Abstract
A multivalent vaccine candidate against hepatitis B virus (HBV) and hepatitis C virus (HCV) infections was constructed on the basis of HBV core (HBc) virus-like particles (VLPs) as carriers. Chimeric VLPs that carried a virus-neutralizing HBV pre-S1 epitope corresponding to amino acids (aa) 20 to 47 in the major immunodominant region (MIR) and a highly conserved N-terminal HCV core epitope corresponding to aa 1 to 60 at the C terminus of the truncated HBcΔ protein (N-terminal aa 1 to 144 of full-length HBc) were produced in Escherichia coli cells and examined for their antigenicity and immunogenicity. The presence of two different foreign epitopes within the HBc molecule did not interfere with its VLP-forming ability, with the HBV pre-S1 epitope exposed on the surface and the HCV core epitope buried within the VLPs. After immunization of BALB/c mice, specific T-cell activation by both foreign epitopes and a high-titer antibody response against the pre-S1 epitope were found, whereas an antibody response against the HBc carrier was notably suppressed. Both inserted epitopes also induced a specific cytotoxic-T-lymphocyte (CTL) response, as shown by the gamma interferon (IFN-γ) production profile.
Genetically engineered virus-like particle (VLP)-based vaccines are one of the most promising tools in modern vaccinology. VLPs from almost all classes of viruses are being evaluated now or have just been adopted to use as carriers for presentation of foreign immunological epitopes (for a review, see references 29 and 31). VLP technologies possess obvious advantages for the generation of safe and efficacious vaccines. First, the repetitive antigenic structure of VLPs makes them highly immunogenic. Second, VLPs lack viral genomes or genes and are noninfectious, although they mimic infectious viruses in their structural and immunological features. Third, VLPs are generated by highly efficient heterologous expression of the cloned viral structural genes with subsequent quantitative in vivo or in vitro self-assembly of their products. Fourth, VLPs can be obtained by simple and efficient purification procedures. VLPs can be used for a broad range of applications, but the generation of vaccines against hepatitis B virus (HBV) and hepatitis C virus (HCV) infections is of special interest.
The HBV core (HBc) protein was first reported as a promising VLP carrier in 1986 and was published in 1987 (6, 10, 24). In many ways, HBc occupies a unique position among the VLP carriers because of its high-level synthesis and efficient self-assembly in virtually all known homologous and heterologous expression systems, including bacteria (for a review, see references 29 to 31). The major HBc B-cell epitopes (c and e1) are localized within the major immunodominant region (MIR), whereas the next important epitope, e2, is localized around amino acid position 130, close to the C-terminal histone-like region (for a review, see reference 30).
The high-resolution spatial structure of HBc icosahedrons (11, 43) shows that the MIR is located on the tip of the spike, around the most protruding region between amino acids (aa) 78 and 82. For this reason, the MIR is generally accepted as the target site of choice for insertion of foreign epitopes (30). The other widely accepted site for insertions is C-terminal position 144, a short stretch after the e2 epitope. For C-terminal insertions, so-called HBcΔ vectors lacking a 39-aa-long positively charged C-terminal histone-like fragment are preferred for their high insertion capacities (up to 741 amino acid residues) (30).
Here, we present the construction and preliminary immunological characterization of a first multivalent HBV and HCV vaccine candidate. As an HBV epitope, we chose the pre-S1 sequence aa 20 to 47, which alone is able to elicit HBV-neutralizing and protective antibodies (23), for insertion into the HBc MIR. Concurrently, we inserted at the C terminus of the HBcΔ vector the N-terminal 60-aa fragment of the HCV core, which is highly conserved among various HCV genotypes with amino acid homology exceeding 95% (12, 14) and therefore is an attractive target for the generation of an HCV vaccine (19, 41). Such a combination of foreign epitopes did not prevent correct self-assembly of chimeric HBc-based particles and provided them with specific HBV and HCV antigenicity and immunogenicity in mice.
MATERIALS AND METHODS
Construction of recombinant plasmids.
Escherichia coli strains RR1 [F− rB− mB− leuB6 proA2 thi-1 araC14 lacY1 galK2 xyl-5 mtl-1 rpsL20 (Strr) glnV44 Δ(mcrC-mrr)] and DH5α [λ− φ80dlacZΔM15 Δ(lacZYA-argF)U169 recA1 endA1 hsdR17(rK− mK−) supE44 thi-1 gyrA relA1] were used for cloning and selection of recombinant plasmids; K802 (F− rK− mK+e14 McrA metB1 lacY1 [or lacI-Y6] galK2 galT22 glnV44 mcrB) and BL21 [F− ompT hsdSB(rB− mB−) gal dcm] were used for expression of recombinant HBc-derived genes.
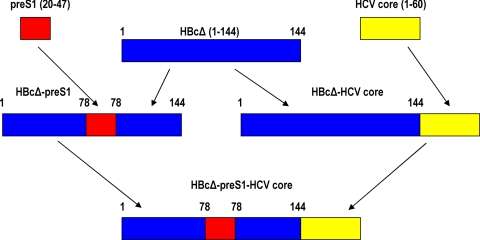
Figure 1 summarizes the construction of all plasmids used in this work. The HBcΔ-pre-S1 plasmid contains a DNA fragment, which encodes pre-S1 aa residues 20 to 47 within the MIR and was constructed by amplification of a pre-S1(20-47) fragment from the pHB320 plasmid (28), using as primers oligonucleotides 5′-AATCCTCTGGGATTCTTTCC-3′ and 5′-GGGATTGAAGTCCCAATCTGG-3′; the fragment obtained was ligated into the Eco72I- and Eco105I-cleaved p2-19 vector (5). The HBcΔ-HCV core was obtained analogously as HBcΔ-HCVcore (aa 1-98 [referred to hereafter as 1-98]) described previously (21). Plasmids HBcΔ-pre-S1 and HBcΔ-HCVcore were cleaved with XbaI and Kpn21I (Fermentas, Lithuania), and after ligation, transformation, analytical restriction, and sequencing, plasmid HBcΔ-pre-S1-HCVcore was obtained. All HBcΔ-derived genes were expressed under the control of the E. coli Trp promoter, which allowed a high expression level without induction. The construction of recombinant HCV core antigen (His-tagged protein 1-98) and its purification using Ni-nitrilotriacetic acid (NTA) resin was described previously (22). The purity of the HCV core (1-98) protein according to Coomassie blue staining of the SDS-PAGE gel was 95%.
