Abstract
Sheep pox and enterotoxemia are important diseases of sheep, and these diseases cause severe economic losses to sheep farmers. The present study was undertaken to evaluate the potential of formaldehyde-inactivated recombinant epsilon toxin as a vaccine candidate. The potency of the recombinant epsilon toxoid with aluminum hydroxide as an adjuvant in sheep was determined. Vaccinated sheep were protected against enterotoxemia, with potency values of >5 IU being protective. Further, the use of this construct in a combination vaccine against sheep pox resulted in the sheep being protected against both sheep pox and enterotoxemia.
Sheep occupy a premier place in the livestock industry and contribute significantly to the world economy. Their populations are threatened by a number of health hazards, the most notable being enterotoxemia (ET) and sheep pox (SP). Both of these diseases inflict substantial losses in terms of reduced productivity and lower quality of wool and leather, thereby posing a major obstacle in the rearing of sheep. Clostridium perfringens is the causative agent of a wide variety of diseases and has been associated with a range of severe enterotoxemic diseases in many species of domestic animals, including sheep (6, 12, 15, 20, 21). Epsilon toxin (Etx), produced by C. perfringens types B and D, is responsible for a rapidly fatal ET in economically important livestock, especially sheep (2, 13). Etx is secreted as a relatively inactive prototoxin which, after treatment with trypsin, is converted to fully active toxin with the loss of an N-terminal peptide (5). The mature toxin is a highly toxic protein with lethal, dermonecrotic, and edematous activities (4). Clinical signs in intoxicated sheep may include colic, diarrhea, and numerous neurological symptoms. Postmortem analysis reveals widespread increases in vascular permeability, with cerebral, cardiac, pulmonary, and kidney edema (23, 26). Etx is classified as a category B overlap select agent by the U.S. Department of Health and Human Services and the U.S. Department of Agriculture. SP is a highly contagious disease of sheep caused by sheep pox virus (SPV). The disease is characterized by pyrexia, generalized skin lesions, internal pox lesions, and lymphoadenopathy. Outbreaks of SP, with very high morbidity and mortality rates, have been reported from different parts of the world regularly (6, 15, 20). It is also an animal bioterrorist agent and is listed as one of the 15 most economically important animal pathogens by Animal World Health Organization (OIE) and is grouped as a risk group II viral agent by Centers for Disease Control and Prevention (CDC), Atlanta, GA. Due to the rapid progression of the diseases among livestock animals, treatment is generally not possible or practical, and the emphasis is placed on prevention by vaccination (1). The commercial vaccine against enterotoxemia includes an inactivated whole-cell vaccine which is known to cause local reactions at the site of inoculation and is sometimes known to fail for reasons of potency. The commercial vaccine against SP is a freeze-dried live attenuated Romanian Fanar strain adapted to the Vero cell line. Live attenuated vaccines provide long-lasting immunity and, hence, are considered the best choice for use in vaccination (14). In this paper, we report for the first time the preparation of a combination recombinant vaccine containing recombinant epsilon toxoid (r-Etox) with aluminum hydroxide as an adjuvant and freeze-dried live attenuated SPV followed by its use as a vaccine in sheep. The combination vaccine protected the sheep against both ET and SP.
MATERIALS AND METHODS
Chemicals and plasmid.
All the fine chemicals used were obtained from Sigma Chemical Company (United States). Aluminum hydroxide (0.05%) used as an adjuvant was obtained from the Production Department of Indian Immunologicals Limited (IIL). pQE605-etx expressing the epsilon toxin gene (etx; ∼0.9 kb) and the M15 strain of Escherichia coli were obtained from the National Institute of Immunology, New Delhi, India. The components for mammalian cell culture were procured from Invitrogen.
Antisera, virus, cells, and bacteria.
