Abstract
Summary: Like for all microbes, the goal of every pathogen is to survive and replicate. However, to overcome the formidable defenses of their hosts, pathogens are also endowed with traits commonly associated with virulence, such as surface attachment, cell or tissue invasion, and transmission. Numerous pathogens couple their specific virulence pathways with more general adaptations, like stress resistance, by integrating dedicated regulators with global signaling networks. In particular, many of nature's most dreaded bacteria rely on nucleotide alarmones to cue metabolic disturbances and coordinate survival and virulence programs. Here we discuss how components of the stringent response contribute to the virulence of a wide variety of pathogenic bacteria.
INTRODUCTION
To monitor and adapt to their environment, bacteria rely on sensory systems to regulate complex physiological processes. For example, bacteria readily modify their stress tolerance and nutrient utilization pathways in response to local cues. Like all microbes, the goal of every pathogen is to survive and replicate. However, to overcome the formidable defenses of their hosts, pathogens are also endowed with traits commonly associated with virulence, such as surface attachment, cell or tissue invasion, and transmission. A wide variety of pathogens couple their specific virulence pathways with more general adaptations, like stress resistance, by integrating dedicated regulators with global signaling networks, including those critical for carbon and nitrogen metabolism (75, 158). Many of nature's most dreaded bacteria rely on nucleotide alarmones to cue metabolic disturbances and coordinate survival and virulence programs.
Over 40 years ago, Cashel and Gallant first visualized guanosine 5′-diphosphate-3′-diphosphate (ppGpp) and guanosine 5′-triphosphate-3′-diphosphate (pppGpp; collectively referred to as ppGpp) by performing two-dimensional thin-layer chromatography of radiolabeled nucleotides from amino acid-starved Escherichia coli cells. The appearance of these “magic spots,” synthesized from GDP, or GTP, by pyrophosphoryl transfer from ATP, correlated with the cessation of rRNA synthesis, a process referred to as the stringent response (160). Subsequent research established that bacterial and plant cells that are experiencing nutritional stress synthesize ppGpp to initiate global physiological changes. Although new roles for ppGpp continue to be discovered, the alarmone generally functions to promote the adaptation and resilience of bacterial cells faced with adversity.
In the heterogeneous environments within mammalian and plant hosts, pathogenic bacteria alter their metabolism and protein repertoire in response to local conditions. Changes in the nutrient supply, alterations in immune responses, or contact with new surfaces can trigger bacterial adaptation. To gain an advantage, pathogenic bacteria may activate specialized secretion systems, motility organelles, or adhesins. Such virulence factors promote survival by equipping microbes to access nutrients, modulate the host cell biology or immune system, or migrate to more favorable locales. In response to local conditions, pathogens utilize dedicated regulators to change their tactics. The expression and activity of many virulence regulators are integrated into a global response mediated by ppGpp, thereby coupling pathogenesis to metabolic status (Table 1). As such, control over cellular ppGpp pools is critical for pathogen survival, replication, and transmission.
TABLE 1.
Stringent response components that contribute to virulence
| Pathogen | Stringent response machinery | Pathogenesis-related phenotype(s) associated with ppGpp | Reference(s) |
|---|---|---|---|
| Gammaproteobacteria | |||
| EHEC | RelA, SpoT,a DksA | Adherence | 143 |
| UPEC | RelA, SpoT,a DksA | Adherence | 1-3 |
| P. aeruginosa | RelA, SpoT,a DksA | Quorum sensing, biofilms, antibiotic tolerance | 18, 24, 27, 63, 202, 205 |
| Y. pestis | RelA, SpoT,a DksAd | Bubonic infection, lung dissemination | 190 |
| V. cholerae | RelA, SpoT,a RelV, DksAd | Mouse colonization | 56, 57, 74, 84, 179 |
| E. carotovora subsp. atrosepticab | RelA, SpoT,a,d DksAd | Rot in potato tubers | 207 |
| S. flexneri | RelA,d SpoT,d DksA | Intercellular spread | 133, 175 |
| S. enterica serovar Typhimurium | RelA, SpoT,a DksA | Invasion (SPI1 dependent), intracellular replication (SPI2 dependent) | 159, 182, 197, 200, 210, 223 |
| S. enterica serovar Gallinarum | RelA, SpoT,a DksAd | Invasion (SPI1 independent), intracellular replication (SPI2 dependent) | 97 |
| L. pneumophila | RelA, SpoT,a DksA | Macrophage transmission | 53, 81 |
| F. tularensis | RelA, SpoT,a DksAd | Phagosome escape in macrophages | 43 |
| Actinobacteria | |||
| M. tuberculosis | RelMtb,a CarD | Persistence in mice | 52, 185 |
| Firmicutes | |||
| L. monocytogenes | RelA,a RelQ,d RelPd | Adherence, intracellular survival | 19, 118, 149, 196 |
| S. aureus | RelA,a RelQ,d RelPd | Essential for viability in vitro | 44, 71, 114 |
| B. anthracis | RelA,a RelQ,d RelPd | Sporulation | 114, 204 |
| C. difficile | RelAa | Antibiotic tolerance | 62 |
| E. faecalis | RelA,a RelQ | Antibiotic tolerance, virulence in C. elegans | 4, 218 |
| S. pyogenes | RelA,a RelQ,d RelPd | 186, 187 | |
| S. pneumoniae | RelSpn,a RelQ | Pulmonary infection of mice | 17 |
| S. mutans | RelA,a RelQ, RelP | Biofilms | 114-116, 145 |
| Alphaproteobacteria | |||
| Brucella sp. | Rsh, DksAd | Macrophage survival, persistence in mice | 59, 104 |
| R. etlic | RelA,a DksAd | Nodule formation and nitrogen fixation | 25, 39, 137 |
| S. melilotic | RelA,a DksAd | Nodule formation | 211, 213, 214 |
| A. tumefaciensb | RelA,a DksAd | Ti plasmid transfer | 206, 221 |
| Epsilonproteobacteria | |||
| C. jejuni | SpoT,a DksA | Adherence, invasion, intracellular survival | 70, 188, 220 |
| H. pylori | SpoTa | Macrophage survival | 138, 212, 224 |
| Spirochetes | |||
| B. burgdorferi | SpoT or RelBbua | Virulence in mice | 34-36 |
Bifunctional synthetase/hydrolase.
Plant pathogen.
Plant symbiont.
Protein encoded by the species but yet to be studied.
METABOLISM OF ppGpp
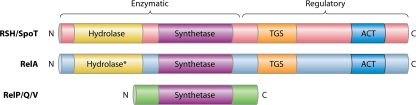
Levels of ppGpp are modulated by two classes of enzymes: monofunctional synthetase-only enzymes and bifunctional synthetase/hydrolase enzymes (Fig. 1). As the nomenclature of the bifunctional enzymes is not standardized, they can be referred to by their E. coli namesakes, RelA (monofunctional) or SpoT (bifunctional), or as RSH (RelA/SpoT homologue) proteins (26, 124, 160, 184). Both the monofunctional RelA- and bifunctional SpoT-like enzymes synthesize ppGpp from either GDP (in the case of guanosine tetraphosphate) or GTP (in the case of guanosine pentaphosphate) and ATP, whereas only the bifunctional enzymes also hydrolyze ppGpp to GDP and pyrophosphate (PPi) and pppGpp to GTP and PPi. Most Gram-negative gammaproteobacteria, like E. coli, Salmonella, Pseudomonas, and Legionella, encode both RelA and SpoT. Without SpoT, bacteria cannot degrade RelA-derived ppGpp, and the unabated accumulation of the nucleotide disrupts cell cycle control. In these species, SpoT function can be studied in the context of relA spoT double mutants (often annotated as ppGpp0 cells), which lack all synthetase activity. Many other pathogenic species encode ppGpp synthetase pathways distinct from the two-enzyme RelA/SpoT paradigm. For example, mycobacteria, alphaproteobacterial Brucella spp., and epsilonproteobacteria each encode a single bifunctional RSH protein (annotated Rel, RSH, and SpoT, respectively), whereas several Gram-positive Firmicutes, such as Bacillus, Listeria, Streptococcus, and Enterococcus, encode not only a single bifunctional RSH protein (alternately termed Rel or RelA) but also other small RelA-like synthetase fragments (termed RelP and RelQ) (Fig. 1). Similarly, the gammaproteobacterium Vibrio cholerae encodes RelV, another truncated synthetase enzyme (57). The existence of multiple enzymes devoted to alarmone synthesis and hydrolysis illustrates that bacteria have evolved versatile mechanisms to control ppGpp levels.
FIG. 1.
Domain structure of the enzymes that modulate bacterial pools of ppGpp. Four functional regions have been identified: the ppGpp synthetase domain, the ppGpp hydrolase domain, and the TGS and ACT regulatory domains. The bifunctional SpoT and RSH (RelA/SpoT homologue) proteins contain both synthetase and hydrolase activities in an N-terminal enzymatic domain. RelA proteins behave as monofunctional synthetases. The RelA and SpoT proteins of E. coli share 31% amino acid identity, and amino acid divergence renders the hydrolase domain inactive (*). The activity of the SpoT/RSH and RelA proteins is controlled through their TGS and ACT domains. RelP, RelQ, and RelV, referred to as small alarmone synthases (SASs), are monofunctional enzymes with little similarity to RelA or each other at the amino acid sequence level. For example, the S. mutans RelP and RelQ proteins share 8% identity with the synthetase domain of E. coli RelA and 29% identity with each other (109).
By regulating the enzyme activities that control the synthesis and degradation of ppGpp, bacteria can coordinate global physiological transformations tailored to distinct metabolic stimuli. It has long been established that the synthetase activity of E. coli RelA is elicited at the ribosome by uncharged tRNAs that accumulate during amino acid starvation (160). Likewise, the bifunctional RSH proteins of bacteria harboring only one ppGpp synthetase are activated by amino acid starvation.
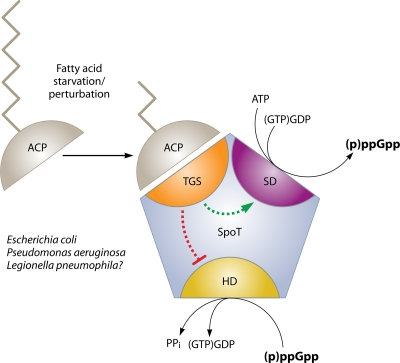
In contrast, for bacteria that also encode RelA, their bifunctional SpoT enzymes respond to a variety of stimuli, including phosphate, carbon, and iron starvation, as well as perturbations in fatty acid metabolism (61, 160). The ability of SpoT to respond to fatty acid biosynthesis inhibition is mediated by acyl carrier protein (ACP) (Fig. 2). Although the mechanistic details remain to be discovered, this physical interaction is influenced by the ratio of unacylated to acylated ACP, enabling bacteria to sense the fatty acid biosynthetic capacity of the cell (160). Direct physical interactions between SpoT and ACP have been demonstrated for the E. coli and Pseudomonas aeruginosa proteins, and genetic evidence suggests that a similar interaction occurs in Legionella pneumophila (17, 53). SpoT-dependent responses can reflect changes in either synthetase or hydrolase activity; thus, the catalytic balance of the bifunctional enzymes may be a critical point of control. Fatty acid and carbon starvation each exert allosteric effects on the monofunctional RelV of V. cholerae, raising the interesting possibility that other monofunctional stringent response enzymes also sense stresses other than amino acid starvation (57). In addition to nutritional cues, enzymes that govern ppGpp metabolism can be regulated both transcriptionally and posttranslationally when pathogens encounter stress during transmission or infection, such as high osmolarity, extreme pH, or chemical onslaughts (4, 149, 212).
FIG. 2.
SpoT activity is regulated through ACP interactions. Gammaproteobacteria that encode both RelA and SpoT have evolved a SpoT-dependent stringent response to fatty acid starvation that is mediated by an interaction between SpoT and ACP. ACP transfers fatty acyl chains to enzymes devoted to phospholipid and secondary metabolite biosynthesis. SpoT interacts with functional acyl-bound ACP at a nonenzymatic region known as the TGS domain. During fatty acid starvation, metabolic signals are transduced through an ACP-SpoT interaction, resulting in an increase in cellular ppGpp pools. It remains to be determined whether, in response to fatty acid stress, the ACP-SpoT interaction specifically modulates the synthetase (SD) or hydrolase (HD) activity of SpoT.
REGULATORY TARGETS OF ppGpp
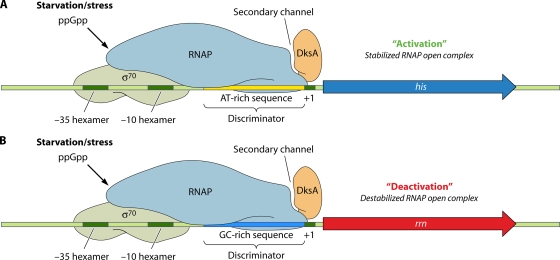
The ppGpp alarmone mediates many of its physiological effects by transcriptional control by either direct or indirect mechanisms. In E. coli, the direct repression of rRNA operons and the direct activation of amino acid biosynthetic operons by ppGpp occur via an interaction between the nucleotide and RNA polymerase (RNAP) that is not fully understood (160). DksA, a small protein that binds in the RNAP secondary channel, potentiates the effects of ppGpp on transcription (85). Whether a given promoter is directly activated or repressed by ppGpp and DksA is dictated by DNA sequence motifs (Fig. 3). Repressed targets are typically GC rich between the −10-box hexamer and the +1 nucleotide (transcriptional start site), a site known as the discriminator region, whereas activated promoters are typically AT rich in this position.
FIG. 3.
ppGpp and DksA control transcription directly. In response to stress, gammaproteobacteria use ppGpp and DksA to control RNAP activity at particular promoters. Although DksA is known to bind at the secondary channel of RNAP, a binding site for ppGpp has not been confirmed. In the presence of DksA and elevated ppGpp levels, transcription can be either activated or deactivated. Whether transcription is stimulated or repressed depends upon intrinsic properties of the promoter. Activated targets such as the E. coli promoter for the histidine biosynthetic (his) operon typically have an AT-rich DNA sequence between the −10 hexamer and the +1 transcriptional start site, known as the discriminator region. Conversely, repressed targets such as the P1 promoter of rRNA (rrn) operons typically have a GC-rich discriminator sequence. Promoters controlled directly by ppGpp and DksA generally depend on the housekeeping/vegetative sigma factor σ70.
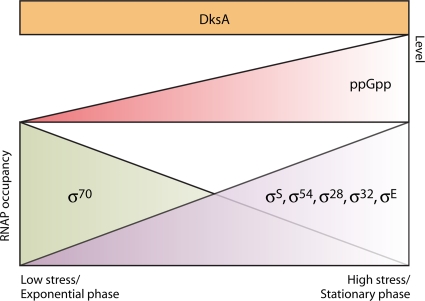
Indirect transcriptional control by ppGpp and DksA can occur through a process known as sigma factor competition (Fig. 4). In gammaproteobacteria, nearly all direct targets of ppGpp require the vegetative housekeeping sigma factor σ70. During a stringent response, alarmone inhibition of strong σ70-dependent promoters, such as rRNA promoters, increases the availability of core RNAP for transcription by alternative sigma factors (20, 49, 80, 194). In this manner, ppGpp indirectly promotes alternative sigma factor-dependent gene regulation by repressing the transcription of rRNA operons. Together, direct and indirect ppGpp-dependent mechanisms are integrated to mediate the global physiological adaptations of the bacterial cell that comprise the stringent response.
FIG. 4.
ppGpp and DksA control transcription indirectly through “sigma factor competition.” During exponential growth or favorable conditions, ppGpp levels are low in gammaproteobacteria, and transcription from strong σ70-dependent promoters such as those of rRNA operons is robust. As bacteria exit the exponential phase, or during high stress, ppGpp levels accumulate. DksA, whose levels remain constant during growth, cooperates with ppGpp to repress the transcription of rRNA operons, liberating RNAP to bind alternative sigma factors (σS, σ54, σ28, σ32, and σE). As a result, transcription from promoters targeted by these sigma factors increases, inducing specialized stress responses. In this manner, ppGpp and DksA contribute indirectly to bacterial adaptation.
Recently, the dogma that ppGpp and DksA always collaborate to regulate E. coli physiology has been challenged. Data from phenotypic studies indicate that the overproduction of DksA by ppGpp0 bacteria can compensate for the lack of the alarmone (160). Additionally, ppGpp and DksA have opposite effects on certain E. coli traits and promoters (1, 125). Furthermore, in vitro assays show opposite and independent regulations of some promoters by these two stringent response components (123, 131). Also, some regulatory effects that are predicted by phenotypic assays, such as the expression of fimbriae and flagella, are not recapitulated when transcriptional regulation by ppGpp and DksA is analyzed in vitro at the promoters of critical regulators, like FimB, FlhDC, and FliA (3, 113). Accordingly, other factors likely influence regulation by ppGpp0 and dksA mutant cells. For example, TraR upregulates transcription from amino acid promoters and downregulates transcription from ribosomal promoters in the absence of ppGpp and DksA (22). Therefore, additional work is required before these complex regulatory interactions are fully understood.
Distinct roles for ppGpp and DksA in the bacterial cell also result from interactions between the alarmone and proteins other than RNAP. For example, in Bacillus subtilis, ppGpp prevents DNA replication elongation by inhibiting DNA primase activity (208). In Salmonella enterica serovar Typhimurium, ppGpp interacts with SlyA, a transcriptional activator of this pathogen's intracellular virulence program, facilitating its dimerization and binding to target promoters (223). In both E. coli and B. subtilis, ppGpp interacts with Obg, a nucleotide binding protein implicated in a number of physiological processes (33, 156). In V. cholerae, the ribosome-associated Obg homologue (“CgtA”) appears to regulate ppGpp synthesis. In particular, CgtA is thought to repress the stringent response under nutrient-replete conditions by interacting with and modulating the activity of SpoT, keeping ppGpp levels low (164). A similar interaction was described for E. coli (215). Therefore, Obg likely contributes to yet another mechanism to regulate ppGpp levels, at least among some pathogenic members of the gammaproteobacteria. Alarmone production also affects the physiology of some Gram-positive bacteria by an indirect mechanism. In B. subtilis, the production of ppGpp is accompanied by a decrease in GTP pools, which affects the activity of GTP binding proteins, including CodY (93). Some cellular GTP is consumed during ppGpp synthesis, but the alarmone itself also inhibits IMP dehydrogenase, an enzyme involved in GTP biosynthesis (68, 119). Since B. subtilis utilizes GTP to initiate transcription from rRNA promoters, by reducing GTP pools, ppGpp indirectly represses rRNA production (109, 110). Thus, ppGpp can operate independently of both DksA and RNAP activity, illustrating the alarmone's far-reaching effects. Although the stringent response pathways of the pathogens that we discuss below (Table 1) have yet to be studied in great detail, several concepts learned from the model microbes E. coli and B. subtilis do apply.
GAMMAPROTEOBACTERIA
Enterohemorrhagic E. coli
In the nutrient-rich upper intestine, enterohemorrhagic E. coli (EHEC) replicates profusely. Descent into the nutrient-limited lower intestine triggers an increase in surface colonization and slower replication by the bacteria. In particular, the harsh environment of the lower intestine cues the timely expression of the EHEC locus of enterocyte effacement (LEE), a 35-kbp pathogenicity island encoding virulence factors necessary for attachment and colonization (98). The hallmark virulence trait of EHEC is the formation of “attaching and effacing” lesions on intestinal epithelial cells (146). This pathology is initiated when adherent EHEC cells secrete effector proteins across the host cell plasma membrane by using a type III secretion system (T3SS) encoded within the LEE region. By injecting factors that co-opt and interfere with cell processes, the bacteria orchestrate the construction, from host materials, of pedestal-like structures, sites of intimate contact that perturb the architecture of the brush border microvilli (201). From this vantage point, EHEC modulates both epithelial cell biology and the local immune response, promoting its survival within the host intestine and inducing symptoms of disease.
EHEC integrates the core ppGpp signaling system with transcriptional regulators of its more recently acquired virulence machinery, presumably to maximize its fitness in the lower intestine. Aside from horizontally acquired elements like the LEE and genes carried by cryptic prophages, EHEC is nearly identical to E. coli K-12 (130). Like this nonpathogenic strain, EHEC encodes a monofunctional RelA enzyme and a bifunctional SpoT enzyme. The accumulation of ppGpp in response to starvation activates LEE gene expression and increases bacterial adherence (143). Along with ppGpp, EHEC employs the transcription factor DksA to regulate LEE expression. Transcriptional targets of ppGpp and DksA include the LEE-encoded regulator (Ler) and the prophage-encoded activators PchA and PchB (143), which amplify ler expression (95). The Ler protein, encoded by the first gene in the LEE operon 1 (LEE1), coordinates the timely activation of the four remaining LEE operons as well as other T3SS effectors encoded elsewhere in the genome (130).
LEE regulation by the stringent response pathway has been analyzed biochemically and genetically. In in vitro transcription assays, ppGpp and DksA are required to activate the pchA, pchB, and LEE1 promoters. Importantly, ppGpp and DksA can mediate promoter activation of LEE1 in the absence of either PchA or PchB. Therefore, EHEC amplifies the starvation stimulus by activating dual systems, ensuring maximal LEE expression (143). In broth cultures, the ppGpp alarmone and DksA protein are required for LEE expression at the onset of stationary phase. However, in late-stationary-phase cells, the activation of the LEE1 promoter still occurs in a dksA mutant but not a ppGpp0 mutant. This delayed activation has been attributed to the DksA-dependent repression of LEE1 during long-term starvation (143). Alternatively, late activation may reflect a ppGpp-mediated bypass of DksA, perhaps via an unidentified transcription factor. By integrating the expression of horizontally acquired virulence factors into its native ppGpp regulon, EHEC amplifies nutritional signals received in the lower intestine to enhance surface colonization, withstand nutrient depletion, and subvert host defenses.
Uropathogenic Escherichia coli
Acute urinary tract infection caused by uropathogenic E. coli (UPEC) can lead to recurrent infections by the same bacterial strain despite antibiotic treatment (168). A mouse model of cystitis suggests that recurrence and resistance may be attributed to the formation of intracellular bacterial communities. The establishment of these biofilm-like communities follows a specific maturation process where loosely associated, rapidly dividing, rod-shaped bacteria mature into slow-growing coccoid bacteria (102). As its microbial intruders replicate, the cytoplasm of the specialized superficial umbrella cell expands, forming protrusions on the bladder epithelium. Here, bacteria begin to express factors critical for motility and flux from the host cell, processes that promote dispersal throughout the luminal space of the urinary tract (102).
Type I fimbriae are adhesive organelles that promote not only bacterial cell-cell contact during biofilm formation but also the colonization and invasion of bladder epithelial cells by binding to particular host receptors (102). Following invasion, type I fimbriae contribute to the maturation of intracellular communities, likely by facilitating physical interactions between resident bacteria (217). To establish an intracellular niche, UPEC requires the timely activation and repression of adhesive fimbriae in response to specific microenvironments encountered at the surface of the bladder epithelium and within host cells.
To ensure the opportune induction of fimbrial gene expression, UPEC relies on ppGpp and DksA to couple the expression of the fim operon with its metabolic status. The control of fimbria expression by ppGpp occurs through the promoter activation of the site-specific recombinase FimB, an enzyme that inverts the 314-bp promoter of the fimAICDFGH operon. Following transcriptional activation, FimB flips the fim promoter from its “off” orientation to its productive “on” position, thereby activating the transcription of genes encoding the structural components of type I fimbriae (2). Entry into the stationary phase results in the ppGpp-mediated activation of both the fimB and fimA promoters, increasing the frequency of adherent cells. An identical response occurs when UPEC starvation is triggered by treatment with serine hydroxamate or when ppGpp synthesis is artificially induced by using a truncated derivative of RelA that is constitutively active (2). Suppressor mutations that restore fimB expression map to the RNAP subunit rpoB and are similar to those that restore the amino acid prototrophy of ppGpp0 mutant bacteria by inducing the expression of the amino acid biosynthetic machinery. Thus, UPEC exploits the classical stringent response alarmone ppGpp to control fimB transcription (2).
