic regulation of the pituitary gland. In addition, the visceromotor network (particularly the vmPFC) is important for altering simple stimulus-reinforcer associations via extinction (Milad et al., 2005; Phelps et al., 2004; Quirk et al., 2000) or reversal learning (Fellows & Farah, 2003) and appears to be useful for decisions based on intuitions and feelings rather than on explicit rules (Dunn et al., 2006; Goel & Dolan, 2003; Shamay-Tsoory et al., 2005), including guesses and familiarity based discriminations (Bechara et al., 1997, 1999; Elliott et al., 1999, 2000; Schnider et al., 2000; Schnyer et al., 2005; Weller et al., 2007).
The circuitry within the neural reference space for core affect binds sensory information from the external world to sensory information from the body, so that every mental state is intrinsically infused with affective content. When core affect is in the background of consciousness, it is perceived as a property of the world, rather than as the person’s reaction to it. It is under these circumstances that scientists usually refer to affect as “unconscious.” We experience a world of facts rather than feelings, and affect gives us a sense of confidence in those facts. This is why a drink tastes delicious or is unappetizing (e.g., Berridge & Winkielman, 2003; Winkielman et al., 2005), why we experience some people as nice, and others as mean; and why some paintings are beautiful whereas others are ugly. When core affect is experienced as a property of the world it acts in stealth by directly translating into a behavior. We have another sip of Bordeaux because it tastes so good. We avoid an acquaintance on the street because he is mean. We stand for hours looking at the details of a painting because it is captivating. When affect is backgrounded in consciousness, we refer to “affective stimuli”—but affect is never a property of a stimulus—it is a feature of a person’s response to that stimulus. An object is said to have affective value precisely because it has the capacity to influence an individual’s core affective state. When core affect is in the foreground of consciousness, it is experienced as a personal reaction to the world: we like or dislike a drink, a person, or a painting. It is at these times that feelings which can be described as pleasant or unpleasant content with some degree of arousal can serve as information for making explicit judgments and decisions (Clore et al., 2005; Schwarz & Clore, 1983).
Finally, we hypothesize that the validity of experience is rooted in core affect. Core affect gives force to our attitudes and beliefs, and provides a sense that what we know is what is right or correct. It seems plausible, then, that core affect would contribute to confidence in our beliefs about political topics (e.g., global warming, abortion, etc.), our world view (e.g., belief in a just world, or in basic moral principles), or even form the core of religious faith (e.g., a strong affective response is how you believe in something that cannot be seen). It is no surprise, then, that the most affectively loaded topics are the ones that produce the most steadfast opinions, even in the face of contrary evidence.
4. The Affective Circumplex: A Descriptive Tool for Representing the Nature of Core Affect
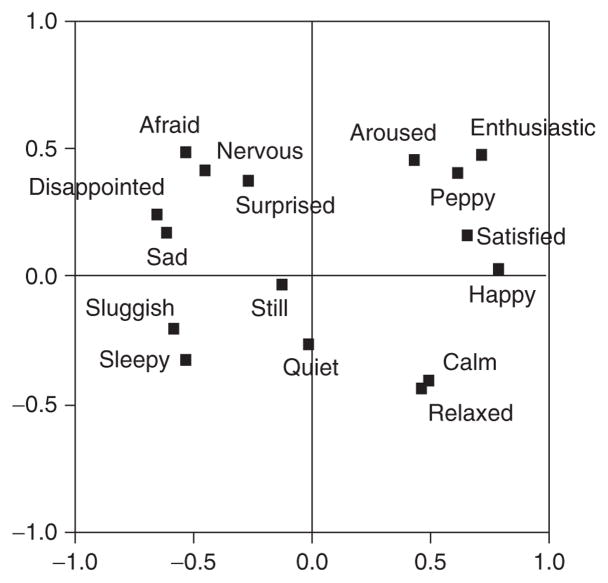
A person’s momentary core affective state (whether it is a simple feeling, part of an emotion, or part of perceiving an image or another person’s face), realized by such complex circuitry in the anterior parts of the human brain, can be psychologically described and represented by a single point on the two dimensional space schematically represented in Fig. 4.3. Many readers will recognize this structure as the affective circumplex (Barrett & Russell, 1999; Feldman, 1995b; Russell, 1980; Russell & Barrett, 1999). The horizontal dimension, hedonic valence, ranges from pleasant states at one end to unpleasant states at the other. The vertical dimension, arousal, ranges from high activity and attention at one end to low activity and sleepiness at the other. Both dimensions are descriptively bipolar (for a discussion, see Russell & Carroll, 1999) and largely independent from one another, meaning that arousal is not merely the intensity of pleasure or displeasure (Kuppens et al., in preparation). In this section, we describe how the circumplex can be used as a research tool for studying the content of core affective states.
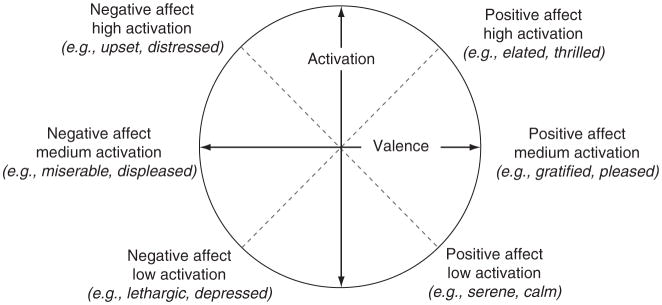
Figure 4.3.
The affective circumplex. Hedonic valence is represented on the horizontal axis and arousal on the vertical axis.
4.1. Deconstructing the affective circumplex
In the most general terms, a circumplex structure is a multipurpose, mathematical tool for representing mental structure through the geometry of the circle (Guttman, 1957). The mental structure can be for any group of objects, items, or stimuli and is assessed by measuring people’s responses to them. For example, researchers typically measure judgments of affect-related words, perceptual judgments of faces depicting emotion, or self-reports of a person’s own momentary feeling state. To create a circumplex, the relations between the judged or rated stimuli are rendered in multidimensional space.
In simple terms, a circumplex, such as the circumplex model of affect, is a circle and a set of axes. The circle depicts the similarity or relatedness between the objects (based on people’s psychological responses to them). The axes represent the psychological properties that quantify what is similar and different about people’s reactions to those objects.
4.1.1. The circle
Most objects in the world are similar to one another (or different from one another) in more than one way. For example, in the interpersonal domain, people differ from one another based on their nurturance (how warm and giving they are) and dominance (the extent to which they prefer to be controlling the outcome of others vs. being controlled by them (Wiggins & Broughton, 1991). Using the terms of psychological measurement, we would say the interpersonal descriptions are heterogeneous—two people cannot be compared to one another using only one property (nurturance) because they simultaneous vary on the other (dominance) as well. If we only compare along only one dimension, we will be making a specification error (leaving some meaningful variance unaccounted for).
When projected into geometric space (using some kind of factor analysis or multidimensional scaling), heterogeneous objects take on a circular shape (Guttman, 1957). In fact, circularity is a kind of statistical test for the descriptive nature of the objects in question. The term “circumplex” literally means “circular order of complexity” to indicate that the psychological objects or events in question are simultaneously similar or different from one another on at least two more basic psychological properties and therefore cannot be easily ordered relative to one another in a simple linear fashion. When objects are homogeneous, and best described by one and only one property, then a circular structure would not appear (instead, when projected into geometric space, would see something more like Thurstone’s simple structure).
When projected into geometric space, measurements of affect almost always take on a circular shape (for a review, see Russell & Barrett, 1999).7 The fact that they arrange in a circular fashion with such regularity reveals that affective objects (be they judgments of words, pictures of faces, or self-report ratings of experience) are similar or different from one another in more than one way (and therefore must be described by more than one fundamental property). For example, both structures in Fig. 4.4 depict circumplex structures of affect in geometric space. The similarity between affective objects is represented solely by their position in the circle. This similarity might be the result of two properties, or three, or even four—the point is there is more than one.
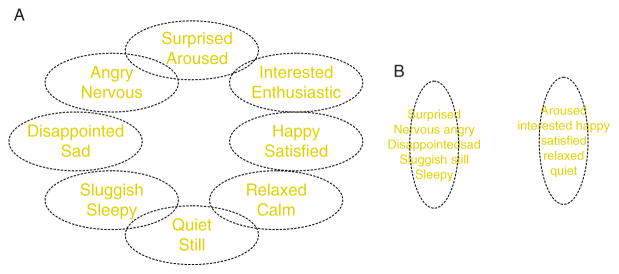
Figure 4.4.
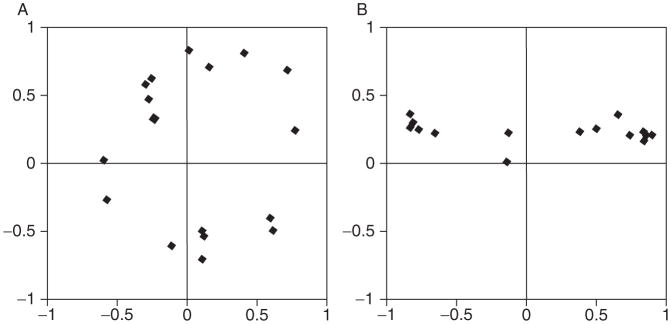
Variations in the affective circumplex. (A) depicts a prototypical affective space insofar as emotions are distributed evenly in a circular structure, with many smaller regions of homogeneity, where each region is psychologically distinct from every other. (B) depicts a nonprototypical affective space with two larger regions, where emotions within a region are highly similar. Figure is adapted from Barrett (2004).
The affective circumplex has an additional feature, over and above a generic circular structure. The qualitative (or ordinal) similarity for two affective objects is reflected in their proximity to one another around the perimeter of the circle. Affective objects that are closer together are more similar, whereas elements separated by an arc distance of 180° are maximally dissimilar (but for an alternative view, see Plutchik, 1980). For example, as the minimal arc distance between elements increases (e.g., “happy” and “enthusiastic”), the degree of similarity decreases (i.e., the correlation becomes smaller), suggesting that the elements are experienced as qualitatively different. Affective objects are separated by an arc distance of 90° (e.g., “happy” and “surprised”) are completely independent. As the arc distance increases to 180° (e.g., “happy” and “sad”), the objects represent bipolar opposites. Past 180°, the objects become increasingly similar again until the original starting point is reached. Over and above these constraints though, objects within space need not be equally spaced around the circle for it to be considered a circumplex (Browne, 1992; Fabrigar et al., 1997; see also Segura & González-Romá, 2003).
4.1.2. The axes
As conceived by Guttman (1957), the circumplex was defined solely in terms of ordinal relationships and so, alone, does not allow a quantitative analysis of the features or properties that psychological responses share—it merely depicts their nonparametric relatedness in geometric space. As is true for some (but not all) circumplexes, it is necessary to embed the affective circle within a two dimensional Euclidean space to discover the multiple properties that best describe how its elements are similar (or different) from one another (see Shepard, 1978). The dimensions represent the salient psychological attributes or features that describe the psychological responses (Davison, 1983). In the affective domain, the specific nature of those attributes has been an issue of great debate for the last half a century.
4.2. Anchoring the affective circumplex
Although valence and arousal are the original set of dimensions that anchored the affective circumplex, other sets of dimensions have been proposed (see Fig. 4.5), including positive and negative activation (e.g., Watson & Tellegen, 1985; Watson et al., 1999), positive and negative affect (Cacioppo et al., 1999), approach and withdrawal (e.g., Davidson, 1992), and tense and energetic activation (Thayer, 1989). In fact, all dimensions can be incorporated within the same circular structure (Carroll et al., 1999; Yik et al., 1999; Fig. 4.6). Still, there have been long debates about which dimensions are the most scientifically useful, with arguments on all sides (for reviews, see Cacioppo et al., 1999; Green et al., 1999; Russell & Barrett, 1999; Watson et al., 1999). A brief discussion of these arguments highlights some important points about the nature of affect.
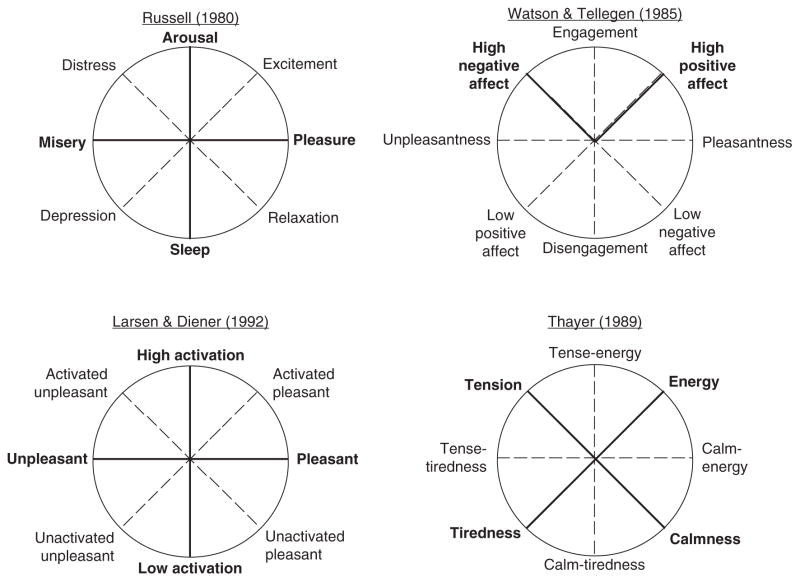
Figure 4.5.
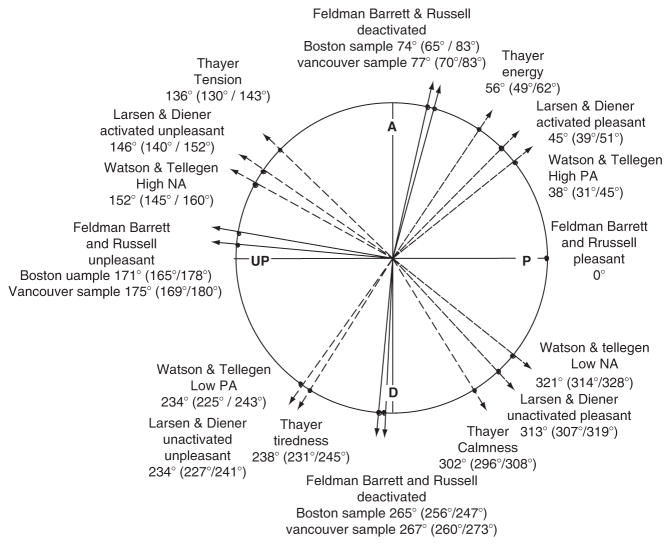
Multiple affective dimensions mapped in circumplex space. Primary (or main) dimensions are indicated in with black solid lines and labeled with capital letters. Secondary dimensions are indicated with gray dotted lines and are labeled in lower case letters. From Barrett and Russell (1999).
Figure 4.6.
A circumplex representation of various affective dimensions plotted according to a CIRCUM analysis. The Russell/Barrett, Larsen/Diener, Thayer, and Watson/Tellegen affective dimensions were measured using separate scales and there position in circular space was estimated using a structural equation modeling program (CIRCUM) that was specifically designed to estimate circumplexity. From Yik et al. (1999).
4.2.1. The great bipolarity debate
One issue that has drawn a good deal of attention is whether a bipolar valence dimension can properly describe affective states. Most typically, this question is asked in terms of whether pleasure and displeasure are truly bipolar opposites. Many studies (relying almost exclusively on zero-order correlation coefficients) have demonstrated that people report feeling both pleasant and unpleasant affective feelings “at the same time,” so that the correlation between the two is nowhere near −1 (which is assumed for bipolar opposites).
