Abstract
In this study, we compared the sequential responses of immunoglobulin G (IgG) subclasses to the diagnostic antigen Em18 in sera from patients with alveolar echinococcosis. A total of 225 sera from 36 patients at different clinical stages according to the WHO-PNM staging system were tested. The antibody responses were measured for cohorts with resected and unresected parasitic lesions by enzyme-linked immunosorbent assays (ELISA). Total IgG and, to a lesser extent, IgG4 antibody levels against Em18 correlated with all PNM stages before treatment, whereas levels of IgG2 were low and IgG3 was undetectable. Antibody kinetics, however, depended on the treatment rather than on the PNM stage. For some patients, after curative surgery, IgG1 antibodies dropped below the cutoff earlier than other antibodies, followed by total IgG and IgG4 within 18 months. For some patients with recurrences after surgery, IgG1 and IgG4 reappeared, whereas patients with unresectable lesions but stable disease showed steady declines in the levels of all antibodies, and IgG1 became undetectable in some patients. Additional testing of IgE responses to Em18 showed constantly low levels at all stages and in all cohorts.
Alveolar echinococcosis (AE) is caused by the vesicular larval stage of the fox tapeworm Echinococcus multilocularis. The helminth causes dangerous infections characterized by infiltrative growth of the larvae in the livers of natural intermediate hosts such as rodents, and rarely in humans. Metastasis formation may also occur. AE is staged according to the World Health Organization (WHO)-PNM (P, parasitic mass in the liver; N, involvement of neighboring organs; M, metastasis) system (10). Radical resection of parasitic lesions is the preferred treatment (1), but most patients are inoperable at the time of diagnosis (5, 13). In a recent serological study, immunoglobulin G (IgG) antibodies directed against Em18, Em10, and Em2plus antigen compositions showed a close relationship between the clinical status and the treatment of patients with AE (16). In direct comparison, antibodies against Em18 demonstrated the greatest dynamic changeability in all patients, cohorts, and PNM stages, irrespective of the individual treatment. Moreover, Em18 indices had shown the best correlation with the PNM stages prior to treatment. These results prompted us to further investigate the IgG subclass and additionally the IgE response against this diagnostic antigen in patients with either resected or unresectable parasitic lesions.
MATERIALS AND METHODS
Patients.
All patients described in this study were seen at the University Hospital and Medical Center Ulm, Ulm, Germany. A total of 36 patients (225 sera) with a history of hepatic AE and a follow-up period of 1.5 to 6.5 years were included in the study. The patients (age range, 17 to 86 years; mean age, 51.2 years; sex ratio [male to female], 0.57:1) were assigned to different clinical WHO-PNM stages of the disease. All patients had acquired AE in Germany and received benzimidazole therapy. Thirteen patients had curatively resected lesions; 4 had recurrences after surgery; 1 had a palliative resection only; 16 had unresectable lesions but stable disease; and 2 had apparently dead, fully calcified lesions (Table 1). All serum samples were tested at the Department of Parasitology, Asahikawa Medical College, Asahikawa, Japan, in a blind test. The classification of curative resection, stable disease, progressive disease, or the presence of an apparently dead, fully calcified lesion was established by magnetic resonance imaging based on lesion size and morphology at the respective follow-up intervals. Ethical approval was obtained from the University of Ulm.
TABLE 1.