As with several other promoters that require ppGpp for direct regulation, fimB promoter activity is also impacted by DksA. However, recent studies of UPEC and E. coli K-12 have challenged the previously ascribed role of DksA during ppGpp-dependent transcriptional regulation (1, 3, 125). DksA not only regulates motility and cell-to-cell adhesion independently of ppGpp but also controls bacterial adherence oppositely of ppGpp (125). Furthermore, whereas ppGpp0 UPEC cells do not express type I fimbriae, dksA mutants are hyperfimbriated (3). These distinct phenotypic patterns for dksA and ppGpp0 cells are also observed at the level of transcription (3).
Differential regulation by ppGpp and DksA at the fimB promoter has been analyzed in detail. The hyperfimbriation of UPEC dksA mutants in broth reflects increased levels of fimB promoter activity. However, when the components are present together in in vitro reactions, ppGpp and DksA stimulate the transcription of fimB independently, exerting co-positive regulation. DksA can also enhance RNAP binding to the fimB promoter independently of ppGpp (3). Discrepancies between the broth phenotypes of dksA mutant UPEC and the DksA activities observed in vitro may be attributed in part to an increased occupancy in vivo of the RNAP secondary channel by structural homologues of DksA, such as the antipausing factors GreA and GreB, which may be missing from in vitro reactions (3). Indeed, increased channel occupancy by GreA and GreB contributes to an elevated level of expression of flagellum-related genes in dksA mutant E. coli K-12 cells (1). The mechanism by which ppGpp and other secondary channel-interacting proteins control the activation of the fimB promoter is highly complex and warrants further study.
Genes other than fimB also illustrate nonoverlapping regulation by ppGpp and DksA. For example, numerous genes required for flagellar biosynthesis and directional motility exhibited a greater-than-5-fold difference in relative transcript levels when ppGpp and dksA mutants were compared (3). It has become clear that direct transcriptional control by ppGpp and DksA in the bacterial cell is more complicated than previously thought.
Recently, TraR, a DksA homologue (30% identity) encoded by the conjugative F plasmid of E. coli K-12, was found to repress rRNA transcription and activate amino acid biosynthetic operons similarly to DksA. However, TraR functions in the absence of ppGpp (22). An understanding of how TraR acts independently of ppGpp will likely provide clues to the mechanisms of ppGpp- and DksA-dependent transcriptional control. Its self-sufficiency and presence on a conjugative plasmid, together with the general propensity of bacteria to integrate ppGpp into the regulation of pathogenicity island genes, make TraR, like other DksA homologues, a candidate activator of specialized bacterial virulence systems (22).
Shigella flexneri
The Gram-negative bacterium Shigella flexneri causes shigellosis in humans. The bacterial invasion of colonic mucosa elicits a robust inflammatory response and results in the destruction of the host epithelium. S. flexneri crosses the epithelial layer through epithelial M cells and accesses the basolateral surface (153), where bacteria induce uptake through membrane ruffling. Following ingestion by host cells, S. flexneri promptly escapes the endocytic vacuole and enters the cytoplasm. Cytosolic bacteria divide rapidly, with generation times of 40 min. To spread to neighboring cells, the pathogen induces a rapid, polar assembly of host actin, which propels Shigella through the cytosol and into double-membrane protrusions formed at the lateral surface of epithelial cells, enabling dissemination to adjacent cells.
Although highly similar to E. coli K-12, S. flexneri has not only lost and gained chromosomally encoded functions but also acquired extrachromosomal elements such as a virulence plasmid, prophage elements, and other insertion sequences that contribute to its fitness in vivo. To control the expression of many of these horizontally acquired virulence determinants, S. flexneri employs DksA.
S. flexneri DksA is 98% identical at the amino acid sequence level to those of E. coli K-12 and Salmonella enterica serovar Typhimurium. In a tissue culture model of infection, S. flexneri specifically requires DksA for intercellular spread but not for invasion or intracellular multiplication (133). DksA apparently controls S. flexneri dissemination via an RpoS-independent mechanism, as rpoS is dispensable for spread. The cell-to-cell transmission of Shigella requires the polar localization of IcsA, a protein that induces the polymerization of the epithelial cell's actin to propel the microbe unidirectionally through the cytosol. The ability of S. flexneri to localize IcsA to a single pole requires DksA, as dksA mutants exhibit an increased propensity to distribute the protein evenly onto its surface, likely contributing to aberrant intercellular spread during infection (175).
Global gene expression analysis during exponential growth in broth demonstrates that DksA exerts positive control over a number of plasmid-carried and chromosomally carried S. flexneri virulence genes. The levels of mRNAs of icsA, virF (encoding a transcriptional regulator of virulence), and certain T3SS genes are each decreased in dksA mutant bacteria relative to wild-type (WT) bacteria. Additionally, DksA positively controls the expression of the chromosomally encoded regulators RpoS, Fur, Hns, and Hfq (175).
To spread from cell to cell, S. flexneri requires DksA for the direct transcriptional activation of Hfq (175), a regulatory protein that promotes the hybridization of small RNAs to their target mRNAs. The exponential-phase activation of hfq transcription by DksA is direct, since the addition of DksA to in vitro reaction mixtures increases hfq transcripts 4-fold (175). DksA-dependent activation is likely enhanced during starvation, because the addition of ppGpp to reaction mixtures results in additional increases in hfq transcript levels. Control over hfq activation is important during the dissemination of S. flexneri, based on the observation that the experimental induction of hfq to physiological protein levels is sufficient to bypass the requirement for dksA during intercellular spread. Thus, DksA activates the transcription of hfq during infection to promote cell-to-cell transmission.
Surprisingly, a role for ppGpp during cell-to-cell spread has not been tested. It will be interesting to determine if ppGpp0 mutant S. flexneri exhibits dissemination defects similar to those of dksA mutant bacteria during infection. If so, starvation may trigger Shigella escape from nutrient-depleted epithelial cells, a strategy employed by L. pneumophila (51).
Pseudomonas aeruginosa
In addition to surviving in a variety of environmental niches, including soil and water, the ubiquitous opportunistic pathogen Pseudomonas aeruginosa infects the lungs of individuals with cystic fibrosis (CF). To do so, P. aeruginosa is thought to persist within biofilms that are recalcitrant to antibiotic treatment (152). As such, the establishment of chronic infection often coincides with the emergence of strains that display a heritable mucoid phenotype characterized by the overproduction of the extracellular biofilm-associated polymer alginate. Also contributing to virulence is an extensive repertoire of exoproduct virulence factors, including toxins, secreted proteases such as elastase, toxic secondary metabolites including pyocyanin, and biofilm-related factors such as rhamnolipid (203).
Like E. coli, P. aeruginosa harbors both a bifunctional SpoT and a RelA, which is required for ppGpp accumulation during amino acid starvation. P. aeruginosa relA mutant strains showed reduced virulence in a Drosophila melanogaster model of infection (63), which may in part reflect a perturbation of quorum sensing (QS). QS equips P. aeruginosa to coordinate the expression of secreted virulence factors with antibiotic and stress tolerance phenotypes. In particular, a hierarchical acylhomoserine lactone (AHL) QS cascade comprised of the Las and Rhl systems contributes to pathogenesis. The Las pathway positively regulates the Rhl system, and they both affect the expression of genes important for biofilm development, motility, and virulence-associated exoprotein expression. P. aeruginosa also harbors a third QS system, mediated by the Pseudomonas quinolone signal (PQS), which intersects with the AHL systems and also controls the stationary-phase production of exoprotein virulence factors. In addition to relA, functional QS systems are required for the full virulence of P. aeruginosa (108, 154).
The stringent response influences QS signaling mechanisms in P. aeruginosa that are necessary for infection. When cultured in minimal medium, differences in levels of AHL production between WT and relA P. aeruginosa strains are negligible. However, under conditions of Mg2+ limitation in minimal medium, conditions that induce relA expression (79) and that may be encountered in the CF lung, P. aeruginosa produced high levels of AHL by a relA-dependent pathway, even at low cell densities (63). In contrast, when amino acid starvation was initiated with serine hydroxamate, PQS levels decreased by a relA-dependent mechanism (63).
Consistent with altered QS signal production, a P. aeruginosa relA mutant dysregulated the expression of QS-controlled virulence factors such as pyocyanin and elastase (60). Specifically, the expression of elastase is controlled by the Las QS system, and a lasR mutant displays an elastase-negative phenotype. Elastase production by lasR mutants is restored by a second-site suppressor mutation that increases the level of expression of the autoinducer synthase gene rhlI. Interestingly, DksA appears to inhibit rhlI expression by this suppressor strain, since multiple copies of dksA abolish its elastase production and reduce rhlI transcript levels (27). The levels of production of rhamnolipid and LasB elastase are also reduced in a dksA mutant; however, this regulation was proposed to occur by a posttranscriptional mechanism (101). Taken together, these observations indicate that ppGpp regulates QS-mediated virulence factor expression in P. aeruginosa, likely by controlling the expression of QS enzymes such as autoinducer synthases.
In P. aeruginosa, the control of biofilm formation appears to be complex, with many regulatory elements interconnected with QS in a manner that depends on environmental cues (105). Some observations suggest that the stringent response pathway also affects P. aeruginosa biofilm development. For example, the contribution of QS to biofilm formation depends on nutrient conditions (178). When nutrients are limiting, bacteria induce the expression of the AHL autoinducer synthases in the exponential phase rather than the early stationary phase (60). Furthermore, ppGpp can induce the expression of QS-regulated virulence factors independent of cell density, causing the premature production of HSL autoinducers (202). Although Erickson et al. did not observe a difference in biofilm formation by relA mutants compared to the WT (63), the strong influence of environmental cues indicates that P. aeruginosa biofilm formation is governed by ppGpp under particular growth conditions that remain to be identified. Alternatively, the basal ppGpp pool resulting from the synthetase or hydrolase activity of SpoT may be more critical than RelA-controlled ppGpp levels for P. aeruginosa biofilm formation. The stringent response pathway may also influence the starvation-induced dispersal of P. aeruginosa biofilms (92, 172).
As expected, ppGpp also affects stress tolerance phenotypes in P. aeruginosa. In particular, SpoT positively regulates the expression of usp genes, encoding universal stress proteins essential for survival under conditions such as the anaerobic stationary phase (24). The response to membrane perturbation also appears to be coordinated by ppGpp, as mutations that affect the membrane composition of P. aeruginosa lead to increased levels of transcription of relA as well as the relA-dependent activation of the AHL QS systems (18).
The stringent response also influences the expression of other regulators of P. aeruginosa virulence. For example, ppGpp may influence stationary-phase survival by controlling the expression of RpoS, since relA mutants contain less RpoS than do WT bacteria (63). P. aeruginosa also requires ppGpp and DksA for expression from RpoN-dependent promoters, possibly due to a stringent repression of σ70 promoters and the subsequent release of RNA polymerase to bind alternative sigma factors (Fig. 4) (194). RpoN-dependent virulence genes include those required for type IV pilus biogenesis, motility, and alginate synthesis (161).
An extensive repertoire of regulatory factors equips P. aeruginosa to detect and respond to numerous environmental conditions. Among these are components of the stringent response, which affect antibiotic and stress tolerance, biofilm formation, and the production of exoproducts associated with virulence. By coupling the stringent response pathway to other regulatory mechanisms, such as QS and alternative sigma factors, this versatile pathogen integrates nutrient cues with virulence factor expression.
Yersinia pestis
Prior to the advent of antimicrobials, plague was a devastating infectious disease affecting mankind. Although the number of cases reported annually has decreased dramatically, Yersinia pestis, the etiological agent of the disease, still threatens humans as a potential agent of bioterrorism. Natural reservoirs for Y. pestis include rodents and fleas, which disseminate bacteria to humans (155). Plague is manifested in bubonic and pneumonic forms. Bubonic plague is caused by the transmission of bacteria into the human lymphatic system by fleas that have fed on infected rodents. Mammals can also transmit bacteria via aerosols, the inhalation of which results in pneumonic infection and rapid mortality (155).
Y. pestis carries three plasmids that contribute to pathogenesis. One virulence plasmid encodes a T3SS required to evade phagocytosis and limit host inflammatory responses (155). Another plasmid is thought to promote survival in fleas (89). The third plasmid encodes factors that facilitate the invasion of host cells and dissemination into the circulatory system of mammals (31, 50). To express a number of plasmid-borne virulence factors, Y. pestis employs ppGpp.
Y. pestis utilizes two ppGpp synthetases to respond to distinct starvation stimuli during growth at flea and mammalian body temperatures. Y. pestis encodes a monofunctional RelA (∼84% identical to E. coli K-12 and S. enterica serovar Typhimurium) and a bifunctional SpoT (∼91% identical to E. coli K-12 and S. enterica serovar Typhimurium). As in E. coli, Y. pestis requires RelA to respond to amino acid starvation and SpoT to respond to carbon starvation (190). A source of ppGpp is critical for optimal Y. pestis growth in heart infusion broth, as relA spoT double mutants, but not relA single mutants, exhibit an increased lag phase and do not achieve WT cell densities at either 26°C (typical of fleas) or 37°C. Double-mutant bacteria also demonstrate increased autoaggregation at 26°C but not at 37°C. Excess autoaggregation is due to the absence of spoT, as the expression of spoT in trans is sufficient to reduce the enhanced autoaggregation phenotype in a dose-dependent manner (190).
Y. pestis also requires a source of ppGpp in a mouse model of bubonic infection. After subcutaneous infection with either WT or relA mutant bacteria, mice die synchronously, with 100% mortality by 8 days postinfection (190). SpoT-dependent ppGpp levels are critical for lethality, as mice infected with relA spoT mutant Y. pestis cells exhibited a decreased mortality rate, with 80% of mice surviving at 6 days. Additionally, the lethal dose of relA spoT mutant bacteria required to kill 50% of mice (LD50) is ∼100,000-fold greater than the LD50 of WT and relA mutant Y. pestis bacteria. Moreover, the experimental induction of spoT fully complements the virulence defect of ppGpp0 bacteria (190). relA spoT mutants exhibit WT colonization patterns in the blood, spleen, and liver; in contrast, the stringent response pathway may play a modest role in dissemination into the lungs, as judged by enumerating CFU in mouse tissues 3 days after infection. Although ppGpp is dispensable for initial colonization, a CFU decrease 7 days postinfection suggests that mice clear relA spoT mutant Y. pestis bacteria.
The defects of Y. pestis relA spoT mutants during mouse infections may reflect decreased quantities of plasmid-encoded virulence proteins. While expression studies by reverse transcription (RT)-PCR analysis of Yops (Yersinia outer proteins) and other T3SS effectors reveal WT patterns of transcription in relA mutant and relA spoT double-mutant bacteria, secreted protein levels are reduced in relA spoT mutants but not relA bacteria (190). Therefore, SpoT is sufficient to elicit a posttranscriptional mechanism that induces the expression of Y. pestis virulence factors directly or indirectly. Specifically, ppGpp induces the expression of three T3SS effectors: YopE and YopH, effectors that disrupt the host cell cytoskeleton and facilitate the resistance of phagocytosis (157, 183, 190), and LcrV, a factor that triggers interleukin-10 (IL-10) release and suppresses the proinflammatory cytokines tumor necrosis factor alpha (TNF-α) and gamma interferon (IFN-γ) (142, 150). As predicted, IL-10 levels in sera of mice inoculated with relA spoT mutants were diminished compared to those in the sera of mice inoculated with the WT. However, relA spoT bacteria may harbor a sufficient amount of LcrV to suppress TNF-α and IFN-γ production, as these cytokines are not detected in mice infected with either WT or alarmone-deficient Yersinia strains. In addition to altered T3SS effector levels, stringent-response-defective Y. pestis exhibits reduced levels of Pla (190), a protease essential during bubonic infection that cleaves host plasminogen (191) and promotes the invasion of epithelial cells (112, 174). Thus, the ability of Y. pestis to disseminate into the lungs and kill its mammalian host during bubonic infection requires the ppGpp-dependent posttranscriptional control of virulence factor production (190).
The phenotypic patterns of Y. pestis relA single-mutant and relA spoT double-mutant bacteria in vivo are similar to those observed for other Gram-negative bacteria that possess both monofunctional and bifunctional stringent response enzymes. As detailed below, relA is dispensable and spoT is sufficient during both Salmonella enterica serovar Typhimurium infection of mice and Legionella pneumophila infection of macrophages. Together, these patterns raise the possibility that a classical, RelA-dependent stringent response to amino acid starvation is not demanded within mammalian hosts. Perhaps, during each of these bacterial infections, stimuli that trigger ppGpp accumulation in vivo are sensed by SpoT specifically. It is also possible that a modest amount of alarmone is sufficient to induce this class of virulence factors, since SpoT is a weaker synthetase than RelA in some bacteria (160). Alternatively, for bacteria to survive in hostile host environments, the ability of SpoT to balance alarmone levels through hydrolysis may outweigh the need for robust ppGpp synthesis.
Vibrio cholerae
As it transits between aquatic reservoirs and the human gastrointestinal tract, enterotoxigenic Vibrio cholerae adapts to each environment by expressing its virulence factors at the appropriate time and place. V. cholerae is thought to persist in aquatic environments by differentiating into stress-tolerant forms, such as biofilms or a viable but nonculturable (VBNC) state (6). Following ingestion, aquatic-adapted V. cholerae induces the expression of a virulence program consisting of toxins such as RTX (repeats in toxin), cholera toxin (CT), hemagglutinin (Hap), hemolysin (HlyA), and the toxin-coregulated pilus (TCP) as well as factors that allow transit through the gastrointestinal tract (128).
While there have been reports of the cell density-dependent expression of virulence factors by QS systems, there is also evidence that V. cholerae integrates particular virulence traits with growth phase regulation programs governed by ppGpp. For example, the RTX toxin is not secreted during the stationary phase, and the promoters of the genes encoding both the toxin and cognate secretion system contain a GC-rich discriminator region, which is a hallmark of negatively controlled stringent promoters (Fig. 3) (23). Furthermore, relA transcripts are upregulated ∼3-fold when cells are in the resilient VBNC state (74). However, while the current body of evidence points to the involvement of ppGpp in V. cholerae pathogenesis, there have been conflicting observations on the extent of the impact of ppGpp in this process. The recent identification a third ppGpp synthetase homologue in V. cholerae, named RelV (57), suggests that this pathogen controls ppGpp levels by using mechanisms not typical of Gram-negative pathogens that harbor only one or two RSH homologues. Although these complex mechanisms remain to be understood, the identification of RelV does provide a potential explanation for the conflicting reports in the literature.
RelA was the first V. cholerae ppGpp synthetase shown to be critical for virulence (84). A relA mutant defective for ppGpp accumulation during amino acid starvation expressed significantly lower levels of two major virulence regulator proteins, ToxR and ToxT. As predicted, the levels of expression of both CT and the TCP were reduced in relA mutants compared to the WT strain under in vitro toxin-inducing conditions; the ratio of the ToxT-regulated porins OmpU and OmpT was also altered. Motility, another virulence trait of V. cholerae, is affected in the relA mutant. Finally, consistent with its lower levels of expression of factors known to be required in vivo, such as TCP, the relA mutant is significantly attenuated for the colonization of suckling mice (79). In contrast, the mutant grows as well as the WT in rich broth. Thus, RelA-dependent ppGpp levels regulate several virulence phenotypes.
RelA also equips V. cholerae to adapt to nutrient limitation, since a relA mutant is also defective for growth in minimal medium (179). However, in contrast to previous work, that same study found that the relA mutant was motile and able to colonize the suckling mouse intestine as well as the WT strain. The mutant also produced normal biofilms and expressed hemagglutinin/protease at WT levels (179, 180); however, the overexpression of RelA did diminish the level of production of this enzyme. Perhaps, differences in SpoT synthetase or hydrolase activities between the two El Tor strains used for these studies are responsible for the conflicting results (179). It is also possible that basal levels of ppGpp, controlled by SpoT (or the newly identified RelV protein [described below]), are sensitive to conditions not yet identified that differ in these studies. As such, the maintenance of basal levels of ppGpp, rather than the RelA-dependent stringent response, may have a greater influence on the expression of virulence factors by V. cholerae (179).
V. cholerae also encodes a third enzyme that generates ppGpp. As predicted by data for E. coli reported in the literature, relA mutant cells do not accumulate ppGpp under conditions of amino acid starvation, but the V. cholerae mutants still respond to carbon source starvation. However, a relA spoT double mutant still accumulates ppGpp during glucose starvation (56). Furthermore, the relA spoT double mutant grows in M9 minimal medium; is resistant to 3-amino-1,2,4-triazole, a reagent that induces histidine limitation; and exerts a stringent control of stable RNA synthesis when glucose is limiting. Since the double mutant lacks several phenotypes expected for a ppGpp0 strain, investigators postulated that V. cholerae encodes another ppGpp synthetase that has yet to be identified. Repeated subculturing of relA spoT double mutants identified a suppressor strain that no longer accumulated ppGpp in response to carbon source starvation (57). The genetic lesion mapped to a locus, now named relV, that encodes a protein homologous to the catalytic domain of E. coli relA and appears to be restricted to the vibrios. Analysis of a relA spoT relV triple mutant confirmed that this novel ppGpp synthetase was the cryptic source of ppGpp in the relA spoT double mutant. Further work is needed to determine whether RelV enables V. cholerae to adapt to environments critical for pathogenesis and whether this third enzyme has contributed to the variations in relA mutant phenotypes reported by different groups. Although both the metabolism of ppGpp and its effect on pathogenesis have proven to be complex, work to date indicates that the alarmone affects the ability of V. cholerae to thrive in animal hosts and in aquatic environments between infections.
Salmonella enterica
Salmonella enterica serovars are responsible for a wide spectrum of human infections, ranging from systemic disease such as typhoid fever to a gastroenteritis known as salmonellosis. Accordingly, the bacterium confronts a variety of environments to which it must adapt. Following oral ingestion within contaminated food or water, Salmonella enterica serovar Typhimurium passes through the stomach and enters the small intestine. Here, environmental cues prompt the activation of a T3SS encoded within the chromosomal Salmonella pathogenicity island 1 (SPI1). When the pathogen encounters specialized epithelial cells known as M cells, the SPI1 T3SS secretes effectors that induce membrane ruffling and the uptake of the bacterium (83). After breaching the epithelium by transcytosis across M cells, salmonellae are ingested by macrophages and trafficked to a hostile, acidic phagosome known as the Salmonella-containing vacuole (83). Conditions within this compartment trigger the activation of a second, chromosomally encoded T3SS within Salmonella pathogenicity island 2 (SPI2), whose effectors convert the vacuole to an environment suitable for replication. As protected macrophage residents, salmonellae then enter the lymphatic system and disseminate to the liver and spleen, where they may cause enteric (typhoid) fever (83).
Its life cycle within the host requires that S. enterica respond to environmental conditions to cue the expression of invasive and intracellular virulence programs: ppGpp signaling coordinates these systems. S. enterica encodes both a monofunctional RelA, responsible for a classic stringent response to amino acid starvation, and a bifunctional SpoT, which responds to carbon starvation (182). In an animal model of systemic disease following oral infection of BALB/c mice, WT and relA mutant S. enterica serovar Typhimurium strains caused morbidity within 7 to 10 days, whereas relA spoT double-mutant bacteria did not trigger signs of illness until 30 days. Furthermore, bacterial colonization of the liver and spleen was completely abolished by the relA spoT mutation (159). In tissue culture models, these ppGpp0 mutants were defective for invasion and replication in intestinal epithelial cells as well as for replication and survival in murine macrophage cell lines (159, 197). Thus, the attenuation of ppGpp0 mutants in vivo may be attributed to defects in virulence programs that are critical for invasion, survival, and replication in host cells. The bifunctional SpoT enzyme is sufficient during infection, suggesting that S. enterica serovar Typhimurium may employ SpoT to both synthesize and balance ppGpp pools through the hydrolysis of the alarmone, a control mechanism to ensure that virulence traits are expressed at appropriate sites within the host.
To coordinate entry into epithelial cells, S. enterica responds to external stimuli within the intestinal lumen by activating the expression of SPI1. In broth, S. enterica serovar Typhimurium induces SPI1 genes in response to ppGpp levels generated by SpoT activity during carbon starvation or exposure to excess short-chain fatty acids (159, 182). Both carbon starvation and oxygen limitation cause the ppGpp-dependent activation of hilA (182, 197), which encodes a transcription regulator of the OmpR/TotT family. In turn, HilA controls the expression of the InvF transcriptional regulator and other factors that together coordinate the expression of SPI1 (55, 121).