When measurement errors are properly controlled, subjective ratings of pleasure and displeasure are strongly negatively correlated (Barrett & Russell, 1998; Green et al., 1993). But to emphasize these negative correlations is to miss the more central point that correlations are statistically inadequate for evaluating bipolarity. Mathematical proofs clearly show that a correlation of −1 is not the gold standard for demonstrating bipolarity (Russell & Carroll, 1999; see also Segura & González-Romá, 2003). This is because the predicted correlation between true bipolar opposites with error-free data, when each is measured on an unambiguous unipolar format Likert-type scale (e.g., “neutral” = 0, “happy” = 6), equals the unintuitive number −.467. This value is based upon assumptions about L-shaped bivariate response distributions (Russell & Carroll, 1999). Item response theory analyses places the correlation for bipolar opposites closer to −.392 (Segura & González-Romá, 2003). Whether the actual value is −.467 or −.392, the point is that zero-order correlations cannot be unambiguously interpreted as supporting either bipolarity or bivalence (independence between pleasure and displeasure). When correlations are more negative than −.467, it is usually the result of systematic measurement error (for a full discussion, see Russell & Carroll, 1999). Consequently, correlational techniques (and statistical methods based on those techniques, such as factor analysis) should never be used to provide evidence for which set of dimensions best anchors the circumplex (cf. Russell & Carroll, 1999; Schimmack, 2001; Schimmack et al., 2002), although scientists routinely ignore this advice and continue to use them for this purpose.
Furthermore, it is not clear what “at the same time” actually means when a person reports feeling happy and sad at the same time. In the timeframe required to render a self-report rating or even a button-press, several different brain states could have occurred. This means that a single button press even when rendered very quickly in behavioral terms, is always a summary of a series of brain states. An equally plausible possibility, then, is that people do not experience two distinct feelings literally at the same time, but instead can alternate back and forth quickly between them, in much the same way that people do when looking the Necker cube illusion (see Fig. 4.7). In this illusion, it is possible to see two different percepts, but it is impossible to see them both at the same time. Instead, they alternate in quick succession. When asked how many configurations you see when you look at Fig. 4.7, you might say two (providing a summary of what you just saw), but you do not actually “see” them simultaneously. The same situation could be happening with affective states.8
Figure 4.7.
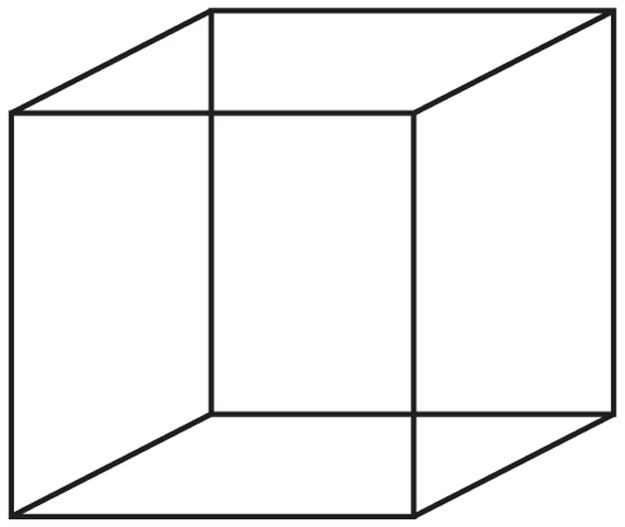
The “Necker” cube illusion.
Some scientists have criticized the valence/arousal model of affect on more causal grounds. Like Titchener, many scientists continue to believe that the descriptive structure of affect should be isomorphic with its causal structure, so that the best affective dimensions are those that are most causally plausible (i.e., the dimensions should reflect the processes that cause affective states). Accordingly, it has been claimed that certain dimensions (e.g., positive and negative affect) are more biologically basic, and therefore should be the preferred anchors of affective space (Ashby et al., 1999; Cacioppo et al., 1997, 1999; Reich et al., 2003). So far, however, the sorts of arguments that have been offered in this regard are problematic, for two reasons.
First, description and explanation usually occur at two different levels of analysis. In the end, a description of psychological content will rarely ever shed light on the processes that caused it, in much the same way that the experience of the sun rising and setting is not evidence that the sun actually revolves around the earth (cf. Barrett, in press).
Second, many of the specific biological arguments that have been offered to support other sets of dimensions do not hold up under closer scrutiny. Most notable is the claim that positive and negative affective states are realized in anatomically different parts of the brain. Sometimes it is claimed that the amygdala is the locus of negative affect, whereas the ventral striatum is the locus of positive affect. As discussed already, neither claim is true. The amygdala is engaged in humans when viewing faces depicting positive expressions (Canli et al., 2002; Mather et al., 2004; Yang et al., 2002) as well as pleasant images (Garavan et al., 2001; Mather et al., 2004); animals with amygdala lesions show impaired stimulus-reward learning (Baxter & Murray, 2001; Baxter et al., 1999, 2000) and are less likely to self-administer rewarding drugs (Robledo & Koob, 1993). And work from Kent Berridge’s lab (e.g., Reynolds & Berridge, 2001, 2002) has clearly shown that neurons in the ventral striatum also code for negativity.
Nor do positive and negative affect consistently show hemispheric specificity. The left dorsolateral prefrontal cortex may somehow support pleasant moods, reactions to pleasant stimuli (e.g., pleasant film clips), and approach behaviors, whereas the right supports unpleasant moods, reactions to unpleasant stimuli (e.g., unpleasant film clips), and withdrawal behaviors (for reviews see Davidson, 1992, 1993, 2004), but this laterality does not extend to other parts of the prefrontal cortex. For example, our own recent meta-analysis of neuroimaging studies of affect and emotion found exactly the opposite lateralization for pleasant and unpleasant affective experiences (particularly in the orbital sector of prefrontal cortex) with positive affective experiences corresponding relatively greater activation on the right and negative experience to relatively greater activation on the left (Wager et al., 2008; see Fig. 4.8). A meta-analysis by Kringebach and Rolls (2004) localized positive affect medially and negative affect laterally within the OFC of both hemispheres (with no differences in lateralization).
Figure 4.8.
Brain areas consistently activated for positive (yellow) and negative (blue) affective experiences. OFC = orbitofrontal cortex; vaINS = ventral anterior insula; Amy = amygdala; vStr = ventral striatum; vGP = ventral globus pallidus; pgACC = pregenual anterior cingulated cortex; rdACC = rostral dorsal anterior cingulate cortex; vmPFC = ventromedial prefrontal cortex; Hy = hypothalamus; Thal = thalamus; PAG/SC = periaquaductal gray/superior colliculus; aINS = anterior insular. From Wager et al. (2008). (See online version of the chapter for color figure).
It is sometimes claimed that positive and negative affective states rely on different neurotransmitter systems (dopamine and serotonin, respectively), but this, too, is debatable. Dopamine is not a reward transmitter (for reviews, see Salamone et al., 2005). Increases in dopamine are observed in rats occur during aversive events, such as tail pinches (Bertolucci-D’Angio et al., 1990), foot shocks (Sorg & Kalivas, 1991; Young et al., 1993), and cold ice baths (Keller et al., 1983). Similarly, serotonin is not a distress neurotransmitter and has been linked to changes in positive affect as well (Barge-Schaapveld et al., 1995; Dichter et al., 2005; Zald & Depue, 2001). Both dopamine and serotonin are what has been called “neuromodulators” in the sense that they originate in the brainstem’s ascending arousal system and tune the firing rates of many different neuronal groups throughout the cortex. Dopamine from both the substantia nigra and ventral tegmental area mark the salience of an event and are important to regulating access to voluntary motor outputs during motivated, effortful behavior; serotonin from the rostral raphe nucleus reduces distractibility and gates the processing of motivationally relevant sensory cues (Mesulam, 2000; Parvizi & Damasio, 2001).
Based on our read of the evidence, valence and arousal are best thought of as the descriptive features of core affect that bear no resemblance to or inform about how affect is caused. Simply put, content does not necessarily tell us anything about process. This means that the structure of felt experience will not correspond to the brain processes that produced those experiences in a one-to-one fashion. It also means that brain structure will not necessarily inform us about which psychological dimensions are best suited to anchor the affective circumplex. Nonetheless, as we demonstrate later in this paper, descriptions can be scientifically useful.
4.2.2. Replicability across affective domains
Wundt’s original properties of hedonic valence and arousal are most replicable across different domains of psychological response (Barrett & Russell, 1999; Russell & Barrett, 1999), and therefore seem to be the best dimensions to anchor the affective circumplex as a descriptive tool. In this section, we briefly review the evidence that judgments of emotion-related language, judgments of facial behaviors, and subjective ratings of emotional episodes, such as anger, sadness, and fear, as well as nonemotional affective states (like fatigue, sleepiness, and placidity) can all be minimally characterized as a combination of hedonic valence and arousal. Other dimensions, such as positive and negative activation, have been identified only for the self-reports of experience, and not for judgments of words or faces.
4.2.3. Judgments of faces
Judgments of emotion in other people’s faces configure as a circumplex that is described by valence and arousal properties. Woodworth (1938) described classification judgments of “facial expressions of emotion” (i.e., emotion caricatures) as well as “judgment errors” (i.e., failures to give the consensual response) using valence and arousal dimensions. Schlosberg (1952) found something similar as well, describing a circular mapping of affect defined first by two dimensions (pleasantness–unpleasantness, attention–rejection), following which he added an intensity dimension (sleep-tension) to produce a cone-like structure (Schlosberg, 1954). Circumplex structures have been identified in perceptions of facial depictions of emotion (e.g., Abelson & Sermat, 1962; Cliff & Young, 1968; Dittman, 1972; Fillenbaum & Rapoport, 1971; Green & Cliff, 1975; Russell et al., 1989; Schlosberg, 1952, 1954; Shepard, 1962a,b), both in adults and in children (Russell & Bullock, 1985; Russell & Ridgeway, 1983). Very young children only seem to make distinctions between facial depictions of pleasant and unpleasant, however (Widen & Russell, 2003).9 Furthermore, event-related potential (ERP) studies general confirm that hedonic valence (and perhaps arousal) is coded early during face perception (as early as 80 ms, but typically between 120 and 180 ms after stimulus onset depending on whether the face is presented fovially or parafoveally; for reviews, see Eimer & Holmes, 2007; Palermo & Rhodes, 2007; Vuilleumier & Pourtois, 2007). Recent neuroimaging evidence also supports the idea that valence is a basic aspect of face perception (e.g., Engell et al., 2007; Todorov, 2008).10
4.2.4. Judgments of words
Multidimensional scaling analyses of similarity judgments (estimates of relatedness) of emotion-related words routinely yield valence and arousal dimensions. Here, valence and arousal represent the basic, semantic properties contained in cognitive maps of emotion language (Barrett & Fossum, 2001; see Fig. 4.9). Circumplex structures anchored by valence and arousal dimensions have been reliably derived from similarity ratings for different sets of affect terms (Barrett, 2004; Block, 1957; Bush, 1973; Feldman, 1995a; Russell, 1980; also, see Fig. 4.10) indexing emotion language in many different cultures (Russell, 1991). These findings are consistent with the semantic differential work by Osgood et al. (1957) who demonstrated that there are three major components of meaning in natural language (evaluation, activity, and potency).
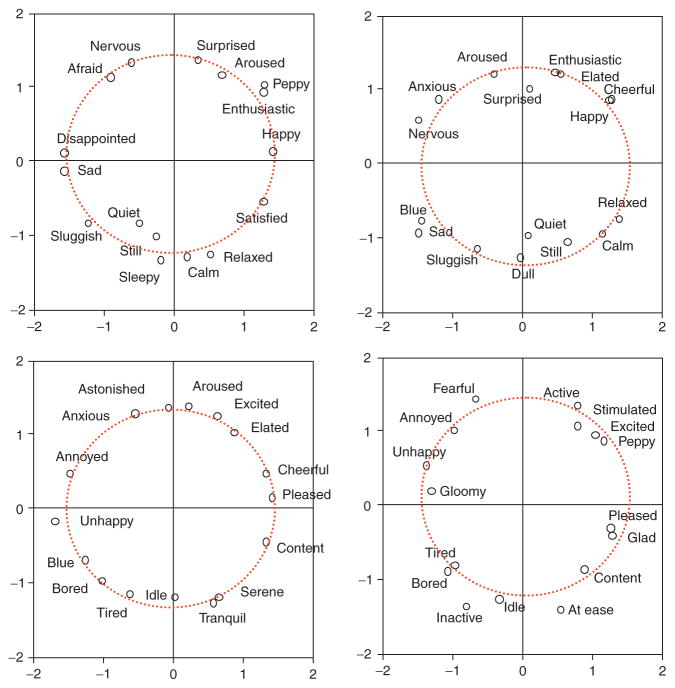
Figure 4.9.
Cognitive maps of affective space. The circumplex structure of affect derived from direct semantic ratings, similarity judgments, and conditional probability judgments of emotion words. Based on data from Barrett and Fossum (2001).
Figure 4.10.
Affective circumplex structures estimated from multidimensional scalings of similarity judgments using different sets of emotion adjectives (Barrett, unpublished data).
4.2.5. Self-report ratings of experience
Ratings of subjective ratings of emotion experience also configure into a circumplex described by valence and arousal. Self-reports of emotion experience taken from a group of individuals at one point in time configure into a circumplex shape anchored by valence and arousal dimensions (Feldman, 1995b; Russell, 1980; Yik et al., 1999; for a review, see Barrett & Russell, 1998; Russell & Barrett, 1999) (see Fig. 4.11). So do idiographic reports that are taken over time and modeled separately for each person (Barrett, 1998, 2004; Feldman, 1995a; Fig. 4.12; see next section for a more detailed description). People are also able to give an explicit account of core affective feelings using a variety of self-rating scales (Barrett & Russell, 1998; Bradley & Lang, 1994; Carroll et al., 1999; Frijda et al., 1989; Kitayama et al., 2000; Lang et al., 1993; Roseman et al., 1996; Russell et al., 1989; Scherer, 1997; Smith & Ellsworth, 1985, 1987; Yik et al., 1999).
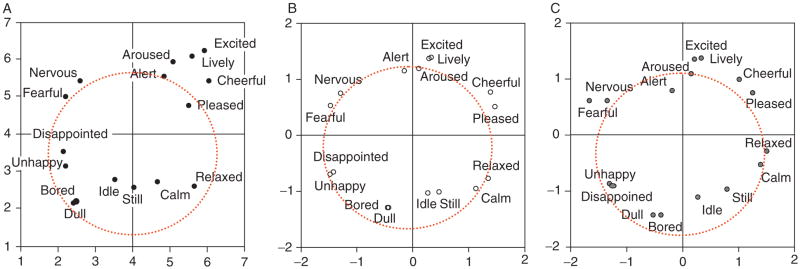
Figure 4.11.
Cross-sectional ratings of emotional experience modeled as a circumplex. Factor loading plot for ratings of emotional experience taken using 16 adjectives. Valence is represented as the horizontal axis and arousal as the vertical axis. Taken from Feldman (1995b).
Figure 4.12.
Idiographic variation in circumplex structure. Examples of idiographic affective circumplexes derived from momentary ratings of emotional experience for two participants. The participant depicted in (A) has a relatively prototypical circumplex with many small regions of homogeneity which reflects high emotional granularity. The participant in (B) has a flatter, more elliptically shaped circumplex which reflects low emotional granularity. Figure reprinted from Feldman (1995b).
All humans, it seems, can tell the difference between a pleasant affective state and an unpleasant affective state. Many, but not all, people also characterize their affective states as high or low in activation. In these studies, valence and arousal dimensions did not reflect the artificial influence of language (for evidence, see Barrett, 2004, 2006b) nor social desirability (Barrett, 1996). Instead, valence and arousal represented the content of experience. In the next section, we discuss how the affective circumplex can be used to model individual differences in the phenomenological experience of valence and arousal.
5. Individual Differences in Core Affect
For about a decade, our lab used a range of experience-sampling procedures to observe how people reported their emotion experiences (using simple English words for emotion) in the course of everyday life over several weeks. Primarily with the use the palm-top computers, we observed hundreds of people reporting their experiences over many occasions. We then treated those reports as verbal behaviors and constructed an affective circumplex structure for each person. We observed significant variation in affective structure across people, and with the use of some novel multivariate techniques (outlined first in Feldman, 1995a), revealed individual differences in core affective experience that was linked to broader differences in the granularity of emotional life.
