Abstract
By use of a mouse mucosal immunization model, LT-IIb(T13I), a nontoxic mutant type II heat-labile enterotoxin, was shown to have potent mucosal and systemic adjuvant properties. In contrast to LT-IIb, which binds strongly to ganglioside receptors decorated with either N-acetylneuraminic acid (NeuAc) or N-glycolylneuraminic acid (NeuGc), LT-IIb(T13I) binds NeuAc gangliosides much less well. Rather, LT-IIb(T13I) binds preferentially to NeuGc gangliosides. To determine if the adjuvant properties of LT-IIb(T13I) are altered in the absence of NeuGc ganglioside receptors, experiments were conducted using a Cmah-null mouse line which is deficient in the synthesis of NeuGc gangliosides. Several immunomodulatory properties of LT-IIb(T13I) were shown to be dependent on NeuGc gangliosides. LT-IIb(T13I) had reduced binding activity for NeuGc-deficient B cells and macrophages; binding to NeuGc-deficient T cells and dendritic cells (DC) was essentially undetectable. Treatment of Cmah-null macrophages with LT-IIb(T13I), however, upregulated the transcription of interleukin-4 (IL-4), IL-6, IL-17, and gamma interferon (IFN-γ), four cytokines important for promoting immune responses. The production of mucosal IgA and serum IgG against an immunizing antigen was augmented in NeuGc-deficient mice administered LT-IIb(T13I) as a mucosal adjuvant. Notably, NeuGc gangliosides are not expressed in humans. Still, treatment of human monocytes with LT-IIb(T13I) induced the secretion of IL-6, an inflammatory cytokine that mediates differential control of leukocyte activation. These results suggested that NeuAc gangliosides are sufficient to mediate the immunomodulatory properties of LT-IIb(T13I) in mice and in human cells. The nontoxic mutant enterotoxin LT-IIb(T13I), therefore, is potentially a new and safe human mucosal adjuvant.
Mucosal vaccines consisting solely of protein antigens (Ag) routinely induce only weak immune responses or tolerance at mucosal surfaces (50). Thus, to generate robust and long-lived immune responses to Ag at mucosal sites, an immune-stimulating agent(s) must be added to mucosal vaccines. These immune-stimulating agents are commonly referred to as mucosal adjuvants.
The strongest mucosal adjuvants described are members of the family of bacterial heat-labile enterotoxins (HLTs). This family is divided into two major groups based on genetic, biochemical, and immunological characteristics (25). A number of heat-labile enterotoxins are members of the type I HLT subfamily; these include cholera toxin (CT), produced by Vibrio cholerae, and LT (referred to below as LT-I), produced by Escherichia coli (25). The type II subfamily of HLT comprises LT-IIa (19, 20), LT-IIb (19, 20), and LT-IIc, a newly identified member of the subfamily (H. F. Nawar and T. D. Connell, unpublished data). When introduced into the gut by the expressing enteric bacteria, members of the type I and type II subfamilies of HLTs induce diarrhea in humans and animals (25).
Type I and type II enterotoxins are oligomeric proteins composed of an A polypeptide that is noncovalently coupled to a pentameric array of B polypeptides. The A polypeptide is enzymatically active and upregulates adenylate cyclase by catalyzing the ADP ribosylation of the Gsα regulatory protein (8, 35). Ribosylation of Gsα decouples regulated expression of adenylate cyclase, thus promoting the accumulation of intracellular cyclic AMP (cAMP). Elevation of cAMP levels indirectly induces the intoxicated cell to secrete chloride ions and likely modulates other processes for which cAMP is a signaling molecule (8, 25). The B polypeptides of the HLTs mediate the binding of the enterotoxins to receptors found on the surfaces of cells. The receptors for LT-IIa, LT-IIb, CT, and LT-I are one or more gangliosides, a heterogeneous family of sialylated glycosphingolipids. Notably, the sialic acid moieties of gangliosides are heterogeneous and include N-acetylneuraminic acid (NeuAc) and N-glycolylneuraminic acid (NeuGc), among many others (44). CT and LT-I bind with high affinity to GM1 (although LT-I also has affinity for an unknown glycoprotein) (40). CT also binds with low affinity (in descending order) to GM2, GD1a, GM3, GT1b, GD1b, and asialo-GM1 (27). LT-IIa binds specifically, in descending order of avidity, to gangliosides GD1b, GM1, GT1b, GQ1b, GM2, GD1a, and GM3. LT-IIb binds most avidly only to GD1a and, at much lower activities, to GM2 and GM3 (17). Differences in the ganglioside-binding properties and specificities of LT-IIa, LT-IIb, LT-I, and CT are hypothesized to be important in determining host, tissue, and cell specificity (25) and, in the context of this study, in promoting the distinguishable patterns of immunomodulatory properties observed in T cells and B cells (2, 34). While it is likely that the immunomodulatory properties of CT (and LT-I) depend on binding to ganglioside GM1 (12, 18), the receptors that mediate toxicity and elicit the potent and unique immunomodulatory properties of LT-IIb (or the other type II HLTs) have not been well described (10, 11).
The mucosal adjuvant activities of CT, LT-I, and LT-IIa, and those of their detoxified mutant enterotoxins, have been extensively characterized in a variety of animals (reviewed in references 10, 14, 16, 21, 32, 39, 41, and 47). Recent studies have shown, however, that one of the most promising mucosal adjuvants is LT-IIb (33). The potent mucosal immunomodulatory properties of LT-IIb have been well established by several studies using intranasal mouse immunization models (33, 38). Unfortunately, two issues have restricted the use of LT-IIb (and the other HLTs) as a human intranasal adjuvant: (i) LT-IIb is intrinsically toxic for most cells (25), and (ii) when used as an intranasal adjuvant, LT-IIb may have a propensity, as has been demonstrated for CT (45, 46), to induce inflammation in the brain after trafficking from the nasal epithelium into the brain via the olfactory nerve. In an attempt to remove these undesirable characteristics, mutant LT-IIb enterotoxins have been engineered (11). Toxicity, as measured using a high-resolution bioassay, was significantly diminished or undetectable in several of these mutant LT-IIb enterotoxins (11). Within this population of mutants, the most promising candidate was LT-IIb(T13I) (38). In addition to exhibiting significantly less toxic activity than the parental enterotoxin, LT-IIb(T13I) had no detectable binding activity in vitro for GD1a, GM2, and GM3, gangliosides that are avidly bound by LT-IIb (11, 38). However, LT-IIb(T13I), when employed as a mucosal adjuvant, augmented both mucosal and systemic antibody responses to a coadministered model Ag at levels equivalent to those induced by the use of wild-type (wt) LT-IIb (38).
Although it failed to bind to commercially available gangliosides (GD1a, GM2, and GM3), LT-IIb(T13I) bound to the surfaces of a variety of immunocompetent cells, including macrophages, CD4+ T cells, CD8+ T cells, and B cells (38). It was clear, therefore, that LT-IIb(T13I) interacted with one or more unknown receptors on those cells and that, in macrophages, those receptors had chemical characteristics compatible with a ganglioside (5). Using thin-layer chromatography (TLC) and immunoblotting methods, the ganglioside receptors for LT-IIb and LT-IIb(T13I) on RAW 264.7 cells and on murine peritoneal macrophages were identified (5). These experiments revealed that LT-IIb had binding affinity for a broader range of gangliosides than had been reported previously (5, 17). Moreover, LT-IIb(T13I) was shown to have preferential binding for NeuGc gangliosides while exhibiting diminished binding activity for NeuAc gangliosides (5).
Interestingly, most, if not all mammals, with the notable exception of humans, express NeuGc gangliosides. In humans, the deficiency is due to a frameshift mutation in Cmah, a gene encoding CMP-Neu5Ac hydroxylase, the enzyme that catalyzes the stepwise conversion of NeuAc to NeuGc (7, 9). The observation that LT-IIb(T13I) binds preferentially to NeuGc gangliosides and that NeuGc gangliosides are not expressed by human cells raised concerns that the potent immunomodulatory properties of LT-IIb(T13I) observed in mice might be lacking when the mutant HLT was used as a mucosal adjuvant in humans. To test this hypothesis, a mouse strain in which NeuGc gangliosides were absent was required. In 2007, Takematsu and colleagues engineered a Cmah-null mouse (36). This mutant mouse, therefore, was deemed an ideal model for evaluating whether NeuGc gangliosides are essential for the immunomodulatory properties of LT-IIb(T13I).
The results obtained in this study indicated that the mucosal and systemic adjuvant properties of LT-IIb(T13I) are, indeed, retained in the absence of NeuGc gangliosides. In combination with the observation that LT-IIb(T13I) exhibits significantly diminished toxicity in human cells, these studies provide strong experimental support for the potential of LT-IIb(T13I) to be a safe and potent adjuvant for use in humans.
MATERIALS AND METHODS
Enterotoxins.