WHO reference standard antitoxin used for the determination of potency of the vaccines was obtained from National Institute for Biological Standards and Control (NIBSC; 1,020 antitoxin units per vial) (catalog no. CPEPSILONAT), United Kingdom. Challenge sheep pox virus (titer, 2 × 106 50% lethal doses [LD50]/ml) and Vero and MDCK cells were obtained from the Quality Control Department of IIL. Cells were propagated in minimum essential medium (MEM; Sigma, United States) supplemented with 10% fetal bovine serum (Sigma, United States). Confluent monolayers (80 to 90%) were used for propagation and titration of SPV. Conventional Etx, prepared from cultures of Clostridium perfringens type D cultures, was obtained from the Production Department of IIL.
Vaccines.
RAKSHA-ET (commercial vaccine against enterotoxemia, containing inactivated whole-cell Clostridium perfringens) and RAKSHA-SP (commercial freeze-dried vaccine against sheep pox, containing the live attenuated Romanian Fanar strain), obtained from the Quality Control Department of IIL, were used as the positive controls in all animal experiments.
Animals.
Swiss Albino mice (17 to 22 g), New Zealand rabbits (1 to 1.5 kg), and Nellore sheep (4 to 6 months old; seronegative for enterotoxemia and sheep pox), obtained from the holding farm of the IIL, were used for immunization experiments.
Expression, purification, and inactivation of r-Etx to generate r-Etox.
Expression and large scale purification of the recombinant Etx (r-Etx) was done as described by Goswami et al. (10). The concentration of the purified protein was estimated using standard procedures (18), diluted to a concentration of 1 mg/ml using a phosphate-buffered diluent (10 mM phosphate buffer, pH 7.2, and 0.85% sodium chloride [PBS]) filter sterilized using 0.2 μm filters and stored at −20°C until further use. Inactivation was done by incubation of r-Etx with two doses of 0.5% formaldehyde (Sigma Chemical Co., United States) and incubation at 30°C for 24 h following each of the doses to yield recombinant epsilon toxoid (r-Etox). Residual toxicity of the r-Etox was determined in mice.
Determination of residual toxicity of r-Etox in mice.
Residual toxicity of the r-Etox was determined according to the procedure mentioned in the European Pharmacopoeia (7a). Briefly, 0.2 ml of r-Etox was inoculated intravenously into 10 mice. Lack of mortality in the mice 72 h postinoculation (hpi) indicated the absence of toxicity. This r-Etox was used in the preparation of the vaccine.
Titration of Etx.
Conventional Etx prepared from cultures of Clostridium perfringens type D was mixed in equal proportions with the varied dilutions of the standard commercial antitoxin, and the mixture was incubated at 30°C for 30 min. Residual toxicity of the mixture mentioned above in mice was determined, and the amount of antitoxin units (IU) of the toxin was calculated according to the method of Reed and Muench (22). The number of antitoxin units (IU) of the toxin was calculated as 3.79 (titrated epsilon toxin [t-Etx]).
Determination of antitoxin units (IU) of test sera by using t-Etx.
The t-Etx was mixed with varied dilutions of the test sera (antisera obtained from both rabbits and sheep) and incubated at 30°C for 30 min, and the residual toxicity in mice was determined by inoculating 0.2 ml of the mixture intravenously into 10 mice. Lack of mortality in the mice 72 hpi indicated neutralization of t-Etx. Based on the dilution factor, the number of antitoxin units (IU) of the test serum was determined, and it was directly correlated to the potency of the vaccine. A value of >5 antitoxin units (IU) per ml of serum was considered to be protective according to the European Pharmacopoeia (7a).
Determination of potency of sheep pox vaccine.
Sheep vaccinated with the SP vaccine were challenged on the 21st day postvaccination (dpv) with 2 × 106 LD50/ml of virulent SPV by the inoculation of 10-fold dilutions of virus at five sites (0.1 ml per site) on the ventral side of their abdomens. The sheep were monitored for pyrexia and clinical signs of sheep pox for a period of 14 days postchallenge (dpc). Potency of the vaccine was calculated according to the method of Reed and Muench (22) and expressed in the form of protective units (PU). A vaccine with a PU of >2.0 was considered potent.
Vaccine formulations using r-Etox.