Within the affective structure for a given person, a local region of homogeneity formed when reports of two experiences are relatively close over time (e.g., “happy” and “satisfied”). Very high correlations reflected the fact that experiences were descriptively similar and are phenomenologically indistinguishable. People whose verbal behaviors produced a prototypical circumplex with a uniform, circular structure show many small regions of homogeneity across the circle. This means that they had many precise domains of experience that were descriptively distinct from one another (like that depicted in Fig. 4.4A; for an example of actual data, see Fig. 4.12A). These individuals were said to be high in emotional granularity because they used different adjectives to represent distinct kinds of experience (e.g., anger and sadness are phenomenologically distinct).
People who produced a structure that is flatter and more elliptical in shape show a small number of broad regions of homogeneity and correspondingly fewer domains of distinct experience (e.g., see Figs. 4.4B and 4.12B). These individuals were lower in emotional granularity, because even though they were using the same set of adjectives to report their experience (as were those higher in emotional granularity), they used these terms to represent only a few general feeling states. For example, they might use words like “angry,” “sad,” and “afraid” to mean “unpleasant,” and words like “excited,” “happy,” and “calm” to mean pleasant. Less frequently, we observed people who use arousal words interchangeably, so that “excited” and “nervous” are experienced as phenomenologically similar, as are “tired” and “calm.”
Individual variation in emotional granularity (represented by the shape of the circle) could be quantified in terms of the emphasis that an individual placed on the hedonic and arousal properties of core affect when reporting his or her experience. Estimating the emphasis (or focus) on valence was accomplished by computing the proportion of variance in the verbal reports of emotion experience due to the valence-based meaning of the words (for a step by step description of the process, see Barrett, 2004; Feldman, 1995a). The emphasis (or focus) on arousal was estimated by computing the proportion of variance in the verbal reports due to the arousal-based meaning of the words. In this procedure, then, the emphasis on core affective properties was measured directly from behavior (as opposed to asking people to report how much they focus or emphasize each feature).
The more that valence-based meaning of the words accounts for variance in the reports of actual experience, the more an individual emphasizes or focuses on valence during the reporting process. Valence focus (VF) represents the amount of information about pleasure or displeasure contained in verbal reports of emotional experience. It does not represent the tendency to report pleasant states, or unpleasant states, but rather reflects the extent to which hedonic valence is an important descriptive property of core affective responding in that individual. Individuals high in VF emphasize pleasure and displeasure in the content of their verbal reports more than do those lower in VF, often at the expense of other properties of affect, like arousal (Barrett, 2004).
Similarly, arousal focus (AF) represents the amount of information about felt activation or deactivation contained in those verbal reports. It does not represent the tendency to report high arousal states, or low arousal states, but rather it reflects the extent to which arousal is an important descriptive property of core affective responding in that individual.
Individuals high in emotional granularity, with perfectly circular affective structures, experienced core affective states that were equally hedonic and arousal-based (VF = AF). In Fig. 4.13, VF is plotted against AF for almost 700 participants who have participated in our experience-samplings studies. Respondents who fell around the diagonal displayed circular affective structures. Individuals lower in emotional granularity, with elliptical structures experience core affective states that were relatively more hedonic (VF > AF) fell below the diagonal. These individuals had difficulty distinguishing between negative states that differed in arousal (such as anger and sadness); the same was true for positive states. Those whose affective states were relatively more arousal-based (AF > VF) fell above the diagonal, and had difficulty distinguishing between high arousal states that differed in hedonic valence (such as nervousness and excitement); the same was true for low arousal states.
Figure 4.13.
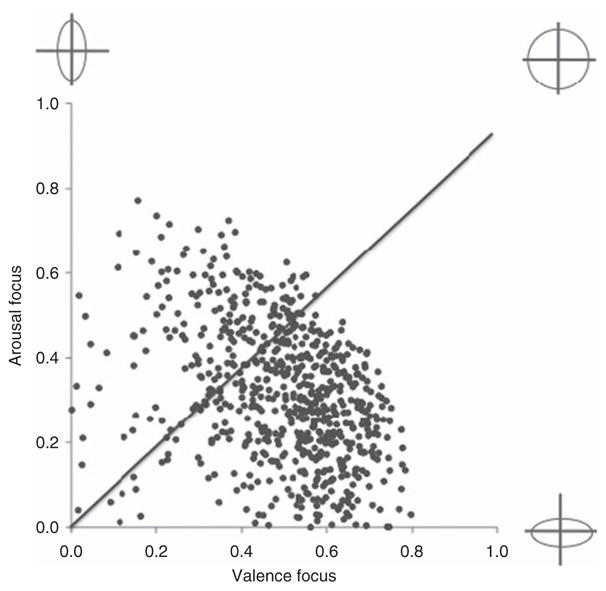
Scatterplot of variation in valence focus and arousal focus. Valence focus plotted against arousal focus for ~700 subjects who completed experience sampling experiments in our laboratory over a 10-year period. Caricatured circumplex structures are plotted on the extremes of the axes. Participants who fall along the diagonal line (where VF = AF) are high in emotional granularity and have a prototypical circumplex structure. Participants who fall above the diagonal like (AF > VF) and below the diagonal line (VF > AF) are less granular and have more elliptical shaped circumplex structures.
Individual differences in both VF and AF relate to other psychological phenomena in a way that establishes their construct validity. For example, people who are more valence focused are also more perceptual sensitive to hedonic information in the face of another person (Barrett & Niedenthal, 2004). Using a Morph Movies task, participants were presented with a series of movies in which actors began with neutral facial expressions and gradually, over the course of one hundred frames, began to express happiness, sadness, or anger. Participants advanced each movie using a cursor at the bottom of the screen and were instructed to stop the cursor at the point at which they first detected any feeling on the actor’s face. Heightened levels of VF predicted earlier detection of the appearance of affective expressions, suggesting that people high in VF have enhanced perceptual sensitivity to valenced information in the environment. People high in VF also described themselves as being more sensitive to hedonic cues, as indexed by reports on a variety of traditional personality measures (e.g., neuroticism and extraversion) (Barrett, 2006c).
Increased sensitivity to hedonically evocative cues has real-world importance for the lives of people high in VF. People high in VF experience a life as a rollercoaster ride filled with drama. They experience a world that is saturated with hedonic value because their threshold for detecting and responding to such cues is comparatively lower than people who are low in VF. We verified this hypothesis in another series of experience sampling studies where we examined the extent to which VF was linked to self-esteem lability. In two event-related experience-sampling studies, participants reported on their social interactions over either a week or two-week period. During each sampling moment, participants reported on their emotional experiences (using the methodology from previous studies and therefore allowing for the computation of VF), their self-esteem at the moment of sampling, and the valenced information in the social interaction (e.g., the amount of positive or negative emotion expressed by the interaction partner(s)). Lability in self-esteem was measured behaviorally in hierarchical linear modeling analyses, as the magnitude of the self-esteem change when faced with positive and negative cues during social interactions. As predicted, individuals who were more valence focused also demonstrated more self-esteem lability—their self-regard was like a ping-pong ball, bouncing around from interaction to interaction (Pietromonaco & Barrett, manuscript under review). People high in VF are not simply perceiving more hedonic information in their environments—they are using that information to shape and change their sense of self.
AF, on the other hand, is related to an enhanced sensitivity to one’s own physical state (Barrett et al., 2004). Participants completed a modified Whitehead heartbeat detection task (Whitehead & Drescher, 1980) during which they were asked to judge whether a series of tones were either in sync or not in sync with their heartbeats. These data were then subjected to a signal detection analysis yielding an index of interoceptive sensitivity. In two studies, people who were higher in AF showed enhanced sensitivity to their own heartbeats. These finding indicated that people who have more awareness of the internal sensory cues coming from their body also experience more variation in the arousal-based property of core affect. They clearly showed that people can, at times, detect specific information in their bodies, and this sensitivity is, in some way, related to the experience of emotion.
Furthermore, the AF-interoception link helps to clarify the relation between interoceptive sensitivity and emotional experience. Most studies have examined the link between heartbeat detection and explicit ratings of the intensity of emotion experience, with inconsistent results (Critchley et al., 2004; Ferguson & Katkin, 1996; Hantas et al., 1982; Wiens et al., 2000). In most studies, respondents rated their experience on a Likert-type scale using a set of adjectives, and those ratings were summed to derive an index of experienced emotion. It is possible; however, that interoceptive sensitivity is better conceptualized as relating to the perception of arousal as a property of experience, rather than to the intensity of experience per se. The feelings of activation and deactivation arising from interoceptive cues may be too impoverished to reliably influence direct, consciously available explicit ratings of emotion. Instead, these background interoceptive cues may manifest in a focus on activation-based aspects of emotional states in a more indirect or nonexplicit way. Presumably, individuals who are more interoceptively sensitive would be more likely to perceive feelings of arousal, and would communicate those feelings in self-report process over time, even if such differences are not apparent in the intensity of explicit reports.
Moreover, the relation between AF and interoceptive sensitivity not only provided validity for the link between interoceptive sensitivity and experienced emotion, but they also provided much needed incremental validity for self-reports of emotional experience more generally. By demonstrating that AF was related to interoceptive sensitivity, we were able to demonstrate that information implicitly contained in self-report ratings (i.e., the extent to which people focus on a property of their experience when reporting it) was associated with a behavioral variable (heartbeat sensitivity). This is a different sort of validity than showing that the levels of self-reported emotional experience (e.g., participants’ ratings of anger, pleasure, etc.) correlate with behavioral or psychophysiological measurements. In addition, many of the studies that provide validity evidence for self-reports of emotional experience examine concurrent relationships between self-reports and validity variables. In contrast, we demonstrated that AF was linked to interoceptive sensitivity when the measurements of each were separated by several weeks time.
6. Future Directions
Taken together, both psychological and neuroscience evidence supports the conclusion that core affect is a basic psychological ingredient in emotion. Studies examining the circumplex structure of affect demonstrate that core affect is a multiproperty phenomenon, and the structure is robust enough to accommodate many different ways of describing affect. Furthermore, the structure is able to represent meaningful individual differences in affective focus and link them to patterns of variation in emotional experience.
More recently, our lab has focused its attention on the hypothesis that core affect is a basic psychological ingredient of mental life more generally. The neuroanatomical studies mapping affective circuitry strongly suggest that core affect plays a formative role in other psychological phenomena that fall outside the traditional boundaries of emotion. In the past several years, we have been investigating role of core affect in two such processes: learning and vision.
6.1. Core affect supports learning
To survive, a person must know to avoid threat and danger and approach reward and nourishment. A person must be able to navigate through the world using affective reactions as a guide. Such navigational skills are critical not only in the physical world (e.g., knowing to avoid a poisonous snake in the desert), but also for survival in the social world (e.g., knowing to avoid a person who has not proven trustworthy in the past). Very few objects and situations (and even fewer people) have the innate or intrinsic power to perturb another person’s core affect. Instead, humans (like all living creatures) must learn what to approach and what to avoid, what to desire and what to ignore. Core affect supports this kind of learning, which we call affective learning.
Affective learning occurs when a stimulus that does not have the capacity to perturb core affect (what colloquially would be called a “neutral” stimulus) acquires that capacity on future occasions. Stimuli acquire affective value by being paired with other stimuli that change in a person’s core affective state. When the two stimuli are paired across a number of experiences, the neutral stimulus begins to itself elicit changes in core affect. In this way, a neutral stimulus is said to have acquired affective value. Examples of associative affective learning include Pavlovian or classical conditioning (i.e., where neutral stimuli are paired with stimuli that cause robust sympathetic nervous system (SNS) reactions; for reviews, see, Delgado et al., 2006; Domjan, 2005; Pearce & Bouton, 2001) and evaluative conditioning (i.e., where neutral stimuli are paired with stimuli that are explicitly evaluated to be liked (or good) or disliked (or bad); for reviews, see De Houwer et al., 2001; Field, 2005). SNS activity is broadly implicated in affective responding (Cacioppo et al., 2000) and changes in people’s SNS responses to a stimulus are taken as an indication that it has the capacity to perturb core affect.
In associative learning studies, affective learning is usually demonstrated by pairing human faces (e.g., Hermans et al., 2002) and pictures of geometric shapes (e.g., LaBar et al., 2004; Lipp et al., 2003) with stimuli that have the capacity to perturb core affect, like high pitched and loud noises (e.g., Büchel et al., 1999; LaBar & Phelps, 2005) and electric shocks (e.g., Grillon, 2002; LaBar et al., 1998). So, for example, a neutral blue square acquires affective value by being paired repeatedly with an aversive (i.e., negative and arousing) electric shock. As it increasing comes to predict the presence of the shock, the blue square elicits the same affective response (typically indexed by SNS activation measured as electrodermal activity (EDA) on the surface of their fingertips). The larger the affective change, (presumably) the easier (and perhaps more robust) the affective learning.
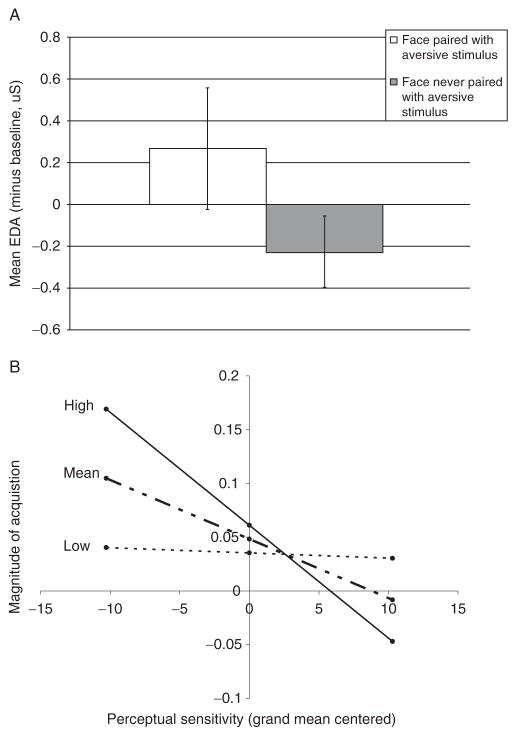
An on-going line of work in our laboratory is investigating how individual differences in affective reactivity support individual variation in affective learning. In an associative learning experiment (Bliss-Moreau et al., manuscript under review), participants were presented with two neutral faces. One picture (the CS+) was consistently paired with a shock (the US) during an acquisition phase of learning, and the other picture was never paired with a shock (the CS−). When participants were shocked (i.e., presented with the US), they generated large sympatric nervous system responses measured as the magnitude of their EDA response. Over time and many pairings, participants began to respond with heightened EDA to the CS + face (paired with the shock) than to the CS – face (never paired with shock), and this response to the CS + face occurred even when the US was not presented (see Fig. 4.14A). With this pattern of findings, we demonstrated, like many other studies before us, that a neutral face acquired affective value and was able to change a person’s affective state based on prior instances where it was paired with a stimulus that easily did so. Most importantly, we found that individual differences in affective reactivity predicted the magnitude of learning in this experiment. Individuals who demonstrated a perceptual sensitivity to affective value (assessed using the Morph Movies task that was related to VF in a prior experiment; Barrett & Niedenthal, 2004) also demonstrated enhanced affective learning. Specifically, as perceptual sensitivity increased, so too did the magnitude of the EDA response to the CS+. This learning effect was further enhanced for individuals who described themselves as high on neuroticism (itself an index of sensitivity to negative value) (see Fig. 4.14B). These findings provide some of the first results to show that individual differences in core affective reactivity are related to variation in negative affective learning.
Figure 4.14.
Variation in affective learning. Sympathetic nervous system response (as indexed by EDA) to face stimuli that were either consistently or were never paired with an aversive electric shock during an associative affective learning paradigm (A). The stimulus that was paired with a shock (CS+) acquired affective value as indicated by a significantly higher EDA response as compared with the EDA response to the stimulus that was never paired with shock (CS−). Individual differences in the acquisition of affective value were related to variation in affective reactivity (B). The relationship between perceptual sensitivity to affective value and the magnitude of affective learning is presented at three levels of neuroticism. From Bliss-Moreau et al. (manuscript under review).