FIG. 1.
Schematic representation of chimeric HBcΔ-derived protein-encoding genes constructed in the current work.
Monoclonal and polyclonal antibodies.
The monoclonal mouse antibodies anti-pre-S1 MA18/7 (37), anti-HBc 13C9 and C1-5 (3), and anti-HCV core HCM-071-5 (Austral Biologicals, San Ramon, CA), as well as the rabbit polyclonal anti-HCV core 34-7 antibody (1), were used in this work.
Cultivation and purification of HBcΔ, HBcΔ-pre-S1, HBcΔ-HCV core, and HBcΔ-preS1-HCV core VLPs.
Cultivation of bacteria and purification of HBcΔ-derived proteins were based generally on the previously described method (11).
Transformed E. coli K802 cells were grown overnight on a shaker at 37°C in 750-ml flasks containing 300 ml of M9 minimal medium supplemented with 1% Casamino Acids (Difco) and 0.2% glucose with a final optical density at 540 nm (OD540) of 2 to 5. The cells were pelleted and lysed by 30 min of incubation on ice in lysis buffer containing 50 mM Tris-HCl (pH 8.0), 5 mM EDTA, 50 μg/ml phenylmethylsulfonyl fluoride (PMSF), and 0.5 M urea and then ultrasonicated 5 times for 15 s each time at 22 kHz. After clarification, the proteins were precipitated with ammonium sulfate at 33% saturation overnight at 4°C. The pellets were resuspended in a phosphate-buffered saline (PBS) buffer containing 0.1% Triton X-100, PMSF, and 0.9 M urea, and 5 ml of the solution was loaded on a Sepharose CL-4B column (2.5 by 85 cm) and eluted with PBS buffer without Triton X-100. The presence of HBc-containing material in fractions was tested by SDS-PAGE. Positive fractions were pooled and concentrated by ammonium sulfate precipitation at 33% saturation for 20 h at 4°C. The pellets were resuspended in 0.9 M urea in PBS to a final concentration of about 5 to 20 mg/ml, dialyzed overnight against 2,000 volumes of the same buffer, and stored at −70°C or at −20°C in 50% glycerol.
The purified proteins were analyzed by Western blotting with mouse monoclonal anti-HBc 13C9 antibodies, mouse monoclonal anti-pre-S1 MA18/7 antibodies, and polyclonal rabbit anti-HCV core 34-7 antibodies, as well as by double radial immunodiffusion using polyclonal rabbit anti-HBc antibodies.
The stability of VLPs was determined by evaluation of fluorescence-based thermal shift in accordance with the previously described methodology (20). The melting points of VLPs in starting buffer and in the presence of 0.5 M NaCl and 20 mM dithiothreitol (DTT) were compared.
Electron microscopy.
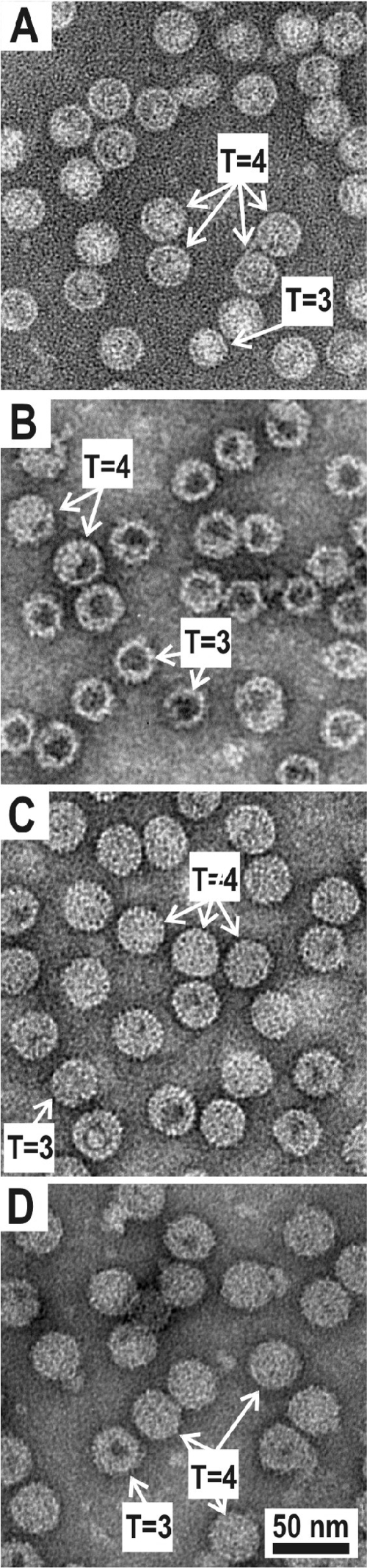
VLPs in suspension were adsorbed to carbon-Formvar-coated copper grids and negatively stained with 1% uranyl acetate aqueous solution. The grids were examined with a JEM-1230 electron microscope (Jeol Ltd., Tokyo, Japan) at 100 kV. The ratio of T=3 and T=4 particles was calculated on different independent electron microscopy images on the basis of diameter measurement.
Mice and immunization schemes.
Female BALB/c (H-2d) mice, 6 to 8 weeks of age and weighing 18 to 20 g, were obtained from the Animal Breeding Centre of the Institute of Virology, Riga, Latvia, and held at the Latvian Biomedical Research and Study Centre. Five mice in each group were immunized subcutaneously with 25 μg of the appropriate protein, HBcΔ, HBcΔ-pre-S1, HBcΔ-HCV core, or HBcΔ-pre-S1-HCV core, in 200 μl of sterile PBS. No adjuvants were used for immunizations. On day 14 after the first immunization, the second 25-μg boost was performed. For monitoring of the humoral response, immune serum was collected 14 days after the first and second immunizations. For T-cell proliferation tests, mice were sacrificed and spleens were obtained at day 28 after the first immunization. The ethics of the mouse immunization experiments for the Latvian Biomedical Research and Study Centre (registration number 025413) were approved in compliance with the Latvian Animal Protection Law (certificate no. 4 from the Food and Veterinary Service, 8 January 2009) on the basis of the decision of the Council for Ethical Treatment of Animals in Latvia.