Recombinant His-tagged LT-IIb and His-tagged LT-IIb(T13I) holotoxins were cloned, expressed, and purified using nickel affinity and gel filtration chromatography (38). Recombinant proteins were analyzed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) to confirm purity. A quantitative Limulus amebocyte lysate assay (Charles River Endosafe, Charleston, SC) was employed to determine the level of incidental endotoxin contamination in the purified preparations; all protein preparations were essentially free of lipopolysaccharide (LPS) (< 0.03 ng/μg of protein).
Gangliosides.
Purified gangliosides were obtained from Matreya, LLC (Pleasant Gap, PA) or were purified from mouse peritoneal macrophages (see below).
Binding of enterotoxins to lymphoid cells.
Single-cell suspensions were obtained from the spleens of naïve mice (38, 39). Total cell yield and viability were determined using a hemocytometer after staining of cells with trypan blue (Sigma-Aldrich, St. Louis, MO). The binding of LT-IIb and LT-IIb(T13I) to lymphoid cells isolated from the spleens of NeuGc-proficient (C57BL/6J) and NeuGc-deficient (Cmah-null) mice was detected by flow cytometry (38, 39). Briefly, 107 splenic cells were treated in vitro with 1.0 μg of LT-IIb or 1.0 μg of LT-IIb(T13I) and with antibodies to CD16/CD32 (PharMingen, San Diego, CA) (to block Fc receptors on the cells). After incubation on ice for 1 h, cells were washed and incubated on ice for 30 min with a pretitrated concentration of a polyclonal rabbit antibody (Ab) to LT-IIb. After a wash, cells were incubated with allophycocyanin (APC)-conjugated goat anti-rabbit immunoglobulin G (IgG) (0.5 μg/ml) to detect bound anti-LT-IIb antibodies and with a fluorescein isothiocyanate (FITC)-conjugated monoclonal antibody (MAb) to CD4, CD8, B220, CD11b, or CD11c (PharMingen, San Diego, CA). After incubation for 30 min on ice, cells were washed and resuspended in fluorescence-activated cell sorter (FACS) buffer containing 0.1 μg/ml of 7-amino-actinomycin D (7-AAD) (Calbiochem, Darmstadt, Germany) to distinguish living cells from dead cells. Data acquisition and analysis were performed using a FACScalibur flow cytometer and CellQuest software (both from Becton Dickinson, Franklin Lakes, NJ). Percentages of enterotoxin-bound cells in mixed cell populations were calculated by gating for FITC+ APC+ 7-AAD− fluorescence. Percentages of cells in cell populations bound by LT-IIb or LT-IIb(T13I) were quantified by correcting for the percentage of control cells exhibiting nonspecific binding of the APC-conjugated antibody.
Animals and immunization.
For immunization experiments, a well-established mouse mucosal immunization model was employed (33, 39). Unanesthetized wt female C57BL/6J mice (The Jackson Laboratory, Bar Harbor, ME) or homozygous Cmah-null mice (36) approximately 8 to 10 weeks of age were immunized by the intranasal (i.n.) route. Groups of 5 to 6 mice were immunized 3 times at 2-week intervals with 10 μg of AgI/II, a streptococcal Ag (42), alone or in combination with 1.0 μg of LT-IIb or 1.0 μg of LT-IIb(T13I). Immunizations were administered in a standardized volume (10.0 μl) that was applied slowly to both external nostrils. Animal experiments were approved by the Institutional Animal Care and Use Committee of the University at Buffalo.
Collection of secretions and sera.
Serum, saliva, and vaginal wash samples were collected from individual mice at a point 1 week before the initial immunization and at 2 weeks after the third immunization, corresponding to the time point of peak production of antigen-specific antibodies (33, 38). Mucosal secretions and serum samples were stored at −70°C.
Antibody analysis.
Levels of isotype-specific antibodies in saliva, sera, and vaginal wash specimens were determined by enzyme-linked immunosorbent assays (ELISA) (38).
Purification of total gangliosides from murine peritoneal macrophages.
Gangliosides were isolated from peritoneal macrophages obtained from thioglycolate-treated C57BL/6J or Cmah-null mice (5, 6).
TLC.
Two-dimensional (2-D) thin-layer chromatography (TLC) was used to resolve gangliosides (5). Standard ganglioside markers (Matreya, LLC) were included in each dimension for reference. Gangliosides on the TLC plates were visualized by spraying the plates with resorcinol-hydrochloric acid reagent (43).
Sialic acid quantification.
The ganglioside-bound sialic acid of murine macrophages was quantified, relative to known quantities (0.5 to 8.0 μg) of sialic acid (Sigma), by densitometric scanning (Molecular Dynamics, Sunnyvale, CA) of thin-layer chromatograms (15). Briefly, sialic acid was visualized by resorcinol spraying, and the sialic acid contents of macrophage gangliosides were quantified using a standard curve (r2, 0.98). Results were expressed as the mean amounts of ganglioside-bound sialic acid per 106 cells ± the standard errors of the means.
Primary cell isolation, culture, and cytokine induction.
Primary human monocytes were purified from peripheral blood by centrifugation over a NycoPrep 1.068 gradient (Axis-Shield, Oslo, Norway) (24). Incidental nonmonocytic cells were removed by magnetic depletion using a cocktail of biotin-conjugated MAbs and magnetic microbeads coupled to anti-biotin MAbs (Monocyte isolation kit II; Miltenyi Biotec, Auburn, CA). Purified monocytes were cultured at 37°C under a 5% CO2 atmosphere in RPMI 1640 (Invitrogen/Gibco) supplemented with 10% heat-inactivated fetal bovine serum (FBS) (Gibco), 2 mM l-glutamine, 10 mM HEPES (Sigma), 100 U/ml penicillin G, 100 μg/ml streptomycin (Gibco), and 0.05 mM 2-mercaptoethanol (Gibco). Human blood was collected in compliance with established federal guidelines and institutional policies.
Thioglycolate-elicited macrophages were isolated from the peritoneal cavities of Cmah-null mice and wt C57BL/6J mice (5, 6). Mouse primary macrophages were cultured in complete RPMI 1640. In comparison to medium-only control treatments, none of the experimental treatments significantly affected cell viability. Human monocytes (2 × 105/well) or mouse macrophages (2 × 106/well) were treated for 16 h with 2.0 μg/ml of either LT-IIb or LT-IIb(T13I). Supernatants of human monocytes were analyzed for interleukin 6 (IL-6) using an IL-6-specific ELISA kit (eBioscience, San Diego, CA).
Total RNA was isolated from mouse macrophages using a Qiagen RNeasy minikit (Qiagen, Valencia, CA). An on-column DNase digestion reaction (Qiagen) was employed to eliminate contaminating DNA. cDNA was synthesized from 1.0 μg of total RNA using an iScript cDNA synthesis kit (Bio-Rad, Hercules, CA). Real-time quantitative PCRs (qRT-PCR) were performed using 50 ng of the synthesized cDNA, specific synthetic oligonucleotide primer sets (Integrated DNA Technologies, Coralville, IA) (Table 1), and the iQ SYBR green Supermix kit (Bio-Rad) with the following reaction conditions: 1 cycle of 95°C for 10 min and 40 cycles of 95°C for 15 s and 60°C for 1 min. Melting curve analyses (55°C to 95°C) were performed using a MyiQ single-color real-time PCR detection system (Bio-Rad). Fold changes in cytokine mRNA levels (treated versus control cells) that are greater than 2 or less than 0.5 are considered to be significant changes.
TABLE 1.
Nucleotide sequences of synthetic oligonucleotide sets used in qRT-PCR
| Cytokinea | Primer direction | Primer sequence |
|---|---|---|
| β-Actin | Forward | 5′-AGAGGGAAATCGTGCGTGAC-3′ |
| Reverse | 5′-CAATAGTGATGACCTGGCCGT-3′ | |
| GAPDH | Forward | 5′-TCACCACCATGGAGAAGGC-3′ |
| Reverse | 5′-GCTAAGCAGTTGGTGGTGCA-3′ | |
| IL-1β | Forward | 5′-CAACCAACAAGTGATATTCTCCATG-3′ |
| Reverse | 5′-GATCCACACTCTCCAGCTGCA-3′ | |
| IL-2 | Forward | 5′-CCTGAGCAGGATGGAGAATTACA-3′ |
| Reverse | 5′-TCCAGAACATGCAGAAGAG-3′ | |
| IL-4 | Forward | 5′-ACAGGAGAAGGG ACGCCAT-3′ |
| Reverse | 5′-GAAGCCCTACAGACGAGGTCA-3′ | |
| IL-6 | Forward | 5′-GAGGATACCACTCCCAACAGACC-3′ |
| Reverse | 5′-AAGTGCATCATCGTTGTTCATACA-3′ | |
| IL-10 | Forward | 5′-GGTTGCCAAGCCTTATCGGA-3′ |
| Reverse | 5′-ACCTGCTCCACTGCCTTGCT-3′ | |
| IL-12p40 | Forward | 5′-GGAAGCACGGCAGCAGAATA-3′ |
| Reverse | 5′-AACTTGAGGGAGAAGTAGGAATGG-3′ | |
| IL-17 | Forward | 5′-GCTCCAGAAGGCCCTCAGA-3′ |
| Reverse | 5′-AGCTTTCCCTCCGCATTGA-3′ | |
| TGF-β | Forward | 5′-TGACGTCACTGGAGTTGTACGG-3′ |
| Reverse | 5′-GGTTCATGTCATGGATGGTGC-3′ | |
| TNF-α | Forward | 5′-CATCTTCTCAAAATTCGAGTGACAA-3′ |
| Reverse | 5′-TGGGAGTAGACAAGGTACAACCC-3′ | |
| IFN-γ | Forward | 5′-TCAAGTG GCATAGATGTGGAAGAA-3′ |
| Reverse | 5′-TGGCTCTGCAGGATTTTCATG-3′ |
GAPDH, glyceraldehyde-3-phosphate dehydrogenase.