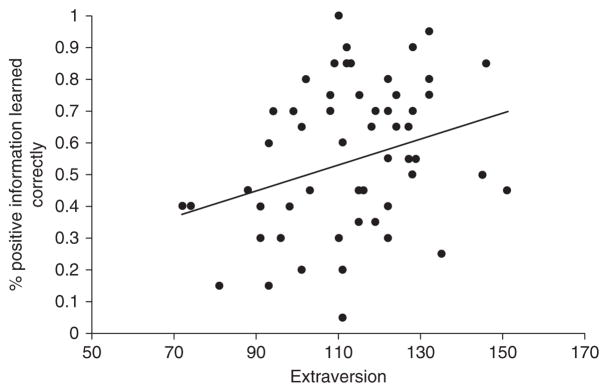
Individual differences in core affective responding also predicted better rule-based affective learning (Bliss-Moreau et al., 2008). Rule-based affective learning occurs when the value of an object is communicated explicitly through symbolic means (e.g., telling someone that another person is threatening) rather than the object being paired in time or space with something of known affective value (as is the case for associative affective learning; for a discussion of rule-based vs. associative processing, see Sloman, 1996; Smith & DeCoster, 2000). We developed a rule-based affective learning paradigm using a modified spontaneous trait inference paradigm (e.g., Todorov & Uleman, 2002, 2003). Participants were asked to learn about the behavior of a series of target people. Participants were shown a series of 60 face target pictures, each of which was paired with a sentence describing a behavior during the learning phase of an experiment. The behaviors were either positive (e.g., “celebrated a friend’s birthday”), negative (e.g., “hit a small child”), or neutral (i.e., “asked the cab driver for directions”) in affective tone. Participants were instructed to imagine the targets performing the behaviors described by the sentences. In a following test phase, participants made explicit judgments of the faces (presented without the sentences) as positive, negative, or neutral. More often than chance, participants categorized the faces according to the affective value of the sentence with which it had been paired during the prior learning phase. In addition, affectively positive learning was enhanced for people who described themselves as particularly reactive to positive affective value (as measured by extraversion). As self-reported levels of extraversion increased, so too did people’s propensity to categorize faces which had been paired with positive sentences as being positive (see Fig. 4.15).
Figure 4.15.
Individual differences in rule-based affective learning. Positive affective learning via rule-based means is predicted by participants’ sensitivity to positive information and propensity to experience positive affect (as indexed by self-reported extra-version). Adapted from Bliss-Moreau et al. (2008, Study 3).
Taken together, these findings suggest that both associative and rule-based affective learning are enhanced for people whose core affective states are often and easily perturbed. These findings have real-world implications for understanding how people come to have such different mental lives. As we noted earlier, people who are those high in VF surf a tumultuous sea of agony and ecstasy, and are easily moved or perturbed by changes in their surroundings. They often react to things that others find devoid of emotional meaning. Others (who are lower in VF) float in a sea of relative tranquility. They live their lives relatively undisturbed and they are generally less affected by the vicissitudes of life. They often do not react to things that others find compelling or evocative, thereby missing events of potential import or significance. What begin as simple temperamental differences in affective reactivity may develop into these very different emotional lives (manifesting in different degrees of VF) because differences in reactivity support differential degrees of affective learning. In what might be considered a classic positive feedback loop, affective learning may proceed more robustly for a person who is more reactive to begin with. The person’s world will become more populated with affectively evocative stimuli (because great numbers of previously neutral stimuli will presumably acquire value), so that the processing of those affective stimuli will serve to maintain shifts in core affect, which in turn promote enhanced affective learning. And so on. Thus, a person who experiences great reactivity in his or her core affect state sets the stage for that reactivity to be maintained through new affective learning throughout the lifespan
6.2. Core affect as a fundamental feature of conscious experience
Neuroanatomical evidence strongly suggests that core affect provides a source of attention in the human brain (where attention is defined as anything that increases or decreases the firing of a neuron). This implies that core affect has an important role to play in normal perceptual functioning, including consciousness. When sensory information from the world sufficiently influences a person’s internal bodily state, the processing of that information is prioritized so that the resulting object is more easily seen (reviewed in Barrett & Bar, in press; Vuilleumier & Driver, 2007) and remembered (reviewed in Kensinger & Schacter, 2008). Put another way, “feeling” and “seeing” (or “hearing” or “smelling” and so on) may not be all that independent of one another.
Core affect has the capacity to influence sensory processing throughout the brain via a number of direct and indirect routes. Parts of the neural reference space for core affect (such as the amygdala and lateral OFC) project directly to all sensory cortices and so can directly influence sensory processing. For example, the basal nucleus of the amygdala projects directly to all portions of the visual ventral stream, serving to modulate neural activity from the association cortex all the way back to the primary visual cortex (or V1) (for a review, see Duncan & Barrett, 2007). The sensory integration network in the central and lateral OFC projects to the visual association areas in the inferior temporal lobe (part of the “what” or ventral visual stream for object recognition) and the visceromotor network in the medial OFC projects to the visual association areas in the inferior parietal lobe (part of the “where” or dorsal visual stream for spatial localization and action preparation) (for a review, see Barrett & Bar, in press). The circuitry that realizes core affect also project indirectly to sensory neurons via three different routes. The amygdala, the visceromotor network of the OFC (including what is sometimes called the medial OFC or vmPFC), and the ventral striatum project to the ascending arousal systems the brainstem and basal forebrain (for a review, see Edelman & Tononi, 2000; Mesulam, 2000; Semba, 2000) that have diffuse, unidirectional afferent projections throughout the cortical mantle, acting as a “leaky garden hose” (Edelman, 2004, p. 25) to control the level of neuronal firing throughout the brain. (In fact, affective circuitry offers the only path by which sensory information from the outside world reaches the brainstem and basal forebrain; Mesulam, 2000). The amygdala and OFC (as well as the brainstem and forebrain nuclei) also project to certain thalamic nuclei that regulate the transmission of sensory information to the cortex and are partly responsible for forming and selecting the groups of neurons that fire in synchrony (called neuronal assemblies) to form conscious percepts (the things people are aware of seeing) (Zikopoulos & Barbas, 2007; for a review, see Duncan & Barrett, 2007). Finally, the lateral portions of OFC project to lateral prefrontal cortex (which is the source of what scientists term a “top-down” or “goal-directed” or “endogenous” source of attention). In these ways, areas involved with establishing a core affective state can indirectly constrain ongoing processing throughout the rest of the cortex and help to select the information that reaches conscious awareness by directing the formation and maintenance of the neuronal assemblies that underlie conscious experience.
Indeed, evidence shows that sensory areas show enhanced neural activity during perceptual states having a strong core affective component. Affectively potent, as compared to neutral, stimuli generate robust responses in the visual cortex (e.g., Lane et al., 1999; Lang et al., 1998; Moll et al., 2002; Morris et al., 1998; Taylor et al., 2000). Activity in the visual cortex was also enhanced for stimuli that recently acquired affective value by being paired with an electric shock as compared to perceptually similar stimuli that never were paired with shock (e.g., for functional neuroimagining evidence using fMRI see Damaraju et al., manuscript under review Pamalaand & Pessoa, 2008; for ERP evidence see Stolarova et al., 2006).
Our own meta-analytic investigation of fMRI and PET studies of emotion confirmed that V1 is consistently activated in response to affectively potent as compared with neutral stimuli (Kober et al., 2008; Wager et al., 2008; see Fig. 4.2). Activity in the visual cortex also appears to be further enhanced when affective stimuli are particularly arousing. When we assessed activation in the visual cortex in studies of negative core affect (using negative pictures, sounds, words, facial expressions, etc.), there was greater neural activity in studies using stimuli that generated core affective states that were high in arousal as compared to lower in arousal (e.g., perception of fear faces vs. neutral faces; experiences of anxiety vs. neutral affect) (Bliss-Moreau et al., 2008).
The pattern of projections from the neural reference space for core affect to visual cortex suggests the intriguing hypothesis that what people literally see in the world around them may in part be determined by their core affective state. In our lab, we are in the process of investigating three specific hypotheses with respect to core affect and vision.
First, we hypothesize that core affect may play a role in basic object perception, even when objects are not affectively evocative per se. As we noted already, the OFC has strong reciprocal projections to both the dorsal “where” and ventral “what” visual streams involved in object perception. Furthermore, when briefly presented objects are successfully recognized, there is more neural activity in OFC as compared to when objects go unrecognized (Bar et al., 2001, 2006). One hypothesis is that OFC provides top-down modulation of basic visual processing necessary for determining both what and where objects are (Barrett & Bar, in press). Feeling something affective about sensory stimulation may make it more likely that you will see an object in the first place.
Second, we are investigating the hypothesis that an individual’s momentary core affective state helps to select the contents of consciousness, so that what you feel literally influences what you see. There is evidence, for example, that the affective content of a visual image can resolve a phenomenon called “binocular rivalry.” Binocular rivalry occurs when two incompatible images are presented to both eyes that cannot be merged into a coherent three dimensional image. Instead of perceiving a mixture of the two images, people experience one image at a time, oscillating back and forth into visual awareness every few seconds. By measuring which percept is seen first, and for how long, it is possible to assess which percepts are selected for subjective awareness. A handful of studies have shown that images with affective meaning tend to be represented in conscious awareness more often than rival images with more neutral content. Valenced scenes have greater perceptual dominance over neutral scenes (Alpers & Pauli, 2006), as do facial depictions of emotion when compared to neutral faces (Alpers & Gerdes, 2007). Stimuli that have recently acquired affective value by being paired with an aversive electric shock in an associative learning paradigm also dominate subjective visual awareness compared to neutral stimuli (Alpers et al., 2005). In our lab, we are currently exploring how changing the core affective state of the perceiver more directly influences subjective visual awareness for objects (such as faces). In our lab, we now have preliminary evidence that affectively-potent objects are selected over neutral objects more often when the perceiver is in a salient affective state.
Third, we are investigating whether individual differences in core affect enhance or diminish blindsight. Blindsight occurs when perceptually blind people (i.e., people who report not being able to see) are able to detect visual stimuli without having any conscious or qualitative awareness that they can do so (Weiskrantz, 1986). Blindsight can be induced in the lab with the brief presentation of an object (e.g., 16 or 33 ms) followed by a backward mask (to prevent the re-entrant feedback processing that is necessary for subjective awareness). Although objects not consciously seen under these conditions, people can still respond to them behaviorally in such a way as to indicate that the objects are being detected at better than chance levels. We hypothesize that a strong core affective state may enhance experimentally-induced blindsight, so that intense core affective feelings may allow people to better detect and act on certain objects or blind them to others, before the sensory information is shaped into a fully formed percept that reaches full subjective awareness.
Although our research on the affect and vision is in its infancy, it will have two important implications if successful. First, this research explores the possibility that there is normal variability in the extent to which the world appears affectively infused, so that the environment may literally look different to different people depending on how they feel. This would translate into different base rates for affective (and potentially emotional) events even when the physical surroundings are held constant. It is highly possible that this variation instantiates individual differences in personality dimensions that are broadly related to mental and physical illness (e.g., neuroticism and introversion). Some people may be affectively wired to see certain types of information better.
Second, and perhaps more importantly, this research will inform an ongoing debate over the distinctiveness between affect and cognition, suggesting that the distinction may not be an ontological distinction that is respected by the brain (cf. Duncan & Barrett, 2007). The most far-reaching implication of this work is that “thinking” (e.g., sensing and categorizing or deliberating on an object) might not be a fundamentally different sort of psychological activity than “affecting” (i.e., constructing a state to represent how the object affects you).
Acknowledgments
Deep and heartfelt thanks to Jim Russell for his wise council and collaborative input into much of the work reported in this paper. Thanks also to Rainer Reisenzein for pointing out the Titchener reference which details Wundt’s changing views on affect. Preparation of this manuscript was supported by the National Institutes of Health Director’s Pioneer Award (DP1OD003312), a National Institute of Mental Health’s Independent Scientist Research Award (K02 MH001981), grants from the National Institute of Aging (AG030311) and the National Science Foundation (BCS 0721260; BCS 0527440), and a contract with the Army Research Institute (W91WAW), as well as by a James McKeen Cattell Award and a Sabbatical Fellowship from the American Philosophical Society to Lisa Feldman Barrett.
Footnotes
In an earlier volume of Physiological Psychology, Wundt argued that affect is an attribute of sensation. In his 1896 Outlines of Psychology, he changed his view and argued that sensations and feelings are complementary elements (Titchener, 1908).
Wundt described how affective and ideational compounds combine via a specific temporal course in a way that strongly foreshadows the kind of stage model described by Schachter and Singer (1962) (and carried forward in some newer constructionist views, e.g., Russell, 2003). According to Wundt, emotions begin with an “inceptive feeling” that is affective in nature. The inceptive feeling is caused either by external sensory stimulation (what Wundt called “outer emotional stimulation”) or internal stimulation arising from associative or apperceptive conditions (what Wundt referred to as “psychical”) (1897/1998b, p. 171). Next, an “ideational process” distinguishes different emotional feelings from one another. Although Wundt did not provide a clear definition of what an ideational process is, his writing is at least suggestive that he is referring to some sort of embodied conceptualization close to that proposed by Barrett (2006b). Finally, there is a terminal feeling, which is basically a more diffuse affective state that remains after the more intense feelings have dissipated—similar to a mood state. Interestingly, Wundt argued that the psychical compounds combine to produce emergent emotional phenomena (in a way that is reminiscent of more recent treatments of emotion, e.g., Barrett, 2006b; Clore & Ortony, 2008). “The attributes of psychical compounds” Wundt wrote “are never limited to those of the elements that enter into them, but new attributes, peculiar to the compounds themselves, always arise as a result of the combination of these elements” (1897/1998b, p. 91).
This may be one reason why the Negative Affectivity/Positive Affectivity model of affect (Watson & Tellegen, 1985) and other similar models are so popular. The empirical basis for this model is grounded largely in self-reports of affective experience.
The acoustical properties that reflect the identity of the sender (reflected in “sonants” and “gruffs”) indirectly influence the affective state of the perceiving animal based on its prior experience with the sender, whether it is animal (Owren & Rendall, 1997) or a human speaker (Bliss-Moreau et al., manuscript under review).
In our view, freezing is not a behavioral index of fear. Freezing can be thought of as an alert, behavioral stance that allows a creature to martial all its attentional resources to quickly learn more about stimulus whose threat value is uncertain (e.g., a tone that is suddenly paired with a footshock).
Our starting assumption is that core affective states are realized in a broadly distributed system within the mammalian brain. This view is inspired by constraint satisfaction logic that represents how the brain works (Barrett et al., 2007; O’Reilly & Munakata, 2000; Spivey, 2007; Wagar & Thagard, 2004), as well as newer evidence on population-based coding and multi voxel pattern analysis where information is contained in spatial patterns of neuronal activity across the brain (Norman et al., 2006). In our view, different instances of core affect (combinations of hedonic valence and arousal) correspond to different brain states (flexible, distributed assemblies of neurons) from moment to moment, but these need not be localized in different parts of the brain. Two specific instance of high arousal, negative affect can be realized in different neuronal assemblies, even within the same person. A given neuron, because it receives input from many other neurons, can participate (in a probabilistic sense) in more than one neuronal assembly at the same time. It is even the case that single neurons can respond to different classes of information, depending on the frequency of firing (or the context) (Basole et al., 2003; Izhikevich et al., 2003; Reynolds & Berridge, 2008), so that even neurons are probably not specific to a single feature or content.
For ease of explication, we will refer to words, pictures, or faces as “affective objects” to denote their ability to change a perceiver’s affective state; or to denote their reflection of that change, as in self-reports of emotion experience.
Alternatively (and much more speculatively), it might even be possible for a person to be in both a positive and a negative state at the same time (in a probabilistic sense). Spivey (2007) argues that the human brain is rarely in a discrete state, and can be described by a fuzzy logic that allows many different states at once (each with some probability of reaching consciousness or causing action). When a certain threshold is crossed (or the probability of a given state is sufficiently high), the brain is said to be “in” that state, resulting in an experience (e.g., “having an positive affective experience” or “having a negative affective experience”) or a behavior (e.g., approaching or avoiding an object). Because the brain can configure itself into several different states in the time it takes to generate one motor response (to indicate a response choice, for e.g.), it is possible that positive and negative affective states, which bear no subjective resemblance to one another, are realized in neuronal assemblies that involve many of the same brain areas. Something (like attention) must bias processing to allow a motor output and/or consciousness of one or the other.