Characteristics of patients with alveolar echinococcosis included in the study
| Patient no. | Stage | PNM codea | Statusa | Age (yr)b | Sex | Follow-up duration (yr) |
|---|---|---|---|---|---|---|
| 1 | I | P1N0M0 | Curative resection | 62 | F | 5.5 |
| 2 | I | P1N0M0 | Curative resection | 24 | F | 5 |
| 3 | I | P1N0M0 | Curative resection | 22 | F | 4 |
| 4 | I | P1N0M0 | Curative resection | 33 | F | 2 |
| 5 | I | P1N0M0 | Apparently dead, fully calcified lesion | 58 | M | 4 |
| 6 | I | P1N0M0 | Unresectable, stable disease | 61 | M | 3.5 |
| 7 | II | P2N0M0 | Curative resection | 38 | F | 3 |
| 8 | II | P2N0M0 | Unresectable, stable disease | 71 | M | 6 |
| 9 | II | P2N0M0 | Unresectable, stable disease | 68 | F | 5.5 |
| 10 | II | P2N0M0 | Unresectable, stable disease | 59 | F | 6.5 |
| 11 | II | P2N0M0 | Unresectable, stable disease | 60 | F | 6 |
| 12 | II | P2N0M0 | Curative resection | 41 | F | 2.5 |
| 13 | II | P2N0M0 | Recurrence after resection | 74 | F | 2.5 |
| 14 | II | P2N0M0 | Unresectable, stable disease | 75 | M | 3 |
| 15 | IIIa | P3N0M0 | Curative resection | 25 | F | 4.5 |
| 16 | IIIa | P3N0M0 | Curative resection | 62 | M | 5.5 |
| 17 | IIIa | P3N0M0 | Recurrence after resection | 17 | F | 3.5 |
| 18 | IIIa | P3N0M0 | Unresectable, stable disease | 69 | F | 6 |
| 19 | IIIa | P3N0M0 | Unresectable, stable disease | 39 | F | 4 |
| 20 | IIIa | P3N0M0 | Apparently dead, fully calcified lesion | 57 | F | 6 |
| 21 | IIIa | P3N0M0 | Unresectable, stable disease | 43 | M | 2 |
| 22 | IIIa | P3N0M0 | Recurrence after resection | 19 | F | 3 |
| 23 | IIIb | P4N0M0 | Unresectable, stable disease | 60 | F | 5 |
| 24 | IIIb | P3N1M0 | Unresectable, stable disease | 49 | F | 5.5 |
| 25 | IIIb | P2N1M0 | Recurrence after resection | 50 | M | 5 |
| 26 | IIIb | P3N1M0 | Progression after palliative resection | 32 | M | 6 |
| 27 | IIIb | P4N0M0 | Unresectable, stable disease | 57 | M | 3 |
| 28 | IIIb | P4N0M0 | Unresectable, stable disease | 86 | F | 5.5 |
| 29 | IV | P4N1M0 | Curative resection | 56 | F | 6.5 |
| 30 | IV | P4N1M0 | Curative resection | 30 | M | 3 |
| 31 | IV | P4N1M0 | Curative resection | 72 | F | 3 |
| 32 | IV | P4N1M0 | Unresectable, stable disease | 71 | F | 5.5 |
| 33 | IV | P4N1M0 | Curative resection | 52 | M | 2.5 |
| 34 | IV | P4N1M0 | Curative resection | 63 | F | 1.5 |
| 35 | IV | P4N1M0 | Unresectable, stable disease | 34 | M | 2.5 |
| 36 | IV | P4N1M0 | Unresectable, stable disease | 54 | M | 4 |
As assessed either by imaging alone (apparently dead lesion, progressive disease, and stable disease) or by imaging and histology (curative resection).
At the time when the first blood sample was drawn.
Methods.
For the Em18 enzyme-linked immunosorbent assay (ELISA), recombinant Em18 antigen (14) was used to coat microtiter plates at a concentration of 100 ng/well. Patients' sera were tested at dilutions of 1:100 for total IgG and IgG subclasses, and 1:10 for IgE, after preabsorption of the wells with 1% casein in 20 mM Tris-HCl (pH 7.4)-150 mM NaCl buffer. Serum IgG bound to echinococcal antigens were detected with horseradish peroxidase (HRP)-conjugated protein G (Zymed) as a secondary antibody by using 2,2′-azinobis(3-ethylbenzthiazolinesulfonic acid) (ABTS; Sigma, Germany) as a chromogenic substrate. For the detection of recombinant Em18-specific IgE and IgG subclasses, HRP-conjugated mouse monoclonal anti-human IgE, IgG1, IgG2, IgG3, or IgG4 antibodies (Zymed) were used. Absorbance was measured after 30 min at 405 nm with a reference wavelength of 630 nm. For the calculation of the cutoff, the mean value of the absorbances of 31 sera from healthy blood donors was added to 3 times the standard deviation (SD) for total IgG and to 5 times the SD for the IgG subclasses and IgE. The index of the individual serum sample was calculated by dividing the sample's absorbance by the cutoff.
