Abstract
Human immunodeficiency virus type 1 (HIV-1) vaccine and natural history studies are critically dependent on the ability to isolate, cryopreserve, and thaw peripheral blood mononuclear cell (PBMC) samples with a high level of quality and reproducibility. Here we characterize the yield, viability, phenotype, and function of PBMC from HIV-1-infected and uninfected Ugandans and describe measures to ascertain reproducibility and sample quality at the sites that perform cryopreservation. We have developed a comprehensive internal quality control program to monitor processing, including components of method validation. Quality indicators for real-time performance assessment included the time from venipuncture to cryopreservation, time for PBMC processing, yield of PBMC from whole blood, and viability of the PBMC before cryopreservation. Immune phenotype analysis indicated lowered B-cell frequencies following processing and cryopreservation for both HIV-1-infected and uninfected subjects (P < 0.007), but all other major lymphocyte subsets were unchanged. Long-term cryopreservation did not impact function, as unstimulated specimens exhibited low background and all specimens responded to staphylococcal enterotoxin B (SEB) by gamma interferon and interleukin-2 production, as measured by intracellular cytokine staining. Samples stored for more than 3 years did not decay with regard to yield or viability, regardless of HIV-1 infection status. These results demonstrate that it is possible to achieve the high level of quality necessary for vaccine trials and natural history studies in a resource-limited setting and provide strategies for laboratories to monitor PBMC processing performance.
Cryopreserved peripheral blood mononuclear cells (PBMC) are critical to studies of HIV-1 infection and for the development of an effective HIV vaccine. High-quality cryopreservation is particularly challenging and important in resource-limited settings, where natural history, therapeutic, and preventative protocols are being developed. Cryopreservation and batch testing allow for simultaneous assessment of critical samples, thereby reducing interassay variability. Since standard method validation measures, such as accuracy, precision, linearity, and reportable ranges, are not easily obtained for PBMC processing, more studies are needed to understand the parameters influencing the reproducibility of processing and quality of cryopreserved PBMC samples in resource-limited settings.
Separation of PBMC should yield a pure population of mononuclear cells (95% ± 5%) (18), with minimal contamination from red blood cells, granulocytes, and platelets. Anticipated yields of mononuclear cells from healthy donors are approximately 1 × 106 to 2 × 106 cells/ml of whole blood, with a purity of 60 to 70% lymphocytes at >95% viability, with a reduction of platelets to <0.5% of the original whole-blood content (25). However, the data on the impact of cryopreservation on lymphocyte phenotypes are inconsistent (4, 31, 34, 39, 41). Additionally, there are differences in hematologic parameters and lymphocyte subsets in African populations compared to populations in the industrialized world (17, 26, 33, 44). Careful examination of the impact of cryopreservation on yield and phenotype is therefore needed for this region. Furthermore, studies indicate an increased ability to preserve the function of cryopreserved PBMC compared to cells in whole blood, as measured by proliferation assays (1, 12, 23, 46, 47), cytokine production (9, 28, 30, 32, 37), apoptosis (40), and HLA tetramer staining (2). This is particularly important for HIV-1, as many vaccine candidates are designed to elicit T-cell responses, and reliable PBMC-based assays such as enzyme-linked immunospot (ELISPOT) assay and multiparameter flow cytometry are required to prioritize candidates for large-scale efficacy testing.
Some studies indicate that the functional integrity of cryopreserved PBMC samples is maintained (22), while other reports suggest a loss of function (38). Preanalytic variables which have been examined closely include time from blood draw to initiation of processing, type of anticoagulant, proper mixing of blood tubes, and transport temperature (9, 13, 28, 45). Analytic variables that impact PBMC processing include separation method, type of cryoprotectant medium, prechilling of the freeze containers, cryopreservation parameters, and temperature of the thawing medium (9, 15, 28, 30, 37). In addition to defining processing parameters, it is important to monitor quality assurance for cryopreserved PBMC assays after cryopreservation (7). However, few studies have addressed the importance of continuous monitoring of the processing and cryopreservation of PBMC themselves but have focused instead on the procedures for thawing of cells in labs that perform end-point assays. We have developed a comprehensive internal quality control program to monitor PBMC processing. This study describes this program and the results achieved in a resource-limited setting. Additionally, we describe procedures to evaluate our laboratory methods for PBMC processing.
MATERIALS AND METHODS
Study participants.
All samples were obtained from participants enrolled in studies conducted in the Kampala (27), Kayunga (19), and Rakai (29) districts in Uganda as previously described. All studies were reviewed and approved by the appropriate institutional review boards in the United States and Uganda, with consent forms signed by all participants.
PBMC processing.