Contrary to popular belief, studies do not conclusively demonstrate that infants distinguish between discrete emotion categories. Infants categorize as distinct faces with different perceptual features (e.g., closed versus toothy smiles) even when they belong to the same emotion category (Bornstein & Arterberry, 2003) and no studies can rule out the alternative explanation that infants are categorizing faces based on the valence, intensity, or novelty (especially in the case of fear) of the facial configurations. For example, infants look longer at fear (or anger or sad) caricatures following habituation to happy caricatures, but this may reflect their ability to distinguish between faces of different valence (e.g., Flom & Bahrick, 2007). Similarly, infants look longer at a sad face following habituation to angry faces (or vice versa), but infants may be categorizing the faces in terms of arousal (e.g., de Rosnay et al., 2004, Experiment 3). Many studies find that infants tend to show biased attention for fear caricatures (e.g., Flom, & Bahrick, 2007), but this is likely driven by the fact that infants rarely see people making these facial configurations.
Although affect is a basic aspect of face perception, it is most likely a learned aspect. For example, in a recent case study, an individual recovering from blindness (following a corneal transplant) could not tell the difference between happiness and sadness in faces that were unfamiliar to him. This problem persisted for several years after he was able to receive visual stimulation in early visual brain areas.
References
- Abelson RP, Sermat V. Multidimensional scaling of facial expressions. Journal of Experimental Psychology. 1962;63:546–554. doi: 10.1037/h0042280. [DOI] [PubMed] [Google Scholar]
- Alpers GW, Gerdes ABM. Here is looking at you: Emotional faces predominate in binocular rivalry. Emotion. 2007;7:495–506. doi: 10.1037/1528-3542.7.3.495. [DOI] [PubMed] [Google Scholar]
- Alpers GW, Pauli P. Emotional pictures predominate in binocular rivalry. Cognition and Emotion. 2006;20:596–607. [Google Scholar]
- Alpers GW, Ruhleder M, Walz N, Mühlberger A, Pauli P. Binocular rivalry between emotional and neutral stimuli: A validation using fear conditioning and EEG. International Journal of Psychophysiology. 2005;57:25–32. doi: 10.1016/j.ijpsycho.2005.01.008. [DOI] [PubMed] [Google Scholar]
- Arnold M. Emotion and personality. New York: Columbia University Press; 1960. [Google Scholar]
- Ashby FG, Isen AM, Turken AU. A neuropsychological theory of positive affect and its influence on cognition. Psychological Review. 1999;106:529–550. doi: 10.1037/0033-295x.106.3.529. [DOI] [PubMed] [Google Scholar]
- Bachorowski J. Vocal expression and perception of emotion. Current Directions in Psychological Science. 1999;8:53–56. [Google Scholar]
- Bar M, Kassam KS, Ghuman AS, Boshyan J, Schmidt AM, Dale AM, et al. Top-down facilitation of visual recognition. Proceedings of the National Academy of Science. 2006;103(2):449–454. doi: 10.1073/pnas.0507062103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bar M, Tootell RB, Schacter DL, Greve DN, Fischl B, Mendola JD, et al. Cortical mechanisms specific to explicit visual object recognition. Neuron. 2001;29:529–535. doi: 10.1016/s0896-6273(01)00224-0. [DOI] [PubMed] [Google Scholar]
- Barad M, Gean P, Lutz B. The role of the amygdala in the extinction of conditioned fear. Biological Psychiatry. 2006;60:322–328. doi: 10.1016/j.biopsych.2006.05.029. [DOI] [PubMed] [Google Scholar]
- Barbas H. Organization of cortical afferent input to orbitofrontal areas in the rhesus monkey. Neuroscience. 1993;56:841–864. doi: 10.1016/0306-4522(93)90132-y. [DOI] [PubMed] [Google Scholar]
- Barbas H. Connections underlying the synthesis of cognition, memory, and emotion in primate prefrontal cortices. Brain Research Bulletin. 2000;52:319–330. doi: 10.1016/s0361-9230(99)00245-2. [DOI] [PubMed] [Google Scholar]
- Barbas H. Flow of information for emotions through temporal and orbitofrontal pathways. Journal of Anatomy. 2007;211:237–249. doi: 10.1111/j.1469-7580.2007.00777.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbas H, De Olmos J. Projections from the amygdala to basoventral and mediodorsal prefrontal regions in the rhesus monkey. Journal of Comparative Neurology. 1990;300(4):549–571. doi: 10.1002/cne.903000409. [DOI] [PubMed] [Google Scholar]
- Barbas H, Pandya DN. Architecture and intrinsic connections of the prefrontal cortex in the rhesus monkey. Journal of Comparative Neurology. 1989;286:353–375. doi: 10.1002/cne.902860306. [DOI] [PubMed] [Google Scholar]
- Barbas H, Saha S, Rempel-Clower N, Ghashghaei T. Serial pathways from primate prefrontal cortex to autonomic areas may influence emotional expression. BMC Neuroscience. 2003;4:25–37. doi: 10.1186/1471-2202-4-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barge-Schaapveld DQCM, Nicolson NA, van der Hoop RG, DeVries MW. Changes in daily life experience associated with clinical improvement in depression. Journal of Affective Disorders. 1995;34:139–154. doi: 10.1016/0165-0327(95)00012-c. [DOI] [PubMed] [Google Scholar]
- Barrett LF. Hedonic tone, perceived arousal, and item desirability: Three components of affective experience. Cognition and Emotion. 1996;10:47–68. [Google Scholar]
- Barrett LF. Discrete emotions or dimensions? The role of valence focus and arousal focus. Cognition and Emotion. 1998;12:579–599. [Google Scholar]
- Barrett LF. Feelings or words? Understanding the content in self-report ratings of emotional experience. Journal of Personality and Social Psychology. 2004;87:266–281. doi: 10.1037/0022-3514.87.2.266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett LF. Emotions as natural kinds? Perspectives on Psychological Science. 2006a;1:28–58. doi: 10.1111/j.1745-6916.2006.00003.x. [DOI] [PubMed] [Google Scholar]
- Barrett LF. Solving the emotion paradox: Categorization and the experience of emotion. Personality and Social Psychology Review. 2006b;10:20–46. doi: 10.1207/s15327957pspr1001_2. [DOI] [PubMed] [Google Scholar]
- Barrett LF. Valence as a basic building block of emotional life. Journal of Research in Personality. 2006c;40:35–55. [Google Scholar]
- Barrett LF. The future of psychology: Connecting mind to brain. Perspectives in Psychological Science. doi: 10.1111/j.1745-6924.2009.01134.x. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett LF, Bar M. See it with feeling: Affective predictions in the human brain. Royal Society Philosophical Transactions, B. 2006c doi: 10.1098/rstb.2008.0312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett LF, Fossum T. Mental representations of affect knowledge. Cognition and Emotion. 2001;15:333–364. [Google Scholar]
- Barrett LF, Lindquist K, Bliss-Moreau E, Duncan S, Gendron M, Mize J, et al. Of mice and men: Natural kinds of emotion in the mammalian brain? Perspectives on Psychological Science. 2007;2:297–312. doi: 10.1111/j.1745-6916.2007.00046.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett LF, Niedenthal PM. Valence focus and the perception of facial affect. Emotion. 2004;4:266–274. doi: 10.1037/1528-3542.4.3.266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett LF, Ochsner KN, Gross JJ. On the automaticity of emotion. In: Bargh J, editor. Social psychology and the unconscious: The automaticity of higher mental processes. New York: Psychology Press; 2007. [Google Scholar]
- Barrett LF, Quigley K, Bliss-Moreau E, Aronson KR. Arousal focus and interoceptive sensitivity. Journal of Personality and Social Psychology. 2004;87:684–697. doi: 10.1037/0022-3514.87.5.684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett LF, Russell JA. Independence and bipolarity in the structure of current affect. Journal of Personality and Social Psychology. 1998;74:967–984. [Google Scholar]
- Barrett LF, Russell JA. Structure of current affect. Current Directions in Psychological Science. 1999;8:10–14. [Google Scholar]
- Basole A, White LE, Fitzpatrick D. Mapping multiple features in the population response of visual cortex. Nature. 2003;423:986–990. doi: 10.1038/nature01721. [DOI] [PubMed] [Google Scholar]
- Baxter MG, Hadfield WS, Murray EA. Rhinal cortex lesions produce mild deficits in visual discrimination learning for an auditory secondary reinforcer in rhesus monkeys. Behavioral Neuroscience. 1999;113:243–252. doi: 10.1037//0735-7044.113.2.243. [DOI] [PubMed] [Google Scholar]
- Baxter MG, Murray EA. Impairments in visual discrimination learning and recognition memory produced by neurotoxic lesions of rhinal cortex in rhesus monkeys. European Journal of Neuroscience. 2001;13:1228–1238. doi: 10.1046/j.0953-816x.2001.01491.x. [DOI] [PubMed] [Google Scholar]
- Baxter MG, Parker A, Lindner CCC, Izquierdo AD, Murray EA. Control of response selection by reinforcer value requires interaction of amygdala and orbital prefrontal cortex. Journal of Neuroscience. 2000;20:4311–4319. doi: 10.1523/JNEUROSCI.20-11-04311.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bechara A, Damasio H, Damasio AR, Lee GP. Different contributions of the human amygdala and ventromedial prefrontal cortex to decision-making. Journal of Neuroscience. 1999;19:5473–5481. doi: 10.1523/JNEUROSCI.19-13-05473.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bechara A, Damasio H, Tranel D, Damasio AR. Deciding advantageously before knowing the advantageous strategy. Science. 1997;275:1293–1295. doi: 10.1126/science.275.5304.1293. [DOI] [PubMed] [Google Scholar]
- Beebe-Center JG. Pleasure and instinct: A study in the psychology of human action. Psychological Bulletin. 1932;29:355–356. [Google Scholar]
- Berridge KC, Robinson TE. What is the role of dopamine in reward: Hedonic impact, reward learning, or incentive salience? Brain Research Reviews. 1998;28:309–369. doi: 10.1016/s0165-0173(98)00019-8. [DOI] [PubMed] [Google Scholar]
- Berridge KC, Winkielman P. What is an unconscious emotion? (the case for unconscious “liking”) Cognition and Emotion. 2003;17:181–211. doi: 10.1080/02699930302289. [DOI] [PubMed] [Google Scholar]
- Bertolucci-D’Angio M, Serrano A, Scatton B. Differential effects of forced locomotion, tail-pinch, immobilization, and methyl-beta-carboline carboxylate on extracellular 3,4-dihydroxyphenylacetic acid levels in the rat striatum, nucleus accumbens, and prefrontal cortex: An in vivo voltammetric study. Journal of Neurochemistry. 1990;55:1208–1215. doi: 10.1111/j.1471-4159.1990.tb03126.x. [DOI] [PubMed] [Google Scholar]
- Bliss-Moreau E, Barrett LF, Horvitz J, Quigley K, Balsam P. Individual variation in the acquisition and recovery of affective value. 2008 Manuscript under review. [Google Scholar]
- Bliss-Moreau E, Barrett LF, Owren M. I like the sound of your voice: Affective learning about voices. doi: 10.1016/j.jesp.2009.12.017. Manuscript under review. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bliss-Moreau E, Barrett LF, Wright CI. Individual differences in learning the affective value of others under minimal conditions. Emotion. 2008;8:479–493. doi: 10.1037/1528-3542.8.4.479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bliss-Moreau E, Wager T, Lindquist KA, Kober H, Joseph J, Davidson M, et al. The valued brain: The neural reference space for core affect. Poster presented at the annual meeting of the Cognitive Neuroscience Society; San Francisco, CA. 2008. [Google Scholar]
- Block J. Studies in the phenomenology of emotions. The Journal of Abnormal and Social Psychology. 1957;54:358–363. doi: 10.1037/h0040768. [DOI] [PubMed] [Google Scholar]
- Bornstein MH, Arterberry ME. Recognition, discrimination and categorization of smiling by 5-month-old infants. Developmental Science. 2003;6:585–599. [Google Scholar]
- Bradley MM, Lang PJ. Measuring emotion: The self-assessment manikin and the semantic differential. Journal of Behavior Therapy and Experimental Psychiatry. 1994;25:49–59. doi: 10.1016/0005-7916(94)90063-9. [DOI] [PubMed] [Google Scholar]
- Bradley MM, Lang PJ. Measuring emotion: Behavior, feeling, and physiology. In: Lane RD, Nadel L, editors. Cognitive neuroscience of emotion. New York: Oxford University Press; 2000. pp. 242–276. [Google Scholar]
- Breiter HC, Etcoff NL, Whalen PJ, Kennedy WA, Rauch SL, Buckner RL. Response and habituation of the human amygdala during visual processing of facial expressions. Neuron. 1996;17:875–877. doi: 10.1016/s0896-6273(00)80219-6. [DOI] [PubMed] [Google Scholar]
- Browne MW. Circumplex models for correlation matrices. Psychometrika. 1992;54:469–497. [Google Scholar]
- Büchel C, Dolan RJ, Armony JL, Friston KJ. Amygdala-hippocampal involvement in human aversive trace conditioning revealed through event-related functional magnetic resonance imaging. Journal of Neuroscience. 1999;19:10869–10876. doi: 10.1523/JNEUROSCI.19-24-10869.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bush LE. Individual differences multidimensional scaling of adjectives denoting feelings. Journal of Personality and Social Psychology. 1973;25:50–57. doi: 10.1037/h0034274. [DOI] [PubMed] [Google Scholar]
- Cabanac M. What is emotion? Behavioural Processes. 2002;60:69–83. doi: 10.1016/s0376-6357(02)00078-5. [DOI] [PubMed] [Google Scholar]
- Cacioppo JT, Berntson GG. Relationship between attitudes and evaluative space: A critical review, with emphasis on the separability of positive and negative substrates. Psychological Bulletin. 1994;115:401–423. [Google Scholar]
- Cacioppo JT, Berntson GG. The affect system and racial prejudice. In: Bargh J, Apsley DK, editors. Unravelling the complexities of social life: A festschrift in honor of Robert B. Zajonc. Washington, DC: American Psychological Association; 2001. pp. 95–110. [Google Scholar]
- Cacioppo JT, Berntson GG, Larsen JT, Poehlmann KM, Ito TA. The psychophysiology of emotion. In: Lewis R, Haviland-Jones JM, editors. The handbook of emotion. 2. New York: Guilford Press; 2000. pp. 173–191. [Google Scholar]
- Cacioppo JT, Gardner WL, Berntson GG. Beyond bipolar conceptualizations and measures: The case of attitudes and evaluative space. Personality and Social Psychology Review. 1997;1:3–25. doi: 10.1207/s15327957pspr0101_2. [DOI] [PubMed] [Google Scholar]
- Cacioppo JT, Gardner WL, Berntson GG. The affect system has parallel and integrative processing components: Form follows function. Journal of Personality and Social Psychology. 1999;76:839–855. [Google Scholar]
- Canli T, Sivers H, Whitfield SL, Gotlib IH, Gabrieli JDE. Amygdala response to happy faces as a function of extraversion. Science. 2002;296:2191. doi: 10.1126/science.1068749. [DOI] [PubMed] [Google Scholar]
- Carmichael ST, Price JL. Limbic connections of the orbital and medial prefrontal cortex in macaque monkeys. Journal of Comparative Neurology. 1995;363:615–641. doi: 10.1002/cne.903630408. [DOI] [PubMed] [Google Scholar]