Binding of enterotoxin to murine macrophage and human monocyte monolayers.
Human monocytes or mouse macrophages were allowed to adhere overnight to the bottoms of 96-well flat-bottom tissue culture plates (BD Biosciences, Franklin Lakes, NJ) (105 cells/well in 100 μl of RPMI 1640 complete medium). Cell monolayers were fixed for 30 min with 2% glutaraldehyde, washed 3 times with phosphate-buffered saline (PBS), and treated with 100 μl of 1.0-μg/ml LT-IIb or LT-IIb(T13I) (diluted in PBS, 1% bovine serum albumin [BSA] [Amresco, Solon, OH], and 0.25% Triton X-100 [Sigma]) per well. Plates were incubated at room temperature (RT) for 2 h to allow sufficient time for binding of the enterotoxins to the cells. After the cells were washed with PBS, 100 μl of rabbit anti-LT-IIb antiserum (diluted 1:5,000 in PBS, 1% BSA, and 0.25% Triton X-100) was added to the wells. Plates were incubated overnight at 4°C and were washed to remove unbound antibodies. A 100-μl aliquot of a 1.0-μg/ml solution of an alkaline phosphatase-conjugated goat anti-rabbit IgG secondary Ab (Southern Biotech, Birmingham, AL) was added to each well. Plates were incubated for 1 h at 37°C, washed with PBS, and immediately developed by adding 100 μl/well of 1.0-mg/ml nitrophenyl phosphate (Amresco, Solon, OH) diluted in diethanolamine buffer (100 ml of diethanolamine, 1 mM MgCl2, and deionized H2O to 1 liter [pH 9.8]). Color reactions were terminated by the addition of 100 μl of 2.0 M NaOH to each well. The optical density of the color reaction was measured at 405 nm using a VersaMax microplate reader (Molecular Devices, Sunnyvale, CA).
Detection of cAMP.
To measure the accumulation of cAMP in human monocytes (38), cells (106 per well) were cultured in triplicate for 7 days in 12-well tissue culture plates (BD Biosciences) at 37°C under a 5% CO2 atmosphere. The culture medium was removed and replaced with 1 ml of fresh culture medium with or without 1.0 μg of LT-IIb, LT-IIb(T13I), or the B pentamer of LT-IIb (LT-IIb-B5). After incubation at 37°C for 4 h, cells were extracted for 20 min at RT with 200 μl of 0.1 M HCl, scraped from the wells, and centrifuged to clear the extracts of cell debris. Levels of cAMP in the extracts were measured in duplicate using a cAMP enzyme immunoassay kit (Cayman Chemical Co., Ann Arbor, MI).
Statistical analyses.
Analysis of variance (ANOVA) and the Dunnett control-comparison test were employed for comparisons of data. Unpaired t tests with Welch corrections were performed to analyze differences between pairs of data. All statistical analyses were performed using InStat software (GraphPad, San Diego, CA).
RESULTS
Binding of enterotoxins to lymphocytes.
The adjuvant properties of LT-IIa and LT-IIb are highly correlated with the binding activities of those HLTs for gangliosides and immunocompetent cells (38, 39). In contrast to LT-IIb, which binds strongly to both NeuGc- and NeuAc-containing gangliosides, LT-IIb(T13I), a nontoxic mutant of LT-IIb that retains adjuvant properties, preferentially binds to NeuGc gangliosides (5). Thus, it was deemed critical for the goal of understanding the adjuvant properties of LT-IIb(T13I) to determine whether LT-IIb(T13I) had the capacity to bind to various immunocompetent cells that lacked NeuGc gangliosides.
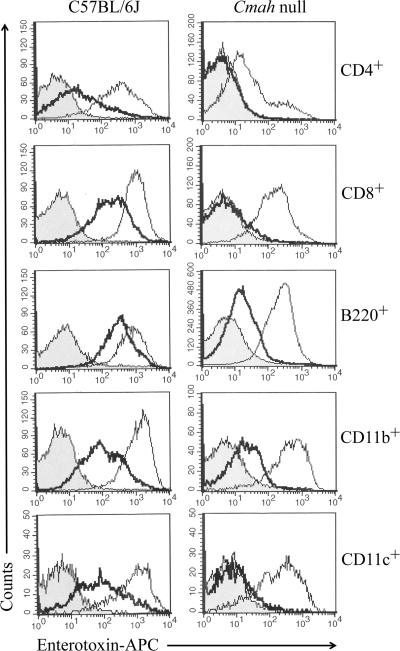
To address this question, flow cytometric analysis of the binding patterns of LT-IIb and LT-IIb(T13I) for immunocompetent cell types was performed. LT-IIb bound to the vast majority of CD4+ T cells (94.9%), CD8+ T cells (98.56%), B cells (94.82%), macrophages (98.47%), and dendritic cells (DC) (97.89%) obtained from C57BL/6J mice (Fig. 1 and Table 2). With the exception of CD4+ T cells (37.05%), LT-IIb(T13I) bound to essentially the same, or slightly lower, numbers of CD8+ T cells, B cells, macrophages, and DC (87.57%, 92.43%, 74.10%, and 77.26%, respectively) as LT-IIb. When cells from Cmah-null mice were evaluated, a different pattern of binding was observed. Again, with the exception of CD4+ T cells (94.9% versus 41.55%), LT-IIb bound to essentially the same numbers of CD8+ T cells, B cells, macrophages, and DC regardless of their genetic derivation (Cmah-null mice or C57BL/6J mice). In contrast, the binding of LT-IIb(T13I) to CD4+ T cells, CD8+ T cells, and DC isolated from Cmah-null mice was reduced to almost undetectable levels (1.76%, 3.75%, and 3.56%, respectively). LT-IIb(T13I) was found to bind a portion of the B cells and macrophages isolated from Cmah-null mice (23.77% and 40.71% of cells, respectively). When the flow cytometric data were evaluated using mean fluorescence intensity (MFI), the patterns of binding of LT-IIb and LT-IIb(T13I) to cells from C57BL/6J or Cmah-null mice were similar to those determined by the percentages of cells binding to enterotoxin (Table 3). In brief, LT-IIb(T13I) bound fewer numbers of immunocompetent cells of each type from Cmah-null mice than did LT-IIb.
FIG. 1.
Patterns of binding of LT-IIb and LT-IIb(T13I) to splenic lymphoid cells isolated from C57BL/6J or Cmah-null mice. Histograms were gated on CD4+ (helper T cells), CD8+ (cytotoxic T cells), B220+ (B cells), CD11b+ (macrophages), or CD11c+ (DC) cells. Dead cells were excluded by staining with 7-AAD. Shaded histograms, control cells (nonspecific binding of antibodies in the absence of enterotoxin); thin-line histograms, LT-IIb; bold-line histograms, LT-IIb(T13I). A shift of the fluorescence intensity to the left indicates a decrease in or absence of binding of enterotoxin to the cells. Histograms are derived from data obtained from one of three independent replicate experiments. APC, allophycocyanin.
TABLE 2.
Percentages of helper T cells, cytotoxic T cells, B cells, macrophages, and DC, isolated from C57BL/6J and Cmah-null mice, that bound to LT-IIb and LT-IIb(T13I)
| Cell typea | % of cells bound by enterotoxin (mean ± 1 SD) (n = 3) for: |
|||
|---|---|---|---|---|
| C57BL/6J mice |
Cmah-null mice |
|||
| LT-IIb | LT-IIb(T13I) | LT-IIb | LT-IIb(T13I) | |
| CD4+ | 94.90 ± 2.45 | 37.05 ± 1.89 | 41.55 ± 2.77 | 1.76 ± 0.98 |
| CD8+ | 98.56 ± 3.02 | 87.57 ± 3.07 | 90.83 ± 3.25 | 3.75 ± 1.08 |
| B220+ | 94.82 ± 2.22 | 92.43 ± 4.01 | 86.50 ± 2.99 | 23.77 ± 4.16 |
| CD11b+ | 98.47 ± 2.09 | 74.10 ± 2.41 | 90.38 ± 4.98 | 40.71 ± 3.66 |
| CD11c+ | 97.89 ± 2.76 | 77.26 ± 1.55 | 87.39 ± 3.63 | 3.56 ± 1.73 |
CD4+, helper T cells; CD8+, cytotoxic T cells; B220+, B cells; CD11b+, macrophages; CD11c+, DC.