Whether the alarmone induces particular SPI1 genes depends on the context or the amount of ppGpp. For example, RelA-dependent ppGpp is not sufficient to activate the expression of the hilA SPI1 activator but is sufficient to activate hisG of the histidine biosynthetic operon (182). The full activation of hilA requires ppGpp, but another mechanism must also contribute, since the overexpression of the SPI1-encoded two-component system HilD/HilC can bypass the ppGpp requirement (159). The only sigma factor known to contribute to the expression of SPI1 genes is the housekeeping sigma factor σ70. On the other hand, both SPI1 and SPI2 regions are AT rich, raising the possibility of direct positive regulation by ppGpp (Fig. 3) rather than passive control through sigma factor competition (Fig. 4) (159).
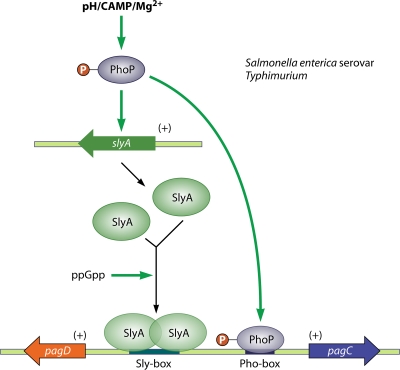
Within its vacuole, S. enterica serovar Typhimurium encounters an acidic, magnesium-limited environment with high levels of cationic antimicrobial peptides. These conditions activate the PhoP/PhoQ two-component system, which represses SPI1 and activates SPI2, resulting in the repression of virulence factors involved in bacterial uptake, increased antimicrobial resistance, and the remodeling of the vacuole to a replicative compartment (83). Although ppGpp does not seem to play a direct role in the repression of SPI1, the bacteria do require the alarmone for the rapid PhoP-dependent activation of SPI2. The PhoP response regulator acts in a feed-forward loop with SlyA, another transcriptional regulator of SPI2 (Fig. 5). PhoP itself activates the transcription of slyA. Together, the PhoP and SlyA proteins then activate the transcription of SPI2 in response to distinct metabolic signals. In particular, low pH, Mg2+, and cationic antimicrobial peptides activate the PhoQ sensor kinase, whereas ppGpp induces SlyA activity (176, 223). For example, in the presence of low Mg2+ and ppGpp, PhoP and SlyA control the transcription of a divergent operon carrying two SPI2 genes, pagC and pagD (223). The alarmone does not impact the level of the PhoP or SlyA protein. Instead, in vitro assays demonstrated that ppGpp interacts directly with SlyA to facilitate its dimerization and binding to target promoters (Fig. 5). Regulation by ppGpp appears to be specific to SlyA, since the alarmone does not affect PhoP-dependent, SlyA-independent gene transcription (223). Ten other loci, including seven divergent operons and three unpaired genes, exhibit both SlyA and PhoP binding motifs as well as ppGpp dependence, demonstrating the breadth of this mechanism (223). Similar physical interactions between ppGpp and DNA binding transcription factors may govern the expression of SPI1 in Salmonella as well as the virulence programs of other pathogens.
FIG. 5.
ppGpp controls dimerization and DNA binding of SlyA. In addition to controlling RNAP activity, ppGpp can directly control transcriptional activators. After phagocytosis, S. enterica serovar Typhimurium encounters an acidic, magnesium-limited environment with high levels of cationic antimicrobial peptides (CAMP), activating the PhoP/PhoQ two-component system. The PhoP response regulator acts with the transcriptional activator SlyA in a feed-forward loop. PhoP itself activates the transcription of the slyA gene; together, the PhoP and SlyA proteins control the expression of SPI2 genes, which are critical for intracellular survival and replication. SPI2 gene expression is intimately linked to ppGpp signaling, as the alarmone facilitates dimerization and DNA binding of SlyA, facilitating the SlyA- and PhoP-dependent activation of several divergent operons that promote the pathogen's intracellular virulence program, including the SPI2 genes pagC and pagD.
S. enterica serovar Gallinarum causes typhoid fever in chickens and seriously threatens the poultry industry in South America and Asia (177). S. enterica serovar Gallinarum encodes SPI1 and SPI2, which share significant homology with other serovars. As in S. enterica serovar Typhimurium, ppGpp controls both invasion and replication in nonphagocytic and phagocytic cells, likely through the regulation of expression of and secretion by both SPI1 and SPI2 T3SSs. In contrast to S. enterica serovar Typhimurium, S. enterica serovar Gallinarum exhibits maximal invasion when cultured under nonstarvation conditions in the presence of oxygen (97). Under these conditions, the expression levels of both hilA and its target SPI1 are low, suggesting that ppGpp controls the invasion of S. enterica serovar Gallinarum by a mechanism that does not require SPI1 (97).
The stringent response transcription factor DksA also contributes to the virulence of Salmonella enterica serovar Typhimurium during infection of both chickens and mice. For example, mutants with transposon insertions in dksA are less often lethal in 1-day-old chicks and poorly colonize the intestines of 3-week-old animals (200). Bacteria lacking DksA also exhibit a 5-log increase in the LD50 in a mouse model of infection (210).
Similar to E. coli, in S. enterica DksA is a component of the general stress response that promotes adaptation to environmental hazards. The DksA protein of S. enterica serovar Typhimurium is 97% identical to that of E. coli K-12. As in E. coli, DksA regulates amino acid biosynthesis and mediates the repression of rRNA expression (200). In addition, DksA promotes the accumulation of the alternative sigma factor RpoS (σS) in the stationary phase and in response to acid stress (210). The σS protein controls the expression of many factors crucial for acid tolerance and is also required during infection (65). Specifically, DksA facilitates the translation of the rpoS transcript, but not mRNA production, to generate sufficient protein for the efficient transcriptional control of the RpoS regulon (210). DksA also increases the levels of expression of several other Salmonella enterica serovar Typhimurium proteins, which accumulate independently of rpoS. Therefore, DksA acts both cooperatively with and independently of RpoS (210). Although a function for DksA in RpoS-mediated regulation in Salmonella enterica serovar Typhimurium has been demonstrated, a direct link between ppGpp and DksA in the control of key virulence factors, like those encoded within SPI1 and SPI2, has not.
The ppGpp alarmone is the first regulatory molecule shown to play a comprehensive role in S. enterica serovar Typhimurium virulence, controlling both SPI1 and SPI2. S. enterica serovar Typhimurium employs ppGpp not only to redirect RNAP during starvation but also to control transcription directly and independently of RNAP by modulating activator binding (223). It is exciting to think that other pathogens may also utilize direct physical interactions of ppGpp with transcriptional regulators of their specialized secretion systems, thereby coordinating the assembly of these virulence machineries with the production of the secreted effectors.
Legionella pneumophila
The inhalation of aerosols from manufactured water systems containing Legionella pneumophila puts immunocompromised individuals at risk of contracting Legionnaires' disease. For millions of years, environmental legionellae have coevolved with freshwater protozoa that graze on biofilms. Selective pressure on phagocytosed bacteria to avoid digestion has driven the evolution of survival strategies that enable L. pneumophila to opportunistically infect human alveolar macrophages. A hallmark virulence trait of L. pneumophila is the ability to differentiate between morphologically and phenotypically distinct states within host cells, including replicative and transmissive cell types (32, 134). In some protozoan hosts, transmissive L. pneumophila differentiates further into “mature intracellular forms” suited for environmental persistence (66).
The regulation of the L. pneumophila life cycle in both protist and mammalian host cells requires a strict control of ppGpp metabolism. Increased levels of ppGpp cue the differentiation of L. pneumophila to a motile, coccoid, transmissive form that exhibits increased resistance to stress and the ability to evade lysosomal degradation (134). In the transmissive state, L. pneumophila effector proteins are increasingly transcribed and translocated into the host cell by the Dot/Icm type IV secretion system (T4SS) (32, 141, 163, 199). In particular, L. pneumophila relies on the Dot/Icm system to avoid fusion with the endosomal pathway and to establish a replication niche in a compartment derived from the endoplasmic reticulum (ER) (94). When nutrients are abundant, transmissive bacteria hydrolyze ppGpp, resulting in the initiation of cell division and repression of transmission factors (53, 135). As a consequence, in mouse macrophages, the block to phagosome-lysosome fusion is relieved, and the replication vacuole matures into an acidic lysosomal vacuole (189). As the replicating bacteria consume nutrients, vacuolar conditions presumably deteriorate and stimulate ppGpp production, prompting the progeny to reenter the transmissive state (53). For example, elevated levels of ppGpp trigger the expression of factors leading to bacterial cytotoxicity to macrophages, motility, stress resistance, and the ability to evade lysosomes, traits which promote the transmission of the pathogens from the exhausted host cell and the infection of naïve ones. Thus, ppGpp potentiates L. pneumophila cell-to-cell transmission.
L. pneumophila is equipped with two ppGpp synthetases that coordinate differentiation to the transmissive state in response to distinct metabolic cues (53, 61). Its monofunctional RelA enzyme is 44% identical and 63% similar to its E. coli K-12 counterpart, and its bifunctional SpoT is 53% identical and 71% similar to the E. coli enzyme. In broth culture, RelA synthesizes ppGpp in response to amino acid starvation. SpoT-dependent ppGpp accumulates following perturbations in fatty acid metabolism. In fact, either the inhibition of fatty acid biosynthesis or the addition of excess short-chain fatty acids triggers SpoT activity in L. pneumophila (61). Alarmone synthesized from either enzyme can be detected at the transition from the exponential to the postexponential phase in rich broth, and the artificial induction of ppGpp synthesis by cells replicating in rich medium is sufficient to trigger rapid differentiation to the transmissive form (53, 81). In macrophages, the alarmone is dispensable for replication (5, 51), but it is essential for transmission to a new host cell, since relA spoT double-mutant L. pneumophila cells replicate intracellularly but are subsequently degraded during the period when WT bacteria undergo transmission.
By virtue of its dual enzymatic activities, SpoT regulates both replicative and transmissive functions in macrophages and in microbiological medium (53). Following uptake by macrophages, a hydrolase-competent SpoT enzyme is essential for transmissive L. pneumophila to initiate replication (Fig. 6). Likewise, when plated onto rich medium, transmissive L. pneumophila bacteria require SpoT to form colonies. Furthermore, the induction of plasmid-borne SpoT, but not RelA, promotes the transmission of relA spoT mutant bacteria between macrophages. In contrast, relA single mutants still transform to motile, lysosome-resistant, transmissive forms in broth, and they exhibit no intracellular growth defects in macrophages (51, 225). Therefore, L. pneumophila bacteria use SpoT first to initiate replication when the vacuole is suitable for propagation and later to engage transmission when vacuolar conditions deteriorate (Fig. 6). Thus, control over ppGpp accumulation and degradation by SpoT is critical for the L. pneumophila life cycle.
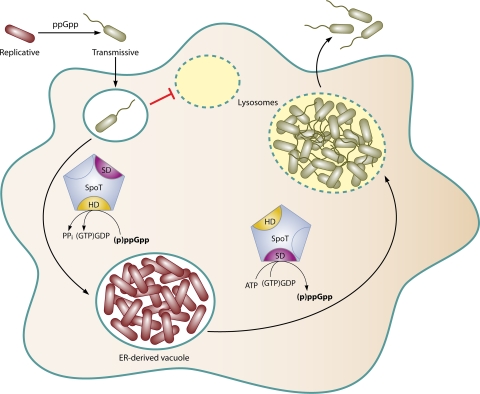
FIG. 6.
SpoT governs the Legionella life cycle in macrophages. During its life cycle, the intracellular pathogen L. pneumophila differentiates between two forms, replicative and transmissive. When nutrients become scarce, ppGpp levels increase, coordinating the differentiation of replicative bacteria to the highly resilient, motile, transmissive form. After phagocytosis, transmissive bacteria inhibit fusion with degradative lysosomes (small, dashed, empty vacuole). To convert to the replicative form, L. pneumophila must sense favorable vacuolar conditions that stimulate the bifunctional SpoT enzyme to reduce alarmone pools via ppGpp hydrolysis (HD). In a vacuole derived from the ER, replicative L. pneumophila cells divide exponentially. Gradually, the replication vacuole acidifies and acquires lysosomal markers. Deteriorating vacuolar conditions elicit SpoT synthetase (SD) activity, cueing replicative bacteria to differentiate back to the transmissive form. Transmissive L. pneumophila cells resist lysosomal degradation and migrate to a naïve host cell, primed to establish a new infection.
L. pneumophila also encodes a DksA homologue that is 72% identical to the DksA proteins of E. coli K-12 and S. enterica serovar Typhimurium that contributes to differentiation in broth and growth in amoebae (54). Genetic analysis revealed that DksA is critical for L. pneumophila differentiation to the transmissive form, including flagellar gene activation, evasion of lysosomes, and cytotoxicity toward macrophages. The roles of DksA and ppGpp depend on the context. For transmission between macrophages, ppGpp is essential, whereas DksA is dispensable; therefore, ppGpp is sufficient to coordinate transmission in macrophage cultures. In broth, DksA promotes differentiation when ppGpp levels increase or in response to fatty acid stress, suggesting that ppGpp-independent signals cue DksA activity. In the flagellar cascade, ppGpp and DksA act both cooperatively and independently. For the basal expression of the alternative sigma factor fliA (σ28) in the exponential phase, DksA functions independently of ppGpp. Furthermore, the experimental induction of dksA expression is sufficient to restore flagellar synthesis and macrophage cytotoxicity to ppGpp0 mutant L. pneumophila cells. When alarmone levels increase, DksA cooperates with ppGpp to control the activation of at least three flagellar gene classes, generating a pulse of early rod transcripts and the prolonged activation of late sigma factor (σ28) and flagellin RNAs. Thus, DksA responds to the level of ppGpp and other stress signals to coordinate the differentiation of replicating L. pneumophila to the transmissive form.
Like other gammaproteobacteria, L. pneumophila may have evolved a SpoT-dependent mechanism to monitor fatty acid metabolism that is mediated by a physical interaction between SpoT and ACP (Fig. 2). ACPs transfer acyl groups to enzymes involved in either phospholipid or secondary-metabolite biosynthesis. The ACP-SpoT interaction is conserved in bacteria possessing two ppGpp synthetase enzymes (i.e., RelA and SpoT) and appears to be highly specific (17). In particular, SpoT interactions are restricted to ACPs that are encoded within fatty acid biosynthesis operons. SpoT binds specifically to functional ACPs, namely, those that have been posttranslationally modified to carry a fatty acid intermediate (16). Furthermore, ACP interacts with SpoT but not RelA. In E. coli, the interaction of ACP with SpoT during growth in nutritionally replete medium appears to inhibit the ppGpp synthetase domain of SpoT, skewing the balance of ppGpp metabolism by this enzyme toward hydrolysis. Upon fatty acid starvation, in some manner, ACP interactions with the TGS domain (a domain found in threonyl-tRNA synthetase, GTPase, and SpoT proteins) of SpoT promote the accumulation of ppGpp in the cell (Fig. 1 and 2). In E. coli, the mutation of specific amino acids within the regulatory domain of SpoT (A404E and S587N) abrogates the ACP interaction and eliminates the cellular response to fatty acid biosynthesis inhibition (16).
A similar SpoT-ACP interaction may govern L. pneumophila differentiation. First, the pathogen requires an identical SpoT amino acid residue (alanine 413) to elicit a stringent response to perturbations in fatty acid biosynthesis (53). In addition, genetic studies indicate that an ACP-SpoT interaction may influence ppGpp hydrolysis in L. pneumophila. When highly expressed, the SpoT(A413E) mutant protein confers a phenotype similar to that caused by RelA overexpression: in both cases, the cells are locked in the transmissive state and are unable to initiate replication in broth or macrophages (53). Thus, some bacteria have evolved mechanisms to monitor fatty acid metabolism and control ppGpp levels through an ACP-SpoT interaction, a scheme analogous to the tRNA-RelA interaction that activates alarmone production when amino acids become scarce.
From atop a complex regulatory cascade, ppGpp exerts both direct and indirect control over downstream activators and repressors of L. pneumophila transmission (134). To respond to elevated ppGpp levels, L. pneumophila requires the LetA/LetS two-component system (82). The mechanism by which ppGpp activates the LetA/LetS system remains unknown. The LetA transcriptional activator binds upstream of two genes encoding small regulatory RNAs, RsmY and RsmZ, initiating their transcription (163, 169). Both RsmY and RsmZ interact with CsrA, an RNA binding protein and repressor of L. pneumophila transmission, relieving its ability to bind transcripts critical for the transmissive phenotype, such as effectors of the Dot/Icm system. Several observations indicate that the ppGpp alarmone likely contributes to the ability of LetA to positively regulate the transcription of the RsmZ regulatory RNA: the activation of the rsmZ promoter requires ppGpp, RsmZ RNA accumulates immediately following ppGpp synthesis, and the overexpression of rsmZ bypasses the requirement for ppGpp to activate motility, toxicity toward macrophages, and lysosome evasion (Z. D. Dalebroux, unpublished data). It is conceivable that ppGpp regulates the ability of LetA to control the activation of rsmZ transcription.
L. pneumophila may also employ ppGpp to control the expression of alternative sigma factors directly and/or to increase their activity indirectly. The stationary-phase sigma factor RpoS (σS), the flagellar sigma factor FliA (σ28), and the alternative sigma factor RpoN (σ54) are each known activators of transmission traits (11, 12, 88, 91, 96). In the transmissive phase, ppGpp is predicted to influence the activity of alternative sigma factors passively. According to studies of E. coli, by deactivating transcription at σ70-dependent rRNA promoters, ppGpp and DksA increase the amount of RNAP available to alternative sigma factors (Fig. 4) (160). Indeed, at the onset of starvation, L. pneumophila RpoN and FliA contribute to flagellar gene expression and synthesis (88, 96). Unlike rpoN, fliA is highly induced in the transmissive phase by ppGpp and DksA (54), making it a likely candidate for direct regulation. Another candidate for direct or indirect regulation by ppGpp and DksA is RpoS. RpoS is essential for the intracellular replication of L. pneumophila in Acanthamoeba castellani and is partially required for growth in macrophages (12, 91). Although RpoS translation efficiency and protein levels in L. pneumophila have not been monitored, the rpoS transcript is more abundant in the exponential phase than in the stationary phase (13). As with E. coli, the transcription of rpoS in L. pneumophila is sensitive to ppGpp pools, since transcript levels increased following the artificial induction of the alarmone (30). Thus, it appears that ppGpp controls RpoS expression to affect transmission and replication in L. pneumophila; however, specifics of the interplay in this complex regulatory mechanism remain to be clarified.
RpoS is also integrated into the L. pneumophila CsrA regulatory system. In particular, this sigma factor is required for maximal rsmY and rsmZ gene expression (91, 163). Therefore, by mechanisms yet to be defined, ppGpp, LetA/LetS, and RpoS cooperate to relieve the CsrA-mediated repression of transmissive transcripts. These and other ppGpp-dependent regulatory mechanisms, such as sigma factor competition, enable L. pneumophila to transition rapidly between replicative and transmissive virulence programs in host cells. Its reliance on ppGpp and the bifunctional SpoT enzyme makes L. pneumophila an attractive experimental model to understand how metabolic cues are transmitted by second-messenger signaling pathways to govern bacterial virulence.
Francisella tularensis
Francisella tularensis, the etiological agent of tularemia, is a Gram-negative, facultative, intracellular bacterium that infects mammals, fish, insects, amphibians, and protozoa. Its ability to disseminate readily among a variety of hosts along with its high infectivity and mortality rates make F. tularensis a bioterrorism threat (170). F. tularensis can infect humans by many routes, including inhalation and skin abrasions. Most commonly, arthropods that have fed on an infected mammal transmit bacteria to humans through bites. To develop treatment and prevention strategies, much effort has focused on understanding the interaction between F. tularensis and the mammalian macrophage, which serves as an intracellular replication niche during infection.
Following macrophage uptake by asymmetric, spacious pseudopod loops, F. tularensis is contained in a specialized phagosome. At 1.5 h postinfection, the bacteria induce the expression of MglA, a protein sharing 20% identity to the stringent starvation transcriptional regulator SspA of E. coli (14). MglA interacts with another SspA homologue (referred to as SspA), and the MglA-SspA complex associates with RNAP to control the transcription of genes within the Francisella pathogenicity island (FPI). Factors encoded by the FPI cue the arrest of phagosome maturation and the escape of bacteria into the cytosol, where F. tularensis replicates. The FPI carries ∼100 genes essential for intracellular survival, including a type VI secretion system (122). To integrate nutritional cues into virulence regulation, F. tularensis uses ppGpp to promote physical interactions between the RNAP-associated MglA-SspA complex and the putative DNA binding factor PigR to control the activation of FPI (43). In this manner, ppGpp contributes to phagosome escape and the intracellular survival of F. tularensis.
F. tularensis encodes both a monofunctional RelA and a bifunctional SpoT enzyme. In the live vaccine strain of F. tularensis subsp. holarctica, mglA mutant and relA spoT double-mutant (ppGpp0) bacteria exhibit overlapping defects in FPI gene activation, growth in macrophages, and virulence in mice, suggesting that ppGpp works with MglA and SspA to control virulence expression (43). A SpoT-dependent ppGpp pool is sufficient for F. tularensis virulence, since relA mutant bacteria show only modest defects relative to WT bacteria. The ppGpp alarmone does not influence the abundance of either MglA or SspA or affect the MglA-SspA-RNAP interaction. Instead, ppGpp controls MglA-SspA-dependent gene expression through PigR, another regulator of FPI critical for intracellular growth and virulence. PigR regulates the MglA-SspA complex in a manner that requires ppGpp, as the interaction was not observed in bacteria lacking the alarmone (43). Furthermore, the autoactivation of pigR requires both ppGpp and the MglA-SspA complex, indicating that the alarmone also amplifies PigR-dependent gene expression. This paradigm can likely be extended to include F. tularensis subsp. novicida, as the PigR orthologue FevR is also required for virulence in macrophages and mice (29). It remains to be determined if ppGpp allosterically impacts the interaction between PigR and the RNAP-associated MglA-SspA complex through the alarmone's ability to control RNAP itself. Alternatively, ppGpp may interact directly with PigR to facilitate its binding to the MglA-SspA complex. In either case, F. tularensis is a striking example of how ppGpp modulates critical regulatory proteins to control virulence expression during infection.
ACTINOBACTERIA
Mycobacterium tuberculosis
A hallmark of the life-style of Mycobacterium tuberculosis is its entry into a state of dormancy that withstands an intense immune response until conditions favor reactivation. When humans inhale tubercle bacilli, alveolar dendritic cells ingest invading bacteria and then initiate a potent cell-mediated immune response. To counter host defenses, M. tuberculosis blocks phagosome-lysosome fusion to establish an intracellular replication niche. Nevertheless, released bacterial products continue to stimulate a local immune response. After several weeks, chronic cytokine stimulation leads to granuloma formation and bacterial containment. Surrounded by a hostile environment, M. tuberculosis transforms to a latent, hypometabolic state (58, 127). During latency, which can extend for decades, bacilli dramatically reduce their replication rate, adopt a granular appearance, and become refractory to acid-fast staining and more resistant to antimicrobial agents (72, 147, 148). If the immune system wanes, the granuloma undergoes caseous necrosis, causing the death of most bacilli and substantial tissue destruction (78, 171). The erosion of air cavities permits access to the respiratory tree, enabling bacteria to spread to other humans through aerosols (72). Worldwide, 2 billion people are infected with latent M. tuberculosis. Reactivation typically occurs in ∼5% of these individuals, but this rate increases to 50% for those coinfected with HIV (171).