- Carmichael ST, Price JL. Connectional networks within the orbital and medial prefrontal cortex of macaque monkeys. Journal of Comparative Neurology. 1996;371:179–207. doi: 10.1002/(SICI)1096-9861(19960722)371:2<179::AID-CNE1>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- Carr HW. The idea of an external world. Symposion. 1925;1:33–39. [Google Scholar]
- Carroll JM, Yik MSM, Russell JA, Barrett LF. On the psychometric principles of affect. Review of General Psychology. 1999;3:14–22. [Google Scholar]
- Cavada C, Company T, Tejedor J, Cruz-Rizzolo RJ, Reinsos-Suarez F. The anatomical connections of the Macaque monkey orbitofrontal cortex: A review. Cerebral Cortex. 2000;10:220–242. doi: 10.1093/cercor/10.3.220. [DOI] [PubMed] [Google Scholar]
- Choi WY, Balsam PD, Horvitz JC. Extended habit training reduces dopamine mediation of appetitive response expression. Journal of Neuroscience. 2005;25:6729–6733. doi: 10.1523/JNEUROSCI.1498-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cliff N, Young FW. On the relation between unidimensional judgments and multidimensional scaling. Organizational Behavior & Human Performance. 1968;3:269–285. [Google Scholar]
- Clore GL, Ortony A. Appraisal theories: How cognition shapes affect into emotion. In: Lewis M, Haviland-Jones JM, Barrett LF, editors. Handbook of emotions. 3. New York: Guilford Press; 2008. pp. 628–642. [Google Scholar]
- Clore GL, Storbeck J, Robinson MD, Centerbar D. Seven sins in the study of unconscious affect. In: Feldman Barrett L, Niedenthal P, Winkielman P, editors. Emotion: Conscious and unconscious. New York, NY: Guilford Press; 2005. pp. 384–408. [Google Scholar]
- Craig AD. Opinion: How do you feel? Interoception: The sense of the physiological condition of the body. Nature Reviews Neuroscience. 2002;3:655–666. doi: 10.1038/nrn894. [DOI] [PubMed] [Google Scholar]
- Critchley HD, Wiens S, Rotshtien P, Ohman A, Dolan RJ. Neural systems supporting interoceptive awareness. Nature Neuroscience. 2004;7:189–195. doi: 10.1038/nn1176. [DOI] [PubMed] [Google Scholar]
- Damaraju E, Huang Y-M, Barrett LF, Pessoa L. Affective learning enhances activity and functional connectivity in early visual cortex. 2008 doi: 10.1016/j.neuropsychologia.2009.04.023. Manuscript under review. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson RJ. Prolegomenon to the structure of emotion: Gleanings from neuropsychology. Cognition and Emotion. 1992;6:245–268. [Google Scholar]
- Davidson RJ. Parsing affective space: Perspectives from neuropsychology and psychophysiology. Neuropsychology. 1993;7:464–475. [Google Scholar]
- Davidson RJ. Affective style, psychopathology, and resilience: Brain mechanisms and plasticity. American Psychologist. 2000;55:1196–1214. doi: 10.1037//0003-066x.55.11.1196. [DOI] [PubMed] [Google Scholar]
- Davidson RJ. Affective style: Causes and consequences. In: Cacioppo JT, Berntson GC, editors. Essays in social neuroscience. Cambridge, MA: MIT Press; 2004. pp. 77–91. [Google Scholar]
- Davidson RJ, Pizzagalli D, Nitschke JB, Putnam K. Depression: Perspectives from affective neuroscience. Annual Review of Psychology. 2002;53:545–574. doi: 10.1146/annurev.psych.53.100901.135148. [DOI] [PubMed] [Google Scholar]
- Davison ML. Introduction to multidimensional scaling and its applications. Applied Psychological Measurement. 1983;7:373–379. [Google Scholar]
- De Houwer J, Thomas S, Baeyens F. Association learning of likes and dislikes: A review of 25 years of research on human evaluative conditioning. Psychological Bulletin. 2001;127:853–869. doi: 10.1037/0033-2909.127.6.853. [DOI] [PubMed] [Google Scholar]
- Delgado MR, Olsson A, Phelps EA. Extending animal models of fear conditioning to humans. Biological Psychology. 2006;73:39–48. doi: 10.1016/j.biopsycho.2006.01.006. [DOI] [PubMed] [Google Scholar]
- de Rosnay M, Pons F, Harris PL, Morrell JMB. A lag between understanding false belief and emotion attribution in young children: Relationships with linguistic ability and mothers’ mental-state language. British Journal of Developmental Psychology. 2004;22:197–218. [Google Scholar]
- Dichter GS, Tomarken AJ, Freid CM, Addington S, Shelton RC. Do venlafaxine XR and paroxetine equally influence negative and positive affect? Journal of Affective Disorders. 2005;85:333–339. doi: 10.1016/j.jad.2004.10.007. [DOI] [PubMed] [Google Scholar]
- Diener E. Introduction to the special section on the structure of emotion. Journal of Personality and Social Psychology. 1999;76:803–804. doi: 10.1037//0022-3514.76.5.803. [DOI] [PubMed] [Google Scholar]
- Dittman AT. Interpersonal messages in emotion. New York: Springer; 1972. [Google Scholar]
- Domjan M. Pavlovian conditioning: A functional perspective. Annual Review of Psychology. 2005;56:179–206. doi: 10.1146/annurev.psych.55.090902.141409. [DOI] [PubMed] [Google Scholar]
- Dubois S, Rossion B, Schiltz C, Bodart JM, Michel C, Bruyer R, et al. Effect of familiarity on the processing of human faces. Neuroimage. 1999;9:278–289. doi: 10.1006/nimg.1998.0409. [DOI] [PubMed] [Google Scholar]
- Duffy E. Is emotion a mere term of convenience? Psychological Review. 1934;41:103–104. [Google Scholar]
- Duncan S, Barrett LF. Affect as a form of cognition: A neurobiological analysis. Cognition and Emotion. 2007;21:1184–1211. doi: 10.1080/02699930701437931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunn BD, Dalgleish T, Lawrence AD. The somatic marker hypothesis: A critical evaluation. Neuroscience and Biobehavioral Reviews. 2006;30:239–271. doi: 10.1016/j.neubiorev.2005.07.001. [DOI] [PubMed] [Google Scholar]
- Eagly AH, Chaiken S. Attitude structure and function. In: Gilbert DT, Fiske ST, Lindzey G, editors. The handbook of social psychology. 4. New York, NY: McGraw-Hill; 1998. pp. 269–322. [Google Scholar]
- Edelman GM. Wider than the sky: The phenomenal gift of consciousness. London: Yale University Press; 2004. [Google Scholar]
- Edelman GM, Tononi G. A universe of consciousness: How matter becomes imagination. New York: Basic Books; 2000. [Google Scholar]
- Eimer M, Holmes A. Event-related brain potential correlates of emotional face processing. Neuropsychologia. 2007;45:15–31. doi: 10.1016/j.neuropsychologia.2006.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elliott R, Friston KJ, Dolan R. Dissociable neural responses in human reward systems. Journal of Neuroscience. 2000;20:6159–6165. doi: 10.1523/JNEUROSCI.20-16-06159.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elliott R, Rees G, Dolan RJ. Ventromedial prefrontal cortex mediates guessing. Neuropsychologia. 1999;37:403–411. doi: 10.1016/s0028-3932(98)00107-9. [DOI] [PubMed] [Google Scholar]
- Engell AD, Haxby JV, Todorov A. Implicit trustworthiness decisions: Automatic coding of face properties in the human amygdala. Journal of Cognitive Neuroscience. 2007;19:1508–1519. doi: 10.1162/jocn.2007.19.9.1508. [DOI] [PubMed] [Google Scholar]
- Fabrigar LR, Visser PS, Browne MW. Conceptual and methodological issues in testing the circumplex structure of data in personality and social psychology. Personality and Social Psychology Review. 1997;1:184–203. doi: 10.1207/s15327957pspr0103_1. [DOI] [PubMed] [Google Scholar]
- Feldman LA. Variations in the circumplex structure of emotion. Personality and Social Psychology Bulletin. 1995a;21:806–817. [Google Scholar]
- Feldman LA. Valence focus and arousal focus: Individual differences in the structure of affective experience. Journal of Personality and Social Psychology. 1995b;69:153–166. [Google Scholar]
- Fellows LK, Farah MJ. Ventromedial frontal cortex mediates affective shifting in humans: Evidence from a reversal learning paradigm. Brain: A Journal of Neurology. 2003;126:1830–1837. doi: 10.1093/brain/awg180. [DOI] [PubMed] [Google Scholar]
- Ferguson ML, Katkin ES. Visceral perception, anhedonia, and emotion. Biological Psychology. 1996;42:131–145. doi: 10.1016/0301-0511(95)05151-1. [DOI] [PubMed] [Google Scholar]
- Field AP. Learning to like (or dislike): Associative learning of preferences. In: Wills AJ, editor. New directions in human associative learning. Mahwah, NJ: Erlbaum; 2005. pp. 221–252. [Google Scholar]
- Fillenbaum S, Rapoport A. Structures in the subjective lexicon. New York: Academic Press; 1971. [Google Scholar]
- Flom R, Bahrick LE. The development of infant discrimination of affect in multimodal and unimodal stimulation: The role of intersensory redundancy. Developmental Psychology. 2007;43:238–252. doi: 10.1037/0012-1649.43.1.238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forgas JP. Mood and judgment: The affect infusion model (AIM) Psychological Bulletin. 1995;117:39–66. doi: 10.1037/0033-2909.117.1.39. [DOI] [PubMed] [Google Scholar]
- Forgas JP. On feeling good and getting your way: Mood effects on negotiator cognition and bargaining strategies. Journal of Personality and Social Psychology. 1998;74:565–577. doi: 10.1037//0022-3514.74.3.565. [DOI] [PubMed] [Google Scholar]
- Forgas JP. Feeling and speaking: Mood effects on verbal communication strategies. Personality and Social Psychology Bulletin. 1999a;25:850–863. [Google Scholar]
- Forgas JP. On feeling good and being rude: Affective influences on language use and request formulations. Journal of Personality and Social Psychology. 1999b;76:928–939. [Google Scholar]
- Forgas JP, Fiedler K. Us and them: Mood effects on intergroup discrimination. Journal of Personality and Social Psychology. 1996;70:28–40. [Google Scholar]
- Frijda NH, Kuipers P, ter Schure E. Relations among emotion, appraisal, and emotional action readiness. Journal of Personality and Social Psychology. 1989;57:212–228. [Google Scholar]
- Gallo LC, Bogart LM, Vranceanu A, Matthews KA. Socioeconomic status, resources, psychological experiences, and emotional responses: A test of the reserve capacity model. Journal of Personality and Social Psychology. 2005;88:386–399. doi: 10.1037/0022-3514.88.2.386. [DOI] [PubMed] [Google Scholar]
- Garavan H, Pendergrass JC, Ross TJ, Stein EA, Risinger RC. Amygdala response to both positively and negatively valenced stimuli. Neuroreport. 2001;12:2779–2783. doi: 10.1097/00001756-200108280-00036. [DOI] [PubMed] [Google Scholar]
- Gemelli A. Orienting concepts in the study of affective states. Part I. Journal of Nervous and Mental Disease. 1949a;110:198–214. [PubMed] [Google Scholar]
- Gemelli A. Orienting concepts in the study of affective states. Part II. Journal of Nervous and Mental Disease. 1949b;110:299–314. [PubMed] [Google Scholar]
- Gendron M, Barrett LF. Reconstructing the past: A century of emotion theorizing in psychology. Emotion Review. doi: 10.1177/1754073909338877. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghashghaei HT, Barbas H. Pathways for emotion: Interactions of prefrontal and anterior temporal pathways in the amygdala of the rhesus monkey. Neuroscience. 2002;115:1261–1279. doi: 10.1016/s0306-4522(02)00446-3. [DOI] [PubMed] [Google Scholar]
- Gilbert DT, Ebert JEJ. Decisions and revisions: The affective forecasting of changeable outcomes. Journal of Personality and Social Psychology. 2002;82:503–514. [PubMed] [Google Scholar]
- Gilbert DT, Pinel EC, Wilson TD, Blumberg SJ, Wheatley T. Immune neglect: A source of durability bias in affective forecasting. Journal of Personality and Social Psychology. 1998;75:617–638. doi: 10.1037//0022-3514.75.3.617. [DOI] [PubMed] [Google Scholar]
- Goel V, Dolan RJ. Explaining modulation of reasoning by belief. Cognition. 2003;87:B11–B22. doi: 10.1016/s0010-0277(02)00185-3. [DOI] [PubMed] [Google Scholar]
- Green RS, Cliff N. Multidimensional comparisons of structures of vocally and facially expressed emotion. Perception and Psychophysics. 1975;17:429–438. [Google Scholar]
- Green DP, Goldman SL, Salovey P. Measurement error masks bipolarity in affect ratings. Journal of Personality and Social Psychology. 1993;64:1029–1041. doi: 10.1037//0022-3514.64.6.1029. [DOI] [PubMed] [Google Scholar]
- Green DP, Salovey P, Truax KM. Static, dynamic, and causative bipolarity of affect. Journal of Personality and Social Psychology. 1999;76:856–867. doi: 10.1037//0022-3514.76.5.856. [DOI] [PubMed] [Google Scholar]
- Greene JD, Sommerville RB, Nystrom LE, Darley JM, Cohen JD. An fMRI investigation of emotional engagement in moral judgment. Science. 2001;293:2105–2108. doi: 10.1126/science.1062872. [DOI] [PubMed] [Google Scholar]
- Grillon C. Associative learning deficits increase symptoms of anxiety in humans. Biological Psychiatry. 2002;51:851–858. doi: 10.1016/s0006-3223(01)01370-1. [DOI] [PubMed] [Google Scholar]
- Guttman L. A new approach to factor analysis: The radex. In: Lazarsfeld PF, editor. Mathematical thinking in the social sciences. New York: Columbia University Press; 1957. pp. 258–348. [Google Scholar]
- Haidt J. The emotional dog and its rational tail: A social intuitionist approach to moral judgment. Psychological Review. 2001;108:814–834. doi: 10.1037/0033-295x.108.4.814. [DOI] [PubMed] [Google Scholar]
- Haidt J. Dialogue between my head and my heart”: Affective influences on moral judgment. Psychological Inquiry. 2002;13:54–56. [Google Scholar]
- Hantas M, Katkin ES, Blascovich J. Relationship between heartbeat discrimination and subjective experience of affective state. Psychophysiology. 1982;19:563. [Google Scholar]
- Hermans D, Vansteenwegen D, Crombez G, Baeyens F, Eelen P. Expectancy-learning and evaluative learning in human classical conditioning: Affective priming as an indirect and unobtrusive measure of conditioned stimulus valence. Behaviour Research and Therapy. 2002;40:217–234. doi: 10.1016/s0005-7967(01)00006-7. [DOI] [PubMed] [Google Scholar]
- Herry C, Bach DR, Esposito F, Di Salle F, Perrig WJ, Scheffler K, et al. Processing of temporal unpredictability in human and animal amygdala. Journal of Neuroscience. 2007;27:5958–5966. doi: 10.1523/JNEUROSCI.5218-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holland PC, Gallagher M. Amygdala circuitry in attentional and representational processes. Trends in Cognitive Science. 1999;3:65–73. doi: 10.1016/s1364-6613(98)01271-6. [DOI] [PubMed] [Google Scholar]
- Horvitz JC. Mesolimbic and nigrostriatal dopamine responses to salient non-reward events. Neuroscience. 2000;96:651–656. doi: 10.1016/s0306-4522(00)00019-1. [DOI] [PubMed] [Google Scholar]
- Horvitz JC. Dopamine gating of glutamatergic sensorimotor and incentive motivational inputs to the striatum. Behavioral Brain Research. 2002;137:65–74. doi: 10.1016/s0166-4328(02)00285-1. [DOI] [PubMed] [Google Scholar]
- Hsu M, Bhatt M, Adolphs R, Tranel D, Camerer CF. Neural systems responding to degrees of uncertainty in human decision-making. Science. 2005;310:1680–1683. doi: 10.1126/science.1115327. [DOI] [PubMed] [Google Scholar]
- Hunt WA. A critical review of current approaches to affectivity. Psychological Bulletin. 1939;36:807–828. [Google Scholar]