TABLE 3.
Mean florescence intensity of enterotoxin-APC bound to splenic lymphoid cells isolated from C57BL/6J or Cmah-null mice
| Cell type | MFI of enterotoxin-APC bound to splenic lymphoid cells (mean ± 1 SD) (n = 3) for: |
|||
|---|---|---|---|---|
| C57BL/6J mice |
Cmah-null mice |
|||
| LT-IIb | LT-IIb(T13I) | LT-IIb | LT-IIb(T13I) | |
| CD4+ | 297.74 ± 22.42 | 78.47 ± 9.78 | 141.78 ± 12.77 | 34.43 ± 4.98 |
| CD8+ | 856.92 ± 36.02 | 171.18 ± 13.07 | 214.58 ± 23.52 | 41.47 ± 7.28 |
| B220+ | 671.79 ± 33.91 | 303.64 ± 19.69 | 291.30 ± 25.37 | 42.69 ± 5.32 |
| CD11b+ | 1,011.12 ± 43.11 | 155.35 ± 18.41 | 569.29 ± 35.59 | 49.33 ± 6.89 |
| CD11c+ | 807.98 ± 26.76 | 256.74 ± 24.95 | 383.72 ± 29.61 | 46.22 ± 7.13 |
Peritoneal macrophage ganglioside expression.
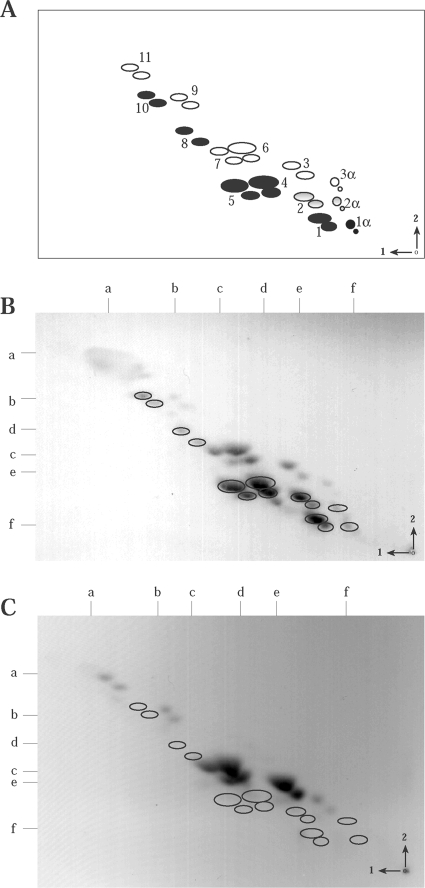
Although LT-IIb(T13I) binds preferentially to NeuGc gangliosides, the mutant enterotoxin retains reasonably strong binding activities for NeuAc gangliosides (5). Thioglycolate-elicited murine peritoneal macrophages, with an array of 28 distinct ganglioside spots of known structures that include broad diversity in carbohydrate structure and sialic acid composition (49), have served as a model for LT-IIb ganglioside binding (5). To verify the absence of NeuGc gangliosides in Cmah-null mice, peritoneal macrophage gangliosides of both C57BL/6J and Cmah-null mice were carefully analyzed by 2-D TLC (Fig. 2). C57BL/6J macrophages contained all previously described murine macrophage ganglioside structures (Fig. 2B) (49). In contrast, macrophages of Cmah-null mice were missing specific ganglioside spots that correlated with the positions of NeuGc gangliosides (NeuGc gangliosides are highlighted by ellipses in Fig. 2B; positions from which those gangliosides are missing are denoted by ellipses in Fig. 2C and are summarized in Table 4).
FIG. 2.
Two-dimensional thin-layer chromatograms for gangliosides isolated from macrophages obtained from thioglycolate-treated C57BL/6J mice (B) or homozygous Cmah-null mice (C) and the corresponding schematic diagram (A). (A) Solid ellipses indicate NeuGc gangliosides; shaded ellipses indicate mixed NeuGc- and NeuAc-containing gangliosides; open ellipses indicate NeuAc gangliosides. Numbers in the schematic diagram (reprinted from reference 49 with permission of the publisher) correspond to structures as follows: 1α, GD1α-NeuGc; 2α, GD1α-NeuAc/NeuGc; 3α, GD1α-NeuAc; 1, GD1a-NeuGc; 2, GDla-NeuAc/NeuGc; 3, GD1a-NeuAc; 4, GM1b-NeuGc; 5, GM1a-NeuGc; 6, GM1b-NeuAc; 7, GM1a-NeuAc; 8, GM2-NeuGc; 9, GM2-NeuAc; 10, GM3-NeuGc; 11, GM3-NeuAc. (B and C) Ellipses denote NeuGc gangliosides expressed in C57BL/6J mice (B) or the positions of the missing NeuGc gangliosides in Cmah-null mice (C). Arrows and numbers in the lower right corner indicate the directions of movement of the first and second solvents. The origin is denoted by a dot in the lower right corner. Ganglioside standards, resolved in each dimension, are noted along the top (solvent 1) and left (solvent 2) of the chromatographs as follows: a, GM3; b, GM2; c, GM1; d, GD3; e, GD1a; f, GD1b.
TABLE 4.
Gangliosides expressed by peritoneal macrophages obtained from thioglycolate-treated Cmah-null micea
| Spot no. | Ganglioside |
|---|---|
| Missing gangliosides | |
| 1α | GD1α-NeuGc |
| 2α | GD1α-NeuAc/NeuGc |
| 1 | GD1a-NeuGc |
| 2 | GD1a-NeuAc/NeuGc |
| 4 | GM1b-NeuGc |
| 5 | GM1a-NeuGc |
| 8 | GM2-NeuGc |
| 10 | GM3-NeuGc |
| Expressed gangliosides | |
| 3α | GD1α-NeuAc |
| 3 | GD1a-NeuAc |
| 6 | GM1b-NeuAc |
| 7 | GM1a-NeuAc |
| 9 | GM2-NeuAc |
| 11 | GM3-NeuAc |
NeuAc gangliosides, however, were relatively overrepresented in macrophages from Cmah-null mice compared with the expression of those gangliosides in macrophages from C57BL/6J mice (Fig. 2C). In fact, macrophages of C57BL/6J and Cmah-null mice did not differ statistically in the amounts of ganglioside-bound sialic acid (243.9 ± 13.3 ng/106 cells versus 287.9 ± 13.6 ng/106 cells, respectively). Therefore, the loss of NeuGc gangliosides in Cmah-null mice is compensated for by increased expression of NeuAc gangliosides, with a relative consistency in total sialic acid quantity. Thus, the binding of LT-IIb(T13I) to Cmah-null cells (Table 2) correlates with the overexpression of NeuAc gangliosides on those cells.
Mucosal and systemic adjuvant properties of LT-IIb(T13I) in Cmah-null mice.
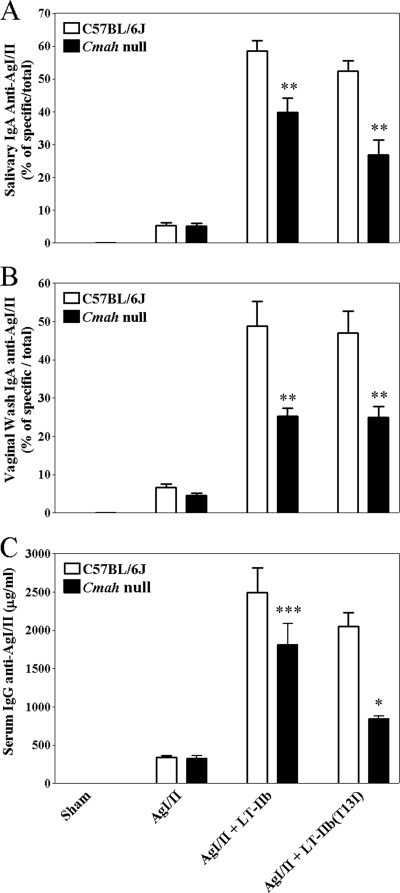
Both LT-IIb and LT-IIb(T13I) have been shown to be potent mucosal and systemic adjuvants in a mucosal immunization model (38). Since LT-IIb(T13I) had diminished binding activity for immunocompetent cells from Cmah-null mice, it was hypothesized that the immunomodulatory properties of LT-IIb(T13I) would be absent or dramatically reduced when that mutant HLT was used as a mucosal adjuvant in a mucosal immunization model employing Cmah-null mice. As expected, LT-IIb, when coadministered with AgI/II, a model antigen from Streptococcus mutans (42), enhanced the levels of Ag-specific IgA in the saliva (58.53% ± 3.16% of total IgA) and in the vaginal vault (48.87% ± 6.41% of total IgA) and the levels of Ag-specific IgG (2,498.72 ± 316.58 μg of IgG/ml) in the sera of C57BL/6J mice (Cmah proficient) over those observed after immunization with AgI/II alone (5.44% ± 0.83% of total salivary IgA; 6.71% ± 0.96% of total vaginal IgA; 339.73 ± 26.63 μg of IgG/ml) (Fig. 3). A similar enhancement of Ag-specific systemic (2,055.02 ± 175.81 μg of IgG/ml) and mucosal (Ag-specific IgA constituted 52.40% ± 3.12% of total salivary IgA and 47.02% ± 5.77% of total vaginal IgA) immune responses to AgI/II was observed when C57BL/6J mice were administered LT-IIb(T13I) as a mucosal adjuvant. Ag-specific immune responses were also elevated when LT-IIb was employed as an adjuvant in Cmah-null mice (1,809.04 ± 278.01 μg of IgG/ml; Ag-specific IgA constituted 39.79% ± 4.35 of total salivary IgA and 25.27% ± 2.06% of total vaginal IgA). Contrary to the hypothesis, however, LT-IIb(T13I) retained potent adjuvant properties when used as a mucosal adjuvant in Cmah-deficient mice. Coadministration of LT-IIb(T13I) with AgI/II elicited levels of anti-AgI/II IgA at proximal (oral cavity) (26.96% ± 4.52% of total salivary IgA) and distal (24.98% ± 2.82% of total vaginal IgA) mucosal sites significantly higher than those induced by administration of AgI/II alone (5.21% ± 0.79% and 4.62% ± 0.58% of total IgA, respectively) (Fig. 3A and B, respectively). The adjuvant activities of LT-IIb(T13I) were not limited to mucosal sites. Higher levels of anti-AgI/II IgG were elicited in the sera of mice (846.32 ± 33.23 μg/ml) when LT-IIb(T13I) was employed as a mucosal adjuvant than when mice were immunized solely with AgI/II (327.56 ± 35.68 μg/ml) (Fig. 3C). In contrast to expectations, these data demonstrated that LT-IIb(T13I) retained potent mucosal and systemic adjuvant properties in mice that do not express NeuGc gangliosides.