To initiate dormancy and establish latent infection, M. tuberculosis employs its stringent response enzyme, RSH. Annotated as M. tuberculosis Rel (RelMtb), this enzyme increases bacterial resilience to antimicrobial mechanisms, alters central metabolism, and modulates the immune response of the host (52). The 738-amino-acid protein exhibits both synthetase and hydrolase activities and is most similar to Rel proteins of the Gram-positive actinomycetes, i.e., Corynebacterium glutamicum (67% identity), Streptomyces antibioticus (66% identity), and Streptococcus equisimilis (62% identity), and less like those of Gram-negative organisms like E. coli (9, 162). After aerosol inoculation of C57BL/6 mice with relMtb-null mutants constructed in the virulent H37Rv strain background, bacterial growth in the lung was normal initially. As expected, the enzyme is also dispensable for replication in the THP-1 macrophage cell line (162). However, RelMtb is essential for chronic persistence in vivo (52). Beginning 5 to 7 weeks after infection, relMtb mutant viability declined drastically in the lungs and spleens of infected mice (52). The persistence defect correlates with reduced histopathology in the lungs of animals 15 and 38 weeks after infection with relMtb mutant bacteria. Therefore, RelMtb function is critical for M. tuberculosis latency.
The requirement for RelMtb at the onset of latency suggests that the enzyme responds to environmental conditions particular to this stage of infection. The level of ppGpp is known to increase during the stationary phase, carbon source starvation (0.2% glucose), and inhibition of the respiratory chain by sodium azide in broth cultures of M. tuberculosis (162). Mutants lacking relMtb are also vulnerable in vitro to long periods of nutrient deprivation (100 days) and hypoxia (24 h). When the lipid dipalmitoylphosphatidylcholine (DPPC) is provided as a nutrient source in synthetic liquid medium, relMtb mutant mycobacteria grow as well as the WT, whereas the mutants are attenuated for growth on either peptone or oleic acid (162). The observation that relMtb is dispensable during replication in macrophages is consistent with the idea that M. tuberculosis exploits lipids as a carbon source during lung infection. Together, current data suggest that after a period of intracellular replication, RelMtb ppGpp synthetase activity is elicited at the onset of hypoxia, perhaps as a result of a major shift in carbon metabolism.
M. tuberculosis also employs an RNAP-interacting protein, CarD, to activate its stringent response by repressing the transcription of components of the translation machinery (185). Transcript levels of carD increase in response to genotoxic, oxidative, and nutritional stress, and M. tuberculosis requires CarD to survive these hostile conditions. Similar to RelMtb, CarD is required for M. tuberculosis to persist in mice. However, unlike relMtb, the carD gene is also essential for M. tuberculosis viability in vitro and replication in mice, indicating that CarD plays a broader role than RelMtb (185). Although CarD can substitute for DksA function to repress E. coli rRNA synthesis, the two proteins regulate RNAP by different mechanisms. Specifically, CarD binds the N terminus of the RNAP β subunit and not the secondary channel. The ability of factors like CarD to exert a transcriptional repression of the ribosomal machinery may explain why dksA homologues are not obvious in the genomes of M. tuberculosis and other nonproteobacteria.
Like the enzyme activity, the transcription of the relMtb gene is induced during stress, ensuring a robust activation of the stringent response regulon to counter nutritional adversity. The transcriptional activation of relMtb requires a complex signaling pathway involving the MprAB two-component system, σE, and polyphosphate (192). Polyphosphate is a ubiquitous, anionic, high-energy molecule that participates in numerous biological processes. In E. coli, ppGpp acts upstream of polyphosphate, inhibiting its degradation by PPX, a polyphosphate hydrolase. As polyphosphate accumulates, it increases both RecA and RpoS levels, thereby activating the SOS response and increasing general stress resistance of the bacteria (111).
In mycobacteria, polyphosphate instead acts upstream of ppGpp (Fig. 7). Under carbon-limiting conditions, Mycobacterium smegmatis rel promoter activity increases dramatically. Both in vitro transcription and transcriptional reporter assays showed that rel induction is directly dependent on σE. In particular, σE and MprAB comprise a positive-feedback loop: the two-component system activates the transcription of sigE, and mprAB itself is part of the σE regulon (192). Polyphosphate can both act as a phosphodonor to MprAB and activate the transcription of mprAB, sigE, and rel. Thus, polyphosphate promotes rel transcription, which then increases ppGpp levels (192). A similar signaling pathway was verified for pathogenic M. tuberculosis by using antisense technology to reduce the level of expression of the gene encoding polyphosphate kinase, ppk1 (192). Mycobacterial latency is a striking illustration of the capacity of bacteria to adapt to stress through an interplay between two-component phosphorelay systems, sigma factors, and the ppGpp alarmone.
FIG. 7.
relMtb expression is induced in response to carbon stress. In addition to controlling the activity of ppGpp synthetases, some bacteria regulate the transcription of the gene that encodes this enzyme. In response to carbon limitation, M. smegmatis and M. tuberculosis accumulate polyphosphate. This high-energy molecule donates phosphate to the MprA/MprB two-component system. MprA/MprB and the alternative sigma factor σE comprise a positive-feedback loop: MprA/MprB activates the transcription of the sigE gene; in return, the σE protein controls the activation of the mprA mprB locus. Together, MprA/MprB and σE activate the transcription of relMtb, thereby increasing levels of ppGpp in the cell. This positive-feedback mechanism amplifies the response to carbon limitation, leading to a robust activation of the stringent response.
In addition to factors regulating RelMtb expression and activity, many downstream M. tuberculosis targets of ppGpp have been identified. In particular, ppGpp alters central metabolism, confers increased resilience to host antimicrobial strategies, and controls the expression of factors that modulate the immune response (52). Consistent with this cellular differentiation, microarray analysis determined that RelMtb downregulates the translational apparatus and influences the expression of factors associated with virulence, including macrophage entry proteins, components required to use nitrate as a terminal electron acceptor, and proteins that engage isocitrate lyase (52). RelMtb also affects the expression of cell wall modification factors, polyketide synthases, proteins critical for antigenic variation, and several potent mycobacterial antigens that actively shape the immune response during infection. Thus, from the long course of coevolution of M. tuberculosis with its human host, RelMtb and the ppGpp alarmone have emerged to coordinate global physiological adaptations not only to survive during latency but also to tolerate and modulate the immune response to secure its place among the human population.
FIRMICUTES
In Gram-positive bacteria, levels of ppGpp are fine-tuned by using mechanisms distinct from those typical of the gammaproteobacteria. For instance, most pathogenic firmicutes encode a bifunctional spoT homologue, sometimes annotated as rel or relA, in addition to one or two small alarmone synthases (SASs) (144), often called relP or relQ (Fig. 1). The SAS proteins account for relA-independent ppGpp synthesis in these bacteria (116). Alarmone synthesis can also reduce the GTP pool in both Gram-negative and -positive bacteria either via consumption during ppGpp synthesis or by the ppGpp-dependent inhibition of IMP dehydrogenase, an enzyme involved in guanine nucleotide biosynthesis (68, 120). Whereas the capacity of ppGpp to control promoter selection by RNA polymerase in Gram-negative bacteria has been well documented, the literature on firmicutes more often describes an indirect control of regulatory proteins by ppGpp. Two such regulators are σB, the sigma factor that controls the general stress regulon (222), and CodY, a global regulatory protein (181).
The bifunctional ppGpp synthetase/hydrolase SpoT is central to the biology of many Gram-positive pathogens. For example, the Staphylococcus aureus spoT homologue (referred to as relA) is essential for viability in vitro (44, 71). A number of the obligately parasitic and genetically streamlined species of the genus Mycoplasma accumulate ppGpp and exhibit stringent control (73). Other members of this phylum rely on the stringent response to regulate global physiological changes implicated in pathogenesis, such as environmental persistence and antibiotic resistance. Bacillus anthracis, causing pulmonary anthrax, survives long periods in the environment as stress-tolerant spores, and its differentiation into spores appears to be relA dependent (204). Clostridium difficile, an increasingly prevalent nosocomial pathogen that is frequently exposed to antimicrobial agents, upregulates a putative ppGpp synthesis/degradation protein in the presence of both amoxicillin and clindamycin (62). Here we discuss more extensive evidence for the direct involvement of ppGpp in the survival and pathogenesis of three firmicutes, the food-borne pathogen Listeria monocytogenes, hospital-acquired Enterococcus faecalis, and the versatile Streptococcus spp.
Listeria monocytogenes
L. monocytogenes is associated with a rare, but potentially fatal, food-borne illness termed listeriosis. Although infection may manifest only as noninvasive gastroenteritis, severe cases can lead to septicemia and meningitis. Such infections likely reflect the potential of this pathogen to invade host cells and promote cell-to-cell spread. L. monocytogenes can induce its uptake by macrophages, which is followed by listeriolysin O-mediated escape from its phagosome and intracellular replication in the cytosol (67). Cell-to-cell spread is then achieved by inducing host actin polymerization, which propels the bacterium into adjacent cells. L. monocytogenes adapts to nutritional challenges posed by replication within the host cytosol. Furthermore, as a food-borne pathogen, L. monocytogenes can also survive in contaminated food products, such as unpasteurized cheeses, where it adapts to high salt and low temperatures. The adherence of L. monocytogenes to surfaces during food preparation and storage also promotes survival (196).
Although relQ and relP homologues have been identified in the genome of Listeria spp. (114), the only enzyme characterized to date is the bifunctional spoT homologue, interchangeably termed Rel or RelA. When amino acid starvation was induced with serine hydroxamate, these relA mutant L. monocytogenes strains still produced ppGpp (149), suggesting that its relQ or relP may contribute. Carbon starvation induced by alpha-methyl glucoside does not affect ppGpp levels in either WT or relA mutant L. monocytogenes (149). On the other hand, genetic screens seeking factors important for tolerance to low temperatures and high osmolarity have implicated RelA function in stress survival (118, 149). L. monocytogenes also tolerates cold temperatures, allowing survival on refrigerated food products. In E. coli, ppGpp levels are negatively correlated with the induction of cold shock genes following a shift to low growth temperatures (99). Likewise, L. monocytogenes strains that harbor a transposon insertion in pghH, encoding a putative metal-dependent phosphohydrolase (118), are cold sensitive and contain increased levels of ppGpp. Furthermore, at 4°C, stationary-phase L. monocytogenes cells contain lower levels of relA transcript than do their log-phase counterparts rather than the higher levels usually associated with stress tolerance (42). Therefore, L. monocytogenes may decrease its ppGpp pool to adapt to the cold temperatures encountered in refrigerated food products. The PghH phosphohydrolase may be a critical component of this mechanism by mediating the degradation of ppGpp in response to low-temperature conditions. Finally, ppGpp has also been implicated in L. monocytogenes biofilm growth: a relA mutant was defective for growth following adherence to a surface, and relA transcription was induced upon surface attachment (196).
Intracellular survival and replication contribute to L. monocytogenes virulence, which sometimes causes fatal septicemia and meningitis. However, the contribution of ppGpp to the in vivo adaptation of L. monocytogenes appears to be complex and involves interactions with the pleiotropic regulator CodY. L. monocytogenes mutants lacking relA exhibit reduced survival in culture cell lines, including Caco-2 intestinal epithelial cells and J774.A1 macrophages (19). The defect appears to be specific to intracellular survival, as the mutant escapes the phagosome and induces host actin polymerization similarly to WT bacteria. The contribution of the stringent response to L. monocytogenes during infection is not yet clear, as two mouse infection studies reported conflicting results (149, 196). However, the relA mutant strain that maintained virulence also retained significant ppGpp synthetase activity (149), whereas the attenuated relA mutant assessed by Taylor et al. exhibited decreased ppGpp production (196). Studies of bacteria grown in broth and in cultured cells also indicated that ppGpp equips L. monocytogenes to adapt to conditions likely encountered during infection of the host (19, 149).
In Gram-positive bacteria, the global regulator CodY negatively regulates genes required for adaptation to low nutrient availability, stationary phase, and sporulation in response to levels of branched-chain amino acids and, in some cases, GTP pools (181). The CodY regulon of L. monocytogenes comprises genes involved in amino acid metabolism, nitrogen assimilation, sugar uptake and incorporation, and virulence; members of the regulon are also induced upon entry into stationary phase in vitro (19). The attenuation of an L. monocytogenes relA mutant may be due to its inability to express the virulence-associated CodY regulon, as this genetic circuit was continually repressed in a relA null mutant (19). Thus, an increase in ppGpp levels mediated by RelA may be necessary for the derepression of the CodY regulon under low-nutrient conditions, likely through the depletion of the GTP pool. However, the attenuation of the relA mutant cannot be explained solely by an inability to derepress the CodY regulon: the introduction of a codY mutation into a relA background does not fully restore virulence, and codY mutants are as fit as WT cells within cultured host cells. Thus, the effect of the ppGpp alarmone on L. monocytogenes pathogenesis appears to be multifactorial.
Enterococcus faecalis
Nosocomial enterococci such as Enterococcus faecalis cause diverse opportunistic infections ranging from urinary tract infections to life-threatening conditions such as meningitis and bacteremia. As such, this pathogen must have the capacity to adapt to many niches within a host. In addition, enterococci can tolerate inhospitable conditions common in clinical settings, such as sanitizer exposure and treatment with antibiotics, including vancomycin. Interestingly, E. faecalis accumulates ppGpp in response to numerous stresses typically encountered in a hospital, such as heat or alkaline shock, vancomycin exposure, and amino acid starvation (4).
E. faecalis harbors a bifunctional SpoT enzyme, termed RelA, and an SAS RelQ homologue. A relA mutant is more susceptible to stress conditions such as heat shock, low pH, high osmolarity, and oxidative stress, indicating that relA-dependent ppGpp synthesis contributes to stress tolerance (4). Since the inactivation of relQ in the relA mutant restores several WT phenotypes, the capacity of this RelA enzyme to hydrolyze ppGpp contributes to adaptation to such stresses. In contrast, vancomycin tolerance is enhanced in the relA mutant but reduced in either a relQ mutant or a relA relQ double mutant (4). Furthermore, the relA mutant is more resistant to bile salts, low pH, and ethanol treatment (218). High ppGpp levels may also reduce tolerance to oxidative stress while increasing vancomycin resistance. Thus, ppGpp likely has reciprocal effects on certain stress phenotypes in E. faecalis. It is conceivable that certain decontamination procedures inadvertently contribute to antimicrobial resistance by modulating ppGpp levels, a notion that warrants further investigation as the burden of nosocomial infections recalcitrant to treatment rises. Nonetheless, ppGpp appears to contribute to the virulence of E. faecalis, as judged by the observation that a ppGpp0 (relA relQ) mutant was attenuated in a Caenorhabditis elegans infection model (4).
Streptococcus pyogenes
Group A streptococci (GAS) also adapt to a wide range of environments in the human host. Their versatility is exemplified by the variety of GAS-mediated infections, including impetigo (affecting skin), pneumonia, and meningitis. To do so, the expression of dedicated virulence factors, including secreted enzymes and exotoxins, is coordinated with factors involved in nutrient metabolism in a growth-phase-dependent manner, possibly utilizing ppGpp (45). Like other Gram-positive species, Streptococcus pyogenes harbors relQ and relP SAS homologues in addition to a bifunctional spoT homologue (114). Accordingly, GAS can mount a relA-independent stringent response (186, 187).
Although its relA transcript is induced 2-fold upon entry into stationary phase (45), much of the work exploring the S. pyogenes stringent response has focused on the relA-independent response to amino acid starvation. This pathway affects the expression of genes important for virulence. Examples include the growth-phase-controlled regulator ropB, which governs the expression of the secreted protease exotoxin B, encoded by speB; the two-component regulatory system covRS that controls the expression of streptolysin S, streptokinase (77), and exotoxin B; factors involved in the uptake and processing of oligopeptides, such as the opp and dpp permease systems; and the pepB processing gene (186). Amino acid starvation also induces the fas operon, which regulates virulence, and the gene encoding the autoinducer-2 production protein (187). Genes under negative stringent control and independent of relA include transporters, metabolic enzymes, and at least two virulence genes (126). Thus, ppGpp levels and their indirect influence on the activity of regulators such as CodY are predicted to modulate a wide array of accessory and dedicated virulence genes. Genetic and biochemical studies of the three stringent response enzymes and their target promoters can now examine the mechanistic details.
Streptococcus pneumoniae
Like enterococci and GAS, alpha-hemolytic streptococci such as Streptococcus pneumoniae and Streptococcus suis also exhibit broad tissue tropism, causing infections ranging from endocarditis to otitis media and bacteremia. S. pneumoniae is a notorious cause of pneumonia, and a signature-tagged mutagenesis screen identified a bifunctional rel gene as being essential for full virulence in a murine model of pneumococcal pneumonia (86). S. pneumoniae also encodes a RelQ homologue that was demonstrated to synthesize ppGpp in a heterologous system (17) but not in S. pneumoniae (103). The rel-dependent stringent response in S. pneumoniae strain D39 includes a significant induction of ply (103). This gene encodes a pneumolysin toxin that contributes to early infection and invasion from pulmonary tissues into the blood by both cytotoxic and proinflammatory mechanisms (46); ply-null S. pneumoniae cells are attenuated following intranasal challenge of mice (21). A rel mutant of D39 was also avirulent in a murine model of pulmonary infection (103), perhaps because of the aberrant expression of multiple genes, including those affecting metabolic enzymes (103). Although ply may also be regulated by other pathways, it is one example of the rel-dependent regulation of an S. pneumoniae virulence factor. The related pathogen S. suis, responsible for both infections of pigs and zoonotic disease of humans, upregulates the relA transcript upon iron limitation. Thus, ppGpp may also mediate the adaptation of S. suis to low-iron conditions encountered within the host (117).
Streptococcus mutans
Streptococcus mutans is a major contributor to dental caries. To adapt to the oral cavity, this pathogen forms biofilms, tolerates low pH and fluctuations in nutrient availability, and is flexible in the sugars that it metabolizes. The complexity of ppGpp metabolism in the firmicutes holds true for S. mutans: appropriate alarmone levels are maintained by an interplay between its three ppGpp synthetases, the monofunctional RelA protein and the bifunctional RelQ and RelP enzymes (114). The bacteria accumulate ppGpp by a relA-mediated pathway in response to isoleucine starvation, which is stimulated by mupirocin, a drug that inhibits isoleucine-tRNA synthetase (145). However, as for E. faecalis, an important role of S. mutans RelA may be to limit, through its hydrolase activity, the amount of ppGpp produced by RelP and RelQ (145).
To grow in the absence of branched-chain amino acids, S. mutans requires that RelP and RelQ maintain basal levels of ppGpp (115). CodY repression likely accounts for the poor growth of relA mutants in medium that lacks branched-chain amino acids, since the mutation of codY suppresses this growth defect. Like L. monocytogenes, S. mutans may utilize ppGpp to repress the CodY regulon (115). Thus, the interaction between ppGpp and the CodY regulator in S. mutans is further evidence for ppGpp control mechanisms that extend beyond the gammaproteobacterium paradigm. However, the molecular mechanism underlying CodY-dependent stringent control remains to be clarified for S. mutans, as its CodY appears to respond only to branched-chain amino acids (87) and not to GTP levels as in L. monocytogenes.
Expression profiling also indicates that ppGpp affects many loci associated with adaptation to the environment and the nutritional stress that S. mutans likely encounters in the oral cavity (145). Specifically, when starved for amino acids by mupirocin treatment, the bacteria alter the expression of genes involved in sugar metabolism, biofilm formation, and competence (145). However, some of these changes are relA independent, as judged by comparing the responses of WT and relA mutant cells (145). On the other hand, the relA mutant is defective for survival in medium containing high concentrations of carbohydrate, and it also dysregulates the catabolism of certain sugar substrates, such as mannose and inulin. The mutant also forms poor biofilms on hydroxyapatite; however, relA mutant biofilms do display increased acid tolerance, a pattern consistent with observations of E. faecalis (116). The intricacies of the interactions between the three ppGpp-metabolizing enzymes and their respective hydrolase and/or synthetase activities can be resolved by more detailed genetic and biochemical studies of S. mutans and other Gram-positive pathogens.
ALPHAPROTEOBACTERIA
Brucella spp.
The brucellae are Gram-negative alpha-2-proteobacteria that cause a disease known as brucellosis. In humans, this infection is characterized by a septicemic, febrile illness that is often persistent and sometimes fatal (76). Brucella can infect a wide variety of mammals ranging from sheep and goats (B. melitensis) to cattle (B. abortus) and hogs (B. suis), often causing abortion in pregnant females and sterility in males. Humans are exposed to Brucella during contact with contaminated products from livestock. As such, Brucella is a significant economic burden on the farm industry.
The ability of Brucella to cause disease requires bacterial replication and survival in host macrophages (76). Long-term survival in macrophages is marked by bacterial adaptation and modification of the vacuole into an ER-like compartment that is also suitable for propagation (41). Specifically, following uptake, Brucella-containing phagosomes avoid fusion with degradative lysosomes. This virulence strategy is mediated in part by the VirB T4SS. An acidic pH in the phagosome stimulates the full expression of VirB (41). Accordingly, it was proposed that decisions for vacuolar trafficking are not preprogrammed prior to entry; rather, they are made in response to conditions encountered within the vacuole. Their intracellular replication niche is thought to be nutrient limiting (107).
B. suis and B. melitensis each encode a 751-amino-acid RSH homologue designated B. suis Rsh (RshBs) and B. melitensis Rsh (RshBm). Each Rsh protein exhibits 36% identity to SpoT and 28% identity to RelA of E. coli K-12. These bifunctional enzymes are critical for intracellular survival, as rsh mutants of either pathogen exhibited intracellular growth defects in macrophages (59). In B. melitensis, Rsh is critical for persistence and adaptation in mice, as fewer rshBm mutant bacteria than WT bacteria are recovered from the spleen at 4 weeks but not after 1 week of infection (59). Like its relatives, B. abortus encodes an Rsh enzyme (RshBa) critical for pathogenesis. Both replication and survival in mouse macrophage cultures require RshBa. Likewise, fewer rshBa mutant bacteria than WT bacteria are recovered from the spleens of mice 10 days after infection (104).
Although the signals triggering Rsh activity remain unknown, acidic vacuolar conditions may impact enzyme activity. In B. abortus, rshBa transcription is induced by acid stress as assessed by RT-PCR analysis of broth cultures (104). Consistent with this model, B. abortus rshBa mutants are more sensitive to acidic conditions in broth than WT bacteria. In contrast, Rsh is not critical for the acid resistance of B. suis and B. melitensis (59). Therefore, the B. abortus model cannot be applied to all species of Brucella.
While the accumulation of the ppGpp alarmone has not been observed directly, Rsh mediates a classical stringent response in Brucella. The rsh genes of B. suis and B. melitensis encode functional ppGpp synthetase enzymes, since the heterologous expression of either gene rescues the histidine auxotrophy of relA mutant Sinorhizobium meliloti (59). As discussed in detail below in the plant symbiont section, S. meliloti utilizes its bifunctional RelA enzyme to control a classical stringent response when growing in broth or in symbiosis with alfalfa (214). Similar functions of RshBs must be required during B. suis infection, as the expression of S. meliloti relA complements the intracellular growth defect of rshBs mutants in macrophages (59).
An important aspect of Brucella pathogenesis is its ability to exert timely control over vacuolar trafficking events using the VirB T4SS. To ensure maximal expression, Brucella couples virB gene activation to ppGpp metabolism. The promoter activity of virB and accumulation of VirB proteins are maximal at the transition from the exponential phase to the stationary phase in broth (59). This starvation-dependent increase in levels of VirB requires ppGpp, as rshBm mutant bacteria exhibit reduced levels of virB promoter activity at this transition, and VirB subunits are not detectable in either rshBs or rshBm mutant cultures. Additionally, WT B. melitensis cells that overexpress rshBm in the exponential phase contain excess VirB. Together, these data suggest that in response to starvation, ppGpp induces VirB expression by increasing transcription at its promoter. Thus, the virulence defects of rsh mutant Brucella strains in mice may be partially attributed to their reduced levels of expression of the T4SS.
The role of ppGpp in VirB expression may provide insight into the phagosomal environment encountered by Brucella and other intravacuolar pathogens upon ingestion. In particular, L. pneumophila also relies on a T4SS-dependent mechanism to avoid degradative lysosomes and establish an ER-derived replication compartment. Perhaps, like Brucella, L. pneumophila also responds to intravacuolar stimuli to control its trafficking in host cells. Indeed, L. pneumophila requires its bifunctional synthetase/hydrolase, SpoT, to establish a replicative intracellular niche in macrophages (53).