Blood was collected into 8.5-ml acid citrose dextran (ACD)-anticoagulated tubes and transported to the lab at room temperature. PBMC were isolated from ACD-whole blood within 8 h of collection by centrifugation at 800 × g for 15 min through Ficoll-Hypaque Plus (Pharmacia, Uppsala, Sweden), using Leucosep tubes (Greiner Bio-One, Frickenhausen, Germany). The PBMC layer was harvested and washed three times with phosphate-buffered saline (PBS) by centrifugation at 250 × g for 10 min. Cell counts and viability were determined with a hemacytometer, using trypan blue exclusion, or by a Guava PCA machine (Guava Technologies, Hayward, CA), using Guava ViaCount reagent. PBMC were cryopreserved at a concentration of 107 cells/ml in freeze medium (20% fetal bovine serum [FBS], 10% dimethyl sulfoxide [DMSO], 1% penicillin-streptomycin, 69% RPMI 1640), using isopropyl “Mr. Frosty” freezing containers (Nalgene Labware, Thermo Fisher, Rochester, NY) to control the rate of freezing overnight in a −80°C freezer, and were transferred the next day for long-term storage in liquid nitrogen vapor at −130°C. In order to assess cryopreserved specimens, cells were rapidly thawed in a 37°C water bath until a small ice crystal remained and were washed with complete medium (CM) (RPMI 1640 supplemented with 10% FBS, 2% l-glutamine, and 1% penicillin-streptomycin) added dropwise to avoid a rapid osmotic change.
Lymphocyte immunophenotyping and hematology.
Lymphocyte immunophenotyping was performed on EDTA-anticoagulated whole blood or PBMC product postthawing. Samples were run on a dual-laser flow cytometer using the single-platform Multitest four-color reagent in combination with TruCount tubes and were analyzed using MultiSET software (Becton Dickinson, San Jose, CA). Absolute numbers and percentages of T (CD3+), helper T (CD4+), cytotoxic T (CD8+), B (CD19+), and NK (CD16+ or CD56+) lymphocytes were determined. Complete blood counts with a five-part differential were performed using a Coulter AcT 5 differential instrument (Beckman Coulter, Fullerton, CA) for whole blood and after processing of cell samples.
ICS assay.
A standard intracellular cytokine staining (ICS) assay was performed on cryopreserved PBMC which had been thawed and rested overnight. PBMC (106) were incubated in 96-well round-bottom plates with costimulatory anti-CD28 and anti-CD49d monoclonal antibodies (Becton Dickinson, San Jose, CA), a peptide pool from cytomegalovirus (CMV), Epstein-Barr virus, and influenza virus (CEF pool) (14), and brefeldin A (Becton Dickinson, San Jose, CA). Samples included a negative control of PBMC stimulated without peptide and a positive control of staphylococcal enterotoxin B (SEB; Sigma-Aldrich, Munich, Germany). Cells were incubated for 6 h at 37°C in a 7% CO2 incubator and were stored at 4°C overnight. The next day, 20 μl of 20 mM EDTA was added to each well and incubated for 15 min. After centrifugation, 1× fluorescence-activated cell sorter (FACS) lysing solution was added for 10 min at room temperature. Cells were then washed once in wash buffer (0.5% bovine serum albumin [BSA], 0.1% sodium azide in PBS), permeabilized in 200 μl 1× FACS permeabilizing solution for 10 min, and washed three times. Cells were incubated for 60 min with a 50-μl antibody cocktail containing anti-CD3-allophycocyanin (anti-CD3-APC), anti-CD4-fluorescein isothiocyanate (anti-CD4-FITC), anti-CD8-PerCpcy5.5, anti-gamma interferon (IFN-γ)-phycoerythrin (PE), and anti-interleukin-2 (IL-2)-PE (Becton Dickinson, San Jose, CA). Cells were then washed three times and fixed in 1% paraformaldehyde. Flow cytometric analysis was performed using a FACSCalibur flow cytometer, with at least 20,000 CD3+ CD8+ and CD3+ CD4+ events (each) being acquired. Samples were analyzed using FlowJo software, version 8.5 (Tree Star, Ashland, OR). A positive ICS response was defined as a response at least 3-fold higher than the mean unstimulated response and above 0.05.
HIV-1 subtype determination.
All HIV-1-seropositive participants' plasma samples were tested for virus subtype by the previously described multiregion hybridization assay (MHA), version 2, for HIV-1 subtypes A, C, and D, recombinants, and dual infections (3, 20, 21). HIV-1 subtype A, C, or D was assigned based on five regions (gag, pol, vpu, env, and gp41) of the HIV-1 genome, based on reactivities of the probes if at least 2 regions had probe reactivity. Samples with probe reactivity for a single subtype in different regions were considered pure subtypes.
Statistical analysis.
All statistical analyses were performed using Graph Pad Prism software, version 5.0a, for Mac OS X (GraphPad Software, La Jolla, CA). Direct comparison between two groups was performed using the nonparametric Mann-Whitney U test for continuous data. For paired observations, the paired t test was used. P values of <0.05 were considered significant. For descriptive statistics, the mean with standard deviation is presented graphically, while the median, mean, and minimum-to-maximum range are reported within the text. Reference range intervals were calculated as the range between the 2.5% and 97.5% limits for the parameters tested. Thaw viability was reported as the percentage of viable cells at the time of thaw, using the ViaCount assay and standard gating excluding apoptotic cells. Overnight viability was reported as the percentage of viable cells after overnight incubation in CM at 37°C, 7% CO2, and 90% relative humidity, using the ViaCount assay and standard gating excluding apoptotic cells. Thaw and overnight yields were calculated as numbers of cells divided by the original vial cell content under similar conditions to those for viability.
RESULTS
Leucosep processing and cryopreservation of PBMC preserve lymphocytes.