FIG. 3.
Effects of LT-IIb and LT-IIb(T13I) on salivary IgA (A), vaginal IgA (B), and serum IgG (C) antibody responses to AgI/II. C57BL/6J and Cmah-null mice were immunized intranasally on days 1, 14, and 28 with 10 μg of AgI/II in the presence or absence of 1 μg of LT-IIb or LT-IIb(T13I) as a mucosal adjuvant. Samples were collected 2 weeks after the second booster immunization at the time of peak Ag-specific antibody response. The data are arithmetic means ± standard errors of the means (n, 5 to 6). Statistical differences from the group immunized only with AgI/II are noted as follows: ***, P < 0.001; **, P < 0.01; *, P < 0.05.
While LT-IIb and LT-IIb(T13I) induced serum anti-HLT IgG in both C57BL/6J and Cmah-null mice, the levels of anti-enterotoxin IgG were lower in Cmah-null mice than in C57BL/6 mice (data not shown).
Cytokine modulation by LT-IIb(T13I) in NeuGc-deficient macrophages.
In the course of an Ag-specific immune response, T cells, antigen-presenting cells, and other immunocompetent cells communicate, in part, by secreting and binding various cytokines (4). To evaluate the capacities of LT-IIb and LT-IIb(T13I) to modulate cytokine expression in immunocompetent cells in the presence or absence of NeuGc gangliosides, a transcriptional approach was chosen. Transcriptional expression levels of IL-1β, IL-2, IL-4, IL-6, IL-10, IL-12p40, IL-17, transforming growth factor β (TGF-β), tumor necrosis factor alpha (TNF-α), and gamma interferon (IFN-γ), 10 cytokines that are important in regulating typical immune responses, were determined in cultured peritoneal macrophages obtained from both NeuGc-proficient (C57BL/6J) and NeuGc-deficient (Cmah-null) mice after treatment with LT-IIb or LT-IIb(T13I).
LT-IIb dramatically modulated the expression of IL-1β, IL-2, IL-6, IL-10, IL-17, TNF-α, and IFN-γ in peritoneal macrophages isolated from C57BL/6J mice. Treatment of the cells with LT-IIb induced elevated transcription of IL-1β, IL-6, and IL-17, and reduced transcription of IL-2, IL-10, TNF-α, and IFN-γ, in comparison with transcriptional levels in untreated cells. The transcription of IL-4, IL-12p40, and TGF-β was not altered by treatment of the cells with LT-IIb. Treatment of the macrophages with LT-IIb(T13I) modulated only the expression of IL-1β, IL-2, IL-10, IL-17, and TNF-α in comparison to the expression of those cytokines in untreated cells. In contrast, the effects of LT-IIb and LT-IIb(T13I) on the transcriptional patterns of the 10 cytokines were more prominent in peritoneal macrophages obtained from Cmah-null mice (Table 5). Expression of IL-1β, IL-6, and IFN-γ was highly induced by either LT-IIb or LT-IIb(T13I) in NeuGc-deficient macrophages. Expression of IL-10 by those NeuGc-deficient cells was suppressed by both enterotoxins. Relative to expression in C57BL/6J cells, treatment of Cmah-null macrophages with LT-IIb elicited hyperresponsive expression of IL-2 and IL-17 (0.22-fold versus 233.96-fold and 4.64-fold versus 226.58-fold, respectively). A similar pattern of hyperresponsive expression of IL-2 and IL-17 was observed for Cmah-null macrophages treated with LT-IIb(T13I) (0.34-fold versus 103.03-fold; 2.05-fold versus 30.94-fold). The transcriptional expression of IL-12p40, TGF-β, and TNF-α by Cmah-null macrophages was essentially unaltered after treatment with either LT-IIb or LT-IIb(T13I).
TABLE 5.
Fold changes in the levels of cytokine mRNAs in peritoneal macrophages treated with LT-IIb or LT-IIb(T13I) from the levels of cytokine mRNAs in untreated control cells
| Cytokine | Fold change in mRNA levela (mean ± SD) (n = 3) |
|||
|---|---|---|---|---|
| C57BL/6J mice |
Cmah-null mice |
|||
| LT-IIb | LT-IIb(T13I) | LT-IIb | LT-IIb(T13I) | |
| IL-1β | 3.27 ± 0.43 | 4.73 ± 1.18 | 32.66 ± 1.48 | 21.67 ± 1.09 |
| IL-2 | 0.22 ± 0.12 | 0.34 ± 0.33 | 233.96 ± 55.33 | 103.03 ± 2.14 |
| IL-4 | 0.92 ± 0.12 | 1.06 ± 0.30 | 2.64 ± 0.43 | 2.68 ± 0.84 |
| IL-6 | 4.06 ± 0.40 | 1.60 ± 0.16 | 40.14 ± 2.65 | 10.16 ± 1.51 |
| IL-10 | 0.16 ± 0.04 | 0.27 ± 0.06 | 0.26 ± 0.01 | 0.42 ± 0.14 |
| IL-12p40 | 1.26 ± 0.06 | 1.14 ± 0.31 | 0.91 ± 0.02 | 1.01 ± 0.08 |
| IL-17 | 4.64 ± 1.29 | 2.05 ± 1.13 | 226.58 ± 57.58 | 30.94 ± 1.51 |
| TGF-β | 0.71 ± 0.01 | 0.66 ± 0.20 | 1.01 ± 0.18 | 0.88 ± 0.14 |
| TNF-α | 0.48 ± 0.06 | 0.46 ± 0.10 | 1.93 ± 0.36 | 1.60 ± 0.10 |
| IFN-γ | 0.57 ± 0.11 | 0.54 ± 0.12 | 12.36 ± 1.74 | 4.97 ± 1.10 |
From the mRNA level in untreated control cells.
Immunomodulatory activity of LT-IIb(T13I) in human monocytes.
In contrast to expression in other mammals, NeuGc gangliosides are not expressed in humans due to a genetic deficiency in Cmah, the gene encoding the enzyme essential for the hydroxylation of NeuAc into NeuGc (7, 9). The observation that LT-IIb(T13I) enhanced the immune response to a coadministered Ag in the Cmah-null mouse, a human mimic with regard to ganglioside decoration with NeuAc, prompted subsequent experiments to determine whether LT-IIb(T13I) retained the capacity to bind to human monocytes and to induce important immunomodulatory activities by those cells.