ELISAs.
Direct and competitive enzyme-linked immunosorbent assays (ELISAs) were performed, in general, as described in reference 36. Recombinant HBc, HCV core (1-98), and pre-S1 peptide aa 20 to 47 were adsorbed overnight at 4°C to 96-well plates (Nunc) at 10 μg/ml in 50 mM sodium carbonate buffer, pH 9.6. After being blocked with PBS containing 1% bovine serum albumin (BSA) for 1 h at 37°C, serial dilutions of mouse sera were added to the plates and incubated for an additional 1 h at 37°C. After being washed 3 times with PBS containing 0.05% Tween 20, an anti-mouse IgG horseradish peroxidase-conjugated antibody (Sigma) was applied at a 1:1,000 dilution. Following 1 h of incubation at 37°C, the plates were washed, and substrate OPD (Sigma) was added for color development. The results were checked in an automatic reader (Multiscan, Sweden) at 492 nm. The endpoint titers were defined as the highest serum dilution that resulted in an absorbance value three times greater than that of negative-control sera derived from nonimmunized mice.
Detection of IgG isotypes.
Detection of total IgG1 and IgG2a from sera of immunized mice was performed using a murine-antibody-isotyping ELISA kit (Sigma).
T-cell proliferation assay.
Murine splenocytes were harvested using red blood cell lysing buffer (Sigma). Single-cell suspensions were prepared (5 × 106 cells/ml) and cocultured in RPMI 1640 (Gibco, Germany) with the appropriate recombinant protein or pre-S1(20-47) peptide with three consecutive 10× dilutions of carrier VLPs or peptide starting from 10 μg/ml. Concanavalin A was used as a positive control at 4 μg/ml. The plates were then incubated for 72 h before the addition of 1 μCi of [3H]thymidine per well (TdR; Amersham). The cells were harvested after 18 h onto cellulose filters, and the radioactivity was measured on a beta counter (Beckman). The results were presented as stimulation indexes (SI), which were calculated as a ratio of the mean cpm values obtained in the presence and absence of a stimulator.
Cytokine test.
For detection of interleukin 2 (IL-2), gamma interferon (IFN-γ), and IL-10 cytokines in cell cultures, the supernatants from the wells were removed at 24 h and at 48 h for IL-2, IL-10, and IFN-γ measurements after the T-cell proliferation tests were started. Analysis for the presence of cytokines in cell supernatants was performed using the commercial tests OptEIA Mouse IFN-γ, IL-2, and IL-10 ELISA Sets from BD Bioscience Pharmingen, according to the manufacturers' instructions.
RESULTS
Structure and self-assembly of VLPs carrying the pre-S1(20-47) and HCV core (1-60) epitopes.
The HBcΔ-pre-S1 fusion protein was constructed by insertion of the pre-S1(20-47) fragment between positions 78 and 79 of the HBcΔ protein with the intention of exposing the pre-S1 fragment on the surface of the HBc particle. The HBcΔ-HCV core fusion protein was constructed by the addition of HCV core fragment (1-60) downstream of amino acid residue 144 to displace the arginine-rich histone-like C terminus of the HBc molecule. The HBcΔ-pre-S1-HCV core fusion protein is HBcΔ with both pre-S1(20-47) and core (1-60) fragments inserted as described above in HBcΔ-pre-S1 and HBcΔ-HCV core constructs, respectively (Fig. 1).
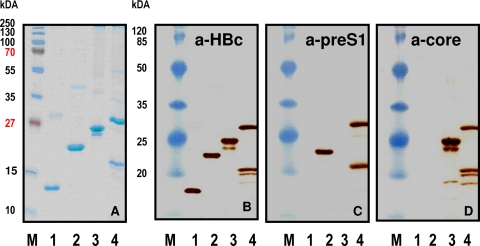
Chimeric HBcΔ-pre-S1, HBcΔ-HCV core, and HBcΔ-pre-S1-HCV core proteins were purified and evaluated by Western blot analysis with mouse monoclonal anti-HBc and anti-pre-S1 antibodies and polyclonal anti-HCV core antibodies (Fig. 2), as well as by electron microscopy with negative staining (Fig. 3). The expression level of chimeric proteins was around 5% of the total intracellular protein. The presence of VLP-like structures was detected by double radial immunodiffusion using polyclonal rabbit anti-HBc antibodies. Chimeric proteins were precipitated by anti-HBc antibodies with the same efficiency as nonchimeric HBcΔ VLPs, suggesting efficient formation of correctly folded VLPs (data not shown). The morphology of particles in the chimeric VLP preparations did not differ significantly from that of the particles in the nonchimeric HBcΔ VLP preparation (Fig. 3). The HBcΔ, HBcΔ-HCV core, and HBcΔ-pre-S1-HCV core preparations demonstrated similar levels of the T=3 particles: 23, 20, and 16%, respectively. It is surprising that HBcΔ-pre-S1 showed a much higher content of T=3 particles (35%).
FIG. 2.
SDS-PAGE analysis of purified HBcΔ-derived proteins. (A) Coomassie blue-stained gel. (B to D) Western blotting with mouse monoclonal anti-HBc (a-HBc) antibody (B), mouse monoclonal anti-pre-S1 antibody (C), and rabbit polyclonal anti-HCV core antibody (D). Lanes: 1, HBcΔ; 2, HBcΔ-pre-S1; 3, HBcΔ-HCV core; 4, HBcΔ-pre-S1-HCV core. Lanes M, Page Ruler Plus Prestained Protein Ladder and Prestained Protein Molecular Mass Marker (both from Fermentas, Lithuania) were used as molecular mass markers on the stained gel and on Western blots, respectively.
FIG. 3.
Electron microscopy of purified HBcΔ-derived VLPs. (A) HBcΔ without insertions. (B) HBcΔ-pre-S1. (C) HBcΔ-HCV core. (D) HBcΔ-pre-S1-HCV core. Typical examples of T=3 and T=4 particles are indicated by arrows. Scale bar, 50 nm.