EPSILONPROTEOBACTERIA
Campylobacter jejuni
Campylobacter jejuni is a major contributor to food-borne illness in humans. The colonization of the gastrointestinal tract with C. jejuni manifests as an intense inflammatory gastroenteritis termed campylobacteriosis. As a zoonotic pathogen, C. jejuni lives commensally in the gastrointestinal tract of avian species but causes significant illness when transmitted to susceptible humans. Thus, C. jejuni survives both outside and inside animals, where it tolerates fluctuations in osmolarity, temperature, and nutrient availability as well as the presence of antimicrobial agents such as bile salts and the host immune system. C. jejuni interacts intimately with host cells through mechanisms that promote adherence, transcytosis across the intestinal epithelium, and the invasion of nonphagocytic epithelial cells (64, 219). Following uptake by a distinct mechanism, C. jejuni resides intracellularly within a vacuole that subverts normal endosome trafficking (209). While it was initially thought that C. jejuni does not survive within host cells, it now appears that internalized bacteria maintain viability for at least 24 h. However, this survival likely requires that the bacteria differentiate to a distinct state, since in vitro culture is optimized by growing C. jejuni in an oxygen-limited environment (209). A physiological switch within host cells is thought to occur, such as a shift in respiration, which allows C. jejuni to adapt to its intracellular niche. Consistent with this, C. jejuni has evolved significant metabolic diversity that influences host colonization and tissue tropism (90).
As a microaerophilic bacterium, C. jejuni is relatively fragile during routine laboratory culture. Furthermore, the streamlined genome of C. jejuni, with a limited repertoire of regulatory genes, points to a host-restricted life-style (136). Nonetheless, because it also survives transmission between animal reservoirs and susceptible human hosts within food and water, C. jejuni must adapt to conditions outside animal hosts. Similar to adaptation to the vacuolar environment within intestinal epithelial cells, global changes in physiology, such as biofilm formation and differentiation into a VBNC form, may explain the surprising resilience of C. jejuni (193). In some manner, growth within a biofilm provides C. jejuni with the resources to tolerate conditions that are normally lethal to free-living cells, such as those in aquatic ecosystems and other intermediate reservoirs during transit between animal and human hosts (37).
Physiological changes that occur upon entry into stationary phase may contribute to the resilience of C. jejuni either in the environment, within a biofilm, or in host cell endosomes. Growth phase analysis of C. jejuni has identified a significant phenotypic switch upon entry into stationary phase. Its differentiation coincides with increased motility, the preferential utilization of specific amino acids, and an acetate switch mechanism (216). Moreover, in response to nutrient starvation, C. jejuni also shows both positive and negative changes in stress tolerance and pathogenicity (106). Taken together, these observations suggest that C. jejuni regulates the expression of survival and virulence factors at the onset of stationary phase. The C. jejuni genome encodes a relative paucity of regulatory elements, lacking alternative sigma factors such as RpoS and obvious cyclic di-GMP-metabolizing enzymes and harboring a limited number of two-component regulatory systems (151). This pattern is characteristic of host-restricted pathogens (136). C. jejuni does, however, encode a single bifunctional SpoT homologue, and a spoT-null mutant exhibits a ppGpp0 phenotype (70). Together, these findings strongly suggest the capacity for a stringent response-mediated regulation of factors contributing to pathogenesis.
Expression profiling provided an early indication that the stringent response contributes to C. jejuni adaptation in vivo. Specifically, the C. jejuni spoT homologue was upregulated in a rabbit ileal-loop model (188), and a spoT mutant was significantly attenuated compared to the WT parental strain in competition under the same conditions (188). The spoT transcript is also upregulated in the presence of INT 407 intestinal epithelial cells, and the gene is potentially coregulated with other previously characterized virulence factors. These expression profiles, together with the ΔspoT mutant functional data described below (70), support the model that ppGpp mediates C. jejuni-host cell interactions.
Analysis of gene expression and host-related phenotypes of a ΔspoT (ppGpp0) mutant likewise indicates that the stringent response is important to the adaptation of C. jejuni during interactions with host cells. The putative SpoT regulon, identified by microarray analysis, includes genes associated with nutritional adaptation, including phosphate uptake genes such as the pstS periplasmic phosphate binding protein. The expressions of numerous loci relating to redox balance and energy production are also affected by ppGpp levels. These include nap, encoding a periplasmic nitrate reductase; nuo, encoding the aerobic respiratory chain NADH dehydrogenase; and the operon composed of Cj0073c-Cj0075c, which includes genes with homology to an iron-sulfur oxidoreductase system. Furthermore, Cj0037, encoding a gene expressed more highly in high-O2-adapted strains, also appears to be positively controlled by spoT. Taken together, these results are consistent with a spoT-dependent expression of genes associated with the adaptation of bacterial metabolism to changes in oxygen availability, such as the switch that was observed previously upon the adaptation of C. jejuni to the environment within host cells (209). Genes present on the putative virulence plasmid pVIR are also downregulated in the spoT-null strain, and stress response genes such as groELS, dnaK, htrA, and clpB are expressed at higher levels in the spoT mutant than in the WT strain (70). Whether ppGpp regulates each of these genes directly can now be investigated.
Consistent with the upregulation of spoT in the presence of epithelial cells, a ΔspoT mutant is defective for certain interactions with host cells, such as adherence, invasion, and intracellular survival in human epithelial cells. Phenotypic analyses also linked the C. jejuni stringent response to other aspects of pathogenesis. For instance, C. jejuni cells accumulate ppGpp upon a shift to nutrient-limited minimal medium in a spoT-dependent fashion (70). The spoT mutant also displays a stationary-phase survival defect, suggesting that some of the prototypical stringent response paradigms are conserved in C. jejuni. It is therefore surprising that the mutant tolerates numerous stresses as well as the WT. Nonetheless, the spoT mutant does exhibit key differences from the WT strain for phenotypes such as survival under low-CO2/high-O2 conditions. As C. jejuni is microaerophilic and capnophilic, the stringent response may regulate phenotypes particular to this pathogen and its life cycle.
Analysis of a dksA-deficient mutant also provides support for a role for ppGpp in controlling pathogenesis-related phenotypes in C. jejuni (220). Transcriptome and proteome comparisons of a dksA mutant to the WT showed changes in the expressions of amino acid- and iron metabolism-related genes. The dksA mutant is also impaired for the invasion of and induction of IL-8 secretion from epithelial cells in vitro, which further demonstrates the importance of the stringent response during the pathogenesis of C. jejuni.
Signal transduction pathways that control stress responses and biofilm formation also appear to be affected by ppGpp in C. jejuni. For example, compared to the WT, the spoT mutant exhibits markedly lower levels of polyphosphate upon entry into the stationary phase, linking ppGpp metabolism to polyphosphate in C. jejuni (40). Furthermore, a ppk1 polyphosphate kinase mutant also accumulates less polyphosphate than the WT and is defective for osmotolerance and intracellular survival. Finally, both the spoT and the ppk mutants showed enhanced biofilm formation, suggesting that ppGpp and polyphosphate may be required to maintain a planktonic-growth life-style or promote biofilm dispersal in response to nutritional conditions in C. jejuni (40, 129). Biofilms have been proposed to contribute to the persistence of C. jejuni in aerobic environments, a trait that is surprising considering its fragility during planktonic growth in the laboratory (37). It follows that ppGpp and downstream regulatory networks contribute to the resilience of this pathogen.
Collectively, a SpoT-mediated stringent response is clearly important to the success of C. jejuni in environments encountered inside and outside animal hosts as well as during transmission to initiate zoonotic infection. Specific pathogenesis-related phenotypes, such as tolerance to aerobic atmospheres and low nutrient availability, appear to be spoT dependent in C. jejuni. Furthermore, the maintenance of ppGpp levels also appears to affect global shifts in bacterial physiology and life-style, which occur during biofilm formation. Thus, the dissemination of C. jejuni between commensal and susceptible hosts is potentially dependent on the stringent response pathway. Finally, gene expression analyses of dksA and ppGpp0 C. jejuni mutants have demonstrated a perturbed regulation of metabolic genes. Therefore, the stringent response may also play a role in the metabolic shift that appears to occur upon adaptation to the endosome environment within host cells.
Helicobacter pylori
Up to 50% of the human population is thought to carry Helicobacter pylori, an epsilonproteobacterium that colonizes the gastric epithelium and leads to cellular changes that can result in ulcers and gastric cancer. In this harsh niche, H. pylori survives low pH, low iron and nutrient availability, and oxidative stress from phagocytic cells before entering the mucus layer, colonizing the epithelium, and interacting with host cells. During long-term colonization, the bacteria also subvert host immune responses. H. pylori adapts to the host environment despite a small genome and a minimal repertoire of regulatory elements. For example, H. pylori lacks alternative sigma factors such as RpoS and encodes few two-component regulatory systems. Furthermore, this pathogen was initially thought to lack a stringent response, as H. pylori cells did not accumulate ppGpp in response to pseudomonic acid-mediated isoleucine starvation (173) despite encoding a single bifunctional RelA/SpoT homologue (referred to as spoT).
H. pylori does exhibit a growth-phase-dependent regulation of virulence phenotypes, including the delivery of the effector protein CagA and inducing the elongation of particular host cells during the transition into stationary phase (198). During this transition, the bacteria downregulate spoT and gpp, a gene encoding a guanosine pentaphosphate phosphohydrolase, as determined by microarray analysis. One potential untested interpretation is that the reduced SpoT hydrolase activity in the cell allows higher ppGpp levels to persist. It is also conceivable that, as a potential consequence of the lower GppA phosphohydrolase activity, a reduced conversion of pppGpp to ppGpp could extend the half-life of the alarmone. To date, ppGpp accumulation by H. pylori has been observed directly only in response to nutrient starvation and stress conditions (212). Nevertheless, the stationary-phase survival and morphology defects exhibited by ΔspoT (ppGpp0) mutants (138) point to the importance of maintaining ppGpp levels during entry into stationary phase. At this transition, H. pylori also induces the expression of virulence factors such as flagellin and napA, encoding a homologue of Dps (DNA protection during starvation) that is thought to protect against oxidative stress damage (48). Therefore, the stringent response pathway likely coordinates virulence factor expression with growth phase.
A direct substantiation of a stringent response in H. pylori was the observation of a rapid accumulation of ppGpp upon nutrient downshift achieved by transferring cells to minimal MOPS (morpholinepropanesulfonic acid)-MGS medium (212). The activation and function of SpoT in H. pylori more closely resemble those of E. coli SpoT rather than those of RelA, as H. pylori spoT readily complements an E. coli relA spoT double mutant, more so than a single relA mutant (138). This attribute may explain the lack of stringent control in response to amino acid starvation previously reported; it also provides insight into the role of ppGpp in the response and adaptation to specific nutrient conditions. H. pylori requires serum for growth, and a spoT mutant grows to higher densities than the WT strain in serum-free medium, a trait mirroring the “relaxed” phenotype of E. coli mutants lacking ppGpp (224). SpoT function is also required to maintain the helical morphology of the bacteria in the absence of serum. Thus, H. pylori may require SpoT to limit its metabolic activity and growth appropriately under conditions of serum starvation, such as those that may be encountered in the microenvironment near gastric epithelial cells (224).
The ppGpp alarmone also influences the adaptation of H. pylori to challenges posed by the gastric environment during pathogenesis, including specific host-related phenotypes. An accumulation of ppGpp is induced during acid shock (212), and genetic studies indicated that SpoT function is required for the survival of acid exposure, stationary phase, and increased levels of oxygen (138). In addition to tolerance to low nutrient availability and high pH, the persistence of H. pylori during chronic infection of the gastric mucosa also depends on the avoidance of host innate immune components. For example, in macrophages, H. pylori interferes with the maturation of phagosomes and promotes the formation of large, more habitable, vacuoles called megasomes (8). Whereas ΔspoT mutants display WT levels of invasion, their ability to survive within macrophages is significantly reduced (224).
Whether a SpoT-mediated stringent response is required for the colonization of the gastric mucosa is unknown. It will be intriguing to learn whether the H. pylori stringent response modulates specific virulence factor genes such as vacA, urease, or the cag pathogenicity island. Reports of a growth-phase-dependent regulation of virulence traits (198) indicate that further analysis of the contribution of ppGpp to H. pylori colonization is warranted. Furthermore, the H. pylori requirement for polyphosphate for optimal colonization of mice (10, 195) suggests that, like in C. jejuni, the stringent response may intersect with polyphosphate metabolism to affect virulence and survival.
SPIROCHETES
Borrelia burgdorferi
Lyme disease, or borreliosis, is characterized by a systemic and sometimes relapsing or persistent infection initiated by the bite of an infected tick. As such, the zoonotic life-style of the B. burgdorferi spirochete involves both commensal relationships with insects and pathogenic interactions in susceptible human hosts. Throughout its life cycle, B. burgdorferi is exposed to many different environments. For example, B. burgdorferi must adapt to low-nutrient conditions present within arthropod hosts, which often go extended periods without feeding. Spirochetes must also tolerate unfavorable physicochemical conditions or evade the immune system during persistent infection of humans.
B. burgdorferi spirochetes can differentiate into cyst forms characterized by low metabolic activity and an altered surface antigen profile, promoting survival until conditions again support replication (28). Cyst formation in B. burgdorferi is stimulated by shifting spirochetes to unfavorable conditions, such as distilled water (28), extreme pH, high temperature, and peroxide (139). Serum starvation also induces differentiation into cysts, which revert to a spirochete morphology when serum is added to the medium (7). Accordingly, the encystation of B. burgdorferi in nutritionally demanding environments may be under regulatory control by ppGpp. Indeed, when spirochetes are cultured under conditions designed to mimic unfed ticks, their transcription profile has features of a stringent response (166). Cyst formation is also inhibited by tetracycline, suggesting that de novo protein synthesis is required for cyst development (7).
The minimal genome of B. burgdorferi harbors a single bifunctional spoT homologue (interchangeably called B. burgdorferi rel [relBbu] and spoT) that is required for virulence in mice, indicating that B. burgdorferi pathogenesis is regulated by ppGpp (35). The alarmone likely contributes to nutritional adaptation, since during the growth of B. burgdorferi in medium with serum or in the presence of tick cells, the levels of both ppGpp and spoT (called relBbu) mRNAs decrease (34). Conversely, spoT transcription is induced when spirochetes are cultured in medium supplemented with tick saliva (47). While this regulatory pattern is consistent with spoT-dependent ppGpp levels repressing growth when suitable nutrients are scarce, neither glucose nor amino acid starvation induces appreciable spoT expression by B. burgdorferi (35, 47). B. burgdorferi relies on serum as a source of fatty acids, which it cannot synthesize de novo. As a result, B. burgdorferi is sensitive to serum starvation (47), a condition that induces at least 20 proteins detectable by proteomic analysis. Therefore, it is conceivable that a global regulatory mechanism mediated by spoT and ppGpp equips the spirochetes to adapt to fatty acid deprivation (7).
Beyond nuances under the conditions that trigger the stringent response of Borrelia spirochetes, the mechanisms of ppGpp-mediated regulation appear to have diverged from those of E. coli. Whereas the B. burgdorferi stringent response appears to contribute to adaptation to nutrient starvation and stationary phase (36), the stringent control of stable RNA synthesis does not correlate with ppGpp accumulation under starvation conditions in tick cells. Consistent with this difference, the region immediately downstream of the −10 hexamer of the 16S rRNA promoter region does not contain a GC discriminator motif characteristic of stringently controlled promoters in other bacteria (Fig. 3) (35). In addition, the accumulation of ppGpp does not affect rpoS expression in B. burgdorferi (36). Moreover, the RpoS sigma factor controls the expression of specific stress-related virulence determinants rather than global adaptation to stationary phase (38). Therefore, B. burgdorferi may have adapted the stringent response to suit needs specific to its unique life cycle (35), including the differential expression of specific proteins that promote adaptation while cycling between hosts (167). Nonetheless, the attenuation of spoT mutants in mice (35) underscores the contribution of ppGpp to B. burgdorferi fitness in the face of challenges encountered during infection.
PLANT PATHOGENS AND SYMBIONTS
Prokaryote-host cell interactions are not limited to those with animal cells or to interactions resulting in disease. Many bacteria form intimate relationships with plant hosts, with interactions ranging from symbiotic to commensal to pathogenic. Many of these interactions have a wide economic impact. Many factors important for plant cell interactions, such as quorum sensing (QS), are coordinated with growth phase and nutritional cues, often by the ppGpp alarmone. Well-characterized examples of such phenomena include those mediated by the single bifunctional SpoT homologues of genera such Rhizobium, Sinorhizobium, and Agrobacterium.
Nitrogen-fixing alphaproteobacteria, such as Rhizobium etli and Sinorhizobium meliloti, form symbiotic relationships with plant roots. In exchange for an environmentally and nutritionally suitable niche, the bacteria contribute nitrogenase activity that provides the plant with fixed nitrogen. R. etli forms nitrogen-fixing nodules on leguminous plants such as the common bean, and Phaseolus vulgaris and S. meliloti often colonize the roots of Medicago spp. such as alfalfa. These symbiotic bacteria are exposed to challenges such as high salinity and transient nutrient availability both prior to infection and during nodulation. In R. etli and S. meliloti, the bifunctional synthetase/hydrolase homologue, termed RelA, affects salt tolerance (25, 211). Furthermore, relA contributes to R. etli survival of heat shock, peroxide stress, and minimal medium (25) as well as nutritional competence (39).
In symbiotic rhizobia, ppGpp also affects phenotypes specifically related to interactions with the plant host during nodule formation. For example, an R. etli relA mutant fails to switch to a coccoid form, a morphology typical of WT nodule-residing bacteroids (25). Nodules produced in the host P. vulgaris by an R. etli relA mutant are smaller than those produced by the WT strain (39). Likewise, S. meliloti relA mutants produce few or no nodules, and the few bacteria that can be recovered from nodules harbor suppressor mutations in rpoBC (213, 214). Given that amino acid substitutions in the RpoBC subunits of E. coli RNA polymerase also suppress phenotypes normally activated by ppGpp (15), these genetic data implicate an alarmone in the regulation of R. etli nodulation. Any relA mutant-derived nodules formed by either symbiont are unable to fix nitrogen, and the R. etli relA mutant nodules exhibit lower nitrogenase activity than those formed by the WT strain (137). These relA mutant phenotypes indicate that ppGpp modulates metabolic pathways that are intimately related to its symbiotic relationship with its host. Specifically, the aberrant nodules may reflect a lower level of expression of rpoN-regulated genes that are required for nitrogen fixation (132), suggesting that ppGpp affects the expression of alternative sigma factors that promote interactions with the host.
During nodulation, the coordination of behavior between bacteria, as well as with the host, is critical. Communication ensures the correct temporal production of specific factors that contribute to host interactions, such as extracellular polymers. In R. etli, RelA positively controls the expression of the cin- and rai-encoded QS systems, thereby contributing to density-dependent adaptive responses related to symbiosis, such as nodulation and nitrogen fixation (137). Plant symbionts also communicate with host cells, initiating nodule formation by the secretion of lipochitooligosaccharide-based Nod factors. Interestingly, an R. etli relA-null mutant also produces Nod factors constitutively (39). The stringent response also coordinately regulates the production of specific effectors thought to stimulate nodule invasion, such as extracellular polysaccharides, as an S. meliloti relA mutant produces excess succinoglycan (214) and more readily forms biofilms (129).
Like symbionts, plant pathogens also utilize the stringent response to coordinate the expression of factors involved in host interactions. Agrobacterium tumefaciens, an alphaproteobacterium implicated in crown gall disease, relies on QS to induce the conjugal transfer of the virulence factor-encoding Ti plasmid. Once cells enter stationary phase, Ti plasmid conjugation is terminated by the AttM-mediated degradation of acyl homoserine lactone (AHL) quormones. A. tumefaciens relA mutants fail to induce the expression of attM upon entry into stationary phase (221). Additionally, the expression of the AldH succinic semialdehyde dehydrogenase, which also promotes quormone degradation through the succinic acid-dependent induction of AttM, appears to be negatively regulated by relA (206). These contrasting observations suggest a complex regulatory mechanism whereby RelA controls Ti plasmid transfer according to environmental conditions. Neighboring nonpathogenic bacteria that reside within the same tumor may provide a secondary source of AHL to modulate the virulence of plant pathogens. For example, coinhabitants of the genus Novosphingobium utilize a ppGpp-dependent pathway to produce AHL, which affects neighboring plant pathogens (69).
Nonalphaproteobacterial plant pathogens also exploit the stringent response to coordinate virulence. One example is the gammaproteobacterium Erwinia carotovora subsp. atroseptica, whose broad host range demands that the bacteria adapt to varied host environments. When nutrients become limiting, an E. carotovora relA mutant is defective for motility, the production of the Pel and Prt exoenzyme virulence factors, and causing rot in potato tubers (207).
ppGpp0 MUTANTS AS LIVE VACCINES
The striking virulence defects of ppGpp0 mutant Salmonella and Yersinia strains in mice has motivated the application of these strains as live vaccines. Live, attenuated vaccines both confer long-lasting immunity and elicit niche-specific immune responses during infection (51, 165). A potential pitfall of using ppGpp0 bacteria is the risk of residual pathogenicity. Nevertheless, work has shown that S. enterica serovar Typhimurium and Y. pestis mutants lacking the stringent response are highly efficacious at triggering immune responses and conferring protection against WT infection.
Oral and intraperitoneal inoculations of female BALB/c mice with ppGpp0 mutant S. enterica serovar Typhimurium elicit a robust antibody response and confer protection against subsequent infection with WT bacteria (140). In these models, the LD50 of ppGpp0 mutant bacteria is 105 times higher than that of WT bacteria irrespective of the oral or intraperitoneal inoculation route. The ppGpp0 bacteria also colonize internal organs at numbers 2 to 3 logs lower than those of the WT. Both routes of infection trigger a significant serum and mucosal antibody response; however, intraperitoneal inoculation generates a more efficient systemic response than the oral route, which triggers a stronger mucosal response. The cell-mediated immune response of ppGpp0-immunized mice is also robust, as inoculated mice subsequently exposed to WT bacterial lysates exhibited increased footpad swelling compared to their naïve counterparts. Finally, immunized mice were protected against WT challenge at 4 weeks postinoculation, as 95% of orally inoculated mice and 85% of intraperitoneally inoculated mice survived challenge with WT bacteria at CFU values 106-fold higher than the LD50 observed during infection of naïve mice (140). In comparison, 100% of nonimmunized control mice reached morbidity by 7 days postinfection. Taken together, these results strongly suggest that live vaccines consisting of ppGpp0 mutant bacteria protect against S. enterica serovar Typhimurium infection.
In a murine model of bubonic plague, immunization with ppGpp0 mutant Y. pestis induces a potent humoral immune response and provides full protection from subsequent bubonic challenge (190). Specifically, subcutaneous inoculation with ppGpp0 mutant bacteria generates a robust serum antibody response to Y. pestis cell lysates. The mouse response to ppGpp0 mutant Y. pestis is slightly biased toward a Th2 (antibody-mediated humoral) response rather than a Th1 (cell-mediated) response, as the levels of Th2-specific anti-Y. pestis antibodies were higher than those of Th1-specific antibodies. Accordingly, it is not surprising that ppGpp0 mutant Y. pestis strains fail to induce the proinflammatory cytokines IFN-γ and TNF-α, as these hallmarks of a Th1 response are required to induce cell-mediated immunity during infection (190). Nevertheless, at 14 days postimmunization, 100% of mice were protected against subcutaneous (bubonic) challenge with WT Y. pestis, while 60% of mice were protected against intranasal (pneumonic) challenge. Hence, ppGpp0 mutants are an effective Y. pestis live vaccine, providing excellent protection against the bubonic form and intermediate protection against pneumonic infection.
Indeed, the prospect of stringent response mutants as attenuated vaccines is promising, and work with Salmonella and Yersinia demonstrates their utility. It will be interesting to determine if ppGpp0 mutants of M. tuberculosis, a pathogen that requires ppGpp for persistence in mice, also confer protection as a live vaccine (52). Whether such attenuated strains stimulate the appropriate arms of the immune system required to clear infection by each pathogen also warrants study. While preliminary work has no doubt provided evidence for the efficacy of ppGpp0 strains in eliciting robust immune responses, further work is necessary to demonstrate the degree of attenuation, and, thus, the safety, of such stringent response-defective strains in humans.