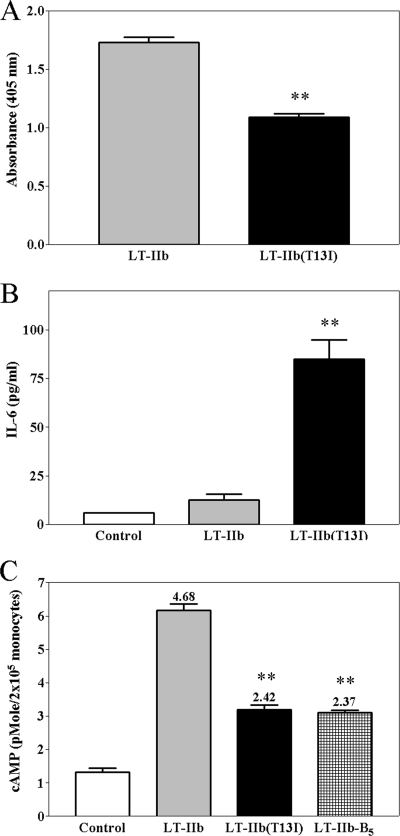
The binding of LT-IIb and LT-IIb(T13I) to primary human monocytes was evaluated using a cell-based ELISA. As expected, based on the ELISA signal intensity, LT-IIb bound avidly to human monocytes (Fig. 4 A). Similarly, LT-IIb(T13I) bound to human monocytes, albeit at a slightly lower ELISA signal intensity.
FIG. 4.
Effects of LT-IIb and LT-IIb(T13I) on human monocytes. (A) Binding of LT-IIb and LT-IIb(T13I) to human monocytes. (B) Induction of IL-6 by human monocytes after treatment with LT-IIb or LT-IIb(T13I). (C) Accumulation of cAMP in human monocytes treated with LT-IIb, LT-IIb(T13I), or LT-IIb-B5. Fold increases (cAMP levels in treated cells/cAMP levels in untreated cells) for each set are given above the bars. The data are arithmetic means ± standard errors of the means (n = 3). Double asterisks indicate statistical differences from the LT-IIb group (P < 0.01).
To determine if the binding of LT-IIb and LT-IIb(T13I) to human monocytes elicited a relevant immunological response, cells were evaluated for the induction of IL-6, an inflammatory cytokine that mediates differential control of leukocyte recruitment, activation, and apoptosis. Of particular relevance, IL-6 has also been recognized as a regulator of the immunological shift from innate to acquired immunity (26), a shift that is routinely enhanced by adjuvants (13). In comparison to no treatment (<6.0 pg of IL-6/ml) or to treatment of monocytes with LT-IIb (12.67 ± 2.91 pg of IL-6/ml), treatment of human monocytes with LT-IIb(T13I) induced a significant elevation in the secretion of IL-6 (85.01 ± 9.82 pg/ml) (P, <0.01) (Fig. 4B).
Elevation of cAMP levels has been shown to activate protein kinase A, which eventually suppresses NF-κB activation and subsequent cytokine production by monocytic cells (22, 31). Thus, the attenuation of IL-6 induction observed in LT-IIb-treated human monocytes was likely elicited by the ADP ribosylation activity of LT-IIb, which is lacking in LT-IIb(T13I) (38). To investigate that possibility, the levels of cAMP in human monocytes treated with either HLT were measured (Fig. 4C). The LT-IIb B pentamer (LT-IIb-B5), the pentameric subunit of LT-IIb that mediates the binding of the enterotoxin to gangliosides, was used as a nontoxic control. Untreated monocytes produced only a small amount of cAMP (1.32 ± 0.14 pmol/2 × 105 monocytes). cAMP levels were highly elevated in monocytes treated with LT-IIb. Treatment of monocytes with LT-IIb(T13I) induced the production of an amount of cAMP no greater than the amount induced by treatment of monocytes with the nontoxic subunit LT-IIb-B5 (3.19 ± 0.14 pmol/2 × 105 monocytes and 3.12 ± 0.06 pmol/2 × 105 monocytes, respectively). The relatively low-level, baseline production of cAMP in LT-IIb-B5-treated cells was likely induced by processes involving prostaglandin E2 (31). These data demonstrated that LT-IIb(T13I) was essentially nontoxic to human monocytes, an observation likely explained by the natural lack of expression of NeuGc gangliosides in human cells.
Collectively, the data strongly suggested that the NeuAc gangliosides are sufficient to mediate the immunomodulatory properties of LT-IIb(T13I). Furthermore, it is likely that this nontoxic yet potent mucosal adjuvant will retain its adjuvant properties when employed in humans.
DISCUSSION
Prior investigations have demonstrated that the functional ligands for LT-IIa and LT-IIb are one or more types of gangliosides (5, 17, 38, 39). Data obtained from various investigations have provided strong evidence that the binding of LT-IIa, LT-IIb, and other HLTs to gangliosides stimulates a series of molecular and cellular events associated with immunomodulation. For example, binding to GM1 is essential for the immunomodulatory properties of CT (37). Since in vitro ELISA-based studies confirmed that LT-IIb bound avidly to ganglioside GD1a, it was reasonable to hypothesize that the immunomodulatory properties and toxicity of LT-IIb were dependent on the binding of the enterotoxin to GD1a. Yet the recent observation that LT-IIb(T13I) is a potent mucosal and systemic adjuvant despite its loss of affinity for GD1a (38), in combination with the observation that exogenous GD1a cannot block the toxic properties of wt LT-IIb in a mouse Y1 adrenal cell assay (17), suggested that LT-IIb likely employs a cell receptor(s) other than, or in addition to, GD1a to mediate adjuvanticity and toxicity. This hypothesis was supported by experiments demonstrating that LT-IIb had affinity for a broad range of gangliosides (5).
Gangliosides are a structurally diverse group of molecules. Diversity within the group is attained in part by differentially incorporating the terminal and/or side chain sugars with NeuAc and NeuGc. Biochemical analyses using enzymes or inhibitors that disrupt the carbohydrate structures of glycolipids indicated that NeuAc and NeuGc are crucial components of gangliosides that mediate the binding affinities of LT-IIb and LT-IIb(T13I) (5). Using purified murine peritoneal macrophage gangliosides, LT-IIb was observed to bind to a broad array of NeuAc- and NeuGc-containing gangliosides (5). In contrast, LT-IIb(T13I) exhibited a preference for NeuGc gangliosides (5). This distinct difference in the binding affinities of LT-IIb and LT-IIb(T13I) to gangliosides decorated with either NeuGc or NeuAc likely explains, in part, the various responses of certain cell types after treatment with either LT-IIa or LT-IIb (30, 38).
Functionally, gangliosides participate in a variety of modulatory responses related to cellular development and function. In the nervous system, gangliosides mediate the induction of neurite outgrowth, the function of receptors, reactions to adhesion, and signal transduction (28). Gangliosides, directly or indirectly, differentially regulate cellular responses to different external stimuli (e.g., platelet-derived growth factor [PDGF], epidermal growth factor [EGF], and insulin [23, 51]). The abilities of LT-IIb and LT-IIb(T13I) to elicit higher levels of expression of a variety of proinflammatory cytokines in cells that are deficient in NeuGc gangliosides than in cells that express both NeuGc gangliosides and NeuAc gangliosides suggest that NeuGc and NeuAc gangliosides are essential components of cellular systems that mediate different cellular responses. Such differential cell responses were also observed for NeuGc-deficient versus NeuGc-proficient B cells (36). In a T-cell-independent immunization study, Cmah-null mice elaborated higher levels of Ag-specific IgM and Ag-specific IgG3 than did isogenic C57BL/6J mice. On the cellular level, B cells isolated from Cmah-null mice had elevated proliferative responses after stimulation with an F(ab′)2 fragment (anti-μ chain) against the B-cell receptor (BCR) than did isogenic C57BL/6J B cells (36). Collectively, these data, in combination with the observations on the patterns of cytokine production, support a model in which NeuGc gangliosides are required for optimal negative regulation of immune cells.
In contrast to those of most, if not all, other mammals, human gangliosides are not decorated with NeuGc, due to an evolutionary mutation in the Cmah gene (7, 9). In this study, Cmah-null mice were confirmed to be deficient in the expression of NeuGc gangliosides. With respect to ganglioside presentation, Cmah-null mice phenotypically mimic humans. Therefore, Cmah-null mice were employed as a model for evaluating the immunomodulatory properties of LT-IIb and LT-IIb(T13I) in humans. The experiments described here are supported by data obtained from other studies evaluating the immunomodulatory properties of LT-IIa and LT-IIb. From studies employing mutant enterotoxins, it is clear that gangliosides mediate the binding of LT-IIa and LT-IIb to immunocompetent cells (5, 38, 39). Mutants of either LT-IIa or LT-IIb that exhibited no detectable binding or diminished binding to purified gangliosides also exhibited no detectable binding or diminished binding to immunocompetent cells (38, 39). The assorted immunomodulatory properties of these mutant HLTs correlated with their binding affinities for immunocompetent cells. Specifically, LT-IIa(T34I) showed no detectable binding to gangliosides or to immunocompetent cells and lacked the immunomodulatory activities associated with wt LT-IIa (2, 38). LT-IIa(T14D) and LT-IIa(T14I), two mutant enterotoxins with diminished binding affinities for gangliosides and for immunocompetent cells, had less-potent, yet still significant adjuvant properties in comparison to those of wt LT-IIa (39). The mutant enterotoxin LT-IIb(T13I), which had diminished binding affinity for NeuAc gangliosides (5), also had diminished binding affinity for immune cells isolated from Cmah-null mice. While the immunomodulatory activity of LT-IIb(T13I) in Cmah-null mice was reduced from the immunomodulatory activities in isogenic C57BL/6J mice, the results clearly demonstrated that LT-IIb(T13I) retained potent adjuvant properties in the absence of expression of NeuGc gangliosides on the surfaces of cells in those mice.