The stability of VLPs was determined by comparing the melting points of VLPs in starting buffer and in the presence of 0.5 M NaCl and 20 mM DTT (not shown). Whereas HBcΔ, HBcΔ-pre-S1, and HBcΔ-HCV core demonstrated similar melting points (77 to 80°C), double-inserted HBcΔ-pre-S1-HCV core VLPs were a bit less stable, with a melting point of 67 to 69°C. For all VLP variants, the melting points did not depend on the presence of 0.5 M NaCl and 20 mM DTT.
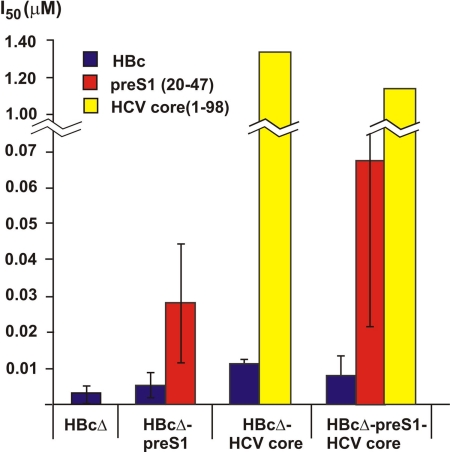
Surface exposure of foreign sequences on VLPs was checked by competitive ELISA (Fig. 4), which showed that the pre-S1(20-47) epitope was well exposed but the HCV core (1-60) epitope was buried within the particles.
FIG. 4.
Exposition of inserted epitopes on the surfaces of chimeric HBcΔ-derived VLPs detected by competitive ELISA. Molar amounts of HBcΔ, HBcΔ-pre-S1, HBcΔ-HCV core, and HBcΔ-pre-S1-HCV core proteins, which are necessary and sufficient for 50% inhibition (I50) of anti-HBc, anti-pre-S1, and anti-HCV core antibody binding to solid phase coated with the appropriate substrates. The error bars indicate standard deviations.
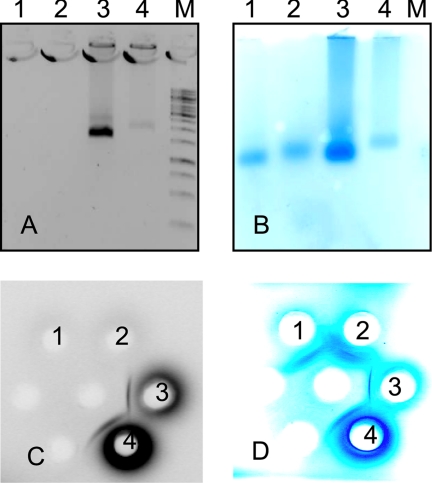
The presence of nucleic acids within chimeric VLPs was determined by native agarose gel electrophoresis of VLPs (Fig. 5). Nucleic acids were present in the HBcΔ-HCV core and HBcΔ-pre-S1-HCV core preparations, but not in the HBcΔ-pre-S1 and nonchimeric HBcΔ preparations.
FIG. 5.
Abilities of chimeric HBcΔ-derived VLPs to package nucleic acids. Native 1% agarose electrophoresis gels in Tris-acetate-EDTA (TAE) buffer (A and B) and Ouchterlony's double radial immunodiffusion (C and D) of purified HBcΔ-derived VLPs stained by ethidium bromide (EtBr) (A and C) and Coomassie blue (B and D). Lanes and spots: 1, HBcΔ; 2, HBcΔ-pre-S1; 3, HBcΔ-HCV core; 4, HBcΔ-pre-S1-HCV core; M, 1-kb ladder (Fermentas, Lithuania).
Humoral response in mice.
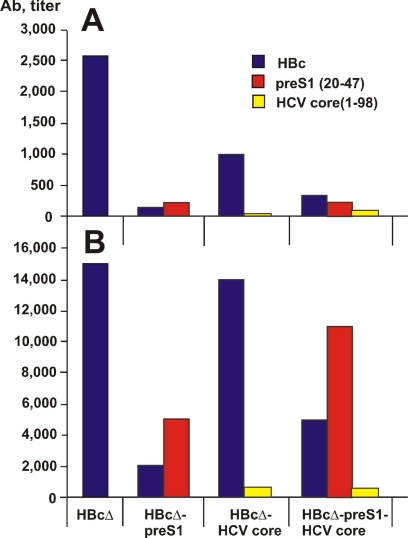
Animals were bled on days 14 and 28 after immunization. The boost on day 14 led to a significant increase of specific antibody response (compare Fig. 6 A and B). Control groups were immunized with the initial HBcΔ without any insertions, which induced anti-HBc antibody response at a titer of 1:15,000.
FIG. 6.
Induction of antibodies (Ab) in mice by chimeric HBcΔ-derived VLPs. Shown are antibody titers in pools of sera from five animals at day 14 (A) and at day 28 (B) after the first immunization. Titers in specimens were determined on plates coated with HBc, pre-S1 peptide (20-47), and HCV core protein (1-98) as shown by different colors.
Insertion of the pre-S1 epitope into the MIR led to a substantial decrease of anti-HBc response (HBcΔ-pre-S1 and HBcΔ-pre-S1-HCV core), whereas C-terminal addition of the HCV core in the HBcΔ-HCV core construct did not affect the anti-HBc response. Surprisingly, the multivalent HBcΔ-pre-S1-HCV core VLPs induced a higher anti-pre-S1 antibody response than HBcΔ-pre-S1 with a single epitope insertion. The humoral anti-HCV core response remained low in both HBcΔ-HCV core and HBcΔ-pre-S1-HCV core VLP immunizations (Fig. 6).
Isotyping of IgG antibodies.
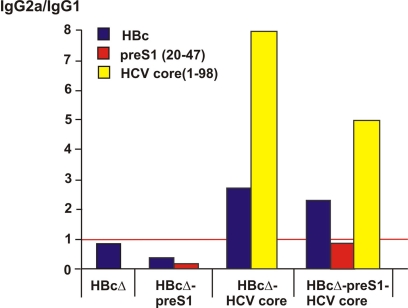
We analyzed specific isotype profiles of observed antibodies of vaccinated mice. Chimeric VLPs containing HCV core induced a predominantly IgG2a antibody response against the HBc carrier, whereas HBcΔ and HBcΔ-pre-S1 induced a predominantly IgG1 antibody response (Fig. 7). In contrast, the antibody response against pre-S1 remained predominantly IgG1. Levels of anti-HCV antibodies were too low to confirm the apparent predominance of IgG2a. These observations were further confirmed by the results of the T-cell proliferation assay, in which all immunized groups of animals showed high T-cell proliferation.