CONCLUDING REMARKS
Investigations of bacteria grown in the laboratory in broth culture have generated a wealth of knowledge of bacterial pathogenesis. However, more recent studies emerging from the fields of cellular microbiology (bacterial-host cell interactions) and “social” microbiology (quorum sensing and biofilms) have significantly expanded our understanding of the pathogenic process. In particular, pathogen behavior is being examined under conditions that more closely resemble those encountered during infection as well as in the natural environment. Studies in these more complex settings have revealed that physiological adaptations to different growth conditions are intimately linked to the resilience and virulence of bacterial pathogens.
The distinct phenotypic profiles displayed by pathogens under different growth conditions indicate that changes in physiology can reflect a marked switch between two specialized cell types. Noteworthy examples include the differentiation between replicative and transmissive forms by facultative intracellular pathogens, such as L. pneumophila, and the shift between biofilm and free-swimming planktonic life-styles in pathogens like P. aeruginosa. The reciprocal pattern of expression of certain traits suggests that an activity that provides benefit during replication may decrease fitness when conditions deteriorate. As such, an efficient spatiotemporal regulation of factors that promote colonization or spread is critical.
The local nutrient supply is one factor that dictates which developmental and physiological programs confer benefit. The global design of the stringent response also allows bacteria to coordinate numerous factors that act in concert. Indeed, many pathogens rely on ppGpp to govern complex pathogenesis traits, such as constituents of the Salmonella SPI1 and SPI2 pathogenicity islands.
While many aspects of the stringent response paradigm discovered in classical studies of laboratory strains of E. coli hold true for bacterial pathogens, it has also become clear that some features of ppGpp-mediated gene regulation by pathogenic bacteria are distinct. For instance, it is generally observed that low ppGpp levels occur under nutrient-replete conditions and allow replication, whereas higher ppGpp levels induce growth arrest and new physiologies to increase tolerance to low-nutrient conditions and hostile environments. Thus, global shifts in physiology confer enhanced resilience and virulence to pathogens. In contrast to this general theme, a trend observed for pathogens that warrants further study is the observation that the appropriate control of pathogenesis-related phenotypes requires basal SpoT-dependent ppGpp pools but often not the RelA-dependent alarmone. An understanding of how the balance between SpoT hydrolase and synthetase activities is regulated, and the extent to which the hydrolase activity contributes to pathogenesis, will elucidate the contributions of SpoT to virulence. Furthermore, determining the degree to which the newly identified SAS enzymes such as RelP, RelQ, and RelV, some of which appear to maintain ppGpp levels during growth under nutrient-replete conditions, contribute to virulence- and survival-related phenotypes may also highlight potentially different roles for either basal or induced ppGpp pools in pathogenesis. Further studies of the diverse mechanisms of ppGpp metabolism, together with studies of specific virulence factors that are regulated by the stringent response, will continue to reveal new insights into transmission strategies and disease etiologies.
Finally, the conservation of mechanisms of ppGpp metabolism among bacteria, including significant pathogens from a variety of taxonomic divisions, together with the link of virulence and survival traits with the stringent response, warrants the consideration of this pathway as an antimicrobial target. RSH homologues are present in most bacterial pathogens that are not obligately intracellular. Furthermore, RelA- or SpoT-like enzymes have not yet been identified in humans. As discussed here, ppGpp-defective mutants have, so far, demonstrated potential as live vaccine strains. The body of work also demonstrates that the resilience of pathogens typically increases upon the induction of the stringent response, likely explaining the paradoxical success of organisms that are fragile when cultured under standard laboratory conditions. By this line of reasoning, antimicrobial agents that provoke the stringent response could inadvertently enhance the resilience of their target. Thus, studies evaluating antimicrobial tolerance mechanisms under stress conditions may be pertinent for an understanding of the emergence of resistance under selective pressure in a clinical setting. Numerous exciting and fruitful avenues of study of the link between virulence and the stringent response remain open. Likewise, regulatory pathways critical for carbon and nitrogen metabolism also impact virulence expression by a variety of pathogens (75, 158). Knowledge of how each pathogen's life-style serves its metabolic demands will both inform studies of pathogenesis and reveal new avenues for pathogen control.
Acknowledgments
Research on the stringent response in the Swanson laboratory is supported by a University of Michigan Rackham predoctoral fellowship (Z.D.D.) and NIH grant 2 R01 AI44212. Research in the Gaynor laboratory is supported by Natural Sciences and Engineering Council CGS-M and CGS-D traineeship awards; a Michael Smith Foundation for Health Research senior graduate traineeship award (S.L.S.); and a Burroughs Wellcome Fund career development award in biomedical sciences, an MSFHR Scholar Award, a Canada Research Chair Award, and Canadian Institutes of Health research operating grant MOP-68981 (E.C.G.).
Biography
 Zachary Dalebroux was born in Wisconsin and grew up in Madison, WI. He received his B.S. in Bacteriology from the University of Wisconsin—Madison in 2005. As an undergraduate research assistant with Dr. Juan de Pablo, he tested the effects of sugar-phosphate mixtures on liposome protection during lyophilization with the goal of developing effective formulas for protecting dehydrated biomaterials. In the laboratory of Dr. Kenneth Hammel, he investigated the mechanism by which brown rot fungi employ extracellular Fenton chemistry to generate hydroxyl radicals for biodegradation of lignocellulose. For his graduate studies at the University of Michigan, Ann Arbor, he joined the laboratory of Dr. Michele Swanson, focusing his research on mechanisms of bacterial gene regulation critical for pathogenesis. For his thesis, he studied the role of guanosine tetraphosphate (ppGpp) and DksA in controlling Legionella pneumophila transmission in macrophages. His work demonstrated that the bifunctional SpoT enzyme balances ppGpp pools to control both the replication and transmission of L. pneumophila in host cells. Subsequently, he determined that ppGpp cooperates and acts independently of DksA to control L. pneumophila virulence expression. After completing his Ph.D. in Microbiology and Immunology in 2010, he moved to the University of Washington, Seattle, for postdoctoral studies in the laboratory of Dr. Samuel Miller.
Zachary Dalebroux was born in Wisconsin and grew up in Madison, WI. He received his B.S. in Bacteriology from the University of Wisconsin—Madison in 2005. As an undergraduate research assistant with Dr. Juan de Pablo, he tested the effects of sugar-phosphate mixtures on liposome protection during lyophilization with the goal of developing effective formulas for protecting dehydrated biomaterials. In the laboratory of Dr. Kenneth Hammel, he investigated the mechanism by which brown rot fungi employ extracellular Fenton chemistry to generate hydroxyl radicals for biodegradation of lignocellulose. For his graduate studies at the University of Michigan, Ann Arbor, he joined the laboratory of Dr. Michele Swanson, focusing his research on mechanisms of bacterial gene regulation critical for pathogenesis. For his thesis, he studied the role of guanosine tetraphosphate (ppGpp) and DksA in controlling Legionella pneumophila transmission in macrophages. His work demonstrated that the bifunctional SpoT enzyme balances ppGpp pools to control both the replication and transmission of L. pneumophila in host cells. Subsequently, he determined that ppGpp cooperates and acts independently of DksA to control L. pneumophila virulence expression. After completing his Ph.D. in Microbiology and Immunology in 2010, he moved to the University of Washington, Seattle, for postdoctoral studies in the laboratory of Dr. Samuel Miller.
 Sarah L. Svensson is a Ph.D. candidate in the Department of Microbiology and Immunology at the University of British Columbia. She was born and raised in Victoria, BC, Canada, and received her bachelor of science in biochemistry from the University of Victoria. While completing the Cooperative Education portion of her degree, she worked in laboratories across Canada on pathogens ranging from mycobacteria to parasites and was introduced to the stringent response while studying the regulation of the E. coli RelA ppGpp synthetase and the RelEB toxin-antitoxin system under Edward Ishiguro at the University of Victoria. She joined the laboratory of Erin Gaynor in Spring 2005 to study the pathogenesis of the food-borne pathogen Campylobacter jejuni, where she has continued her interest in the relationship between bacterial stress tolerance and pathogenesis. Specifically, she studies both environmental gene regulation by two-component regulatory systems and the mechanisms of biofilm formation in C. jejuni in the hopes of understanding the paradoxical success of an apparently fastidious bacterial pathogen.
Sarah L. Svensson is a Ph.D. candidate in the Department of Microbiology and Immunology at the University of British Columbia. She was born and raised in Victoria, BC, Canada, and received her bachelor of science in biochemistry from the University of Victoria. While completing the Cooperative Education portion of her degree, she worked in laboratories across Canada on pathogens ranging from mycobacteria to parasites and was introduced to the stringent response while studying the regulation of the E. coli RelA ppGpp synthetase and the RelEB toxin-antitoxin system under Edward Ishiguro at the University of Victoria. She joined the laboratory of Erin Gaynor in Spring 2005 to study the pathogenesis of the food-borne pathogen Campylobacter jejuni, where she has continued her interest in the relationship between bacterial stress tolerance and pathogenesis. Specifically, she studies both environmental gene regulation by two-component regulatory systems and the mechanisms of biofilm formation in C. jejuni in the hopes of understanding the paradoxical success of an apparently fastidious bacterial pathogen.
 Erin C. Gaynor is an Associate Professor and Canada Research Chair in the Department of Microbiology and Immunology at the University of British Columbia. Originally from California, she obtained her bachelor's degree from the University of California at San Diego (UCSD), majoring in Cell Biology/Biochemistry and Literature. Following graduation, she spent two years working as a research associate for Hybritech Incorporated, helping to develop monoclonal antibody technology for therapeutic applications. She then entered the UCSD Biology Department Ph.D. program and earned her graduate degree with Scott Emr, studying signals and molecules involved in Golgi-ER trafficking in Saccharomyces cerevisiae. She next combined her interests in molecular cell biology and human health by pursuing postdoctoral research in bacterial pathogenesis with Stanley Falkow at Stanford University. At Stanford, she initiated studies on the enteric pathogen Campylobacter jejuni, including those which led her, her colleagues, and her group at the University of British Columbia to identify and characterize the stringent response in both C. jejuni and the related human pathogen Helicobacter pylori. Her laboratory continues to explore the pathogenesis and stress survival mechanisms of C. jejuni, with the ultimate goal of unraveling how this prevalent pathogen causes such severe and widespread human disease.
Erin C. Gaynor is an Associate Professor and Canada Research Chair in the Department of Microbiology and Immunology at the University of British Columbia. Originally from California, she obtained her bachelor's degree from the University of California at San Diego (UCSD), majoring in Cell Biology/Biochemistry and Literature. Following graduation, she spent two years working as a research associate for Hybritech Incorporated, helping to develop monoclonal antibody technology for therapeutic applications. She then entered the UCSD Biology Department Ph.D. program and earned her graduate degree with Scott Emr, studying signals and molecules involved in Golgi-ER trafficking in Saccharomyces cerevisiae. She next combined her interests in molecular cell biology and human health by pursuing postdoctoral research in bacterial pathogenesis with Stanley Falkow at Stanford University. At Stanford, she initiated studies on the enteric pathogen Campylobacter jejuni, including those which led her, her colleagues, and her group at the University of British Columbia to identify and characterize the stringent response in both C. jejuni and the related human pathogen Helicobacter pylori. Her laboratory continues to explore the pathogenesis and stress survival mechanisms of C. jejuni, with the ultimate goal of unraveling how this prevalent pathogen causes such severe and widespread human disease.
 Michele S. Swanson, a Professor in the Department of Microbiology and Immunology at the University of Michigan Medical School, was born and raised in the Midwest. She earned a B.S. in Biology from Yale, where she also played collegiate field hockey and softball. As a research technician at the Rockefeller University, she was introduced to the exciting world of experimental science in the laboratory of Samuel C. Silverstein, an expert in leukocyte cell biology who contributed to seminal studies of Legionella pneumophila growth in macrophages. She developed her love of genetics as a graduate student, using Saccharomyces cerevisiae as a tool to study gene expression with Marian Carlson at Columbia and Fred Winston at Harvard. After a brief hiatus devoted to her two children, she trained as a postdoctoral fellow with Ralph Isberg at Tufts and Howard Hughes Medical Institute (HHMI), where she developed cell biological methods to analyze the fate of L. pneumophila in macrophages. In addition to exploiting this pathogen as a genetic probe of macrophage function, her laboratory investigates how metabolic cues govern its virulence expression. At the University of Michigan, Dr. Swanson has had the privilege and pleasure of mentoring several gifted Ph.D. students; together, they developed the L. pneumophila paradigm described here.
Michele S. Swanson, a Professor in the Department of Microbiology and Immunology at the University of Michigan Medical School, was born and raised in the Midwest. She earned a B.S. in Biology from Yale, where she also played collegiate field hockey and softball. As a research technician at the Rockefeller University, she was introduced to the exciting world of experimental science in the laboratory of Samuel C. Silverstein, an expert in leukocyte cell biology who contributed to seminal studies of Legionella pneumophila growth in macrophages. She developed her love of genetics as a graduate student, using Saccharomyces cerevisiae as a tool to study gene expression with Marian Carlson at Columbia and Fred Winston at Harvard. After a brief hiatus devoted to her two children, she trained as a postdoctoral fellow with Ralph Isberg at Tufts and Howard Hughes Medical Institute (HHMI), where she developed cell biological methods to analyze the fate of L. pneumophila in macrophages. In addition to exploiting this pathogen as a genetic probe of macrophage function, her laboratory investigates how metabolic cues govern its virulence expression. At the University of Michigan, Dr. Swanson has had the privilege and pleasure of mentoring several gifted Ph.D. students; together, they developed the L. pneumophila paradigm described here.
REFERENCES
- 1.Aberg, A., J. Fernandez-Vazquez, J. D. Cabrer-Panes, A. Sanchez, and C. Balsalobre. 2009. Similar and divergent effects of ppGpp and DksA deficiencies on transcription in Escherichia coli. J. Bacteriol. 191:3226-3236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aberg, A., V. Shingler, and C. Balsalobre. 2006. (p)ppGpp regulates type 1 fimbriation of Escherichia coli by modulating the expression of the site-specific recombinase FimB. Mol. Microbiol. 60:1520-1533. [DOI] [PubMed] [Google Scholar]
- 3.Aberg, A., V. Shingler, and C. Balsalobre. 2008. Regulation of the fimB promoter: a case of differential regulation by ppGpp and DksA in vivo. Mol. Microbiol. 67:1223-1241. [DOI] [PubMed] [Google Scholar]
- 4.Abranches, J., A. R. Martinez, J. K. Kajfasz, V. Chavez, D. A. Garsin, and J. A. Lemos. 2009. The molecular alarmone (p)ppGpp mediates stress responses, vancomycin tolerance, and virulence in Enterococcus faecalis. J. Bacteriol. 191:2248-2256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Abu-Zant, A., R. Asare, J. E. Graham, and Y. Abu Kwaik. 2006. Role for RpoS but not RelA of Legionella pneumophila in modulation of phagosome biogenesis and adaptation to the phagosomal microenvironment. Infect. Immun. 74:3021-3026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Alam, M., M. Sultana, G. B. Nair, A. K. Siddique, N. A. Hasan, R. B. Sack, D. A. Sack, K. U. Ahmed, A. Sadique, H. Watanabe, C. J. Grim, A. Huq, and R. R. Colwell. 2007. Viable but nonculturable Vibrio cholerae O1 in biofilms in the aquatic environment and their role in cholera transmission. Proc. Natl. Acad. Sci. U. S. A. 104:17801-17806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Alban, P. S., P. W. Johnson, and D. R. Nelson. 2000. Serum-starvation-induced changes in protein synthesis and morphology of Borrelia burgdorferi. Microbiology 146(Pt. 1):119-127. [DOI] [PubMed] [Google Scholar]
- 8.Allen, L. A., L. S. Schlesinger, and B. Kang. 2000. Virulent strains of Helicobacter pylori demonstrate delayed phagocytosis and stimulate homotypic phagosome fusion in macrophages. J. Exp. Med. 191:115-128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Avarbock, D., J. Salem, L. S. Li, Z. M. Wang, and H. Rubin. 1999. Cloning and characterization of a bifunctional RelA/SpoT homologue from Mycobacterium tuberculosis. Gene 233:261-269. [DOI] [PubMed] [Google Scholar]
- 10.Ayraud, S., B. Janvier, A. Labigne, C. Ecobichon, C. Burucoa, and J. L. Fauchere. 2005. Polyphosphate kinase: a new colonization factor of Helicobacter pylori. FEMS Microbiol. Lett. 243:45-50. [DOI] [PubMed] [Google Scholar]
- 11.Bachman, M. A., and M. S. Swanson. 2004. Genetic evidence that Legionella pneumophila RpoS modulates expression of the transmission phenotype in both the exponential phase and the stationary phase. Infect. Immun. 72:2468-2476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bachman, M. A., and M. S. Swanson. 2001. RpoS co-operates with other factors to induce Legionella pneumophila virulence in the stationary phase. Mol. Microbiol. 40:1201-1214. [DOI] [PubMed] [Google Scholar]
- 13.Bachman, M. A., and M. S. Swanson. 2004. The LetE protein enhances expression of multiple LetA/LetS-dependent transmission traits by Legionella pneumophila. Infect. Immun. 72:3284-3293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Barker, J. R., and K. E. Klose. 2007. Molecular and genetic basis of pathogenesis in Francisella tularensis. Ann. N. Y. Acad. Sci. 1105:138-159. [DOI] [PubMed] [Google Scholar]
- 15.Bartlett, M. S., T. Gaal, W. Ross, and R. L. Gourse. 1998. RNA polymerase mutants that destabilize RNA polymerase-promoter complexes alter NTP-sensing by rrn P1 promoters. J. Mol. Biol. 279:331-345. [DOI] [PubMed] [Google Scholar]
- 16.Battesti, A., and E. Bouveret. 2006. Acyl carrier protein/SpoT interaction, the switch linking SpoT-dependent stress response to fatty acid metabolism. Mol. Microbiol. 62:1048-1063. [DOI] [PubMed] [Google Scholar]
- 17.Battesti, A., and E. Bouveret. 2009. Bacteria possessing two RelA/SpoT-like proteins have evolved a specific stringent response involving the acyl carrier protein-SpoT interaction. J. Bacteriol. 191:616-624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Baysse, C., M. Cullinane, V. Denervaud, E. Burrowes, J. M. Dow, J. P. Morrissey, L. Tam, J. T. Trevors, and F. O'Gara. 2005. Modulation of quorum sensing in Pseudomonas aeruginosa through alteration of membrane properties. Microbiology 151:2529-2542. [DOI] [PubMed] [Google Scholar]
- 19.Bennett, H. J., D. M. Pearce, S. Glenn, C. M. Taylor, M. Kuhn, A. L. Sonenshein, P. W. Andrew, and I. S. Roberts. 2007. Characterization of relA and codY mutants of Listeria monocytogenes: identification of the CodY regulon and its role in virulence. Mol. Microbiol. 63:1453-1467. [DOI] [PubMed] [Google Scholar]
- 20.Bernardo, L. M., L. U. Johansson, D. Solera, E. Skarfstad, and V. Shingler. 2006. The guanosine tetraphosphate (ppGpp) alarmone, DksA and promoter affinity for RNA polymerase in regulation of sigma-dependent transcription. Mol. Microbiol. 60:749-764. [DOI] [PubMed] [Google Scholar]
- 21.Berry, A. M., J. Yother, D. E. Briles, D. Hansman, and J. C. Paton. 1989. Reduced virulence of a defined pneumolysin-negative mutant of Streptococcus pneumoniae. Infect. Immun. 57:2037-2042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Blankschien, M. D., K. Potrykus, E. Grace, A. Choudhary, D. Vinella, M. Cashel, and C. Herman. 2009. TraR, a homolog of a RNAP secondary channel interactor, modulates transcription. PLoS Genet. 5:e1000345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Boardman, B. K., B. M. Meehan, and K. J. Fullner Satchell. 2007. Growth phase regulation of Vibrio cholerae RTX toxin export. J. Bacteriol. 189:1827-1835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Boes, N., K. Schreiber, and M. Schobert. 2008. SpoT-triggered stringent response controls usp gene expression in Pseudomonas aeruginosa. J. Bacteriol. 190:7189-7199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Braeken, K., M. Fauvart, M. Vercruysse, S. Beullens, I. Lambrichts, and J. Michiels. 2008. Pleiotropic effects of a rel mutation on stress survival of Rhizobium etli CNPAF512. BMC Microbiol. 8:219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Braeken, K., M. Moris, R. Daniels, J. Vanderleyden, and J. Michiels. 2006. New horizons for (p)ppGpp in bacterial and plant physiology. Trends Microbiol. 14:45-54. [DOI] [PubMed] [Google Scholar]
- 27.Branny, P., J. P. Pearson, E. C. Pesci, T. Kohler, B. H. Iglewski, and C. Van Delden. 2001. Inhibition of quorum sensing by a Pseudomonas aeruginosa dksA homologue. J. Bacteriol. 183:1531-1539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Brorson, O., and S. H. Brorson. 1998. In vitro conversion of Borrelia burgdorferi to cystic forms in spinal fluid, and transformation to mobile spirochetes by incubation in BSK-H medium. Infection 26:144-150. [DOI] [PubMed] [Google Scholar]
- 29.Brotcke, A., and D. M. Monack. 2008. Identification of fevR, a novel regulator of virulence gene expression in Francisella novicida. Infect. Immun. 76:3473-3480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Brown, L., D. Gentry, T. Elliott, and M. Cashel. 2002. DksA affects ppGpp induction of RpoS at a translational level. J. Bacteriol. 184:4455-4465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Brubaker, R. R., E. D. Beesley, and M. J. Surgalla. 1965. Pasteurella pestis: role of pesticin I and iron in experimental plague. Science 149:422-424. [DOI] [PubMed] [Google Scholar]