Collectively, the data reported here and from prior studies using LT-IIa mutants as adjuvants (38, 39) strongly support a model in which the adjuvant properties of LT-IIa and LT-IIb, and those of their respective mutants, depend on the binding activities of the enterotoxins for their ganglioside receptors.
Either LT-IIb or LT-IIb(T13I), when used as a mucosal adjuvant, induces a balanced TH1/TH2 immune response to a coadministered Ag (33, 38). This balanced immune response is believed to be the culmination of the pattern of cytokines induced by the HLT (1, 2, 22, 34). It is likely that LT-IIb and LT-IIb(T13I) have the capacity to induce a balanced TH1/TH2 immune response even in the absence of NeuGc gangliosides. IL-4 and IFN-γ are required to establish TH2 and TH1 responses, respectively (48). Expression of IL-4 and expression of IFN-γ were enhanced in Cmah-deficient macrophages after treatment of the cells with either LT-IIb or LT-IIb(T13I). Yet the immunoregulatory properties of LT-IIb and LT-IIb(T13I) are probably not limited to effects induced by the elaboration of IL-4 and IFN-γ. In previous studies, CT established a TH17 response in an IL-6-dependent manner after intranasal administration (29). The results reported here show that LT-IIb and LT-IIb(T13I) induce, to various degrees, the transcriptional expression of IL-6 and IL-17 in macrophages isolated from either C57BL/6J or Cmah-null mice. An IL-6 knockout mouse is currently being employed to determine whether LT-IIb and LT-IIb(T13I) have the capacity to induce a TH17 response.
The use of HLTs as mucosal adjuvants in human vaccines has been restricted by their intrinsic toxicity and by their propensity to traffic to the brain via the olfactory nerve (45, 46). It is assumed that neuronal trafficking requires the binding of the HLT to olfactory neurons and that the brain inflammation which occurs after trafficking is promoted by the inherent toxicity of the HLT (3). The preferential affinity of LT-IIb(T13I) for NeuGc gangliosides, which are not expressed in human cells, and the diminished toxicity of LT-IIb(T13I) in human (and murine) cells are likely to be useful and exploitable characteristics in that regard. While mouse models are informative, human trials will be required in order to firmly determine whether LT-IIb(T13I) has utility as a human mucosal adjuvant. Regardless, LT-IIb(T13I) is indisputably a powerful molecular tool that can be used to investigate the cellular and molecular components of ganglioside-dependent immunomodulatory signaling cascades.
In conclusion, the data from these experiments demonstrated that NeuAc gangliosides are sufficient to mediate the adjuvant properties of LT-IIb(T13I). The data also suggest that NeuGc gangliosides are not required for the toxic activity of LT-IIb. Finally, these results suggest the existence of divergent mechanisms for immune regulation and cellular responses that are mediated through the binding of external ligands to NeuGc and NeuAc gangliosides.
Acknowledgments
This work was supported by National Institutes of Health research grants DE013833 (T.D.C.), DE014357 (T.D.C.), HL6654901 (C.S.B.), and DE017138 (G.H.).
Footnotes
Published ahead of print on 14 April 2010.
REFERENCES
- 1.Arce, S., H. F. Nawar, G. Muehlinghaus, M. W. Russell, and T. D. Connell. 2007. In vitro induction of immunoglobulin A (IgA)- and IgM-secreting plasma blasts by cholera toxin depends on T-cell help and is mediated by CD154 up-regulation and inhibition of gamma interferon synthesis. Infect. Immun. 75:1413-1423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Arce, S., H. F. Nawar, M. W. Russell, and T. D. Connell. 2005. Differential binding of Escherichia coli enterotoxins LT-IIa and LT-IIb and of cholera toxin elicits differences in apoptosis, proliferation, and activation of lymphoid cells. Infect. Immun. 73:2718-2727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Armstrong, M. E., E. C. Lavelle, C. E. Loscher, M. A. Lynch, and K. H. Mills. 2005. Proinflammatory responses in the murine brain after intranasal delivery of cholera toxin: implications for the use of AB toxins as adjuvants in intranasal vaccines. J. Infect. Dis. 192:1628-1633. [DOI] [PubMed] [Google Scholar]
- 4.Belardelli, F. 1995. Role of interferons and other cytokines in the regulation of the immune response. APMIS 103:161-179. [DOI] [PubMed] [Google Scholar]
- 5.Berenson, C. S., H. F. Nawar, H. C. Yohe, S. A. Castle, D. J. Ashline, V. N. Reinhold, G. Hajishengallis, and T. D. Connell. 2010. Mammalian cell ganglioside-binding specificities of E. coli enterotoxins LT-IIb and variant LT-IIb(T13I). Glycobiology 20:41-54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Berenson, C. S., H. C. Yohe, and J. L. Ryan. 1989. Factors mediating lipopolysaccharide-induced ganglioside expression in murine peritoneal macrophages. J. Leukoc. Biol. 45:221-230. [DOI] [PubMed] [Google Scholar]
- 7.Brinkman-Van der Linden, E. C., E. R. Sjoberg, L. R. Juneja, P. R. Crocker, N. Varki, and A. Varki. 2000. Loss of N-glycolylneuraminic acid in human evolution. Implications for sialic acid recognition by siglecs. J. Biol. Chem. 275:8633-8640. [DOI] [PubMed] [Google Scholar]
- 8.Cassel, D., and Z. Selinger. 1977. Mechanism of adenylate cyclase activation by cholera toxin: inhibition of GTP hydrolysis at the regulatory site. Proc. Natl. Acad. Sci. U. S. A. 74:3307-3311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chou, H. H., H. Takematsu, S. Diaz, J. Iber, E. Nickerson, K. L. Wright, E. A. Muchmore, D. L. Nelson, S. T. Warren, and A. Varki. 1998. A mutation in human CMP-sialic acid hydroxylase occurred after the Homo-Pan divergence. Proc. Natl. Acad. Sci. U. S. A. 95:11751-11756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Connell, T. D. 2007. Cholera toxin, LT-I, LT-IIa and LT-IIb: the critical role of ganglioside binding in immunomodulation by type I and type II heat-labile enterotoxins. Expert Rev. Vaccines 6:821-834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Connell, T. D., and R. K. Holmes. 1995. Mutational analysis of the ganglioside-binding activity of the type II Escherichia coli heat-labile enterotoxin LT-IIb. Mol. Microbiol. 16:21-31. [DOI] [PubMed] [Google Scholar]
- 12.de Haan, L., I. K. Feil, W. R. Verweij, M. Holtrop, W. G. Hol, E. Agsteribbe, and J. Wilschut. 1998. Mutational analysis of the role of ADP-ribosylation activity and GM1-binding activity in the adjuvant properties of the Escherichia coli heat-labile enterotoxin towards intranasally administered keyhole limpet hemocyanin. Eur. J. Immunol. 28:1243-1250. [DOI] [PubMed] [Google Scholar]
- 13.Dempsey, P. W., M. E. D. Allison, S. Akkaraju, C. C. Goodnow, and D. T. Fearon. 1996. C3d of complement as a molecular adjuvant: bridging innate and acquired immunity. Science 271:348-350. [DOI] [PubMed] [Google Scholar]
- 14.Elson, C. O., and M. T. Dertzbaugh. 2005. Mucosal adjuvants, p. 967-986. In J. Mestecky, M. E. Lamm, W. Strober, J. Bienenstock, J. R. McGhee, and L. Mayer (ed.), Mucosal immunology, 3rd ed., vol. 1. Elsevier Academic Press, San Diego, CA.