FIG. 7.
IgG isotype distribution in serum pools after immunization of mice by chimeric HBcΔ-derived VLPs. The red line depicts the equivalent distribution of IgG2a and IgG1 isotypes.
T-cell proliferative responses and cytokine profile.
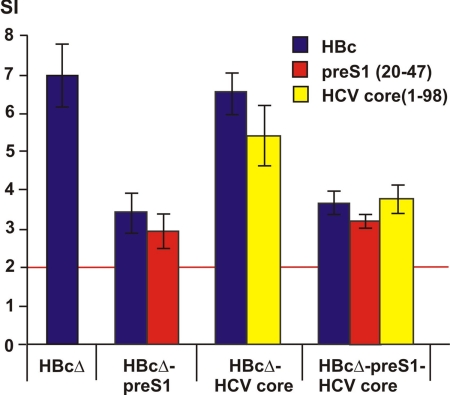
Lymphocytes of mice immunized with all studied VLPs demonstrated detectable proliferation in vitro by HBc carriers, with the highest level for HBcΔ-pre-S1-HCV core induced by recombinant HBc at day 28 after the first immunization (Fig. 8). Proliferation in vitro with the pre-S1 peptide (20-47) resulted in SI levels similar to the appropriate SI levels for proliferation with HBc. Proliferation in vitro with HCV core protein (1-98) demonstrated the highest SI levels in the case of the HBcΔ-HCV core.
FIG. 8.
T-cell proliferation after immunization of mice by chimeric HBcΔ-derived VLPs. SI were measured in response to stimulation of T cells with HBc, pre-S1 peptide (20-47), and HCV core protein (1-98). The red line depicts the basal level of negative stimulation. The error bars indicate standard deviations.
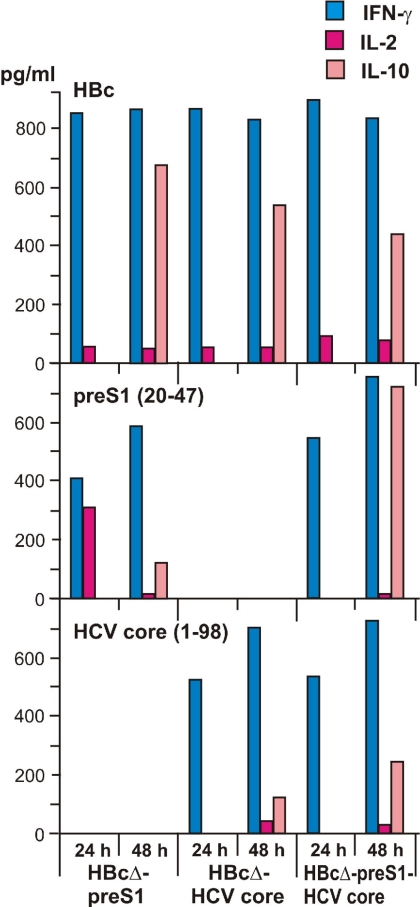
Figure 9 shows direct measurements of the Th1 cell-derived cytokines IL-2, IFN-γ, and IL-10 after appropriate stimulation. In general, these data follow the proliferation response data. The highest production of IFN-γ was detected for all VLP variants after stimulation with HBc. Lower but highly specific stimulation of IFN-γ production was achieved after stimulation with the pre-S1 peptide (20-47) or HCV core protein (1-98).
FIG. 9.
Cytokines produced by T cells after immunization of mice with chimeric HBcΔ-derived VLPs. Cell supernatants were removed and analyzed at 24 h and 48 h for IFN-γ, IL-2, and IL-10 production after stimulation with HBc, pre-S1 peptide (20-47), and HCV core protein (1-98).
Production of IL-2 was very low, whereas production of IL-10 was detectable in all cases. We also performed direct measurement of the Th2 cell-derived cytokine IL-4. Due to the low sensitivity level of the test, not all VLP variants demonstrated IL-4 production (data not shown).
DISCUSSION
VLPs carrying foreign epitopes gave one of the first impulses to the idea of universal, self-assembling, and noninfectious carriers for foreign epitopes, which are now widely used in VLP technology (29, 31). HBc VLPs have been used as the carrier of choice for the construction of many successful vaccine candidates against foot and mouth disease, malaria, human papilloma, hantavirus infection, and influenza, but first of all against HBV and HCV infections (for references, see reference 31).
The target of the present work was to combine HBV and HCV epitopes within the same VLP for the first time with the aim of developing a novel multivalent vaccine. HBcΔ (1-144) vector carrying all HBc T-helper cell and cytotoxic-T-lymphocyte (CTL) epitopes but lacking the C-terminal arginine-rich region was chosen for epitope insertions as an efficient vaccine scaffold. The HBc MIR region and C terminus of such shortened HBcs were selected as the best places for insertions of HBV and HCV epitopes, respectively (30).
The selection of the pre-S1 (20-47) fragment, a virus-neutralizing target of the HBV large envelope protein (LHBsAg) for insertion into the MIR of HBcΔ, was based on extensive previous experience. The pre-S1 (20-47) fragment, which is involved directly in virus attachment to hepatocytes (23, 26, 27), shows efficient immunogenicity at the B-cell level. A dominant T-cell recognition site was identified within residues 21 to 28 (serotype adw) of the pre-S1 sequence (13, 15), and in vitro binding studies have defined pre-S1 (25-33) as a promising helper T-cell epitope (17). Recently, it was demonstrated that a mutant LHBsAg bearing a deletion in the 26- to 30-amino-acid sequence of the pre-S1 receptor binding site was noninfectious and hence deficient in viral entry (4), further supporting the importance of this domain as an infectivity determinant in hepatitis B virus.
The HBc MIR had already been used as a target for insertion of numerous pre-S1 variants containing elements of the 20-to-47 region: aa 27 to 53 (34), aa 31 to 35 (18), and aa 31 to 36 (5). Numerous variants of the pre-S1 (21-47) insertions into the MIR with and without internal MIR deletions in HBc and HBcΔ were studied (25, 38). Further, three tandem copies of pre-S1 (21-47) were inserted at the MIR (44). Moreover, practically full-length pre-S1, with deletion of the inner hydrophobic membrane-targeting fragment, was exposed on the surfaces of the HBc and HBcΔ VLPs (35). In addition, numerous variants of mosaic HBc particles carrying the complete pre-S sequence at the MIR have been constructed with and without MIR deletions (16).