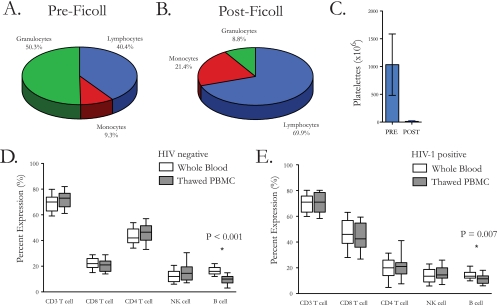
In order to assess the technique of laboratory staff and the efficiency of the PBMC processing procedure using Leucosep tubes, 52 samples were assessed for whole-blood and postprocessing concentrations of granulocytes, lymphocytes, monocytes, and platelets. Basophils, eosinophils, and neutrophils were considered together as granulocytes. The composition of white blood cells in whole blood was 50.3% (range, 28.4% to 78.9%) granulocytes, 40.4% (17.0% to 58.7%) lymphocytes, and 9.3% (2.0% to 42.5%) monocytes (Fig. 1A). After Ficoll separation using Leucosep tubes, the mean percentages were 8.8% (1.2% to 45.8%) granulocytes, 69.9% (29.4% to 92.6%) lymphocytes, and 21.4% (4.9% to 56.3%) monocytes (Fig. 1B). Initial whole-blood product (27 ml whole blood) contained 103 × 106 (35 × 106 to 223 × 106) total platelets per ml, which decreased to 12 × 106 platelets/ml (0 to 100 × 106) following processing (Fig. 1C).
FIG. 1.
PBMC were isolated from whole blood by use of Leucosep tubes, and the quality of the procedure was assessed. (A) Pie chart showing mean (range) proportions of granulocytes (50.3% [28.4% to 78.9%]), lymphocytes (40.4% [17.0% to 58.7%]), and monocytes (9.3% [2.0% to 42.5%]) from 52 whole-blood samples before processing. (B) Pie chart showing mean (range) proportions of granulocytes (8.8% [1.2% to 45.8%]), lymphocytes (69.9% [29.4% to 92.6%]), and monocytes (21.4% [4.9% to 56.3%]) from 52 samples after PBMC processing. (C) Bar chart showing the mean concentration of platelets (106) in whole blood (1,034 [35 to 223]) or after processing (12 [0 to 100]). The effect of processing and cryopreservation on the immune phenotype was assessed and compared to peripheral blood concentrations. (D) Box and whisker plots showing the median and 10th and 90th percentiles for major immune cell subsets in 40 normal healthy individuals. A statistically significant difference was observed for the percentage of B cells (P < 0.001). (E) Box and whisker plots showing the median and 10th and 90th percentiles for major immune cell subsets in 28 HIV-1-positive individuals. A statistically significant decrease was observed for the percentage of B cells (P = 0.007).
In order to assess the effect of processing and cryopreservation on PBMC phenotype, we studied PBMC from freshly drawn peripheral blood and from thawed samples that had been stored in the vapor phase of liquid nitrogen for approximately 1.5 years from 40 uninfected and 28 chronically HIV-1-infected donors. Whole-blood immune cell subsets in uninfected subjects were 70% (47% to 81%) (median [range]) CD3+, 22% (13% to 47%) CD8+, 42% (29% to 57%) CD4+, 12% (5% to 33%) CD16+ or CD56+, and 16% (9% to 26%) CD19+. Immediately following thawing and counting, PBMC immune subsets were compared to those of whole blood, and a statistically significant difference was observed only for the percentage of B cells (P < 0.001) (Fig. 1D). Whole-blood immune cell subsets in HIV-1-infected individuals were 71% (47% to 83%) (median [range]) CD3+, 46% (27% to 71%) CD8+, 20% (2% to 43%) CD4+, 14% (4% to 35%) CD16+ or CD56+, and 14% (6% to 24%) CD19+. Comparing cell subsets between thawed PBMC samples and the paired fresh PBMC samples, a statistically significant decrease was again observed only for the B-cell subset (P = 0.007) (Fig. 1E).
Harvesting plasma before or after processing does not affect quality.
In order to assess the effects of harvesting plasma prior to and following Ficoll separation, 10 uninfected donor specimens (94 ml whole blood [each]) were processed in parallel (47 ml for each sample) to determine the final PBMC yield, viability, and purity. PBMC yield and viability were not different between samples when plasma was collected before or after Ficoll separation (P = 0.506 and 0.085, respectively [data not shown]). The median yields (ranges) for plasma collection before and after Ficoll separation were 54 × 106 PBMC (24 × 106 to 67 × 106 PBMC) and 53 × 106 PBMC (27 × 106 to 71 × 106 PBMC), respectively. The median viability (range) was 95% (90% to 100%) for plasma collection before Ficoll separation and 94% (91% to 98%) for plasma collection after Ficoll separation. No difference was observed in platelet concentration for harvest of plasma before and after Ficoll separation (67 × 106 [22 × 106 to 128 × 106]/ml and 69 × 106 [17 × 106 to 133 × 106]/ml, respectively [P = 0.489]). Similarly, the contaminating granulocyte concentration was 0.6 × 106 (0.4 × 106 to 2.6 × 106)/ml before Ficoll separation and 0.7 × 106 (0.5 × 106 to 1.1 × 106)/ml after Ficoll separation, with no statistical difference (P = 0.706).