Different amounts of the r-Etox in PBS (50, 100, 200, 300, and 500 μg) were supplemented with 0.05% aluminum hydroxide gel (algel) as an adjuvant and blended into doses of 1 ml each with 0.01% thiomersal as a preservative. The vaccine was stored at 4°C until further use.
Combination vaccine formulation incorporating the r-Etox and SP vaccine (combination vaccine).
A 50-dose equivalent of the r-Etox (200 μg/dose) with algel as an adjuvant in 50 ml was used as a diluent to reconstitute a vial of 50-dose freeze-dried RAKSHA-SP. Sheep were vaccinated intramuscularly with a 1-ml dose.
Determination of safety and potency of the r-Etox in rabbits and sheep.
The safety and potency of r-Etox with aluminum hydroxide as an adjuvant in both rabbits and sheep were determined. Eight groups of animals, each containing 5 rabbits and 10 sheep, were immunized subcutaneously (s.c.) with 2 doses of vaccine each containing 50, 100, 200, 300, or 500 μg of the r-Etox, RAKSHA-ET, algel alone, and PBS. All the animals were vaccinated twice at an interval of 3 weeks and bled at weekly intervals until the 35th day, followed by bleeding on the 60th and 90th days. The sera were stored at −20°C until further analysis.
Determination of potency of combination vaccine in sheep.
Six groups of 10 sheep each were immunized with 2 doses of vaccine each containing the combination vaccine (groups 1 and 2), RAKSHA-ET (group 3), RAKSHA-SP (group 4), algel alone (group 5), or PBS (group 6) subcutaneously. All the animals were vaccinated twice at an interval of 3 weeks and bled at weekly intervals until the 35th day, followed by bleeding on 60 and 90 dpv. The serum was stored at −20°C and used for determination of potency against ET, and the animals except those in group 1 were challenged for determination of potency against sheep pox.
RESULTS
Expression and purification of the r-Etx in E. coli and inactivation of the r-Etx to generate r-Etox.
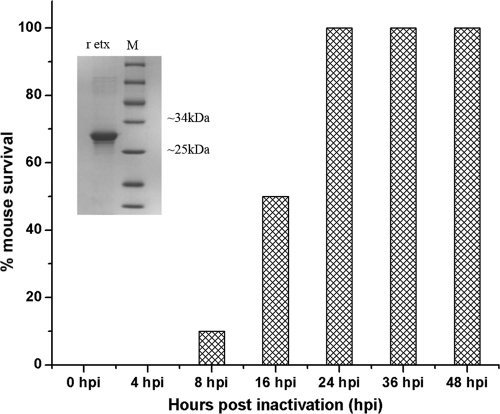
The r-Etx cloned into the bacterial expression vector pQE60-etx was expressed in strain M15 of E. coli. The protein was purified according to the method of Goswami et al. (10). Purification of the protein resulted in an ∼32-kDa protein with yields ranging from 10 to 12 mg/liter of culture (inset of Fig. 1). Following inactivation of r-Etx with formaldehyde, no residual toxicity was observed in mice 24 hpi (Fig. 1).
FIG. 1.
Determination of residual toxicity of r-Etox in mice. The inset shows purified r-Etx run on a 12% SDS-PAGE gel. Lane r-etox indicates the purified protein (∼32 kDa), and lane M shows the protein ladder (MBI Fermentas).
Determination of potency of r-Etox in rabbits and sheep.