In regard to the immunogenicity of the pre-S1 epitope in our present study, the surprising thing is that the bivalent chimera HBcΔ-pre-S1-HCV core induces stronger anti-pre-S1 antibody response than the monovalent HBcΔ-pre-S1. At the same time, the anti-pre-S1 antibody responses gained here are definitely not lower than those in the long list of previous studies, especially taking into account differences in the lengths and compositions of pre-S1 fragments, immunization schemes and doses, adjuvants, and methodologies of titer calculations.
A strong therapeutic potential was shown with the HBcΔ (aa 1-to-155) variant bearing the C-terminally fused pre-S1 (aa 3-to-55) peptide, which was found to be surface accessible (9), although C-terminally added epitopes are mostly buried within HBc VLPs (for a review, see references 29 and 31). It encouraged us to try C-terminal addition of the HCV epitope within the planned multivalent vaccine candidate.
The N-terminal part of the HCV core was chosen, since it was found to contain three distinct antibody binding sites, including the previously reported site at residues 7 to 18 (7). The other two are located at residues 19 to 26 and residues 29 to 34 (33). A highly conservative T-cell epitope (HLA-A2 type) of the HCV core region is located within aa 35 to 44 (2). Attempts to insert the epitope into the HBc MIR and to use it as a DNA vaccine were made earlier (8).
Nevertheless, in our multivalent vaccine candidate, C-terminally added HCV core epitope (1-60) was neither exposed on the VLP surface nor able to induce remarkable antibody response, in contrast to the pre-S1 epitope (20-47). The latter showed good accessibility on the VLP surface and high-titer induction of specific antibodies. HCV core is known to be a relatively weak inducer of antibody response after insertion into chimeric VLPs (for a review, see reference 31). Remarkably, in this study, the immunogenicity of the HCV core epitope within the bivalent chimera HBcΔ-pre-S1-HCV core was not lower than that within the monovalent HBcΔ-HCV core. Moreover, HCV epitope (1-60) showed sufficient T-cell immunogenicity, which supports the idea of further elucidation of the multivalent construction.
Elimination of the C terminus of the HBc in the HBcΔ particles drastically reduced the Th1-biased immunogenicity of the HBc (32). This finding can be explained by the presence of CpG-like sequences in the bacterial RNA, which is packaged within the full-length HBc-derived particles. Synthetic oligodeoxynucleotides containing CpG motifs (CpG ODNs) are known as strong Th1 cell and CTL activity inducers when administered together with HBs (42) and HBc (39, 40) VLPs. According to our data, HBcΔ VLPs carrying HCV core epitope (1-60) restored the ability to package nucleic acids and switched the Th2 type of anti-HBc immune response to the Th1 type. In earlier work, we showed that HCV core (1-98) His-tagged protein was able to induce Th1-mediated response (1).
Therefore, bivalent VLPs carrying various foreign epitopes at different places are able to induce an immunological response to inserted epitopes that is higher, or at least not lower, than the response against individual epitopes within the corresponding monovalent VLP chimeras. One of the further activities in the application of the multivalent VLP constructions could be connected to a combination of DNA prime and protein boost vaccination schemes.
Acknowledgments
We thank Juris Ozols, Larisa Kovalevskaya, Lilija Auzina, Inara Akopjana, and Irina Stahovskaya for excellent technical assistance and Andris Zeltins, who suggested the method for stability measurements.
This work was supported by grants from the Latvian Council of Science 05.1626, ERAF VPD1/ERAF/CFLA/05/APK/2.5.1./000021/010, and EU 05-1000004-7748; ESF grant 1DP/1.1.1.2.0/09/APIA/VIAA/150; and the New Visby program of the Swedish Institute.
Footnotes
Published ahead of print on 21 April 2010.
REFERENCES
- 1.Alekseeva, E., I. Sominskaya, D. Skrastina, I. Egorova, E. Starodubova, E. Kushners, M. Mihailova, N. Petrakova, R. Bruvere, T. Kozlovskaya, M. Isaguliants, and P. Pumpens. 2009. Enhancement of the expression of HCV core gene does not enhance core-specific immune response in DNA immunization: advantages of the heterologous DNA prime, protein boost immunization regimen. Genet. Vaccines Ther. 7:7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Battegay, M., J. Fikes, A. M. Di Bisceglie, P. A. Wentworth, A. Sette, E. Celis, W. M. Ching, A. Grakoui, C. M. Rice, K. Kurokohchi, J. A. Berzofsky, J. H. Hoofnagle, S. M. Feinstone, and T. Akatsuka. 1995. Patients with chronic hepatitis C have circulating cytotoxic T cells which recognize hepatitis C virus-encoded peptides binding to HLA-A2.1 molecules. J. Virol. 69:2462-2470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bichko, V., F. Schodel, M. Nassal, E. Gren, I. Berzinsh, G. Borisova, S. Miska, D. L. Peterson, E. Gren, P. Pushko, and H. Will. 1993. Epitopes recognized by antibodies to denatured core protein of hepatitis B virus. Mol. Immunol. 30:221-231. [DOI] [PubMed] [Google Scholar]
- 4.Blanchet, M., and C. Sureau. 2007. Infectivity determinants of the hepatitis B virus pre-S domain are confined to the N-terminal 75 amino acid residues. J. Virol. 81:5841-5849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Borisova, G., O. Borschukova, D. Skrastina, A. Dislers, V. Ose, P. Pumpens, and E. Grens. 1999. Behavior of a short preS1 epitope on the surface of hepatitis B core particles. Biol. Chem. 380:315-324. [DOI] [PubMed] [Google Scholar]