Establishing quality indicators for PBMC processing in Uganda.
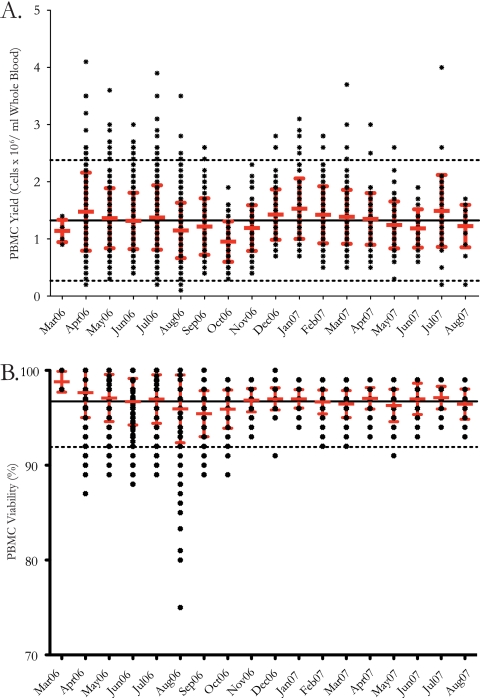
In order to monitor the quality and performance of the processing laboratory in Uganda during the conduct of a phase I/II HIV-1 vaccine trial, the following four processing quality indicators were acquired daily and collated monthly during the study: (i) total time from blood draw to cryopreservation, (ii) time for actual PBMC processing, (iii) PBMC yield per ml of whole blood collected before cryopreservation, and (iv) PBMC viability before cryopreservation. A total of 2,310 samples from both HIV-1-infected (n = 115) and uninfected (n = 2,195) subjects were received in the laboratory for PBMC processing. Samples were processed and cryopreserved from March 2006 through August 2007. The median (range) total time from blood draw to cryopreservation was 3.7 h (3.0 to 7.2 h), while the median (range) process time from blood receipt in the lab to cryopreservation was 2.7 h (2.2 to 6.0 h). The maximum number of samples processed in a month was 313. The cell yield per ml of whole blood collected before cryopreservation was 1.3 × 106 (0.1 × 106 to 4.1 × 106) PBMC/ml whole blood (Fig. 2A). Viability before cryopreservation was assessed, and the overall median (range) was 97% (75% to 100%) (Fig. 2B).
FIG. 2.
Quality indicators were measured for PBMC processing conducted during a phase I/II clinical trial from March 2006 through August 2007 (n = 2,310). PBMC were received in the laboratory, processed using Leucosep tubes, and cryopreserved in liquid nitrogen vapor. (A) Aligned dot plot showing the mean and standard deviation for PBMC yield (cells [106] per ml of whole blood processed) for samples processed each month over the conduct of the clinical trial. The mean for all samples processed is shown with a solid line, and the level of 2 standard deviations is shown with dotted lines. (B) Aligned dot plot showing the mean and standard deviation for PBMC percent viability after processing of samples each month over the conduct of the clinical trial. The mean for all samples processed is shown with a solid line, and the level of 2 standard deviations is shown with a dotted line.
Assessment of PBMC processing shows preservation of viability and function.
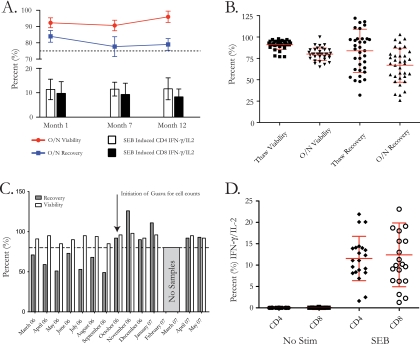
Assessments of PBMC processing, cryopreservation, and storage in liquid nitrogen were made over the course of 1 year by assessing longevity and interval precision in selected samples. Samples from three uninfected individuals were assessed for longevity following processing and cryopreservation by thawing of samples at months 1, 7, and 12. Thawed samples were counted using the Guava ViaCount assay, and cell concentration and viability were determined. Samples were rested overnight, and then cell counts and viability determinations were repeated. Cells were assessed by ICS to measure the amounts of IFN-γ and IL-2 within the CD4 and CD8 T-cell compartments in response to the superantigen SEB. Mean viability after the overnight rest was 93%, 92%, and 98% at months 1, 7, and 12, respectively, while mean recovery was 85%, 79%, and 80%, respectively (Fig. 3A). CD4 T-cell responses to SEB were 9.1%, 10.4%, and 10.0%, while CD8 T-cell responses were 10.7%, 9.6%, and 9.1% at months 1, 7, and 12 postcryopreservation, respectively (Fig. 3A). Precision was assessed by examining PBMC from three individuals, distributed into at least 3 vials each, at 10 × 106 cells per aliquot. All samples showed coefficients of variation of <2% for thaw and overnight viabilities and recovery yields (data not shown).
FIG. 3.