- Hunt WA. Recent developments in the field of emotion. Psychological Bulletin. 1941;38:249–276. [Google Scholar]
- Hurliman E, Nagode JC, Pardo JV. Double dissociation of exteroceptive and interoceptive feedback systems in the orbital and ventromedial prefrontal cortex of humans. Journal of Neuroscience. 2005;258:4641–4648. doi: 10.1523/JNEUROSCI.2563-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito TA, Cacioppo JT. Affect and attitudes: A social neuroscience approach. In: Forgas JP, editor. Handbook of affect and social cognition. Mahwah, NJ: Erlbaum; 2001. pp. 50–74. [Google Scholar]
- Izhikevich EM, Desai NS, Walcott EC, Hoppensteadt FC. Bursts as a unit of neural information: Selective communication via resonance. Trends in Neuroscience. 2003;2:161–167. doi: 10.1016/S0166-2236(03)00034-1. [DOI] [PubMed] [Google Scholar]
- James W. The principals of psychology. New York: Holt; 1890. [Google Scholar]
- Keller RW, Stricker EM, Zigmond MJ. Environmental stimuli but not homeostatic challenges produce apparent increases in dopaminergic activity in the striatum: An analysis by in vivo voltammetry. Brain Research. 1983;279:159–170. doi: 10.1016/0006-8993(83)90174-9. [DOI] [PubMed] [Google Scholar]
- Kensinger EA, Schacter DL. Memory and emotion. In: Lewis M, Haviland-Jones JM, Barrett LF, editors. The handbook of emotion. 3. New York: Guilford Press; 2008. [Google Scholar]
- Kitayama S, Markus HR, Kurokawa M. Culture, emotion and well-being: Good feelings in Japan and the United States. Cognition and Emotion. 2000;14:93–124. [Google Scholar]
- Kober H, Barrett LF, Joseph J, Bliss-Moreau E, Lindquist KA, Wager TD. Functional networks and cortical–subcortical interactions in emotion: A meta-analysis of neuroimaging studies. Neuroimage. 2008;42:998–1031. doi: 10.1016/j.neuroimage.2008.03.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kringelbach ML. Linking reward to hedonic experience. Nature Reviews Neuroscience. 2005;6:691–702. doi: 10.1038/nrn1747. [DOI] [PubMed] [Google Scholar]
- Kringelbach ML, Rolls ET. The functional neuroanatomy of the human orbitofrontal cortex: Evidence from neuroimaging and neuropsychology. Progress in Neurobiology. 2004;72:341–372. doi: 10.1016/j.pneurobio.2004.03.006. [DOI] [PubMed] [Google Scholar]
- LaBar KS, Cook CA, Torpey DC, Welsh-Bohmer KA. Impact of healthy aging on awareness and fear conditioning. Behavioral Neuroscience. 2004;118:905–915. doi: 10.1037/0735-7044.118.5.905. [DOI] [PubMed] [Google Scholar]
- LaBar KS, Gatenby JC, Gore JC, LeDoux JE, Phelps EA. Human amygdala activation during conditioned fear acquisition and extinction: A mixed-trial fMRI study. Neuron. 1998;20:937–945. doi: 10.1016/s0896-6273(00)80475-4. [DOI] [PubMed] [Google Scholar]
- LaBar KS, Phelps EA. Reinstatement of conditioned fear in humans is context dependent and impaired in amnesia. Behavioral Neuroscience. 2005;119:677–686. doi: 10.1037/0735-7044.119.3.677. [DOI] [PubMed] [Google Scholar]
- Lane RD, Chua PM, Dolan RJ. Common effects of emotional valence, arousal and attention on neural activation during visual processing of pictures. Neuropsychologia. 1999;37:989–997. doi: 10.1016/s0028-3932(99)00017-2. [DOI] [PubMed] [Google Scholar]
- Lang PJ, Bradley MM, Fitzsimmons JR, Cuthbert BN, Scott JD, Moulder B, et al. Emotional arousal and activation of the visual cortex: An fMRI analysis. Psychophysiology. 1998;35:199–210. [PubMed] [Google Scholar]
- Lang PJ, Greenwald M, Bradley M, Hamm A. Looking at pictures: Affective, facial, visceral, and behavioral reactions. Psychophysiology. 1993;30:261–273. doi: 10.1111/j.1469-8986.1993.tb03352.x. [DOI] [PubMed] [Google Scholar]
- Lewis M. Emergence of human emotions. In: Lewis M, Haviland-Jones JM, editors. Handbook of emotions. 2. New York, NY: Guilford Press; 2000. pp. 253–322. [Google Scholar]
- Lipp OV, Oughton N, LeLievre J. Evaluative learning in human Pavlovian conditioning: Extinct, but still there? Learning and Motivation. 2003;34:219–239. [Google Scholar]
- Mackie DM, Hamilton DL. Affect, cognition, and stereotyping: Interactive processes in group perception. San Diego, CA: Academic Press; 1993. [Google Scholar]
- Mandler G. Mind and emotion. New York: Wiley; 1975. [Google Scholar]
- Mather M, Canli T, English T, Whitfield S, Wais P, Ochsner K, et al. Amygdala responses to emotionally valenced stimuli in older and younger adults. Psychological Science. 2004;15:259–263. doi: 10.1111/j.0956-7976.2004.00662.x. [DOI] [PubMed] [Google Scholar]
- McCullough LD, Salamone JD. Involvement of nucleus accumbens dopamine in the motor activity induced by periodic food presentation: A microdialysis and behavioral study. Brain Research. 1992;592:29–36. doi: 10.1016/0006-8993(92)91654-w. [DOI] [PubMed] [Google Scholar]
- McDonald AJ. Cortical pathways to the mammalian amygdala. Progress in Neurobiology. 1998;55:257–332. doi: 10.1016/s0301-0082(98)00003-3. [DOI] [PubMed] [Google Scholar]
- Mesquita B. Emotions as dynamic cultural phenomena. In: Goldsmith H, Davidson R, Scherer K, editors. Handbook of the affective sciences. New York: Oxford; 2003. pp. 871–890. [Google Scholar]
- Mesulam M. Principles of behavioral and cognitive neurology. 2. New York, NY: Oxford University Press; 2000. [Google Scholar]
- Milad MR, Quinn BT, Pitman RK, Orr SP, Fischl B, Rauch SL. Thickness of ventromedial prefrontal cortex in humans is correlated with extinction memory. Proceedings of the National Academy of Sciences of the United States of America. 2005;102:10706–10711. doi: 10.1073/pnas.0502441102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mirenowicz J, Schultz W. Importance of unpredictability for reward responses in primate dopamine neurons. Journal of Neurophysiology. 1994;72:1024–1027. doi: 10.1152/jn.1994.72.2.1024. [DOI] [PubMed] [Google Scholar]
- Moll J, de Oliviera-Souza R, Eslinger PJ, Bramati IE, Mourao-Miranda J, Andreiuolo PA, et al. The neural correlates of moral sensitivity: A functional magnetic resonance imaging investigation of basic and moral emotions. Journal of Neuroscience. 2002;22:2730–2736. doi: 10.1523/JNEUROSCI.22-07-02730.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreno KN, Bodenhausen GV. Intergroup affect and social judgement: Feelings as inadmissible information. Group Processes and Intergroup Relations. 2001;4:21–29. [Google Scholar]
- Morris JS, Ohman A, Dolan RJ. A subcortical pathway to the right amygdala mediating “unseen” fear. Proceedings of the National Academy of Sciences. 1998;96:1680–1685. doi: 10.1073/pnas.96.4.1680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nauta W. The problem of the frontal lobe: A reinterpretation. Journal of Psychiatric Research. 1971;8:167–187. doi: 10.1016/0022-3956(71)90017-3. [DOI] [PubMed] [Google Scholar]
- Norman KA, Polyn SM, Detre GJ, Haxby JV. Beyond mind-reading: Multi-voxel pattern analysis of fMRI data. Trends in Cognitive Sciences. 2006;10:424–430. doi: 10.1016/j.tics.2006.07.005. [DOI] [PubMed] [Google Scholar]
- Nygaard LC, Lunders ER. Resolution of lexical ambiguity by emotional tone of voice. Memory and Cognition. 2002;30:583–593. doi: 10.3758/bf03194959. [DOI] [PubMed] [Google Scholar]
- Nygaard LC, Queen JS. Communicating emotion: Linking affective prosody and word meaning. Journal of Experimental Psychology: Human Perception and Performance. 2008;34:1017–1030. doi: 10.1037/0096-1523.34.4.1017. [DOI] [PubMed] [Google Scholar]
- Ongur D, An X, Price JL. Prefrontal cortical projections to the hypothalamus in macaque monkeys. Journal of Comparative Neurology. 1998;401:480–505. [PubMed] [Google Scholar]
- Ongur D, Ferry AT, Price JL. Architectonic subdivision of the human orbital and medial prefrontal cortex. Journal of Comparative Neurology. 2003;460:425–449. doi: 10.1002/cne.10609. [DOI] [PubMed] [Google Scholar]
- Ongur D, Price JL. The organization of networks within the orbital and medial prefrontal cortex of rats, monkeys and humans. Cerebral Cortex. 2000;10:206–219. doi: 10.1093/cercor/10.3.206. [DOI] [PubMed] [Google Scholar]
- O’Reilly RC, Munakata Y. Computational explorations in cognitive neuroscience: Understanding the mind by simulating the brain. Cambridge, MA: The MIT Press; 2000. [Google Scholar]
- Osgood CE, Suci GJ, Tannenbaum PH. The measurement of meaning. Champaign, IL: University of Illinois Press; 1957. [Google Scholar]
- Owren MJ, Rendall D. An affect-conditioning model of nonhuman primate vocal signaling. In: Owings DH, Beecher MD, Thompson NS, editors. Perspectives in ethology. Vol. 12. New York: Plenum; 1997. pp. 299–346. Communication. [Google Scholar]
- Owren MJ, Rendall D. Sound on the rebound: Bringing form and function back to the forefront in understanding nonhuman primate vocal signaling. Evolutionary Anthropology. 2001;10:58–71. [Google Scholar]
- Padmala S, Pessoa L. Affective learning enhances visual detection and responses in primary visual cortex. Journal of Neuroscience. 2008;28:6202–6210. doi: 10.1523/JNEUROSCI.1233-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palermo R, Rhodes G. Are you always on my mind? A review of how face perception and attention interact. Neuropsychologia. 2007;45:75–92. doi: 10.1016/j.neuropsychologia.2006.04.025. [DOI] [PubMed] [Google Scholar]
- Parvizi J, Damasio A. Consciousness and the brainstem. Cognition. 2001;79:135–159. doi: 10.1016/s0010-0277(00)00127-x. [DOI] [PubMed] [Google Scholar]
- Pearce JM, Bouton ME. Theories of associative learning in animals. Annual Review of Psychology. 2001;52:111–139. doi: 10.1146/annurev.psych.52.1.111. [DOI] [PubMed] [Google Scholar]
- Peters HN. The judgmental theory of pleasantness and unpleasantness. Psychological Review. 1935;42:354–386. [Google Scholar]
- Phelps EA, Delgado MR, Nearing KI, LeDoux JE. Extinction learning in humans: Role of the amygdala and vmPFC. Neuron. 2004;43:897–905. doi: 10.1016/j.neuron.2004.08.042. [DOI] [PubMed] [Google Scholar]
- Pietromonaco PR, Barrett LF. Valence focus and self-esteem lability: Reacting to hedonic cues in the social environment. 2008 doi: 10.1037/a0015691. Manuscript under review. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plutchik R. A general psychoevolutionary theory of emotion. In: Plutchik R, Kellerman H, editors. Emotion: Theory, research, and experience: Vol. 1. Theories of emotion. New York: Academic; 1980. pp. 3–33. [Google Scholar]
- Prather MD, Lavenex P, Mauldin-Jourdain ML, Mason WA, Capitanio JP, Mendoza SP, et al. Increased social fear and decreased fear of objects in monkeys with neonatal amygdala lesions. Neuroscience. 2001;106:653–658. doi: 10.1016/s0306-4522(01)00445-6. [DOI] [PubMed] [Google Scholar]
- Price JL. Connections of orbital cortex. In: Zald DH, Rauch SL, editors. The orbitofrontal cortex. New York: Oxford University Press; 2007. pp. 38–56. [Google Scholar]
- Quirk GJ, Russo GK, Barron JL, Lebron K. The role of ventromedial prefrontal cortex in the recovery of extinguished fear. Journal of Neuroscience. 2000;20:6225–6231. doi: 10.1523/JNEUROSCI.20-16-06225.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reich JW, Zautra AJ, Davis M. Dimensions of affect relationships: Models and their integrative implications. Review of General Psychology. 2003;7:66–83. [Google Scholar]
- Rempel-Clower NL, Barbas H. Topographic organization of connections between the hypothalamus and prefrontal cortex in the rhesus monkey. Journal of Comparative Neurology. 1998;398:393–419. doi: 10.1002/(sici)1096-9861(19980831)398:3<393::aid-cne7>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- Revelle W. Personality processes. Annual Review of Psychology. 1995;46:295–328. [Google Scholar]
- Reynolds SM, Berridge KC. Fear and feeding in the nucleus accumbens shell: Rostrocaudal segregation of GABA elicitation of defensive behavior and eating behavior. Journal of Neuroscience. 2001;21:3261–3270. doi: 10.1523/JNEUROSCI.21-09-03261.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds SM, Berridge KC. Positive and negative motivation in nucleus accumbens shell: Bivalent rostrocaudal gradients for GABA-elictied eating, taste “liking”/“disliking” reactions, place preference/avoidance, and fear. Journal of Neuroscience. 2002;22:7308–7320. doi: 10.1523/JNEUROSCI.22-16-07308.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds SM, Berridge SM. Glutamate motivational ensembles in nucleus accumbens: Rostrocaudal shell gradients of fear and feeding. European Journal of Neuroscience. 2003;17:2187–2200. doi: 10.1046/j.1460-9568.2003.02642.x. [DOI] [PubMed] [Google Scholar]
- Reynolds SM, Berridge KC. Emotional environments retune the valence of appetitive versus fearful functions in nucleus accumbens. Nature Neuroscience. 2008;11:423–425. doi: 10.1038/nn2061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robledo P, Koob GF. Two discrete nucleus accumbens projection areas differentially mediate cocaine self-administration in the rat. Behavioural Brain Research. 1993;55:159–166. doi: 10.1016/0166-4328(93)90112-4. [DOI] [PubMed] [Google Scholar]
- Roseman IJ, Antoniou AA, Jose PE. Appraisal determinants of emotions: Constructing a more accurate and comprehensive theory. Cognition and Emotion. 1996;10:241–277. [Google Scholar]
- Ruckmick CA. The psychology of feeling and emotion. New York, NY: McGraw-Hill; 1936. [Google Scholar]
- Russell JA. A circumplex model of affect. Journal of Personality and Social Psychology. 1980;39:1161–1178. doi: 10.1037//0022-3514.79.2.286. [DOI] [PubMed] [Google Scholar]
- Russell JA. Culture and the categorization of emotion. Psychological Bulletin. 1991;110:426–450. doi: 10.1037/0033-2909.110.3.426. [DOI] [PubMed] [Google Scholar]
- Russell JA. Core affect and the psychological construction of emotion. Psychological Review. 2003;110:145–172. doi: 10.1037/0033-295x.110.1.145. [DOI] [PubMed] [Google Scholar]
- Russell JA, Bachorowski J, Fernandez-Dols JM. Facial and vocal expressions of emotion. Annual Review of Psychology. 2003;54:329–349. doi: 10.1146/annurev.psych.54.101601.145102. [DOI] [PubMed] [Google Scholar]
- Russell JA, Barrett LF. Core affect, prototypical emotional episodes, and other things called emotion: Dissecting the elephant. Journal of Personality and Social Psychology. 1999;76:805–819. doi: 10.1037//0022-3514.76.5.805. [DOI] [PubMed] [Google Scholar]
- Russell JA, Bullock M. Multidimensional scaling of emotional facial expressions: Similarity from preschoolers to adults. Journal of Personality and Social Psychology. 1985;48:1290–1298. [Google Scholar]
- Russell JA, Carroll JM. On the bipolarity of positive and negative affect. Psychological Bulletin. 1999b;125:3–30. doi: 10.1037/0033-2909.125.1.3. [DOI] [PubMed] [Google Scholar]
- Russell JA, Lewicka M, Niit T. A cross-cultural study of a circumplex model of affect. Journal of Personality and Social Psychology. 1989;57:848–856. [Google Scholar]