- 6.Borisova, G., M. Bundule, E. Grinstein, D. Dreilina, A. Dreimane, J. Kalis, T. Kozlovskaya, V. Loseva, V. Ose, P. Pumpen, P. Pushko, D. Snikere, E. Stankevica, V. Tsibinogin, and E. I. Gren. 1987. Recombinant capsid structures for exposure of protein antigenic epitopes. Mol. Gen. (Life Sci. Adv.) 6:169-174. [Google Scholar]
- 7.Carlos, M. P., Y. Yamamura, Q. Vu, K. Conzen, D. E. Anderson, and J. V. Torres. 2004. Humoral immunity to immunodominant epitopes of hepatitis C virus in individuals infected with genotypes 1a or 1b. Clin. Immunol. 111:22-27. [DOI] [PubMed] [Google Scholar]
- 8.Chen, J. Y., and F. Li. 2006. Development of hepatitis C virus vaccine using hepatitis B core antigen as immuno-carrier. World J. Gastroenterol. 12:7774-7778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen, X., M. Li, X. Le, W. Ma, and B. Zhou. 2004. Recombinant hepatitis B core antigen carrying preS1 epitopes induce immune response against chronic HBV infection. Vaccine 22:439-446. [DOI] [PubMed] [Google Scholar]
- 10.Clarke, B. E., S. E. Newton, A. R. Carroll, M. J. Francis, G. Appleyard, A. D. Syred, P. E. Highfield, D. J. Rowlands, and F. Brown. 1987. Improved immunogenicity of a peptide epitope after fusion to hepatitis B core protein. Nature 330:381-384. [DOI] [PubMed] [Google Scholar]
- 11.Crowther, R. A., N. A. Kiselev, B. Bottcher, J. A. Berriman, G. P. Borisova, V. Ose, and P. Pumpens. 1994. Three-dimensional structure of hepatitis B virus core particles determined by electron cryomicroscopy. Cell 77:943-950. [DOI] [PubMed] [Google Scholar]
- 12.Davis, G. L. 1999. Hepatitis C virus genotypes and quasispecies. Am. J. Med. 107:21S-26S. [DOI] [PubMed] [Google Scholar]
- 13.Ferrari, C., A. Penna, A. Bertoletti, A. Cavalli, A. Valli, C. Schianchi, and F. Fiaccadori. 1989. The preS1 antigen of hepatitis B virus is highly immunogenic at the T cell level in man. J. Clin. Invest. 84:1314-1319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Houghton, M., M. Selby, A. Weiner, and Q. L. Choo. 1994. Hepatitis C virus: structure, protein products and processing of the polyprotein precursor. Curr. Stud. Hematol. Blood Transfus. 61:1-11. [DOI] [PubMed] [Google Scholar]
- 15.Jin, Y., W. K. Shih, and I. Berkower. 1988. Human T cell response to the surface antigen of hepatitis B virus (HBsAg). Endosomal and nonendosomal processing pathways are accessible to both endogenous and exogenous antigen. J. Exp. Med. 168:293-306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kazaks, A., G. Borisova, S. Cvetkova, L. Kovalevska, V. Ose, I. Sominskaya, P. Pumpens, D. Skrastina, and A. Dislers. 2004. Mosaic hepatitis B virus core particles presenting the complete preS sequence of the viral envelope on their surface. J. Gen. Virol. 85:2665-2670. [DOI] [PubMed] [Google Scholar]
- 17.Kim, J. H., J. H. Park, Y. J. Lee, E. W. Cho, Y. S. Bae, and K. L. Kim. 2000. In vitro binding analysis of hepatitis B virus preS-derived putative helper T-cell epitopes to MHC class II molecules using stable HLA-DRB1*0405/DRA*0101 transfected cells. IUBMB Life 50:379-384. [DOI] [PubMed] [Google Scholar]
- 18.Lachmann, S., H. Meisel, C. Muselmann, D. Koletzki, H. R. Gelderblom, G. Borisova, D. H. Kruger, P. Pumpens, and R. Ulrich. 1999. Characterization of potential insertion sites in the core antigen of hepatitis B virus by the use of a short-sized model epitope. Intervirology 42:51-56. [DOI] [PubMed] [Google Scholar]
- 19.Lagging, L. M., K. Meyer, D. Hoft, M. Houghton, R. B. Belshe, and R. Ray. 1995. Immune responses to plasmid DNA encoding the hepatitis C virus core protein. J. Virol. 69:5859-5863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lo, M. C., A. Aulabaugh, G. Jin, R. Cowling, J. Bard, M. Malamas, and G. Ellestad. 2004. Evaluation of fluorescence-based thermal shift assays for hit identification in drug discovery. Anal. Biochem. 332:153-159. [DOI] [PubMed] [Google Scholar]
- 21.Mihailova, M., M. Boos, I. Petrovskis, V. Ose, D. Skrastina, M. Fiedler, I. Sominskaya, S. Ross, P. Pumpens, M. Roggendorf, and S. Viazov. 2006. Recombinant virus-like particles as a carrier of B- and T-cell epitopes of hepatitis C virus (HCV). Vaccine 24:4369-4377. [DOI] [PubMed] [Google Scholar]
- 22.Mihailova, M., M. Fiedler, M. Boos, I. Petrovskis, I. Sominskaya, M. Roggendorf, S. Viazov, and P. Pumpens. 2006. Preparation of hepatitis C virus structural and non-structural protein fragments and studies of their immunogenicity. Protein Expr. Purif. 50:43-48. [DOI] [PubMed] [Google Scholar]
- 23.Neurath, A. R., S. B. Kent, K. Parker, A. M. Prince, N. Strick, B. Brotman, and P. Sproul. 1986. Antibodies to a synthetic peptide from the preS 120-145 region of the hepatitis B virus envelope are virus neutralizing. Vaccine 4:35-37. [DOI] [PubMed] [Google Scholar]
- 24.Newton, S. E., B. E. Clarke, G. Appleyard, M. J. Francis, A. R. Carroll, D. J. Rowlands, J. Skehel, and F. Brown. 1987. New approaches to FMDV antigen presentation using vaccinia virus, p. 12-21. In R. M. Chanock, R. A. Lerner, F. Brown, and H. Ginsberg (ed.), Modern approaches to new vaccines: prevention of AIDS and other viral, bacterial and parasitic diseases. Cold Spring Harbor Laboratory, Cold Spring Harbor, NY.
- 25.Page, M., J.-L. Li, P. Pumpens, and G. Borisova. 15 March 2006. Modification of hepatitis B core antigen. European patent EP1294893.