Statistical analysis.
Statistical analyses were performed with the free software environment “R” for statistical computing. Nonparametric data were analyzed using Spearman's rank test for the correlation of the clinical stage and the ELISA index of the respective antibody (subclass) responses. Ρ values of <0.005 were regarded as significant.
RESULTS
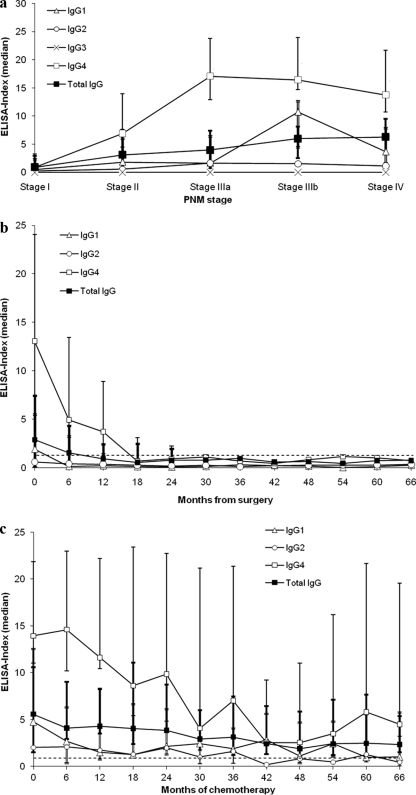
The height of the ELISA index of the antibodies tested showed a weak correlation of total IgG and IgG4 with all clinical PNM stages (stages I to IV) prior to treatment (Fig. 1 a). IgG4 showed a close-to-linear correlation only with the first three stages (stages I to IIIa). The correlation of IgG1 was poor. Since IgE and IgG2 antibodies showed weak reactivities, and IgG3 antibodies were undetectable, no correlation with the clinical stage could be established for these isotypes/subclasses at all. The clinical PNM stage had no influence on the kinetics of antibody levels per se. Rather, antibody levels depended on the treatment the patients underwent at each stage (Fig. 1b and c).
FIG. 1.
(a) Correlation of the PNM stage and the ELISA-index prior to treatment. Median index values of the respective antibodies against Em18 are displayed for each PNM stage. The highest median index in all stages was demonstrated for IgG4, whereas IgG3 was completely unreactive. The lowest positive median indices in all stages were demonstrated for IgE (data not shown) and IgG2. A constant increase in the median values of the total IgG index in parallel with the clinical stage is visible. IgG4 indices showed a close-to-linear increase for stages I to IIIa only. Error bars represent ranges for total IgG (bold lines), IgG4 (thin single lines), and IgG1 (thin double lines). Spearman's rank correlation coefficients (ρ) for total IgG, IgG4, and IgG1 with all PNM stages (stages I to IV) were 0.532 (Ρ, <0.001), 0.499 (Ρ, <0.002), and 0.358 (Ρ, <0.05), respectively. (b) Antibody profiles of patients after curative resection. Median index values from all assays (except those for IgG3 and IgE) for 13 patients at different clinical stages are presented. Rapid declines in the antibody indices of total IgG, IgG1, and IgG4 are clearly visible. Error bars represent ranges (until month 24) for total IgG, IgG4, and IgG1 as explained for panel a. Serological data were accumulated for intervals of 6 months. At each given interval, data from 2 to 13 patients were available. The cutoff level is 1, represented by the dashed line. The time of resection is month zero. (c) Antibody profiles of patients with stable disease and unresectable lesions. Median index values from all assays (except those for IgG3 and IgE) for 16 patients at different clinical stages are presented. A slow decline in the antibody indices is visible. Error bars represent ranges for total IgG, IgG4, and IgG1 as explained for panel a. Serological data were accumulated for intervals of 6 months. At each given interval, data from 5 to 16 patients were available. The cutoff level is 1, represented by the dashed line. The start of chemotherapy is at month zero.