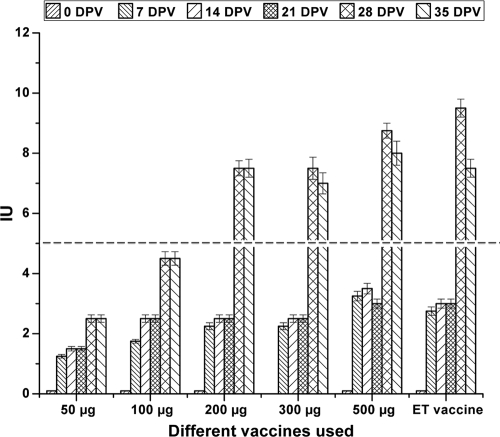
Serum samples collected from the animals were analyzed for potency. Vaccination of the rabbits and sheep led to a rise in serum potency of about ∼2 IU until the 21st dpv. Following the booster, all the animals except those in the groups vaccinated with 50 and 100 μg showed a rise in serum potency values ranging from approximately 7 to 8 IU on the 28th and 35th dpv (Fig. 2 and 3). Analysis of potencies of all samples on the 60th and 90th dpv showed values in the range of approximately 6 to 8 IU, indicating that the vaccine was potent enough to generate an immune response similar to the commercial vaccine (data not shown). The negative controls showed no rise in serum potency and remained negative throughout the experiment. Two-hundred micrograms of the r-Etox was used as the vaccine dose in all further experiments.
FIG. 2.
Determination of the potency of r-Etox in rabbits. Booster was given on the 21st dpv. The dashed line indicates the protective titers against r-Etox (a value of >5 IU is considered protective).
FIG. 3.
Determination of the potency of r-Etox in sheep. Booster was given on the 21st dpv. The dashed line indicates the protective titers against r-Etox (a value of >5 IU is considered protective).
Determination of potency of the combination vaccine in sheep.
Serum samples collected from sheep were used for determination of potency against ET. The negative controls showed no seroconversion and remained negative throughout the experiment. All the animals in groups 1, 2, and 3 (RAKSHA-ET) showed an increase in titers (>5 IU) following booster vaccination (Fig. 4). The sheep of groups 2 to 6 were challenged with virulent SPV on the 21st dpv and observed for 14 dpc for clinical manifestation of the disease. No pyrexia or any systemic or local reactions were observed in the vaccinated animals. All the vaccinated animals except those in groups 3, 5, and 6 withstood challenge. The potency of the vaccine was determined to be >3.0 PU (Fig. 5).
FIG. 4.
Determination of the potency of the combination vaccine. Booster was given on the 21st dpv. The dashed line indicates the protective titers against r-Etox (a value of >5 IU is considered protective).
FIG. 5.
Determination of the potency of the combination vaccine. The dashed line indicates the protection units following challenge against sheep pox virus (a value of >2 PU is considered protective).
DISCUSSION
Inactivated bacterial whole-cell vaccines have been the most widely studied prophylactic mode of treatment for infectious diseases. Though they offer an economical, potentially safe, and effective means of preventing disease, they also possess major disadvantages, which include parenteral administration causing adverse reactions in vaccinated animals, the use of high doses, and the resultant short-term immunity. These vaccines are prepared by a variety of methods, which include bacterial culture and inactivation. A variety of liquid culture media and, in some cases, solid agar media has been used to prepare the bacterial cell mass. However, culture conditions usually are geared towards quantitative (as opposed to qualitative) aspects of the yield. Time of culture has also varied from a few hours to 24 hours, resulting in bacteria harvested during early to late log phase or stationary growth phase (3, 8, 9, 11). Most often, bacteria are cultured at 37°C, and studies have addressed the significance of the culture method to the composition and antigenicity of bacteria utilized in vaccines (7, 17, 24, 25). This is in contrast to important information available in the scientific literature describing the effects of various factors that may be encountered in vivo by pathogens, which regulate expression of virulence factors and production of protective antigens (16, 19). Clearly, if a bacterium has not produced a protective antigen(s) at the time of inactivation, it is unlikely that a vaccine comprising this bacterium will be protective. Herein lies the principal reason for the unacceptable performance of a number of bacterial whole-cell vaccines.
The present investigation has demonstrated the use of the r-Etox as a vaccine candidate against enterotoxemia in sheep. The r-Etx was expressed in E. coli and purified to apparent homogeneity with a high yield. Inactivation of the r-Etx by using formaldehyde yielded the r-Etox, which was highly immunogenic in both rabbits and sheep. Repetition of this procedure a number of times yielded proteins that were similar with respect to yield and potency, indicating the standardization of the process. The present study clearly demonstrates the potential advantages of recombinant proteins as vaccines, which include low production costs, high yields, enhanced safety, and less antigenic competition, since only a few components are included in the vaccine. The concept of using a combined vaccine is not new and has become a regular practice with respect to vaccination in human health. With respect to animal health, combined vaccines already exist for foot-and-mouth disease and a number of bacterial diseases.