- 26.Petit, M. A., S. Dubanchet, F. Capel, P. Voet, C. Dauguet, and P. Hauser. 1991. HepG2 cell binding activities of different hepatitis B virus isolates: inhibitory effect of anti-HBs and anti-preS1(21-47). Virology 180:483-491. [DOI] [PubMed] [Google Scholar]
- 27.Pontisso, P., M. A. Petit, M. J. Bankowski, and M. E. Peeples. 1989. Human liver plasma membranes contain receptors for the hepatitis B virus pre-S1 region and, via polymerized human serum albumin, for the pre-S2 region. J. Virol. 63:1981-1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pumpen, P. P., A. V. Dishler, T. M. Kozlovskaia, V. V. Bychko, and E. I. Gren. 1981. Cloning of hepatitis B virus DNA in Escherichia coli. Dokl. Akad. Nauk SSSR. 260:1022-1024. (In Russian.) [PubMed] [Google Scholar]
- 29.Pumpens, P., and E. Grens. 2002. Artificial genes for chimeric virus-like particles, p. 249-327. In I. Khudiakov and H. A. Fields (ed.), Artificial DNA: methods and applications. CRC Press, Boca Raton, FL.
- 30.Pumpens, P., and E. Grens. 2001. HBV core particles as a carrier for B cell/T cell epitopes. Intervirology 44:98-114. [DOI] [PubMed] [Google Scholar]
- 31.Pumpens, P., R. Ulrich, K. Sasnauskas, A. Kazaks, V. Ose, and E. Grens. 2008. Construction of novel vaccines on the basis of the virus-like particles: hepatitis B virus proteins as vaccine carriers, p. 205-248. In Y. E. Khudyakov (ed.), Medicinal protein engineering. CRC Press Taylor & Francis Group, Boca Raton, FL.
- 32.Riedl, P., D. Stober, C. Oehninger, K. Melber, J. Reimann, and R. Schirmbeck. 2002. Priming Th1 immunity to viral core particles is facilitated by trace amounts of RNA bound to its arginine-rich domain. J. Immunol. 168:4951-4959. [DOI] [PubMed] [Google Scholar]
- 33.Sällberg, M., P. Pumpen, Z. X. Zhang, P. Lundholm, I. Gusars, U. Ruden, B. Wahren, and L. O. Magnius. 1994. Locations of antibody binding sites within conserved regions of the hepatitis C virus core protein. J. Med. Virol. 43:62-68. [DOI] [PubMed] [Google Scholar]
- 34.Schödel, F., A. M. Moriarty, D. L. Peterson, J. A. Zheng, J. L. Hughes, H. Will, D. J. Leturcq, J. S. McGee, and D. R. Milich. 1992. The position of heterologous epitopes inserted in hepatitis B virus core particles determines their immunogenicity. J. Virol. 66:106-114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Skrastina, D., A. Bulavaite, I. Sominskaya, L. Kovalevska, V. Ose, D. Priede, P. Pumpens, and K. Sasnauskas. 2008. High immunogenicity of a hydrophilic component of the hepatitis B virus preS1 sequence exposed on the surface of three virus-like particle carriers. Vaccine 26:1972-1981. [DOI] [PubMed] [Google Scholar]
- 36.Sominskaya, I., W. Paulij, J. Jansons, D. Sobotta, D. Dreilina, C. Sunnen, H. Meisel, W. H. Gerlich, and P. Pumpens. 2002. Fine-mapping of the B-cell epitope domain at the N-terminus of the preS2 region of the hepatitis B surface antigen. J. Immunol. Methods 260:251-261. [DOI] [PubMed] [Google Scholar]
- 37.Sominskaya, I., P. Pushko, D. Dreilina, T. Kozlovskaya, and P. Pumpen. 1992. Determination of the minimal length of preS1 epitope recognized by a monoclonal antibody which inhibits attachment of hepatitis B virus to hepatocytes. Med. Microbiol. Immunol. 181:215-226. [DOI] [PubMed] [Google Scholar]
- 38.Sominskaya, I., D. Skrastina, E. Alekseeva, J. Jansons, and P. Pumpens. 2003. HBc-preS1 VLPs as potential vaccine candidates, abstr. 9-3-0007, p. 190. In A. McLachlan (ed.), Int. meeting of the molecular biology of hepatitis B viruses, 7 to 10 September 2003, Bergamo, Italy. [Abstract.]
- 39.Storni, T., F. Lechner, I. Erdmann, T. Bachi, A. Jegerlehner, T. Dumrese, T. M. Kundig, C. Ruedl, and M. F. Bachmann. 2002. Critical role for activation of antigen-presenting cells in priming of cytotoxic T cell responses after vaccination with virus-like particles. J. Immunol. 168:2880-2886. [DOI] [PubMed] [Google Scholar]
- 40.Storni, T., C. Ruedl, K. Schwarz, R. A. Schwendener, W. A. Renner, and M. F. Bachmann. 2004. Nonmethylated CG motifs packaged into virus-like particles induce protective cytotoxic T cell responses in the absence of systemic side effects. J. Immunol. 172:1777-1785. [DOI] [PubMed] [Google Scholar]
- 41.Tokushige, K., T. Wakita, C. Pachuk, D. Moradpour, D. B. Weiner, V. R. Zurawski, Jr., and J. R. Wands. 1996. Expression and immune response to hepatitis C virus core DNA-based vaccine constructs. Hepatology 24:14-20. [DOI] [PubMed] [Google Scholar]
- 42.Weeratna, R. D., M. J. McCluskie, L. Comanita, T. Wu, and H. L. Davis. 2000. Optimization strategies for DNA vaccines. Intervirology 43:218-226. [DOI] [PubMed] [Google Scholar]
- 43.Wynne, S. A., R. A. Crowther, and A. G. Leslie. 1999. The crystal structure of the human hepatitis B virus capsid. Mol. Cell 3:771-780. [DOI] [PubMed] [Google Scholar]
- 44.Yang, H. J., M. Chen, T. Cheng, S. Z. He, S. W. Li, B. Q. Guan, Z. H. Zhu, Y. Gu, J. Zhang, and N. S. Xia. 2005. Expression and immunoactivity of chimeric particulate antigens of receptor binding site-core antigen of hepatitis B virus. World J. Gastroenterol. 11:492-497. [DOI] [PMC free article] [PubMed] [Google Scholar]