In the cohort of 13 patients who underwent curative resection, levels of all antibodies decreased rapidly after resection of the parasitic lesions. Antibody levels dropped below the cutoff level after some time (Fig. 1b). The index of IgG4 directed against Em18 showed the most marked decline of all antibody (sub)types during the first follow-up interval in all patients and PNM stages in this cohort (Fig. 1b). IgG1 antibodies were the first to drop below the cutoff in some patients, followed by total IgG and IgG4. IgG1 fell below the cutoff in 8 patients (patients 2, 7, 12, 15, 16, 29, 33, and 34) after 30, 12, 24, 6, 12, 12, 6, and 6 months, respectively; total IgG fell below the cutoff in 6 patients (patients 2, 7, 12, 15, 29, and 34) at 54, 12, 24, 6, 12, and 18 months, respectively; and IgG4 fell below the cutoff in 6 patients (patients 2, 7, 12, 15, 29, and 34) after 30, 12, 24, 6, 48, and 18 months, respectively. In some patients, seroreversion in different assays was seen at the same time. Once seroreversion was seen, antibodies remained undetectable throughout the observation period. Follow-up imaging demonstrated that the drop below the cutoff level reflected serologically the clinical regression in this patient cohort.
In the 5 patients with noncurative resection, antibody levels of total IgG, IgG1, and IgG4 against Em18 decreased at first but increased again during the follow-up period. Total IgG never fell below the cutoff level, whereas IgG1 became negative in 2 patients (patients 22 and 26) after 6 and 36 months and increased again after 24 and 54 months, respectively. IgG4 became negative in 1 patient (patient 22) and showed the same kinetics as IgG1 in this patient. Recurrence was demonstrated by follow-up imaging in all patients.
In the cohort of 16 patients with unresectable lesions and stable disease under benzimidazole therapy, total-IgG, IgG1, IgG2, and IgG4 levels showed slow but steady declines (Fig. 1c). IgE levels were very low. No differences in antibody kinetics between different PNM stages in this patient cohort were observed. Overall, the IgG4 index demonstrated the most pronounced decrease in all patients and PNM stages of this cohort. Total IgG showed the smoothest decline, whereas IgG1 and IgG4 displayed undulating decreases. IgG1, IgG2, and IgG4 antibodies dropped below the cutoff in 5 patients (patients 8, 10, 11, 18, and 19) after 12, 36, 72, 18, and 42 months, respectively; in 2 patients (patients 11 and 32) after 12 and 42 months, respectively; and in 1 patient (patient 11) after 72 months. Once seroreversion was seen, antibodies remained undetectable throughout the observation period. The decrease in the antibody levels paralleled serologically the clinically stable disease in this patient cohort as assessed by follow-up imaging.
In the 2 patients with apparently dead lesions, levels of all antibodies against Em18 either were completely below the cutoff or showed a steady decline until total IgG, IgG1, and IgG4 became undetectable at the same time (data not shown). The apparently dead, fully calcified lesions correlated with no detectable growth during follow-up imaging. Testing of IgE responses to Em18 showed constantly low levels in all stages and cohorts (data not shown).