In this paper, we report for the first time the use of a combination vaccine consisting of r-Etox with algel as an adjuvant and a live attenuated SPV vaccine wherein the r-Etox is used as a diluent of the freeze-dried sheep pox vaccine. The vaccine yielded similar potency values of >5 IU with respect to the r-Etox and >2.0 PU with respect to the sheep pox vaccine, compared to the individual vaccines, thereby reiterating the fact that the combined vaccine could protect sheep from both diseases studied. The combination vaccines offer a practical way to overcome the constraints of multiple injections and improve timely vaccination coverage. Other potential advantages of combination vaccines include (i) reducing the cost of stocking and administering separate vaccines, (ii) reducing the cost for extra health care visits, and (iii) facilitating the addition of new vaccines into immunization programs. Besides, the combination vaccine represents a better economic value when one considers the direct and indirect costs of extra injections, delayed or missed vaccinations, and additional handling and storage.
The results presented in this paper not only strengthen the concept that the use of recombinant combined vaccines is a reality for the animal industry but also open a number of avenues for the use of the r-Etox in the development of other combination vaccines. Efforts are presently being directed toward the development of a glycoconjugate vaccine against Brucella spp., incorporating lipopolysaccharide from Brucella melitensis and r-Etox as a carrier protein.
Footnotes
Published ahead of print on 28 April 2010.
REFERENCES
- 1.Aiello, S. E. (ed.). 2003. Merck veterinary manual, 8th ed. Merck and Co., Inc., Whitehouse Station, NJ.
- 2.Arya, S. N., and D. K. Bhatia. 1992. Incidence of some livestock diseases in Tamil Nadu. Indian J. Anim. Res. 26:41-43. [Google Scholar]
- 3.Baqar, S., L. A. Applebee, and A. L. Bourgeois. 1995. Immunogenicity and protective efficacy of a prototype Campylobacter killed whole-cell vaccine in mice. Infect. Immun. 83:3731-3735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Batty, I., and A. T. Glenny. 1947. Titration of Clostridium welchii epsilon-toxin and antitoxin. Br. J. Exp. Pathol. 28:110-126. [PMC free article] [PubMed] [Google Scholar]
- 5.Bhown, A. S., and A. F. S. A. Habeeb. 1977. Structural studies on epsilon-prototoxin of Clostridium perfringens type D. Localization of the site of tryptic scission necessary for activation to epsilon-toxin. Biochem. Biophys. Res. Commun. 78:889-896. [DOI] [PubMed] [Google Scholar]
- 6.Carn, V. M. 1993. Control of capripox virus infections. Vaccine 11:1275-1279. [DOI] [PubMed] [Google Scholar]
- 7.Cripps, A. W., M. L. Dunkley, and R. L. Clancy. 1994. Mucosal and systemic immunizations with killed Pseudomonas aeruginosa protect against acute respiratory infection in rats. Infect. Immun. 62:1427-1436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7a.EDQM. 2008. European Pharmacopoeia, p. 363. Clostridium perfringens vaccines for veterinary use. EDQM, Strasbourg, France.