- Russell JA, Ridgeway D. Dimensions underlying children’s emotion concepts. Developmental Psychology. 1983;19:795–804. [Google Scholar]
- Russell JA, Weiss A, Mendelsohn GA. Affect grid: A single-item scale of pleasure and arousal. Journal of Personality and Social Psychology. 1989;57:493–502. [Google Scholar]
- Salamone JD, Carlson BB, Rios C, Lentini E, Correa M, Wisniecki A, et al. Dopamine agonists suppress cholinomimetic-induced tremulous jaw movements in an animal model of parkinsonism: Tremorolytic effects of pergolide, ropinirole and CY 208–243. Behavioural Brain Research. 2005;156:173–179. doi: 10.1016/j.bbr.2004.05.019. [DOI] [PubMed] [Google Scholar]
- Salamone JD, Correa M, Farrar A, Mingote SM. Effort-related functions of nucleus accumbens dopamine and associated forebrain circuits. Psychopharmacology. 2007;191:461–482. doi: 10.1007/s00213-006-0668-9. [DOI] [PubMed] [Google Scholar]
- Salamone JD, Correa M, Mingote SM, Weber SM. Beyond the reward hypothesis: Alternative functions of nucleus accumbens dopamine. Current Opinion in Pharmacology. 2005;5:34–41. doi: 10.1016/j.coph.2004.09.004. [DOI] [PubMed] [Google Scholar]
- Schachter S, Singer JE. Cognitive, social, and physiological determinants of emotional state. Psychological Review. 1962;69:379–399. doi: 10.1037/h0046234. [DOI] [PubMed] [Google Scholar]
- Scherer KR. Profiles of emotion-antecedent appraisal: Testing theoretical predictions across cultures. Cognition and Emotion. 1997;11:113–150. [Google Scholar]
- Schimmack U. Pleasure, displeasure, and mixed feelings: Are semantic opposites mutually exclusive? Cognition and Emotion. 2001;15:81–97. [Google Scholar]
- Schimmack U, Böckenholt U, Reisenzein R. Response styles in affect ratings: Making a mountain out of a molehill. Journal of Personality Assessment. 2002;78:461–483. doi: 10.1207/S15327752JPA7803_06. [DOI] [PubMed] [Google Scholar]
- Schirmer A, Kotz SA. ERP evidence for a sex-specific stroop effect in emotional speech. Journal of Cognitive Neuroscience. 2003;15:1135–1148. doi: 10.1162/089892903322598102. [DOI] [PubMed] [Google Scholar]
- Schlosberg H. The description of facial expressions in terms of two dimensions. Journal of Experimental Psychology. 1952;44:229–237. doi: 10.1037/h0055778. [DOI] [PubMed] [Google Scholar]
- Schlosberg H. Three dimensions of emotion. Psychological Review. 1954;61:81–88. doi: 10.1037/h0054570. [DOI] [PubMed] [Google Scholar]
- Schnider A, Treyer V, Buck A. Selection of currently relevant memories by the human posterior medial orbitofrontal cortex. Journal of Neuroscience. 2000;20:5880–5884. doi: 10.1523/JNEUROSCI.20-15-05880.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnyer DM, Nicholls L, Verfaellie M. The role of VMPC in metamemorial judgments of content retrievability. Journal of Cognitive Neuroscience. 2005;17:832–846. doi: 10.1162/0898929053747694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultz W, Apicella P, Ljungberg T. Responses of monkey dopamine neurons to reward and conditioned stimuli during successive steps of learning a delayed response task. Journal of Neuroscience. 1993;13:900–913. doi: 10.1523/JNEUROSCI.13-03-00900.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz CE, Wright CI, Shin LM, Kagan J, Whalen PJ, McMullin KG, et al. Differential amygdalar response to novel versus newly familiar neutral faces: A functional MRI probe developed for studying inhibited temperament. Biological Psychiatry. 2003;53:854–862. doi: 10.1016/s0006-3223(02)01906-6. [DOI] [PubMed] [Google Scholar]
- Schwarz N, Clore GL. Mood, misattribution, and judgments of well-being: Informative and directive functions of affective states. Journal of Personality and Social Psychology. 1983;45(3):513–523. [Google Scholar]
- Segura SL, González-Romá V. How do respondents construe ambiguous response formats of affect items? Journal of Personality and Social Psychology. 2003;85:956–968. doi: 10.1037/0022-3514.85.5.956. [DOI] [PubMed] [Google Scholar]
- Semba K. Multiple output pathways of the basal forebrain: Organization, chemical heterogeneity, and roles in vigilance. Behavioural Brain Research. 2000;115:117–141. doi: 10.1016/s0166-4328(00)00254-0. [DOI] [PubMed] [Google Scholar]
- Seo M, Barrett LF, Bartunek JM. The role of affective experience in work motivation. Academy of Management. 2004;29:423–439. [PMC free article] [PubMed] [Google Scholar]
- Shamay-Tsoory SG, Tomer R, Berger BD, Goldsher D, Aharon-Peretz J. Impaired “affective theory of mind” is associated with right ventromedial prefrontal damage. Cognitive and Behavioral Neurology. 2005;18:55–67. doi: 10.1097/01.wnn.0000152228.90129.99. [DOI] [PubMed] [Google Scholar]
- Shepard RN. The analysis of proximities: Multidimensional scaling with an unknown distance function. Part I. Psychometrika. 1962a;27:125–140. [Google Scholar]
- Shepard RN. The analysis of proximities: Multidimensional scaling with an unknown distance function. Part II. Psychometrika. 1962b;27:219–246. [Google Scholar]
- Shepard RN. The mental image. American Psychologist. 1978;33:125–137. [Google Scholar]
- Sloman SA. The empirical case for two systems of reasoning. Psychological Bulletin. 1996;119:3–22. [Google Scholar]
- Slovic P, Finucane M, Peters E, MacGregor DG. Rational actors or rational fools: Implications of the affect heuristic for behavioral economics. The Journal of Socio-Economics. 2002;31:329–342. [Google Scholar]
- Smith ER, DeCoster J. Dual-process models in social and cognitive psychology: Conceptual integration and links to underlying memory systems. Personality and Social Psychology Review. 2000;4:108–131. [Google Scholar]
- Smith CA, Ellsworth PC. Patterns of cognitive appraisal in emotion. Journal of Personality and Social Psychology. 1985;48:813–838. [PubMed] [Google Scholar]
- Smith CA, Ellsworth PC. Patterns of appraisal and emotion related to taking an exam. Journal of Personality and Social Psychology. 1987;52:475–488. doi: 10.1037//0022-3514.52.3.475. [DOI] [PubMed] [Google Scholar]
- Sorg BA, Kalivas PW. Effects of cocaine and footshock stress on extracellular dopamine levels in the ventral striatum. Brain Research. 1991;559:29–36. doi: 10.1016/0006-8993(91)90283-2. [DOI] [PubMed] [Google Scholar]
- Spivey M. The continuity of mind. New York, NY: Oxford University Press; 2007. [Google Scholar]
- Stolarova M, Keil A, Moratti S. Modulation of the C1 visual event-related component by conditioned stimuli: Evidence for sensory plasticity in early affective perception. Cerebral Cortex. 2006;16:876–887. doi: 10.1093/cercor/bhj031. [DOI] [PubMed] [Google Scholar]
- Taylor SF, Liberzon I, Koeppe RA. The effect of graded aversive stimuli on limbic and visual activation. Neuropsychologia. 2000;38:1415–1425. doi: 10.1016/s0028-3932(00)00032-4. [DOI] [PubMed] [Google Scholar]
- Thayer RE. The biopsychology of mood and arousal. New York, NY: Oxford University Press; 1989. [Google Scholar]
- Titchener EB. Lectures on the elementary psychology of feeling and attention. New York: Macmillian; 1908. [Google Scholar]
- Titchener EB. Experimental psychology of the thought processes. Oxford, England: Macmillan; 1909. [Google Scholar]
- Todorov A. Evaluating faces on trustworthiness: An extension of systems for recognition of emotions signaling approach/avoidance behaviors. In: Kingstone A, Miller MB, editors. The year in cognitive neuroscience 2008. Annals of the New York Academy of Sciences. Malden, MA: Blackwell; 2008. pp. 208–224. [DOI] [PubMed] [Google Scholar]
- Todorov A, Uleman JS. Spontaneous trait inferences are bound to actors’ faces: Evidence from a false recognition paradigm. Journal of Personality and Social Psychology. 2002;83:1051–1065. [PubMed] [Google Scholar]
- Todorov A, Uleman JS. The efficiency of binding spontaneous trait inferences to actors’ faces. Journal of Experimental Social Psychology. 2003;39:549–562. [Google Scholar]
- Vuilleumier P, Driver J. Modulation of visual processing by attention and emotion: Windows on causal interactions between human brain regions. Philosophical Transactions of the Royal Society B. 2007;362:837–855. doi: 10.1098/rstb.2007.2092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vuilleumier P, Pourtois G. Distributed and interactive brain mechanisms during emotion face perception: Evidence from functional neuroimaging. Neuropsychologia. 2007;45:174–194. doi: 10.1016/j.neuropsychologia.2006.06.003. [DOI] [PubMed] [Google Scholar]
- Wager T, Barrett LF, Bliss-Moreau E, Lindquist K, Duncan S, Kober H, et al. The neuroimaging of emotion. Chapter to appear. In: Lewis M, Haviland-Jones JM, Barrett LF, editors. The handbook of emotion. 3. New York: Guilford; 2008. pp. 249–271. [Google Scholar]
- Wagar BM, Thagard P. Spiking phineas gage: A neurocomputational theory of cognitive-affective integration in decision making. Psychological Review. 2004;111:67–79. doi: 10.1037/0033-295X.111.1.67. [DOI] [PubMed] [Google Scholar]
- Watson D. Mood and temperament. Emotions and social behavior. New York, NY: Guilford Press; 2000. [Google Scholar]
- Watson D, Tellegen A. Toward a consensual structure of mood. Psychological Bulletin. 1985;98:219–223. doi: 10.1037//0033-2909.98.2.219. [DOI] [PubMed] [Google Scholar]
- Watson D, Wiese D, Vaidya J, Tellegen A. The two general activation systems of affect: Structural findings, evolutionary considerations, and psychobiological evidence. Journal of Personality and Social Psychology. 1999;76:820–838. [Google Scholar]
- Wedig MM, Rauch SL, Albert MS, Wright CI. Differential amygdala habituation to neutral faces in young and elderly adults. Neuroscience Letters. 2005;38:114–119. doi: 10.1016/j.neulet.2005.05.039. [DOI] [PubMed] [Google Scholar]
- Weiskrantz L. Blindsight: A case study and its implications. Oxford: Oxford University Press; 1986. [Google Scholar]
- Weller JA, Levin IP, Shiv B, Bechara A. Neural correlates of adaptive decision making for risky gains and losses. Psychological Science. 2007;18:958–964. doi: 10.1111/j.1467-9280.2007.02009.x. [DOI] [PubMed] [Google Scholar]
- Whalen PJ. Fear, vigilance, and ambiguity: Initial neuroimaging studies of the human amygdala. Current Directions in Psychological Science. 1998;7:177–188. [Google Scholar]
- Whitehead WE, Drescher VM. Perception of gastric contractions and self-control of gastric motility. Psychophysiology. 1980;17:552–558. doi: 10.1111/j.1469-8986.1980.tb02296.x. [DOI] [PubMed] [Google Scholar]
- Widen SC, Russell JA. A closer look at preschoolers’ freely produced labels for facial expressions. Developmental Psychology. 2003;39:114–128. doi: 10.1037//0012-1649.39.1.114. [DOI] [PubMed] [Google Scholar]
- Wiens S, Mezzacappa ES, Katkin ES. Heartbeat detection and the experience of emotions. Cognition and Emotion. 2000;14:417–427. [Google Scholar]
- Wiggins JS, Broughton R. A geometric taxonomy of personality scales. European Journal of Personality. 1991;5:343–365. [Google Scholar]
- Wilson FA, Rolls ET. Neuronal responses related to reinforcement in the primate basal forebrain. Brain Research. 1990;509:213–231. doi: 10.1016/0006-8993(90)90546-n. [DOI] [PubMed] [Google Scholar]
- Winkielman P, Berridge KC, Wilbarger JL. Emotion, behavior, and conscious experience: Once more without feeling. In: Barrett LF, Niedenthal PM, Winkielman P, editors. Emotion and consciousness. New York, NY: Guilford Press; 2005. pp. 335–362. [Google Scholar]
- Woodworth RS. Experimental psychology. Oxford, England: Holt; 1938. [Google Scholar]
- Wright CI, Dickerson BC, Feczko E, Negeira A, Williams D. A functional magnetic resonance imaging study of amygdala responses to human faces in aging and mild alzheimer’s disease. Biological Psychiatry. 2007;62:1388–1395. doi: 10.1016/j.biopsych.2006.11.013. [DOI] [PubMed] [Google Scholar]
- Wright CI, Fischer H, Whalen PJ, McInerney SC, Shin LM, Rauch SL. Differential prefrontal cortex and amygdala habituation to repeatedly presented emotional stimuli. Neuroreport. 2001;12:379–383. doi: 10.1097/00001756-200102120-00039. [DOI] [PubMed] [Google Scholar]
- Wright CI, Martis M, Schwartz CE, Shin LM, Fischer HH, McMullin K, et al. Novelty responses and differential effects of order in the amygdala, substantia innominata and inferior temporal cortex. Neuroimage. 2003;18:660–669. doi: 10.1016/s1053-8119(02)00037-x. [DOI] [PubMed] [Google Scholar]
- Wright CI, Negreira A, Gold AL, Britton JC, Williams D, Barrett LF. Neural correlates of novelty and face-age effects in young and elderly adults. Neuroimage. 2008;42:956–968. doi: 10.1016/j.neuroimage.2008.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright CI, Williams D, Barrett LF, Dickerson B, Wedig MW. Neuroanatomical correlates of extraversion and neuroticism. Cerebral Cortex. 2006;16:1809–1819. doi: 10.1093/cercor/bhj118. [DOI] [PubMed] [Google Scholar]
- Wundt W. In: Lectures on human and animal psychology. Creigton SE, Titchener EB, translators. New York: Macmillian; 1998a. (Original work published 1894) [Google Scholar]
- Wundt W. In: Outlines of psychology. Judd CH, translator. Bristol, UK: Thoemmes Press; 1998b. (Original work published 1897) [Google Scholar]
- Wurm LH, Vakoch DA, Strasser MR, Calin-Jageman R, Ross SE. Speech perception and vocal expression of emotion. Cognition and Emotion. 2001;15:831–852. [Google Scholar]
- Yang TT, Menon V, Eliez S, Blasey C, White CD, Reid AJ, et al. Amygdalar activation associated with positive and negative facial expressions. Neuroreport. 2002;13:1737–1741. doi: 10.1097/00001756-200210070-00009. [DOI] [PubMed] [Google Scholar]
- Yik MSM, Russell JA, Ahn C, Fernández-Dols JM, Suzuki N. Relating the five-factor model of personality to a circumplex model of affect: A five language study. In: McCrae RR, Allik J, editors. The five-factor model of personality across cultures. New York, NY: Kluwer/Plenum; 2002. pp. 79–104. [Google Scholar]
- Yik MSM, Russell JA, Barrett LF. Integrating four structures of current mood into a circumplex: Integration and beyond. Journal of Personality and Social Psychology. 1999;77:600–619. [Google Scholar]
- Young PT. Emotion in man and animal; its nature and relation to attitude and motive. Oxford, England: Wiley; 1943. [Google Scholar]
- Young AMJ, Joseph MH, Gray JA. Latent inhibition of conditioned dopamine release in rat nucleus accumbens. Neuroscience. 1993;54:5–9. doi: 10.1016/0306-4522(93)90378-s. [DOI] [PubMed] [Google Scholar]
- Zald DH, Depue RA. Serotonergic functioning correlates with positive and negative affect in psychiatrically healthy males. Personality and Individual Differences. 2001;30:71–86. [Google Scholar]
- Zikopoulos B, Barbas H. Circuits for multisensory integration and attentional modulation through the prefrontal cortex and the thalamic reticular nucleus in primates. Reviews in the Neurosciences. 2007;18:417–438. doi: 10.1515/revneuro.2007.18.6.417. [DOI] [PMC free article] [PubMed] [Google Scholar]