DISCUSSION
In this study, we performed a serological follow-up of patients with AE grouped according to the WHO-PNM staging system. The recombinant diagnostic antigen Em18 was used in different ELISAs, which measured total IgG, IgG subclasses, and IgE in a German patient cohort. Six different assays were run in parallel. In a previous study, the height of antibody levels prior to treatment was dependent on the PNM stage, and indices of anti-Em18 IgG showed the highest correlation of all antigen compositions used (16). Here, a similar correlation was shown for total IgG against Em18. When the IgG subclass responses were analyzed, however, only IgG4 displayed a comparable correlation with all clinical PNM stages before treatment was begun. IgG4 also exhibited the highest ELISA indices in all patient subcohorts and stages, suggesting a higher diagnostic sensitivity than those for other subclasses or total IgG (7). Accordingly, earlier studies have shown that IgG4 was the predominant IgG subclass responding to Em18 (8). In another study, IgG4 and IgG1 isotype levels were significantly elevated in the sera of AE patients (18), and these isotypes also displayed the most sensitive antibody response to a crude parasite extract containing a 17.5- to 18-kDa antigen in an ELISA (18) and immunoblot analysis (4, 20). In our study, IgG1 levels against Em18 were also increased at all clinical stages, whereas IgG2 demonstrated the lowest positive median indices at all stages, and IgG3 was completely undetectable. Since Em18 is a pure protein antigen, and IgG2 has often been associated with anticarbohydrate immune responses in humans (17), it is understandable that only a low IgG2 response was obtained. Why IgG3 was undetectable in our study is less clear. A greater-than-expected proportion of carbohydrate-specific IgG antibody responses in humans can have a non-IgG2 subclass origin (17), possibly also encompassing IgG3. Hypothetically, a suppression of IgG3 responses toward this diagnostic antigen by the parasite might also be possible. In a previous study by others, the sensitivities of IgG2 and IgG3 directed against a crude parasite extract were also significantly lower than those of the other IgG subclasses in AE patients (19). Levels of IgE in response to Em18 were very low in all stages and patient cohorts. These data indicate that Em18 is a good candidate for parasite-specific total-IgG, IgG4, and IgG1 analysis of AE patients at all WHO stages. Em18 might be an unsuitable antigen for IgE and IgG2 testing, and an unsuitable antigen as a pure protein for IgG3 serology as well.
In the follow-up investigations, IgG4 antibodies against Em18 demonstrated the greatest dynamic changeability in all patients, cohorts, and PNM stages, irrespective of the individual treatment. The data obtained from the patient cohort with curatively resected lesions are consistent with previous findings that antibodies directed against various antigen compositions, including Em18, can drop below the cutoff (2, 6, 15, 16). In this study, IgG1 antibodies were the first to drop below the cutoff, followed by total IgG and IgG4 at 6-month intervals, reflecting the curative resection. In contrast, in a previous follow-up study of cured or improved patients, levels of IgG4 antibodies directed against a crude parasite extract tended to decrease earlier than total-IgG levels (18). However, IgG4 antibodies became negative 1 year after successful treatment, as seen in our study.
For patients with recurrences, the initial decrease in the levels of total IgG against Em18, followed by a later increase, has previously been demonstrated in other studies using total IgG in the Em18 ELISA (6, 16), Em18 Western blotting (12), and other assays with different diagnostic antigens (16). Levels of IgG1 and IgG4 against Em18 followed these kinetics, but these antibodies became intermittently negative in a few patients in this study. Accordingly, specific IgG4 responses to a crude antigen extract reappeared in recurrences, as shown in a survey by others (18).
Our results for the patient cohort with unresectable lesions and stable AE are consistent with previous findings that levels of antibody against Em18 (6, 12, 16), and also other diagnostic antigens (2, 12, 16), decrease slowly under antiparasitic chemotherapy. In our study, total IgG against Em18 showed the smoothest decline, whereas median indices of IgG1 and IgG4 demonstrated undulating decreases over time. In a previous study by others, levels of IgG1 and IgG4 against Em18 fell to zero in some patients treated with albendazole (12), whereas these IgG subclasses showed unchanged levels against a crude parasite antigen extract in patients with stabilized AE (18). In our study, seroreversion was also demonstrable for IgG1 in some patients and for IgG2 and IgG4 in a few patients. Most patients were at an early stage of the disease (stage II). In this patient group, a regression of lesions toward nonviability of the parasite might be possible. However, the observation period might not have been long enough to demonstrate seroreversion in the other IgG subclasses or total IgG.
The kinetics of antibody levels in patients who have apparently dead and fully calcified lesions were very similar to those for the cohort with stable disease, and the results are consistent with those of a previous report (16).
Whether the IgG subclass effect described here is directly related to the Em18 antigen or is a more general effect of AE serology remains to be elucidated. However, several previous studies found that the recombinant Em18 antigen may be one of the best antigens for AE serology (3, 9, 11, 14, 16). Antibody responses to recombinant proteins are much clearer, and thus easier to analyze, than responses to native proteins, since the latter may contain variable epitopes, which may lead to more complex immunological responses.