The Makerere University Walter Reed Project laboratory follows standard procedures for assessing quality of PBMC collected and cryopreserved during protocol vaccine trials, including internal assessment of longevity, precision, and functionality. (A) PBMC from 3 subjects were assessed for the impact of cryopreservation on recovery, viability, and functionality by thawing the same samples at 1, 7, and 12 months postcryopreservation. The bar and line charts show the means and standard deviations for overnight viability (red), overnight recovery (blue), and control positive responses (IFN-γ/IL-2) to SEB for CD4 (white bars) and CD8 (black bars) cells. (B) Thirty-five samples were processed, cryopreserved (for approximately 1 month), thawed, and assessed for recovery and viability after thawing and overnight rest as part of a monthly internal quality assurance procedure. The scatter plot shows the cumulative mean and standard deviation for all 35 samples. (C) Bar chart showing the mean and standard deviation for thaw recovery and viability for the 35 samples monitored, by month (no samples were processed in February and March 2007). (D) Thirty-five samples were thawed and assessed for function after thaw and overnight rest in response to SEB or in the absence of stimulation. The scatter plot shows the cumulative mean and standard deviation for all 35 samples for CD4 T cells and CD8 T cells.
Monthly assessments of processing and cryopreservation were performed using 2 to 6 samples that were cryopreserved at the beginning of the month and thawed at the end of the month. Cell counts and viabilities were determined both at the time of thaw and after an overnight rest. At the time of thaw, the median (range) yield was 62% (32% to 143%) and viability was 93% (77% to 98%), while after overnight rest, the median (range) yield was 69% (26% to 103%) and viability was 80% (65% to 100%) (Fig. 3B). Stratifying the responses by month showed fluctuations in mean recovery, from the low of 49% in September 2006 to the high of 126% in November 2006. The range of viability was from the low of 85% in May and September 2006 to the high of 98% in November 2006 (Fig. 3C). The Guava ViaCount assay was implemented in October 2006 in order to reduce the operator variation from hemacytometer counting. No samples were processed for quality monitoring during the months of February and March 2007.
Monthly samples were also assessed for functional responses by conducting a 6-hour stimulation in the presence of the CEF peptide pool or SEB or in the absence of stimulation, and ICS was performed to assess IFN-γ and IL-2 levels in CD4 and CD8 T cells. Cryopreserved specimens exhibited low background with no stimulation and retained functional responses to SEB. The median (range) combination IFN-γ/IL-2 response in the absence of stimulation was 0.02% (0% to 0.17%) in CD4 T cells and 0% (0% to 0.13%) in CD8 T cells. All 35 specimens (100%) had positive responses to SEB. The median (range) IFN-γ/IL-2 response to SEB stimulation was 10.10% (2.15% to 20.10%) in CD4 T cells and 8.35% (1.03% to 19.40%) in CD8 T cells (Fig. 3D). Positive responses to the CEF peptide pool were seen in 6/35 (17%) and 24/35 (69%) specimens from the CD4 T-cell and CD8 T-cell compartments, respectively. The median (range) positive IFN-γ/IL-2 response to CEF stimulation was 0.11% (0.06% to 0.26%) in CD4 T cells and 0.36% (0.05% to 7.17%) in CD8 T cells.
PBMC maintain viability after long-term storage.
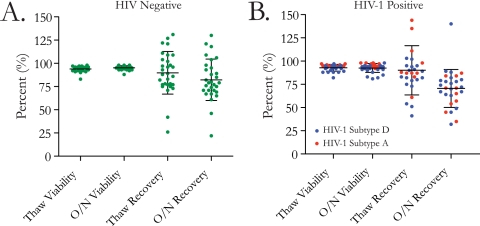
The effect of long-term cryopreservation (>3 years) was assessed in samples from uninfected (n = 30) and HIV-1-infected (n = 29) individuals. Overall, the median (range) percent recovery was 88% (26% to 173%) and the viability was 94% (82% to 98%) at the time of thaw. After overnight rest, the median (range) percent recovery was 76% (22% to 140%) and the viability was 95% (81% to 98%) (Fig. 4A). For the uninfected subjects after overnight rest of samples, the median (range) percent recovery was 79% (22% to 130%) and the viability was 96% (88% to 98%) (Fig. 4A). For 10 HIV-1 subtype A-infected subjects, the median (range) percent PBMC recovery was 68% (35% to 87%) and the viability was 97% (92% to 98%) after overnight rest (Fig. 4B). For 19 HIV-1 subtype D-infected subjects, the median (range) percent recovery was 74% (32% to 140%) and the viability was 92% (81% to 98%) after overnight rest (Fig. 4D). No statistically significant difference in recovery and viability was observed between the HIV-1-infected and uninfected individuals or between the HIV-1 subtype A- and subtype D-infected study participants.
FIG. 4.
The effect of long-term cryopreservation (storage for >3 years) was assessed for HIV-infected and uninfected individuals. (A) Scatter plot showing the cumulative mean and standard deviation for recovery and viability after thaw and overnight (O/N) rest for 30 uninfected samples. (B) Scatter plot showing the cumulative mean and standard deviation for recovery and viability after thaw and overnight rest for 59 HIV-1-infected samples with HIV-1 subtype A (n = 10) (red) and HIV-1 subtype D (n = 19) (blue).