- 32.Bruggemann, H., A. Hagman, M. Jules, O. Sismeiro, M. A. Dillies, C. Gouyette, F. Kunst, M. Steinert, K. Heuner, J. Y. Coppee, and C. Buchrieser. 2006. Virulence strategies for infecting phagocytes deduced from the in vivo transcriptional program of Legionella pneumophila. Cell. Microbiol. 8:1228-1240. [DOI] [PubMed] [Google Scholar]
- 33.Buglino, J., V. Shen, P. Hakimian, and C. D. Lima. 2002. Structural and biochemical analysis of the Obg GTP binding protein. Structure 10:1581-1592. [DOI] [PubMed] [Google Scholar]
- 34.Bugrysheva, J., E. Y. Dobrikova, H. P. Godfrey, M. L. Sartakova, and F. C. Cabello. 2002. Modulation of Borrelia burgdorferi stringent response and gene expression during extracellular growth with tick cells. Infect. Immun. 70:3061-3067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bugrysheva, J., E. Y. Dobrikova, M. L. Sartakova, M. J. Caimano, T. J. Daniels, J. D. Radolf, H. P. Godfrey, and F. C. Cabello. 2003. Characterization of the stringent response and rel(Bbu) expression in Borrelia burgdorferi. J. Bacteriol. 185:957-965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bugrysheva, J. V., A. V. Bryksin, H. P. Godfrey, and F. C. Cabello. 2005. Borrelia burgdorferi rel is responsible for generation of guanosine-3′-diphosphate-5′-triphosphate and growth control. Infect. Immun. 73:4972-4981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Buswell, C. M., Y. M. Herlihy, L. M. Lawrence, J. T. McGuiggan, P. D. Marsh, C. W. Keevil, and S. A. Leach. 1998. Extended survival and persistence of Campylobacter spp. in water and aquatic biofilms and their detection by immunofluorescent-antibody and -rRNA staining. Appl. Environ. Microbiol. 64:733-741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Caimano, M. J., C. H. Eggers, K. R. Hazlett, and J. D. Radolf. 2004. RpoS is not central to the general stress response in Borrelia burgdorferi but does control expression of one or more essential virulence determinants. Infect. Immun. 72:6433-6445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Calderon-Flores, A., G. Du Pont, A. Huerta-Saquero, H. Merchant-Larios, L. Servin-Gonzalez, and S. Duran. 2005. The stringent response is required for amino acid and nitrate utilization, nod factor regulation, nodulation, and nitrogen fixation in Rhizobium etli. J. Bacteriol. 187:5075-5083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Candon, H. L., B. J. Allan, C. D. Fraley, and E. C. Gaynor. 2007. Polyphosphate kinase 1 is a pathogenesis determinant in Campylobacter jejuni. J. Bacteriol. 189:8099-8108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Celli, J., and J. P. Gorvel. 2004. Organelle robbery: Brucella interactions with the endoplasmic reticulum. Curr. Opin. Microbiol. 7:93-97. [DOI] [PubMed] [Google Scholar]
- 42.Chan, Y. C., S. Raengpradub, K. J. Boor, and M. Wiedmann. 2007. Microarray-based characterization of the Listeria monocytogenes cold regulon in log- and stationary-phase cells. Appl. Environ. Microbiol. 73:6484-6498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Charity, J. C., L. T. Blalock, M. M. Costante-Hamm, D. L. Kasper, and S. L. Dove. 2009. Small molecule control of virulence gene expression in Francisella tularensis. PLoS Pathog. 5:e1000641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chaudhuri, R. R., A. G. Allen, P. J. Owen, G. Shalom, K. Stone, M. Harrison, T. A. Burgis, M. Lockyer, J. Garcia-Lara, S. J. Foster, S. J. Pleasance, S. E. Peters, D. J. Maskell, and I. G. Charles. 2009. Comprehensive identification of essential Staphylococcus aureus genes using transposon-mediated differential hybridisation (TMDH). BMC Genomics 10:291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chaussee, M. A., A. V. Dmitriev, E. A. Callegari, and M. S. Chaussee. 2008. Growth phase-associated changes in the transcriptome and proteome of Streptococcus pyogenes. Arch. Microbiol. 189:27-41. [DOI] [PubMed] [Google Scholar]
- 46.Cockeran, R., R. Anderson, and C. Feldman. 2002. The role of pneumolysin in the pathogenesis of Streptococcus pneumoniae infection. Curr. Opin. Infect. Dis. 15:235-239.12015456 [Google Scholar]
- 47.Concepcion, M. B., and D. R. Nelson. 2003. Expression of spoT in Borrelia burgdorferi during serum starvation. J. Bacteriol. 185:444-452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Cooksley, C., P. J. Jenks, A. Green, A. Cockayne, R. P. Logan, and K. R. Hardie. 2003. NapA protects Helicobacter pylori from oxidative stress damage, and its production is influenced by the ferric uptake regulator. J. Med. Microbiol. 52:461-469. [DOI] [PubMed] [Google Scholar]
- 49.Costanzo, A., H. Nicoloff, S. E. Barchinger, A. B. Banta, R. L. Gourse, and S. E. Ades. 2008. ppGpp and DksA likely regulate the activity of the extracytoplasmic stress factor sigmaE in Escherichia coli by both direct and indirect mechanisms. Mol. Microbiol. 67:619-632. [DOI] [PubMed] [Google Scholar]
- 50.Cowan, C., H. A. Jones, Y. H. Kaya, R. D. Perry, and S. C. Straley. 2000. Invasion of epithelial cells by Yersinia pestis: evidence for a Y. pestis-specific invasin. Infect. Immun. 68:4523-4530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Curtiss, R., III, S. M. Kelly, and J. O. Hassan. 1993. Live oral avirulent Salmonella vaccines. Vet. Microbiol. 37:397-405. [DOI] [PubMed] [Google Scholar]
- 52.Dahl, J. L., C. N. Kraus, H. I. Boshoff, B. Doan, K. Foley, D. Avarbock, G. Kaplan, V. Mizrahi, H. Rubin, and C. E. Barry III. 2003. The role of RelMtb-mediated adaptation to stationary phase in long-term persistence of Mycobacterium tuberculosis in mice. Proc. Natl. Acad. Sci. U. S. A. 100:10026-10031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Dalebroux, Z. D., R. L. Edwards, and M. S. Swanson. 2009. SpoT governs Legionella pneumophila differentiation in host macrophages. Mol. Microbiol. 71:640-658. [DOI] [PubMed] [Google Scholar]
- 54.Dalebroux, Z. D., B. F. Yagi, T. Sahr, C. Buchrieser, and M. S. Swanson. 2010. Distinct roles of ppGpp and DksA in Legionella pneumophila differentiation. Mol. Microbiol. 76:200-219. [DOI] [PMC free article] [PubMed]
- 55.Darwin, K. H., and V. L. Miller. 2000. The putative invasion protein chaperone SicA acts together with InvF to activate the expression of Salmonella typhimurium virulence genes. Mol. Microbiol. 35:949-960. [DOI] [PubMed] [Google Scholar]
- 56.Das, B., and R. K. Bhadra. 2008. Molecular characterization of Vibrio cholerae ΔrelA ΔspoT double mutants. Arch. Microbiol. 189:227-238. [DOI] [PubMed] [Google Scholar]
- 57.Das, B., R. R. Pal, S. Bag, and R. K. Bhadra. 2009. Stringent response in Vibrio cholerae: genetic analysis of spoT gene function and identification of a novel (p)ppGpp synthetase gene. Mol. Microbiol. 72:380-398. [DOI] [PubMed] [Google Scholar]
- 58.De Voss, J. J., K. Rutter, B. G. Schroeder, and C. E. Barry III. 1999. Iron acquisition and metabolism by mycobacteria. J. Bacteriol. 181:4443-4451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Dozot, M., R. A. Boigegrain, R. M. Delrue, R. Hallez, S. Ouahrani-Bettache, I. Danese, J. J. Letesson, X. De Bolle, and S. Kohler. 2006. The stringent response mediator Rsh is required for Brucella melitensis and Brucella suis virulence, and for expression of the type IV secretion system virB. Cell. Microbiol. 8:1791-1802. [DOI] [PubMed] [Google Scholar]
- 60.Duan, K., and M. G. Surette. 2007. Environmental regulation of Pseudomonas aeruginosa PAO1 Las and Rhl quorum-sensing systems. J. Bacteriol. 189:4827-4836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Edwards, R. L., Z. D. Dalebroux, and M. S. Swanson. 2009. Legionella pneumophila couples fatty acid flux to microbial differentiation and virulence. Mol. Microbiol. 71:1190-1204. [DOI] [PubMed] [Google Scholar]
- 62.Emerson, J. E., R. A. Stabler, B. W. Wren, and N. F. Fairweather. 2008. Microarray analysis of the transcriptional responses of Clostridium difficile to environmental and antibiotic stress. J. Med. Microbiol. 57:757-764. [DOI] [PubMed] [Google Scholar]
- 63.Erickson, D. L., J. L. Lines, E. C. Pesci, V. Venturi, and D. G. Storey. 2004. Pseudomonas aeruginosa relA contributes to virulence in Drosophila melanogaster. Infect. Immun. 72:5638-5645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Everest, P. H., H. Goossens, J. P. Butzler, D. Lloyd, S. Knutton, J. M. Ketley, and P. H. Williams. 1992. Differentiated Caco-2 cells as a model for enteric invasion by Campylobacter jejuni and C. coli. J. Med. Microbiol. 37:319-325. [DOI] [PubMed] [Google Scholar]
- 65.Fang, F. C., S. J. Libby, N. A. Buchmeier, P. C. Loewen, J. Switala, J. Harwood, and D. G. Guiney. 1992. The alternative sigma factor katF (rpoS) regulates Salmonella virulence. Proc. Natl. Acad. Sci. U. S. A. 89:11978-11982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Faulkner, G., S. G. Berk, E. Garduno, M. A. Ortiz-Jimenez, and R. A. Garduno. 2008. Passage through Tetrahymena tropicalis triggers a rapid morphological differentiation in Legionella pneumophila. J. Bacteriol. 190:7728-7738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Freitag, N. E., G. C. Port, and M. D. Miner. 2009. Listeria monocytogenes—from saprophyte to intracellular pathogen. Nat. Rev. Microbiol. 7:623-628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Gallant, J., J. Irr, and M. Cashel. 1971. The mechanism of amino acid control of guanylate and adenylate biosynthesis. J. Biol. Chem. 246:5812-5816. [PubMed] [Google Scholar]
- 69.Gan, H. M., L. Buckley, E. Szegedi, A. O. Hudson, and M. A. Savka. 2009. Identification of an rsh gene from a Novosphingobium sp. necessary for quorum-sensing signal accumulation. J. Bacteriol. 191:2551-2560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Gaynor, E. C., D. H. Wells, J. K. MacKichan, and S. Falkow. 2005. The Campylobacter jejuni stringent response controls specific stress survival and virulence-associated phenotypes. Mol. Microbiol. 56:8-27. [DOI] [PubMed] [Google Scholar]
- 71.Gentry, D., T. Li, M. Rosenberg, and D. McDevitt. 2000. The rel gene is essential for in vitro growth of Staphylococcus aureus. J. Bacteriol. 182:4995-4997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Gill, W. P., N. S. Harik, M. R. Whiddon, R. P. Liao, J. E. Mittler, and D. R. Sherman. 2009. A replication clock for Mycobacterium tuberculosis. Nat. Med. 15:211-214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Glaser, G., A. Razin, and S. Razin. 1981. Stable RNA synthesis and its control in Mycoplasma capricolum. Nucleic Acids Res. 9:3641-3646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Gonzalez-Escalona, N., A. Fey, M. G. Hofle, R. T. Espejo, and C. A. Guzman. 2006. Quantitative reverse transcription polymerase chain reaction analysis of Vibrio cholerae cells entering the viable but non-culturable state and starvation in response to cold shock. Environ. Microbiol. 8:658-666. [DOI] [PubMed] [Google Scholar]
- 75.Gorke, B., and J. Stulke. 2008. Carbon catabolite repression in bacteria: many ways to make the most out of nutrients. Nat. Rev. Microbiol. 6:613-624. [DOI] [PubMed] [Google Scholar]
- 76.Gorvel, J. P., and E. Moreno. 2002. Brucella intracellular life: from invasion to intracellular replication. Vet. Microbiol. 90:281-297. [DOI] [PubMed] [Google Scholar]
- 77.Graham, M. R., L. M. Smoot, C. A. Migliaccio, K. Virtaneva, D. E. Sturdevant, S. F. Porcella, M. J. Federle, G. J. Adams, J. R. Scott, and J. M. Musser. 2002. Virulence control in group A Streptococcus by a two-component gene regulatory system: global expression profiling and in vivo infection modeling. Proc. Natl. Acad. Sci. U. S. A. 99:13855-13860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Grosset, J. 2003. Mycobacterium tuberculosis in the extracellular compartment: an underestimated adversary. Antimicrob. Agents Chemother. 47:833-836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Guina, T., S. O. Purvine, E. C. Yi, J. Eng, D. R. Goodlett, R. Aebersold, and S. I. Miller. 2003. Quantitative proteomic analysis indicates increased synthesis of a quinolone by Pseudomonas aeruginosa isolates from cystic fibrosis airways. Proc. Natl. Acad. Sci. U. S. A. 100:2771-2776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Gummesson, B., L. U. Magnusson, M. Lovmar, K. Kvint, O. Persson, M. Ballesteros, A. Farewell, and T. Nystrom. 2009. Increased RNA polymerase availability directs resources towards growth at the expense of maintenance. EMBO J. 28:2209-2219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Hammer, B. K., and M. S. Swanson. 1999. Co-ordination of Legionella pneumophila virulence with entry into stationary phase by ppGpp. Mol. Microbiol. 33:721-731. [DOI] [PubMed] [Google Scholar]
- 82.Hammer, B. K., E. S. Tateda, and M. S. Swanson. 2002. A two-component regulator induces the transmission phenotype of stationary-phase Legionella pneumophila. Mol. Microbiol. 44:107-118. [DOI] [PubMed] [Google Scholar]
- 83.Haraga, A., M. B. Ohlson, and S. I. Miller. 2008. Salmonellae interplay with host cells. Nat. Rev. Microbiol. 6:53-66. [DOI] [PubMed] [Google Scholar]
- 84.Haralalka, S., S. Nandi, and R. K. Bhadra. 2003. Mutation in the relA gene of Vibrio cholerae affects in vitro and in vivo expression of virulence factors. J. Bacteriol. 185:4672-4682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Haugen, S. P., W. Ross, and R. L. Gourse. 2008. Advances in bacterial promoter recognition and its control by factors that do not bind DNA. Nat. Rev. Microbiol. 6:507-519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Hava, D. L., and A. Camilli. 2002. Large-scale identification of serotype 4 Streptococcus pneumoniae virulence factors. Mol. Microbiol. 45:1389-1406. [PMC free article] [PubMed] [Google Scholar]
- 87.Hendriksen, W. T., H. J. Bootsma, S. Estevao, T. Hoogenboezem, A. de Jong, R. de Groot, O. P. Kuipers, and P. W. Hermans. 2008. CodY of Streptococcus pneumoniae: link between nutritional gene regulation and colonization. J. Bacteriol. 190:590-601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Heuner, K., C. Dietrich, C. Skriwan, M. Steinert, and J. Hacker. 2002. Influence of the alternative sigma(28) factor on virulence and flagellum expression of Legionella pneumophila. Infect. Immun. 70:1604-1608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Hinnebusch, J., P. Cherepanov, Y. Du, A. Rudolph, J. D. Dixon, T. Schwan, and A. Forsberg. 2000. Murine toxin of Yersinia pestis shows phospholipase D activity but is not required for virulence in mice. Int. J. Med. Microbiol. 290:483-487. [DOI] [PubMed] [Google Scholar]
- 90.Hofreuter, D., V. Novik, and J. E. Galan. 2008. Metabolic diversity in Campylobacter jejuni enhances specific tissue colonization. Cell Host Microbe 4:425-433. [DOI] [PubMed] [Google Scholar]
- 91.Hovel-Miner, G., S. Pampou, S. P. Faucher, M. Clarke, I. Morozova, P. Morozov, J. J. Russo, H. A. Shuman, and S. Kalachikov. 2009. SigmaS controls multiple pathways associated with intracellular multiplication of Legionella pneumophila. J. Bacteriol. 191:2461-2473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Hunt, S. M., E. M. Werner, B. Huang, M. A. Hamilton, and P. S. Stewart. 2004. Hypothesis for the role of nutrient starvation in biofilm detachment. Appl. Environ. Microbiol. 70:7418-7425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Inaoka, T., and K. Ochi. 2002. RelA protein is involved in induction of genetic competence in certain Bacillus subtilis strains by moderating the level of intracellular GTP. J. Bacteriol. 184:3923-3930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Isberg, R. R., T. J. O'Connor, and M. Heidtman. 2009. The Legionella pneumophila replication vacuole: making a cosy niche inside host cells. Nat. Rev. Microbiol. 7:13-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Iyoda, S., and H. Watanabe. 2004. Positive effects of multiple pch genes on expression of the locus of enterocyte effacement genes and adherence of enterohaemorrhagic Escherichia coli O157:H7 to HEp-2 cells. Microbiology 150:2357-2571. [DOI] [PubMed] [Google Scholar]
- 96.Jacobi, S., R. Schade, and K. Heuner. 2004. Characterization of the alternative sigma factor sigma54 and the transcriptional regulator FleQ of Legionella pneumophila, which are both involved in the regulation cascade of flagellar gene expression. J. Bacteriol. 186:2540-2547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Jeong, J. H., M. Song, S. I. Park, K. O. Cho, J. H. Rhee, and H. E. Choy. 2008. Salmonella enterica serovar Gallinarum requires ppGpp for internalization and survival in animal cells. J. Bacteriol. 190:6340-6350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Jerse, A. E., J. Yu, B. D. Tall, and J. B. Kaper. 1990. A genetic locus of enteropathogenic Escherichia coli necessary for the production of attaching and effacing lesions on tissue culture cells. Proc. Natl. Acad. Sci. U. S. A. 87:7839-7843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Jones, P. G., M. Cashel, G. Glaser, and F. C. Neidhardt. 1992. Function of a relaxed-like state following temperature downshifts in Escherichia coli. J. Bacteriol. 174:3903-3914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Reference deleted.
- 101.Jude, F., T. Kohler, P. Branny, K. Perron, M. P. Mayer, R. Comte, and C. van Delden. 2003. Posttranscriptional control of quorum-sensing-dependent virulence genes by DksA in Pseudomonas aeruginosa. J. Bacteriol. 185:3558-3566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Kau, A. L., D. A. Hunstad, and S. J. Hultgren. 2005. Interaction of uropathogenic Escherichia coli with host uroepithelium. Curr. Opin. Microbiol. 8:54-59. [DOI] [PubMed] [Google Scholar]
- 103.Kazmierczak, K. M., K. J. Wayne, A. Rechtsteiner, and M. E. Winkler. 2009. Roles of rel in stringent response, global regulation and virulence of serotype 2 Streptococcus pneumoniae D39. Mol. Microbiol. 72:590-611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Kim, S., K. Watanabe, H. Suzuki, and M. Watarai. 2005. Roles of Brucella abortus SpoT in morphological differentiation and intramacrophagic replication. Microbiology 151:1607-1617. [DOI] [PubMed] [Google Scholar]
- 105.Kirisits, M. J., and M. R. Parsek. 2006. Does Pseudomonas aeruginosa use intercellular signalling to build biofilm communities? Cell. Microbiol. 8:1841-1849. [DOI] [PubMed] [Google Scholar]
- 106.Klancnik, A., B. Guzej, P. Jamnik, D. Vuckovic, M. Abram, and S. S. Mozina. 2009. Stress response and pathogenic potential of Campylobacter jejuni cells exposed to starvation. Res. Microbiol. 160:345-352. [DOI] [PubMed] [Google Scholar]
- 107.Kohler, S., V. Foulongne, S. Ouahrani-Bettache, G. Bourg, J. Teyssier, M. Ramuz, and J. P. Liautard. 2002. The analysis of the intramacrophagic virulome of Brucella suis deciphers the environment encountered by the pathogen inside the macrophage host cell. Proc. Natl. Acad. Sci. U. S. A. 99:15711-15716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Kohler, T., A. Buckling, and C. van Delden. 2009. Cooperation and virulence of clinical Pseudomonas aeruginosa populations. Proc. Natl. Acad. Sci. U. S. A. 106:6339-6344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Krasny, L., and R. L. Gourse. 2004. An alternative strategy for bacterial ribosome synthesis: Bacillus subtilis rRNA transcription regulation. EMBO J. 23:4473-4483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Krasny, L., H. Tiserova, J. Jonak, D. Rejman, and H. Sanderova. 2008. The identity of the transcription +1 position is crucial for changes in gene expression in response to amino acid starvation in Bacillus subtilis. Mol. Microbiol. 69:42-54. [DOI] [PubMed] [Google Scholar]
- 111.Kuroda, A., H. Murphy, M. Cashel, and A. Kornberg. 1997. Guanosine tetra- and pentaphosphate promote accumulation of inorganic polyphosphate in Escherichia coli. J. Biol. Chem. 272:21240-21243. [DOI] [PubMed] [Google Scholar]
- 112.Lahteenmaki, K., M. Kukkonen, and T. K. Korhonen. 2001. The Pla surface protease/adhesin of Yersinia pestis mediates bacterial invasion into human endothelial cells. FEBS Lett. 504:69-72. [DOI] [PubMed] [Google Scholar]
- 113.Lemke, J. J., T. Durfee, and R. L. Gourse. 2009. DksA and ppGpp directly regulate transcription of the Escherichia coli flagellar cascade. Mol. Microbiol. 74:1368-1379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Lemos, J. A., V. K. Lin, M. M. Nascimento, J. Abranches, and R. A. Burne. 2007. Three gene products govern (p)ppGpp production by Streptococcus mutans. Mol. Microbiol. 65:1568-1581. [DOI] [PubMed] [Google Scholar]
- 115.Lemos, J. A., M. M. Nascimento, V. K. Lin, J. Abranches, and R. A. Burne. 2008. Global regulation by (p)ppGpp and CodY in Streptococcus mutans. J. Bacteriol. 190:5291-5299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Lemos, J. A. C., T. A. Brown, Jr., and R. A. Burne. 2004. Effects of RelA on key virulence properties of planktonic and biofilm populations of Streptococcus mutans. Infect. Immun. 72:1431-1440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Li, W., L. Liu, H. Chen, and R. Zhou. 2009. Identification of Streptococcus suis genes preferentially expressed under iron starvation by selective capture of transcribed sequences. FEMS Microbiol. Lett. 292:123-133. [DOI] [PubMed] [Google Scholar]
- 118.Liu, S., D. O. Bayles, T. M. Mason, and B. J. Wilkinson. 2006. A cold-sensitive Listeria monocytogenes mutant has a transposon insertion in a gene encoding a putative membrane protein and shows altered (p)ppGpp levels. Appl. Environ. Microbiol. 72:3955-3959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Lopez, J. M., A. Dromerick, and E. Freese. 1981. Response of guanosine 5′-triphosphate concentration to nutritional changes and its significance for Bacillus subtilis sporulation. J. Bacteriol. 146:605-613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Lopez, J. M., C. L. Marks, and E. Freese. 1979. The decrease of guanine nucleotides initiates sporulation of Bacillus subtilis. Biochim. Biophys. Acta 587:238-252. [DOI] [PubMed] [Google Scholar]
- 121.Lucas, R. L., C. P. Lostroh, C. C. DiRusso, M. P. Spector, B. L. Wanner, and C. A. Lee. 2000. Multiple factors independently regulate hilA and invasion gene expression in Salmonella enterica serovar Typhimurium. J. Bacteriol. 182:1872-1882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Ludu, J. S., O. M. de Bruin, B. N. Duplantis, C. L. Schmerk, A. Y. Chou, K. L. Elkins, and F. E. Nano. 2008. The Francisella pathogenicity island protein PdpD is required for full virulence and associates with homologues of the type VI secretion system. J. Bacteriol. 190:4584-4595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Lyzen, R., M. Kochanowska, G. Wegrzyn, and A. Szalewska-Palasz. 2009. Transcription from bacteriophage lambda pR promoter is regulated independently and antagonistically by DksA and ppGpp. Nucleic Acids Res. 37:655-664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Magnusson, L. U., A. Farewell, and T. Nystrom. 2005. ppGpp: a global regulator in Escherichia coli. Trends Microbiol. 13:236-242. [DOI] [PubMed] [Google Scholar]
- 125.Magnusson, L. U., B. Gummesson, P. Joksimovic, A. Farewell, and T. Nystrom. 2007. Identical, independent, and opposing roles of ppGpp and DksA in Escherichia coli. J. Bacteriol. 189:5193-5202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Malke, H., K. Steiner, W. M. McShan, and J. J. Ferretti. 2006. Linking the nutritional status of Streptococcus pyogenes to alteration of transcriptional gene expression: the action of CodY and RelA. Int. J. Med. Microbiol. 296:259-275. [DOI] [PubMed] [Google Scholar]