In conclusion, our data indicate that the diagnostic antigen Em18 is suitable for parasite-specific serology employing total-IgG, IgG1, and IgG4 assays. Total IgG and IgG4 mirror the clinical PNM stage best before treatment. IgG4 shows the greatest changes in all patient cohorts, clinical stages, and treatments; therefore, IgG4 might be the most sensitive antibody subclass for AE serology employing the Em18 antigen. IgG1 antibody kinetics reflect curative resection, recurrence, and possibly the death of the parasite better than any other antibody (sub)class directed against Em18.
Acknowledgments
We thank Christoph Schoen (Germany) for assistance with the statistical analysis.
Serology in Japan was financially supported in part by the Infection Matrix Fund (2007 to 2008) and the Hokkaido Translational Research Project (2007 to 2012) from the Ministry of Education, Japan, and by the Japan Society for the Promotion of Science (21256003) (to A.I.).
Footnotes
Published ahead of print on 14 April 2010.
REFERENCES
- 1.Ammann, R. W., and J. Eckert. 1996. Cestodes. Echinococcus. Gastroenterol. Clin. North Am. 25:655-689. [DOI] [PubMed] [Google Scholar]
- 2.Ammann, R. W., E. C. Renner, B. Gottstein, F. Grimm, J. Eckert, and E. L. Renner; Swiss Echinococcosis Study Group. 2004. Immunosurveillance of alveolar echinococcosis by specific humoral and cellular immune tests: long-term analysis of the Swiss chemotherapy trial (1976-2001). J. Hepatol. 41:551-559. [DOI] [PubMed] [Google Scholar]
- 3.Bart, J. M., M. Piarroux, Y. Sako, F. Grenouillet, S. Bresson-Hadni, R. Piarroux, and A. Ito. 2007. Comparison of several commercial serologic kits and Em18 serology for detection of human alveolar echinococcosis. Diagn. Microbiol. Infect. Dis. 59:93-95. [DOI] [PubMed] [Google Scholar]
- 4.Dreweck, C. M., C. G. Lüder, P. T. Soboslay, and P. Kern. 1997. Subclass-specific serological reactivity and IgG4-specific antigen recognition in human echinococcosis. Trop. Med. Int. Health 2:779-787. [DOI] [PubMed] [Google Scholar]
- 5.Eckert, J., P. Jacquier, D. Baumann, and P. A. Raeber. 1995. Echinokokkose des Menschen in der Schweiz, 1984-1992. Schweiz. Med. Wochenschr. 125:1989-1998. [PubMed] [Google Scholar]
- 6.Fujimoto, Y., A. Ito, Y. Ishikawa, M. Inoue, Y. Suzuki, M. Ohhira, T. Ohtake, and Y. Kohgo. 2005. Usefulness of recombinant Em18-ELISA to evaluate efficacy of treatment in patients with alveolar echinococcosis. J. Gastroenterol. 40:426-431. [DOI] [PubMed] [Google Scholar]
- 7.Ishikawa, Y., Y. Sako, S. Itoh, T. Ohtake, Y. Kohgo, T. Matuno, Y. Ohsaki, N. Miyokawa, M. Nakao, K. Nakaya, and A. Ito. 2009. Serological monitoring of progression of alveolar echinococcosis with multiorgan involvement by use of recombinant Em18. J. Clin. Microbiol. 47:3191-3196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ito, A., P. M. Schantz, and J. F. Wilson. 1995. Em18, a new serodiagnostic marker for differentiation of active and inactive cases of alveolar hydatid disease. Am. J. Trop. Med. Hyg. 52:41-44. [DOI] [PubMed] [Google Scholar]