- 15.Fakih, M. G., T. F. Murphy, M. A. Pattoli, and C. S. Berenson. 1997. Specific binding of Haemophilus influenzae to minor gangliosides of human respiratory epithelial cells. Infect. Immun. 65:1695-1700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Freytag, L. C., and J. D. Clements. 2005. Mucosal adjuvants. Vaccine 23:1804-1813. [DOI] [PubMed] [Google Scholar]
- 17.Fukuta, S., J. L. Magnani, E. M. Twiddy, R. K. Holmes, and V. Ginsburg. 1988. Comparison of the carbohydrate-binding specificities of cholera toxin and Escherichia coli heat-labile enterotoxins LTh-I, LT-IIa, and LT-IIb. Infect. Immun. 56:1748-1753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Guidry, J., L. Cardenas, E. Cheng, and J. Clements. 1997. Role of receptor binding in toxicity, immunogenicity, and adjuvanticity of Escherichia coli heat-labile enterotoxin. Infect. Immun. 65:4943-4950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Guth, B. E., C. L. Pickett, E. M. Twiddy, R. K. Holmes, T. A. Gomes, A. A. Lima, R. L. Guerrant, B. D. Franco, and L. R. Trabulsi. 1986. Production of type II heat-labile enterotoxin by Escherichia coli isolated from food and human feces. Infect. Immun. 54:587-589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Guth, B. E., E. M. Twiddy, L. R. Trabulsi, and R. K. Holmes. 1986. Variation in chemical properties and antigenic determinants among type II heat-labile enterotoxins of Escherichia coli. Infect. Immun. 54:529-536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hajishengallis, G., S. Arce, C. M. Gockel, T. D. Connell, and M. W. Russell. 2005. Immunomodulation with enterotoxins for the generation of secretory immunity or tolerance: applications for oral infections. J. Dent. Res. 84:1104-1116. [DOI] [PubMed] [Google Scholar]
- 22.Hajishengallis, G., H. Nawar, R. I. Tapping, M. W. Russell, and T. D. Connell. 2004. The type II heat-labile enterotoxins LT-IIa and LT-IIb and their respective B pentamers differentially induce and regulate cytokine production in human monocytic cells. Infect. Immun. 72:6351-6358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hakomori, S. 1993. Structure and function of sphingoglycolipids in transmembrane signalling and cell-cell interactions. Biochem. Soc. Trans. 21(Pt. 3):583-595. [DOI] [PubMed] [Google Scholar]
- 24.Harokopakis, E., M. H. Albzreh, M. H. Martin, and G. Hajishengallis. 2006. TLR2 transmodulates monocyte adhesion and transmigration via Rac1- and PI3K-mediated inside-out signaling in response to Porphyromonas gingivalis fimbriae. J. Immunol. 176:7645-7656. [DOI] [PubMed] [Google Scholar]
- 25.Holmes, R. K., M. G. Jobling, and T. D. Connell. 1995. Cholera toxin and related enterotoxins of gram-negative bacteria, p. 225-255. In J. Moss, B. Iglewski, M. Vaughan, and A. T. Tu (ed.), Handbook of natural toxins, vol. 8. Bacterial toxins and virulence factors in disease. Marcel Dekker, Inc., New York, NY. [Google Scholar]
- 26.Jones, S. A. 2005. Directing transition from innate to acquired immunity: defining a role for IL-6. J. Immunol. 175:3463-3468. [DOI] [PubMed] [Google Scholar]
- 27.Kuziemko, G. M., M. Stroh, and R. C. Stevens. 1996. Cholera toxin binding affinity and specificity for gangliosides determined by surface plasmon resonance. Biochemistry 35:6375-6384. [DOI] [PubMed] [Google Scholar]
- 28.Ledeen, R. W., and G. Wu. 2002. Ganglioside function in calcium homeostasis and signaling. Neurochem. Res. 27:637-647. [DOI] [PubMed] [Google Scholar]
- 29.Lee, J.-B., J.-E. Jang, M. K. Song, and J. Chang. 2009. Intranasal delivery of cholera toxin induces Th17-dominated T-cell response to bystander antigens. PLoS One 4:e5190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Liang, S., M. Wang, R. I. Tapping, V. Stepensky, H. F. Nawar, M. Triantafilou, K. Triantafilou, T. D. Connell, and G. Hajishengallis. 2007. Ganglioside GD1a is an essential coreceptor for Toll-like receptor 2 signaling in response to the B subunit of type IIb enterotoxin. J. Biol. Chem. 282:7532-7542. [DOI] [PubMed] [Google Scholar]
- 31.Liang, S., M. Wang, K. Triantafilou, M. Triantafilou, H. F. Nawar, M. W. Russell, T. D. Connell, and G. Hajishengallis. 2007. The A subunit of type IIb enterotoxin (LT-IIb) suppresses the proinflammatory potential of the B subunit and its ability to recruit and interact with TLR2. J. Immunol. 178:4811-4819. [DOI] [PubMed] [Google Scholar]
- 32.Lycke, N. 2005. Targeted vaccine adjuvants based on modified cholera toxin. Curr. Mol. Med. 5:591-597. [DOI] [PubMed] [Google Scholar]
- 33.Martin, M., D. J. Metzger, S. M. Michalek, T. D. Connell, and M. W. Russell. 2000. Comparative analysis of the mucosal adjuvanticity of the type II heat-labile enterotoxins LT-IIa and LT-IIb. Infect. Immun. 68:281-287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Martin, M., D. J. Metzger, S. M. Michalek, T. D. Connell, and M. W. Russell. 2001. Distinct cytokine regulation by cholera toxin and type II heat-labile toxins involves differential regulation of CD40 ligand on CD4(+) T cells. Infect. Immun. 69:4486-4492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Moss, J., and M. Vaughan. 1977. Choleragen activation of solubilized adenylate cyclase: requirement for GTP and protein activator for demonstration of enzymatic activity. Proc. Natl. Acad. Sci. U. S. A. 74:4396-4400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Naito, Y., H. Takematsu, S. Koyama, S. Miyake, H. Yamamoto, R. Fujinawa, M. Sugai, Y. Okuno, G. Tsujimoto, T. Yamaji, Y. Hashimoto, S. Itohara, T. Kawasaki, A. Suzuki, and Y. Kozutsumi. 2007. Germinal center marker GL7 probes activation-dependent repression of N-glycolylneuraminic acid, a sialic acid species involved in the negative modulation of B-cell activation. Mol. Cell. Biol. 27:3008-3022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nashar, T. O., N. A. Williams, and T. R. Hirst. 1998. Importance of receptor binding in the immunogenicity, adjuvanticity and therapeutic properties of cholera toxin and Escherichia coli heat-labile enterotoxin. Med. Microbiol. Immunol. (Berl.) 187:3-10. [DOI] [PubMed] [Google Scholar]
- 38.Nawar, H. F., S. Arce, M. W. Russell, and T. D. Connell. 2005. Mucosal adjuvant properties of mutant LT-IIa and LT-IIb enterotoxins that exhibit altered ganglioside-binding activities. Infect. Immun. 73:1330-1342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nawar, H. F., S. Arce, M. W. Russell, and T. D. Connell. 2007. Mutants of type II heat-labile enterotoxin LT-IIa with altered ganglioside-binding activities and diminished toxicity are potent mucosal adjuvants. Infect. Immun. 75:621-633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Orlandi, P. A., D. R. Critchley, and P. H. Fishman. 1994. The heat-labile enterotoxin of Escherichia coli binds to polylactosaminoglycan-containing receptors in CaCo-2 human intestinal epithelial cells. Biochemistry 33:12886-12895. [DOI] [PubMed] [Google Scholar]
- 41.Rappuoli, R., M. Pizza, G. Douce, and G. Dougan. 1999. Structure and mucosal adjuvanticity of cholera and Escherichia coli heat-labile enterotoxins. Immunol. Today 20:493-500. [DOI] [PubMed] [Google Scholar]
- 42.Russell, M. W., L. A. Bergmeier, E. D. Zanders, and T. Lehner. 1980. Protein antigens of Streptococcus mutans: purification and properties of a double antigen and its protease-resistant component. Infect. Immun. 28:486-493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Svennerholm, L. 1957. Quantitative estimation of sialic acids. II. A colorimetric resorcinol-hydrochloric acid method. Biochim. Biophys. Acta 24:604-611. [DOI] [PubMed] [Google Scholar]
- 44.Svennerholm, L. 1980. Structure and function of gangliosides. Plenum, New York, NY.
- 45.van Ginkel, F. W., R. J. Jackson, N. Yoshino, Y. Hagiwara, D. J. Metzger, T. D. Connell, H. L. Vu, M. Martin, K. Fujihashi, and J. R. McGhee. 2005. Enterotoxin-based mucosal adjuvants alter antigen trafficking and induce inflammatory responses in the nasal tract. Infect. Immun. 73:6892-6902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.van Ginkel, F. W., R. J. Jackson, Y. Yuki, and J. R. McGhee. 2000. The mucosal adjuvant cholera toxin redirects vaccine proteins into olfactory tissues. J. Immunol. 165:4778-4782. [DOI] [PubMed] [Google Scholar]
- 47.Williams, N. A., T. R. Hirst, and T. O. Nashar. 1999. Immune modulation by the cholera-like enterotoxins: from adjuvant to therapeutic. Immunol. Today 20:95-101. [DOI] [PubMed] [Google Scholar]
- 48.Wynn, T. A. 2005. TH-17: a giant step from TH1 and TH2. Nat. Immunol. 6:1069-1070. [DOI] [PubMed] [Google Scholar]
- 49.Yohe, H. C., S. Ye, B. B. Reinhold, and V. N. Reinhold. 1997. Structural characterization of the disialogangliosides of murine peritoneal macrophages. Glycobiology 7:1215-1227. [DOI] [PubMed] [Google Scholar]
- 50.Yuki, Y., and H. Kiyono. 2003. New generation of mucosal adjuvants for the induction of protective immunity. Rev. Med. Virol. 13:293-310. [DOI] [PubMed] [Google Scholar]
- 51.Zeller, C. B., and R. B. Marchase. 1992. Gangliosides as modulators of cell function. Am. J. Physiol. 262:C1341-C1355. [DOI] [PubMed] [Google Scholar]