- 127.Manabe, Y. C., and W. R. Bishai. 2000. Latent Mycobacterium tuberculosis—persistence, patience, and winning by waiting. Nat. Med. 6:1327-1329. [DOI] [PubMed] [Google Scholar]
- 128.Matson, J. S., J. H. Withey, and V. J. DiRita. 2007. Regulatory networks controlling Vibrio cholerae virulence gene expression. Infect. Immun. 75:5542-5549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.McLennan, M. K., D. D. Ringoir, E. Frirdich, S. L. Svensson, D. H. Wells, H. Jarrell, C. M. Szymanski, and E. C. Gaynor. 2008. Campylobacter jejuni biofilms up-regulated in the absence of the stringent response utilize a calcofluor white-reactive polysaccharide. J. Bacteriol. 190:1097-1107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Mellies, J. L., A. M. Barron, and A. M. Carmona. 2007. Enteropathogenic and enterohemorrhagic Escherichia coli virulence gene regulation. Infect. Immun. 75:4199-4210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Merrikh, H., A. E. Ferrazzoli, and S. T. Lovett. 2009. Growth phase and (p)ppGpp control of IraD, a regulator of RpoS stability, in Escherichia coli. J. Bacteriol. 191:7436-7446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Michiels, J., M. Moris, B. Dombrecht, C. Verreth, and J. Vanderleyden. 1998. Differential regulation of Rhizobium etli rpoN2 gene expression during symbiosis and free-living growth. J. Bacteriol. 180:3620-3628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Mogull, S. A., L. J. Runyen-Janecky, M. Hong, and S. M. Payne. 2001. dksA is required for intercellular spread of Shigella flexneri via an RpoS-independent mechanism. Infect. Immun. 69:5742-5751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Molofsky, A. B., and M. S. Swanson. 2004. Differentiate to thrive: lessons from the Legionella pneumophila life cycle. Mol. Microbiol. 53:29-40. [DOI] [PubMed] [Google Scholar]
- 135.Molofsky, A. B., and M. S. Swanson. 2003. Legionella pneumophila CsrA is a pivotal repressor of transmission traits and activator of replication. Mol. Microbiol. 50:445-461. [DOI] [PubMed] [Google Scholar]
- 136.Moran, A. P., M. M. Prendergast, and E. L. Hogan. 2002. Sialosyl-galactose: a common denominator of Guillain-Barre and related disorders? J. Neurol. Sci. 196:1-7. [DOI] [PubMed] [Google Scholar]
- 137.Moris, M., K. Braeken, E. Schoeters, C. Verreth, S. Beullens, J. Vanderleyden, and J. Michiels. 2005. Effective symbiosis between Rhizobium etli and Phaseolus vulgaris requires the alarmone ppGpp. J. Bacteriol. 187:5460-5469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Mouery, K., B. A. Rader, E. C. Gaynor, and K. Guillemin. 2006. The stringent response is required for Helicobacter pylori survival of stationary phase, exposure to acid, and aerobic shock. J. Bacteriol. 188:5494-5500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Murgia, R., and M. Cinco. 2004. Induction of cystic forms by different stress conditions in Borrelia burgdorferi. APMIS 112:57-62. [DOI] [PubMed] [Google Scholar]
- 140.Na, H. S., H. J. Kim, H. C. Lee, Y. Hong, J. H. Rhee, and H. E. Choy. 2006. Immune response induced by Salmonella typhimurium defective in ppGpp synthesis. Vaccine 24:2027-2034. [DOI] [PubMed] [Google Scholar]
- 141.Nagai, H., J. C. Kagan, X. Zhu, R. A. Kahn, and C. R. Roy. 2002. A bacterial guanine nucleotide exchange factor activates ARF on Legionella phagosomes. Science 295:679-682. [DOI] [PubMed] [Google Scholar]
- 142.Nakajima, R., and R. R. Brubaker. 1993. Association between virulence of Yersinia pestis and suppression of gamma interferon and tumor necrosis factor alpha. Infect. Immun. 61:23-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Nakanishi, N., H. Abe, Y. Ogura, T. Hayashi, K. Tashiro, S. Kuhara, N. Sugimoto, and T. Tobe. 2006. ppGpp with DksA controls gene expression in the locus of enterocyte effacement (LEE) pathogenicity island of enterohaemorrhagic Escherichia coli through activation of two virulence regulatory genes. Mol. Microbiol. 61:194-205. [DOI] [PubMed] [Google Scholar]
- 144.Nanamiya, H., K. Kasai, A. Nozawa, C. S. Yun, T. Narisawa, K. Murakami, Y. Natori, F. Kawamura, and Y. Tozawa. 2008. Identification and functional analysis of novel (p)ppGpp synthetase genes in Bacillus subtilis. Mol. Microbiol. 67:291-304. [DOI] [PubMed] [Google Scholar]
- 145.Nascimento, M. M., J. A. Lemos, J. Abranches, V. K. Lin, and R. A. Burne. 2008. Role of RelA of Streptococcus mutans in global control of gene expression. J. Bacteriol. 190:28-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Nataro, J. P., and J. B. Kaper. 1998. Diarrheagenic Escherichia coli. Clin. Microbiol. Rev. 11:142-201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Nyka, W. 1967. Method for staining both acid-fast and chromophobic tubercle bacilli with carbolfuschsin. J. Bacteriol. 93:1458-1460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Nyka, W. 1974. Studies on the effect of starvation on mycobacteria. Infect. Immun. 9:843-850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Okada, Y., S. Makino, T. Tobe, N. Okada, and S. Yamazaki. 2002. Cloning of rel from Listeria monocytogenes as an osmotolerance involvement gene. Appl. Environ. Microbiol. 68:1541-1547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Overheim, K. A., R. W. Depaolo, K. L. Debord, E. M. Morrin, D. M. Anderson, N. M. Green, R. R. Brubaker, B. Jabri, and O. Schneewind. 2005. LcrV plague vaccine with altered immunomodulatory properties. Infect. Immun. 73:5152-5159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Parkhill, J., B. W. Wren, K. Mungall, J. M. Ketley, C. Churcher, D. Basham, T. Chillingworth, R. M. Davies, T. Feltwell, S. Holroyd, K. Jagels, A. V. Karlyshev, S. Moule, M. J. Pallen, C. W. Penn, M. A. Quail, M. A. Rajandream, K. M. Rutherford, A. H. van Vliet, S. Whitehead, and B. G. Barrell. 2000. The genome sequence of the food-borne pathogen Campylobacter jejuni reveals hypervariable sequences. Nature 403:665-668. [DOI] [PubMed] [Google Scholar]
- 152.Parsek, M. R., and P. K. Singh. 2003. Bacterial biofilms: an emerging link to disease pathogenesis. Annu. Rev. Microbiol. 57:677-701. [DOI] [PubMed] [Google Scholar]
- 153.Parsot, C. 2005. Shigella spp. and enteroinvasive Escherichia coli pathogenicity factors. FEMS Microbiol. Lett. 252:11-18. [DOI] [PubMed] [Google Scholar]
- 154.Pearson, J. P., M. Feldman, B. H. Iglewski, and A. Prince. 2000. Pseudomonas aeruginosa cell-to-cell signaling is required for virulence in a model of acute pulmonary infection. Infect. Immun. 68:4331-4334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Perry, R. D., and J. D. Fetherston. 1997. Yersinia pestis—etiologic agent of plague. Clin. Microbiol. Rev. 10:35-66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Persky, N. S., D. J. Ferullo, D. L. Cooper, H. R. Moore, and S. T. Lovett. 2009. The ObgE/CgtA GTPase influences the stringent response to amino acid starvation in Escherichia coli. Mol. Microbiol. 73:253-266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Persson, C., R. Nordfelth, A. Holmstrom, S. Hakansson, R. Rosqvist, and H. Wolf-Watz. 1995. Cell-surface-bound Yersinia translocate the protein tyrosine phosphatase YopH by a polarized mechanism into the target cell. Mol. Microbiol. 18:135-150. [DOI] [PubMed] [Google Scholar]
- 158.Pfluger-Grau, K., and B. Gorke. Regulatory roles of the bacterial nitrogen-related phosphotransferase system. Trends Microbiol., in press. [DOI] [PubMed]
- 159.Pizarro-Cerda, J., and K. Tedin. 2004. The bacterial signal molecule, ppGpp, regulates Salmonella virulence gene expression. Mol. Microbiol. 52:1827-1844. [DOI] [PubMed] [Google Scholar]
- 160.Potrykus, K., and M. Cashel. 2008. (p)ppGpp: still magical? Annu. Rev. Microbiol. 62:35-51. [DOI] [PubMed] [Google Scholar]
- 161.Potvin, E., F. Sanschagrin, and R. C. Levesque. 2008. Sigma factors in Pseudomonas aeruginosa. FEMS Microbiol. Rev. 32:38-55. [DOI] [PubMed] [Google Scholar]
- 162.Primm, T. P., S. J. Andersen, V. Mizrahi, D. Avarbock, H. Rubin, and C. E. Barry III. 2000. The stringent response of Mycobacterium tuberculosis is required for long-term survival. J. Bacteriol. 182:4889-4898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Rasis, M., and G. Segal. 2009. The LetA-RsmYZ-CsrA regulatory cascade, together with RpoS and PmrA, post-transcriptionally regulates stationary phase activation of Legionella pneumophila Icm/Dot effectors. Mol. Microbiol. 72:995-1010. [DOI] [PubMed] [Google Scholar]
- 164.Raskin, D. M., N. Judson, and J. J. Mekalanos. 2007. Regulation of the stringent response is the essential function of the conserved bacterial G protein CgtA in Vibrio cholerae. Proc. Natl. Acad. Sci. U. S. A. 104:4636-4641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Raupach, B., and S. H. Kaufmann. 2001. Bacterial virulence, proinflammatory cytokines and host immunity: how to choose the appropriate Salmonella vaccine strain? Microbes Infect. 3:1261-1269. [DOI] [PubMed] [Google Scholar]
- 166.Revel, A. T., A. M. Talaat, and M. V. Norgard. 2002. DNA microarray analysis of differential gene expression in Borrelia burgdorferi, the Lyme disease spirochete. Proc. Natl. Acad. Sci. U. S. A. 99:1562-1567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Roberts, D. M., M. Caimano, J. McDowell, M. Theisen, A. Holm, E. Orff, D. Nelson, S. Wikel, J. Radolf, and R. T. Marconi. 2002. Environmental regulation and differential production of members of the Bdr protein family of Borrelia burgdorferi. Infect. Immun. 70:7033-7041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Russo, T. A., A. Stapleton, S. Wenderoth, T. M. Hooton, and W. E. Stamm. 1995. Chromosomal restriction fragment length polymorphism analysis of Escherichia coli strains causing recurrent urinary tract infections in young women. J. Infect. Dis. 172:440-445. [DOI] [PubMed] [Google Scholar]
- 169.Sahr, T., H. Bruggemann, M. Jules, M. Lomma, C. Albert-Weissenberger, C. Cazalet, and C. Buchrieser. 2009. Two small ncRNAs jointly govern virulence and transmission in Legionella pneumophila. Mol. Microbiol. 72:741-762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Santic, M., M. Molmeret, K. E. Klose, and Y. Abu Kwaik. 2006. Francisella tularensis travels a novel, twisted road within macrophages. Trends Microbiol. 14:37-44. [DOI] [PubMed] [Google Scholar]
- 171.Saunders, B. M., and W. J. Britton. 2007. Life and death in the granuloma: immunopathology of tuberculosis. Immunol. Cell Biol. 85:103-111. [DOI] [PubMed] [Google Scholar]
- 172.Schleheck, D., N. Barraud, J. Klebensberger, J. S. Webb, D. McDougald, S. A. Rice, and S. Kjelleberg. 2009. Pseudomonas aeruginosa PAO1 preferentially grows as aggregates in liquid batch cultures and disperses upon starvation. PLoS One 4:e5513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Scoarughi, G. L., C. Cimmino, and P. Donini. 1999. Helicobacter pylori: a eubacterium lacking the stringent response. J. Bacteriol. 181:552-555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Sebbane, F., C. O. Jarrett, D. Gardner, D. Long, and B. J. Hinnebusch. 2006. Role of the Yersinia pestis plasminogen activator in the incidence of distinct septicemic and bubonic forms of flea-borne plague. Proc. Natl. Acad. Sci. U. S. A. 103:5526-5530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Sharma, A. K., and S. M. Payne. 2006. Induction of expression of hfq by DksA is essential for Shigella flexneri virulence. Mol. Microbiol. 62:469-479. [DOI] [PubMed] [Google Scholar]
- 176.Shi, Y., T. Latifi, M. J. Cromie, and E. A. Groisman. 2004. Transcriptional control of the antimicrobial peptide resistance ugtL gene by the Salmonella PhoP and SlyA regulatory proteins. J. Biol. Chem. 279:38618-38625. [DOI] [PubMed] [Google Scholar]
- 177.Shivaprasad, H. L. 2000. Fowl typhoid and pullorum disease. Rev. Sci. Tech. 19:405-424. [DOI] [PubMed] [Google Scholar]
- 178.Shrout, J. D., D. L. Chopp, C. L. Just, M. Hentzer, M. Givskov, and M. R. Parsek. 2006. The impact of quorum sensing and swarming motility on Pseudomonas aeruginosa biofilm formation is nutritionally conditional. Mol. Microbiol. 62:1264-1277. [DOI] [PubMed] [Google Scholar]
- 179.Silva, A. J., and J. A. Benitez. 2006. A Vibrio cholerae relaxed (relA) mutant expresses major virulence factors, exhibits biofilm formation and motility, and colonizes the suckling mouse intestine. J. Bacteriol. 188:794-800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Silva, A. J., and J. A. Benitez. 2004. Transcriptional regulation of Vibrio cholerae hemagglutinin/protease by the cyclic AMP receptor protein and RpoS. J. Bacteriol. 186:6374-6382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Sonenshein, A. L. 2005. CodY, a global regulator of stationary phase and virulence in Gram-positive bacteria. Curr. Opin. Microbiol. 8:203-207. [DOI] [PubMed] [Google Scholar]
- 182.Song, M., H. J. Kim, E. Y. Kim, M. Shin, H. C. Lee, Y. Hong, J. H. Rhee, H. Yoon, S. Ryu, S. Lim, and H. E. Choy. 2004. ppGpp-dependent stationary phase induction of genes on Salmonella pathogenicity island 1. J. Biol. Chem. 279:34183-34190. [DOI] [PubMed] [Google Scholar]
- 183.Sory, M. P., and G. R. Cornelis. 1994. Translocation of a hybrid YopE-adenylate cyclase from Yersinia enterocolitica into HeLa cells. Mol. Microbiol. 14:583-594. [DOI] [PubMed] [Google Scholar]
- 184.Srivatsan, A., and J. D. Wang. 2008. Control of bacterial transcription, translation and replication by (p)ppGpp. Curr. Opin. Microbiol. 11:100-105. [DOI] [PubMed] [Google Scholar]
- 185.Stallings, C. L., N. C. Stephanou, L. Chu, A. Hochschild, B. E. Nickels, and M. S. Glickman. 2009. CarD is an essential regulator of rRNA transcription required for Mycobacterium tuberculosis persistence. Cell 138:146-159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Steiner, K., and H. Malke. 2000. Life in protein-rich environments: the relA-independent response of Streptococcus pyogenes to amino acid starvation. Mol. Microbiol. 38:1004-1016. [DOI] [PubMed] [Google Scholar]
- 187.Steiner, K., and H. Malke. 2001. relA-independent amino acid starvation response network of Streptococcus pyogenes. J. Bacteriol. 183:7354-7364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Stintzi, A., D. Marlow, K. Palyada, H. Naikare, R. Panciera, L. Whitworth, and C. Clarke. 2005. Use of genome-wide expression profiling and mutagenesis to study the intestinal lifestyle of Campylobacter jejuni. Infect. Immun. 73:1797-1810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Sturgill-Koszycki, S., and M. S. Swanson. 2000. Legionella pneumophila replication vacuoles mature into acidic, endocytic organelles. J. Exp. Med. 192:1261-1272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Sun, W., K. L. Roland, C. G. Branger, X. Kuang, and R. Curtiss III. 2009. The role of relA and spoT in Yersinia pestis KIM5 pathogenicity. PLoS One 4:e6720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Suomalainen, M., J. Haiko, P. Ramu, L. Lobo, M. Kukkonen, B. Westerlund-Wikstrom, R. Virkola, K. Lahteenmaki, and T. K. Korhonen. 2007. Using every trick in the book: the Pla surface protease of Yersinia pestis. Adv. Exp. Med. Biol. 603:268-278. [DOI] [PubMed] [Google Scholar]
- 192.Sureka, K., S. Dey, P. Datta, A. K. Singh, A. Dasgupta, S. Rodrigue, J. Basu, and M. Kundu. 2007. Polyphosphate kinase is involved in stress-induced mprAB-sigE-rel signalling in mycobacteria. Mol. Microbiol. 65:261-276. [DOI] [PubMed] [Google Scholar]
- 193.Svensson, S. L., E. Frirdich, and E. C. Gaynor. 2008. Survival strategies of Campylobacter jejuni: stress responses, the viable but nonculturable state, and biofilms, p. 571-590. In I. Nachamkin, C. M. Szymanski, and M. J. Blaser (ed.), Campylobacter, 3rd ed. ASM Press, Washington, DC.
- 194.Szalewska-Palasz, A., L. U. Johansson, L. M. Bernardo, E. Skarfstad, E. Stec, K. Brannstrom, and V. Shingler. 2007. Properties of RNA polymerase bypass mutants: implications for the role of ppGpp and its co-factor DksA in controlling transcription dependent on sigma54. J. Biol. Chem. 282:18046-18056. [DOI] [PubMed] [Google Scholar]
- 195.Tan, S., C. D. Fraley, M. Zhang, D. Dailidiene, A. Kornberg, and D. E. Berg. 2005. Diverse phenotypes resulting from polyphosphate kinase gene (ppk1) inactivation in different strains of Helicobacter pylori. J. Bacteriol. 187:7687-7695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Taylor, C. M., M. Beresford, H. A. S. Epton, D. C. Sigee, G. Shama, P. W. Andrew, and I. S. Roberts. 2002. Listeria monocytogenes relA and hpt mutants are impaired in surface-attached growth and virulence. J. Bacteriol. 184:621-628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Thompson, A., M. D. Rolfe, S. Lucchini, P. Schwerk, J. C. Hinton, and K. Tedin. 2006. The bacterial signal molecule, ppGpp, mediates the environmental regulation of both the invasion and intracellular virulence gene programs of Salmonella. J. Biol. Chem. 281:30112-30121. [DOI] [PubMed] [Google Scholar]
- 198.Thompson, L. J., D. S. Merrell, B. A. Neilan, H. Mitchell, A. Lee, and S. Falkow. 2003. Gene expression profiling of Helicobacter pylori reveals a growth-phase-dependent switch in virulence gene expression. Infect. Immun. 71:2643-2655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Tiaden, A., T. Spirig, S. S. Weber, H. Bruggemann, R. Bosshard, C. Buchrieser, and H. Hilbi. 2007. The Legionella pneumophila response regulator LqsR promotes host cell interactions as an element of the virulence regulatory network controlled by RpoS and LetA. Cell. Microbiol. 9:2903-2920. [DOI] [PubMed] [Google Scholar]
- 200.Turner, A. K., M. A. Lovell, S. D. Hulme, L. Zhang-Barber, and P. A. Barrow. 1998. Identification of Salmonella typhimurium genes required for colonization of the chicken alimentary tract and for virulence in newly hatched chicks. Infect. Immun. 66:2099-2106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Vallance, B. A., and B. B. Finlay. 2000. Exploitation of host cells by enteropathogenic Escherichia coli. Proc. Natl. Acad. Sci. U. S. A. 97:8799-8806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.van Delden, C., R. Comte, and A. M. Bally. 2001. Stringent response activates quorum sensing and modulates cell density-dependent gene expression in Pseudomonas aeruginosa. J. Bacteriol. 183:5376-5384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Van Delden, C., and B. H. Iglewski. 1998. Cell-to-cell signaling and Pseudomonas aeruginosa infections. Emerg. Infect. Dis. 4:551-560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.van Schaik, W., J. Prigent, and A. Fouet. 2007. The stringent response of Bacillus anthracis contributes to sporulation but not to virulence. Microbiology 153:4234-4239. [DOI] [PubMed] [Google Scholar]
- 205.Viducic, D., T. Ono, K. Murakami, H. Susilowati, S. Kayama, K. Hirota, and Y. Miyake. 2006. Functional analysis of spoT, relA and dksA genes on quinolone tolerance in Pseudomonas aeruginosa under nongrowing condition. Microbiol. Immunol. 50:349-357. [DOI] [PubMed] [Google Scholar]
- 206.Wang, C., H. B. Zhang, L. H. Wang, and L. H. Zhang. 2006. Succinic semialdehyde couples stress response to quorum-sensing signal decay in Agrobacterium tumefaciens. Mol. Microbiol. 62:45-56. [DOI] [PubMed] [Google Scholar]
- 207.Wang, J., N. Gardiol, T. Burr, G. P. Salmond, and M. Welch. 2007. RelA-dependent (p)ppGpp production controls exoenzyme synthesis in Erwinia carotovora subsp. atroseptica. J. Bacteriol. 189:7643-7652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Wang, J. D., G. M. Sanders, and A. D. Grossman. 2007. Nutritional control of elongation of DNA replication by (p)ppGpp. Cell 128:865-875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Watson, R. O., and J. E. Galan. 2008. Campylobacter jejuni survives within epithelial cells by avoiding delivery to lysosomes. PLoS Pathog. 4:e14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Webb, C., M. Moreno, M. Wilmes-Riesenberg, R. Curtiss III, and J. W. Foster. 1999. Effects of DksA and ClpP protease on sigma S production and virulence in Salmonella typhimurium. Mol. Microbiol. 34:112-123. [DOI] [PubMed] [Google Scholar]
- 211.Wei, W., J. Jiang, X. Li, L. Wang, and S. S. Yang. 2004. Isolation of salt-sensitive mutants from Sinorhizobium meliloti and characterization of genes involved in salt tolerance. Lett. Appl. Microbiol. 39:278-283. [DOI] [PubMed] [Google Scholar]
- 212.Wells, D. H., and E. C. Gaynor. 2006. Helicobacter pylori initiates the stringent response upon nutrient and pH downshift. J. Bacteriol. 188:3726-3729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Wells, D. H., and S. R. Long. 2003. Mutations in rpoBC suppress the defects of a Sinorhizobium meliloti relA mutant. J. Bacteriol. 185:5602-5610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Wells, D. H., and S. R. Long. 2002. The Sinorhizobium meliloti stringent response affects multiple aspects of symbiosis. Mol. Microbiol. 43:1115-1127. [DOI] [PubMed] [Google Scholar]
- 215.Wout, P., K. Pu, S. M. Sullivan, V. Reese, S. Zhou, B. Lin, and J. R. Maddock. 2004. The Escherichia coli GTPase CgtAE cofractionates with the 50S ribosomal subunit and interacts with SpoT, a ppGpp synthetase/hydrolase. J. Bacteriol. 186:5249-5257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Wright, J. A., A. J. Grant, D. Hurd, M. Harrison, E. J. Guccione, D. J. Kelly, and D. J. Maskell. 2009. Metabolite and transcriptome analysis of Campylobacter jejuni in vitro growth reveals a stationary-phase physiological switch. Microbiology 155:80-94. [DOI] [PubMed] [Google Scholar]
- 217.Wright, K. J., P. C. Seed, and S. J. Hultgren. 2007. Development of intracellular bacterial communities of uropathogenic Escherichia coli depends on type 1 pili. Cell. Microbiol. 9:2230-2241. [DOI] [PubMed] [Google Scholar]
- 218.Yan, X., C. Zhao, A. Budin-Verneuil, A. Hartke, A. Rince, M. S. Gilmore, Y. Auffray, and V. Pichereau. 2009. The (p)ppGpp synthetase RelA contributes to stress adaptation and virulence in Enterococcus faecalis V583. Microbiology 155:3226-3237. [DOI] [PubMed] [Google Scholar]
- 219.Young, K. T., L. M. Davis, and V. J. DiRita. 2007. Campylobacter jejuni: molecular biology and pathogenesis. Nat. Rev. Microbiol. 5:665-679. [DOI] [PubMed] [Google Scholar]
- 220.Yun, J., B. Jeon, Y.-W. Barton, P. Plummer, Q. Zhang, and S. Ryu. 2008. Role of the DksA-like protein in the pathogenesis and diverse metabolic activity of Campylobacter jejuni. J. Bacteriol. 190:4512-4520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Zhang, H. B., C. Wang, and L. H. Zhang. 2004. The quormone degradation system of Agrobacterium tumefaciens is regulated by starvation signal and stress alarmone (p)ppGpp. Mol. Microbiol. 52:1389-1401. [DOI] [PubMed] [Google Scholar]
- 222.Zhang, S., and W. G. Haldenwang. 2003. RelA is a component of the nutritional stress activation pathway of the Bacillus subtilis transcription factor sigma B. J. Bacteriol. 185:5714-5721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Zhao, G., N. Weatherspoon, W. Kong, R. Curtiss III, and Y. Shi. 2008. A dual-signal regulatory circuit activates transcription of a set of divergent operons in Salmonella typhimurium. Proc. Natl. Acad. Sci. U. S. A. 105:20924-20929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Zhou, Y. N., W. G. Coleman, Jr., Z. Yang, Y. Yang, N. Hodgson, F. Chen, and D. J. Jin. 2008. Regulation of cell growth during serum starvation and bacterial survival in macrophages by the bifunctional enzyme SpoT in Helicobacter pylori. J. Bacteriol. 190:8025-8032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Zusman, T., O. Gal-Mor, and G. Segal. 2002. Characterization of a Legionella pneumophila relA insertion mutant and roles of RelA and RpoS in virulence gene expression. J. Bacteriol. 184:67-75. [DOI] [PMC free article] [PubMed] [Google Scholar]