- 9.Ito, A., N. Xiao, M. Liance, M. O. Sato, Y. Sako, W. Mamuti, Y. Ishikawa, M. Nakao, H. Yamasaki, K. Nakaya, K. Bardonnet, S. Bresson-Hadni, and D. A. Vuitton. 2002. Evaluation of an enzyme-linked immunosorbent assay (ELISA) with affinity-purified Em18 and an ELISA with recombinant Em18 for differential diagnosis of alveolar echinococcosis: results of a blind test. J. Clin. Microbiol. 40:4161-4165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kern, P., H. Wen, N. Sato, D. A. Vuitton, B. Grüner, Y. Shao, E. Delabrousse, W. Kratzer, and S. Bresson-Hadni. 2006. WHO classification of alveolar echinococcosis: principles and application. Parasitol. Int. 55:S283-S287. [DOI] [PubMed] [Google Scholar]
- 11.Li, T., A. Ito, X. Chen, Y. Sako, J. Qiu, N. Xiao, D. Qiu, M. Nakao, T. Yanagida, and P. S. Craig. 2010. Specific IgG responses to recombinant antigen B and Em18 in cystic and alveolar echinococcosis in China. Clin. Vaccine Immunol. 17:470-475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ma, L., A. Ito, Y. H. Liu, X. G. Wang, Y. G. Yao, D. G. Yu, and Y. T. Chen. 1997. Alveolar echinococcosis: Em2 plus-ELISA and Em18-Western blots for follow-up after treatment with albendazole. Trans. R. Soc. Trop. Med. Hyg. 91:476-478. [DOI] [PubMed] [Google Scholar]
- 13.Reuter, S., A. Buck, O. Grebe, K. Nussle-Kugele, P. Kern, and B. J. Manfras. 2003. Salvage treatment with amphotericin B in progressive human alveolar echinococcosis. Antimicrob. Agents Chemother. 47:3586-3591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sako, Y., M. Nakao, K. Nakaya, H. Yamasaki, B. Gottstein, M. W. Lightowlers, P. M. Schantz, and A. Ito. 2002. Alveolar echinococcosis: characterization of diagnostic antigen Em18 and serological evaluation of recombinant Em18. J. Clin. Microbiol. 40:2760-2765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schantz, P. M., J. F. Wilson, S. P. Wahlquist, L. P. Boss, and R. L. Rausch. 1983. Serologic tests for diagnosis and post-treatment evaluation of patients with alveolar hydatid disease (Echinococcus multilocularis). Am. J. Trop. Med. Hyg. 32:1381-1386. [DOI] [PubMed] [Google Scholar]
- 16.Tappe, D., M. Frosch, Y. Sako, S. Itoh, B. Grüner, S. Reuter, M. Nakao, A. Ito, and P. Kern. 2009. Close relationship between clinical regression and specific serology in the follow-up of patients with alveolar echinococcosis in different clinical stages. Am. J. Trop. Med. Hyg. 80:792-797. [PubMed] [Google Scholar]
- 17.von Gunten, S., D. F. Smith, R. D. Cummings, S. Riedel, S. Miescher, A. Schaub, R. G. Hamilton, and B. S. Bochner. 2009. Intravenous immunoglobulin contains a broad repertoire of anticarbohydrate antibodies that is not restricted to the IgG2 subclass. J. Allergy Clin. Immunol. 123:1268-1276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wen, H., S. Bresson-Hadni, D. A. Vuitton, D. Lenys, B. M. Yang, Z. X. Ding, and P. S. Craig. 1995. Analysis of immunoglobulin G subclass in the serum antibody responses of alveolar echinococcosis patients after surgical treatment and chemotherapy as an aid to assessing the outcome. Trans. R. Soc. Trop. Med. Hyg. 89:692-697. [DOI] [PubMed] [Google Scholar]
- 19.Wen, H., and P. S. Craig. 1994. Immunoglobulin G subclass responses in human cystic and alveolar echinococcosis. Am. J. Trop. Med. Hyg. 51:741-748. [DOI] [PubMed] [Google Scholar]
- 20.Wen, H., P. S. Craig, A. Ito, D. A. Vuitton, S. Bresson-Hadni, J. C. Allan, M. Rogan, E. Paollilo, and M. Shambesh. 1995. Immunoblot evaluation of IgG and IgG subclass antibody responses for immunodiagnosis of human alveolar echinococcosis. Ann. Trop. Med. Parasitol. 89:485-495. [DOI] [PubMed] [Google Scholar]