- 8.Finch, J. M., A. W. Hill, T. R. Field, and J. A. Leigh. 1994. Local vaccination with killed Streptococcus uberis protects the bovine mammary gland against experimental intramammary challenge with the homologous strain. Infect. Immun. 62:3599-3603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gilmour, N. J. L., W. Donachie, A. D. Sutherland, J. S. Gilmour, G. E. Jones, and M. Quirie. 1991. Vaccine containing iron-regulated proteins of Pasteurella haemolytica A2 enhances protection against experimental pasteurellosis in lambs. Vaccine 9:137-140. [DOI] [PubMed] [Google Scholar]
- 10.Goswami, P. P., P. Rupa, N. S. Prihar, and L. C. Garg. 1996. Molecular cloning of Clostridium perfringens epsilon-toxin gene and its high level expression in E. coli. Biochem. Biophys. Res. Commun. 226:735-740. [DOI] [PubMed] [Google Scholar]
- 11.Greco, D., S. Salmaso, P. Mastrantonio, M. Giuliano, A. E. Tozzi, A. Anemona, M. L. Ciofi Degli Atti, A. Giammanco, P. Panei, W. G. Blockwelder, D. L. Klein, S. G. F. Wassilak, and the Progetto Pertosse Working Group. 1996. A controlled trial of two acellular vaccines and one whole-cell vaccine against pertussis. N. Engl. J. Med. 334:341-348. [DOI] [PubMed] [Google Scholar]
- 12.Harbola, P. C., and A. A. Kumar. 1990. Clostridial infections in animals. Indian Vet. Med. J. 14:162-166. [Google Scholar]
- 13.Hunter, S. E., I. N. Clarke, D. C. Kelly, and R. W. Titball. 1992. Cloning and nucleotide sequencing of the Clostridium perfringens epsilon-toxin gene and its expression in Escherichia coli. Infect. Immun. 60:102-110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kitching, R. P. 1983. Progress towards sheep and goat pox vaccines. Vaccine 1:4-9. [DOI] [PubMed] [Google Scholar]
- 15.Kitching, R. P. 1986. The control of sheep and goat pox. Rev. Sci. Tech. 5:503-511. [DOI] [PubMed] [Google Scholar]
- 16.Konkel, M. E., D. J. Mead, and W. Cieolak, Jr. 1993. Kinetic and antigenic characterization of altered protein synthesis by Campylobacter jejuni during cultivation with human epithelial cells. J. Infect. Dis. 168:948-954. [DOI] [PubMed] [Google Scholar]
- 17.Lodinová-Zádníková, R., B. Korych, and K. Gajdlostikova. 1990. Local and systemic antibody response in infants after oral administration of inactivated enteropathogenic E. coli serotype 0111 and 055. Folia Microbiol. 35:155-162. [DOI] [PubMed] [Google Scholar]
- 18.Lowry, O. H., N. J. Rosebrough, A. L. Farr, and R. J. Randall. 1951. Protein measurement with the Folin phenol reagent. J. Biol. Chem. 193:265-275. [PubMed] [Google Scholar]
- 19.Mekalanos, J. J. 1992. Environmental signals controlling expression of virulence determinants in bacteria. J. Bacteriol. 174:1-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mullick, S. G. 1988. A preliminary study on the epidemiological aspect of sheep pox in some organised farms in India. Indian J. Comp. Microbiol. Immunol. Infect. Dis. 9:186-195. [Google Scholar]
- 21.Narayan, K. G. 1988. Clostridial infections in sheep and goats—a review. In N. Singh and S. C. Dubey (ed.), Proceedings of the second national seminar on sheep and goat diseases. CSWIRI, Avikanagar, Rajasthan, India.
- 22.Reed, L. J., and H. Muench. 1938. A simple method for estimating fifty percent end points. Am. J. Hyg. (Lond.) 27:493-497. [Google Scholar]
- 23.Songer, J. G. 1996. Clostridial enteric diseases of domestic animals. Clin. Microbiol. Rev. 9:216-234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Szalay, G., C. H. Ladel, and S. H. E. Kaufmann. 1995. 1995. Stimulation of protective CD8+T lymphocytes by vaccination with nonliving bacteria. Proc. Natl. Acad. Sci. U. S. A. 92:12389-12392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Thatte, J., S. Rath, and V. Bal. 1995. Analysis of immunization route-related variation in the immune response to heat-killed Salmonella typhimurium in mice. Infect. Immun. 63:99-103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Uzal, F. A., W. R. Kelly, W. E. Morris, J. Bermudez, and M. Baison. 2004. The pathology of peracute experimental Clostridium perfringens type D enterotoxemia in sheep. J. Vet. Diagn. Invest. 16:403-411. [DOI] [PubMed] [Google Scholar]