DISCUSSION
Many of the T-cell-based assays used to assess potential HIV-1 candidate vaccines have been standardized and evaluated to characterize vaccine immunogenicity (7, 22, 43) but may not define correlates of protection (8, 35). T cells are linked to control of HIV-1 infection, and specific functional profiles are associated with slow HIV-1 disease progression in infected individuals (6). Common practice for many multicenter vaccine trials is to cryopreserve cells and ship them to a central laboratory to reduce interlaboratory variation in assay performance. Additionally, cryopreserved cells allow for carefully planned longitudinal analysis and case-control studies. Hematological parameters have previously been shown to differ for populations in Uganda compared to populations in industrialized countries (17, 26, 33, 44), and this could adversely affect protocol end points designed to utilize white blood cells for immunologic phenotype or functional analysis. Here we characterized the function, phenotype, viability, and yield of PBMC from HIV-1-infected and uninfected Ugandans and introduced a number of quality measures that can be used to monitor the performance of collection, processing, cryopreservation, and storage of PBMC in real time.
Studies from Uganda have used cryopreserved PBMC for cellular immunology assessment; however, minimal data have been presented on processing cell yields and postthaw recoveries (5, 10, 11, 24). In a recent study conducted on PBMC from Tanzanian subjects, a yield of 1.1 × 106 cells was harvested per ml of whole blood, with 96% viability (37), compared to the 1.3 × 106 cells per ml of whole blood and 97% viability we observed in Ugandan subjects, using similar procedures and reagents. Our study summarizes results for a much larger cohort of 2,195 healthy individuals and proposes 95% reference intervals (Table 1) for processing parameters in an effort to validate this methodology in accordance with Clinical and Laboratory Standards Institute (CLSI) guidelines, although for granulocytes, monocytes, lymphocytes, and platelets, the recommended number of 120 samples was not achieved (36). Additionally, we observed similar yields and viabilities for HIV-1-infected individuals, although some donors with advanced disease exhibited a high level of lymphopenia and poor separation from red blood cells in the Ficoll gradient, resulting in extremely low yields and viabilities.
TABLE 1.
PBMC processing parameter 95% reference intervals
| Parameter (n) | Median | Mean (SD) | Range |
|---|---|---|---|
| Lymphocytes (%) (52) | 71.7 | 69.9 (12.9) | 32.9-90.1 |
| Monocytes (%) (52) | 20.2 | 21.4 (9.7) | 6.5-48.4 |
| Granulocytes (%) (52) | 4.9 | 8.8 (8.3) | 1.5-35.6 |
| Platelets (106 cells) (52) | 10.0 | 12.1 (16.6) | 0.0-88.2 |
| Yield (cells/ml whole blood) (2,195) | 1.3 | 1.3 (0.5) | 0.4-2.6 |
| Viability (%) (2,195) | 97.0 | 96.7 (2.4) | 91.0-100 |
Separated PBMC should consist of a pure population of mononuclear cells (95%) (18) with yields of approximately 1 × 106 to 2 × 106 cells/ml of whole blood, consisting of 60 to 70% lymphocytes at >95% viability, with a reduction of platelets to <0.5% of the original whole-blood content (25). Performance characteristics obtained using a porous high-density polyethylene barrier “frit” like a Leucosep tube or equivalent Accuspin tube should enable higher accuracy in harvesting mononuclear cells, yielding a final product of 88% lymphocytes, 9% monocytes, and 3% granulocytes at a viability of 98% (42). We did observe slightly higher platelet contamination in the final product, with a median reduction to 1.16% of the total original blood content, but in general we showed that processing with Leucosep tubes gives results similar to manufacturer specifications, allowing for accurate preparation and planning for clinical studies requiring PBMC.
In order to assess the effects of processing and cryopreservation on immune phenotype, we compared T (CD4+ and CD8+ T cells), B, and NK lymphocytes in fresh whole blood or after Ficoll processing, cryopreservation, and thawing. Interestingly, the proportions of most subsets were preserved, with the exception of B cells, consistent with a previous multicenter study conducted in the United States in which a reduction in the percentage of B cells was observed after processing and cryopreservation (39). Other studies have shown increases or static levels of B cells (4, 31, 34, 41). Our data support the observation of reduced B-cell numbers after cryopreservation for both HIV-1-infected and uninfected individuals. One potential explanation is that B cells may have a different density and could be centrifuged through the Ficoll gradient and be included in the red blood cell pellet. Another explanation could be that B cells are more susceptible to the effects of cryopreservation and subsequently die. This loss is of great importance to B-cell immunologists who plan to study this subset from cryopreserved PBMC.
Processing of PBMC is straightforward, but many parameters need to be optimized in order to maximize yield, recovery, viability, and function. One important finding is that contaminating granulocytes increase cell clumping upon thawing (16) and adversely affect PBMC yield, function, and assay background responses (13). Another important processing parameter is the time from blood draw to the time the PBMC are cryopreserved. Several studies indicate that a processing time of 24 h or greater negatively impacts the viability, recovery, and function of PBMC and suggest that processing and cryopreservation need to occur within 8 h of blood draw (9, 13, 16, 28). We confirmed the feasibility of large-scale processing in a resource-limited setting with transport to the laboratory from an off-site clinic, with a time of blood draw to cryopreservation of under 7.5 h and a median time from receipt in the laboratory to cryopreservation of 3.7 h. Additional parameters that have minimal impact on PBMC include shipping, centrifugal force for washing, concentration of cells in the vial, anticoagulant used in collection of whole blood, use of a classical overlay procedure, and the size of the conical wash tube (9, 15, 37, 45), and here we showed that harvesting the plasma before or after the Ficoll gradient step also did not affect the overall quality of PBMC, eliminating one centrifugation step and reducing the processing time.
Other studies suggest that it is not the processing and cryopreservation procedures that adversely affect PBMC, but how they are thawed and if they are rested overnight (13, 28, 30), and that viability needs to be >70 to 75% for consistent functional assessment (15, 46, 47). In our study, function was maintained in response to superantigen, and background responses remained low under unstimulated conditions. While unstimulated and superantigen-exposed PBMC were consistent in function, the antigen-specific CEF peptide set was not consistent in generating positive responses. This may be due in part to differences in the HLA alleles in African populations and the epitopes selected for the CEF peptide pool. The lab has since transitioned to using a commercially available CMV pp65 peptide set that generates a higher frequency of consistent responses in the ICS assay, making for a better positive control.
Preservation of PBMC for HIV vaccine trials is evaluated primarily by centralized laboratories, which perform immunogenicity assays. Some groups focus on a central laboratory to serve as a repository and evaluation unit (9, 28), while others develop a quality assurance scheme that revolves around sample collection and subsequent evaluation performed centrally (16). While these methods are a necessary component to good PBMC practice (GPP), real-time assessments of PBMC processing and cryopreservation are needed. The network of labs in the U.S. Military HIV Research Program all adhere to centralized standard operating procedures for processing, cryopreservation, and shipping of PBMC and participate in training across sites within the network. Additionally, a new site goes through a validation and initialization period, during which a site must prove proficiency in processing and cryopreservation. Once qualified to conduct PBMC processing, a site is monitored both externally and internally. While our PBMC quality management plan includes shipping to external sites for assessment several times a year, here we outline several indicators that can be monitored by the actual processing units, including a plan to thaw PBMC and evaluate recoveries and viabilities at regular intervals. It is critical that these procedures be done in such a manner that they do not impact the end-point analysis for protocols, and they should be developed early in study design to include blood for processing and cryopreservation assessment. Table 2 summarizes the quality control measures developed within our laboratory.
TABLE 2.
PBMC quality measures
| Parameter | Target | Impact | Reference(s) |
|---|---|---|---|
| Analytical parameters | |||
| Granulocytes (%) | 3.0 ± 2.7 | High | 40 |
| Lymphocytes (%) | 60-70 (with Leucosep tubes, 87.6 ± 4.3) | High | 24, 40 |
| Monocytes (%) | 9.1 ± 3.8 | Low | 40 |
| Platelets (%) | 0-88.2 (<0.5% of original content) | High | 24 |
| Viability (%) | >95 | Medium | 24 |
| Yield (cells/ml whole blood) | 1 × 106-2 × 106 | Medium | 24 |
| Postanalytical parameters | |||
| ICS | Various targets, including low background, consistently strong positive controls, and preserved antigen-specific responses | High | 1, 9, 28 |
| Precision | Code of variation of <5% | Medium | |
| Long-term storage in liquid nitrogen (yr) | <10 is optimal, but indefinite storage has been suggested | Low | 12, 22 |
| Transport | Maintain cold chain | High | 13 |
| Overnight viability (%) | >70-75 | High | 46 |
| Overnight recovery (%) | >50 | High |
In summary, we show here that a standard procedure for processing of PBMC by use of Leucosep tubes in the resource-limited setting of Uganda is practical and feasible for conduct of cellular immunology research studies. Through extant laboratory capacity, it is possible to monitor parameters that may adversely affect PBMC functionality, such as granulocyte and platelet contamination. We also provide indicators to track the performance of technical staff and preanalytical and analytical parameters, including time from venipuncture to cryopreservation, processing time, yield of PBMC/ml of whole blood, and viability. We also show comparable functions, phenotypes, viabilities, and yields of PBMC from HIV-1-infected and uninfected Ugandans with preservation over time. Taken together, these data suggest a prudent methodology to both validate and monitor the method for processing and cryopreservation of PBMC.
Acknowledgments
We thank Kayunga, Rakai, and Kampala District research participants for their valuable contributions and support during the conduct of these studies. We thank the Makerere University Walter Reed Project staff for continued dedication toward development of a safe and effective HIV-1 vaccine, despite numerous hurdles. Jeff Currier and the lab at MHRP have given invaluable scientific, technical, and logistical support.
Material has been reviewed by the Walter Reed Army Institute of Research. There is no objection to its presentation and/or publication. The views expressed here are those of the authors and should not be construed to represent the positions of the U.S. Army or DOD.
This work was supported by a cooperative agreement (W81XWH-07-2-0067) between the Henry M. Jackson Foundation for the Advancement of Military Medicine, Inc., and the U.S. Department of Defense (DOD). This research was funded in part by the U.S. National Institute of Allergy and Infectious Diseases.
Footnotes
Published ahead of print on 3 March 2010.
REFERENCES
- 1.Allsopp, C. E., S. J. Nicholls, and J. Langhorne. 1998. A flow cytometric method to assess antigen-specific proliferative responses of different subpopulations of fresh and cryopreserved human peripheral blood mononuclear cells. J. Immunol. Methods 214:175-186. [DOI] [PubMed] [Google Scholar]
- 2.Appay, V., S. Reynard, V. Voelter, P. Romero, D. E. Speiser, and S. Leyvraz. 2006. Immuno-monitoring of CD8+ T cells in whole blood versus PBMC samples. J. Immunol. Methods 309:192-199. [DOI] [PubMed] [Google Scholar]
- 3.Arroyo, M. A., W. B. Sateren, D. Serwadda, R. H. Gray, M. J. Wawer, N. K. Sewankambo, N. Kiwanuka, G. Kigozi, F. Wabwire-Mangen, M. Eller, L. A. Eller, D. L. Birx, M. L. Robb, and F. E. McCutchan. 2006. Higher HIV-1 incidence and genetic complexity along main roads in Rakai District, Uganda. J. Acquir. Immune Defic. Syndr. 43:440-445. [DOI] [PubMed] [Google Scholar]
- 4.Ashmore, L. M., G. M. Shopp, and B. S. Edwards. 1989. Lymphocyte subset analysis by flow cytometry. Comparison of three different staining techniques and effects of blood storage. J. Immunol. Methods 118:209-215. [DOI] [PubMed] [Google Scholar]
- 5.Baker, C. A., K. McEvers, R. Byaruhanga, R. Mulindwa, D. Atwine, J. Nantiba, N. G. Jones, I. Ssewanyana, and H. Cao. 2008. HIV subtypes induce distinct profiles of HIV-specific CD8(+) T cell responses. AIDS Res. Hum. Retroviruses 24:283-287. [DOI] [PubMed] [Google Scholar]
- 6.Betts, M. R., M. C. Nason, S. M. West, S. C. De Rosa, S. A. Migueles, J. Abraham, M. M. Lederman, J. M. Benito, P. A. Goepfert, M. Connors, M. Roederer, and R. A. Koup. 2006. HIV nonprogressors preferentially maintain highly functional HIV-specific CD8+ T cells. Blood 107:4781-4789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Boaz, M. J., P. Hayes, T. Tarragona, L. Seamons, A. Cooper, J. Birungi, P. Kitandwe, A. Semaganda, P. Kaleebu, G. Stevens, O. Anzala, B. Farah, S. Ogola, J. Indangasi, P. Mhlanga, M. Van Eeden, M. Thakar, A. Pujari, S. Mishra, N. Goonetilleke, S. Moore, A. Mahmoud, P. Sathyamoorthy, J. Mahalingam, P. R. Narayanan, V. D. Ramanathan, J. H. Cox, L. Dally, D. K. Gill, and J. Gilmour. 2009. Concordant proficiency in measurement of T-cell immunity in human immunodeficiency virus vaccine clinical trials by peripheral blood mononuclear cell and enzyme-linked immunospot assays in laboratories from three continents. Clin. Vaccine Immunol. 16:147-155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Buchbinder, S. P., D. V. Mehrotra, A. Duerr, D. W. Fitzgerald, R. Mogg, D. Li, P. B. Gilbert, J. R. Lama, M. Marmor, C. Del Rio, M. J. McElrath, D. R. Casimiro, K. M. Gottesdiener, J. A. Chodakewitz, L. Corey, and M. N. Robertson. 2008. Efficacy assessment of a cell-mediated immunity HIV-1 vaccine (the Step Study): a double-blind, randomised, placebo-controlled, test-of-concept trial. Lancet 372:1881-1893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bull, M., D. Lee, J. Stucky, Y. L. Chiu, A. Rubin, H. Horton, and M. J. McElrath. 2007. Defining blood processing parameters for optimal detection of cryopreserved antigen-specific responses for HIV vaccine trials. J. Immunol. Methods 322:57-69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cao, H., P. Kaleebu, D. Hom, J. Flores, D. Agrawal, N. Jones, J. Serwanga, M. Okello, C. Walker, H. Sheppard, R. El-Habib, M. Klein, E. Mbidde, P. Mugyenyi, B. Walker, J. Ellner, R. Mugerwa, et al. 2003. Immunogenicity of a recombinant human immunodeficiency virus (HIV)-canarypox vaccine in HIV-seronegative Ugandan volunteers: results of the HIV Network for Prevention Trials 007 Vaccine Study. J. Infect. Dis. 187:887-895. [DOI] [PubMed] [Google Scholar]
- 11.Cao, H., I. Mani, R. Vincent, R. Mugerwa, P. Mugyenyi, P. Kanki, J. Ellner, and B. D. Walker. 2000. Cellular immunity to human immunodeficiency virus type 1 (HIV-1) clades: relevance to HIV-1 vaccine trials in Uganda. J. Infect. Dis. 182:1350-1356. [DOI] [PubMed] [Google Scholar]
- 12.Costantini, A., S. Mancini, S. Giuliodoro, L. Butini, C. M. Regnery, G. Silvestri, and M. Montroni. 2003. Effects of cryopreservation on lymphocyte immunophenotype and function. J. Immunol. Methods 278:145-155. [DOI] [PubMed] [Google Scholar]
- 13.Cox, J. H., G. Ferrari, R. T. Bailer, and R. A. Koup. 2004. Automating procedures for processing, cryopreservation, storage, and manipulation of human peripheral blood mononuclear cells. J. Assoc. Lab. Autom. 9:16-23. [Google Scholar]
- 14.Currier, J. R., E. G. Kuta, E. Turk, L. B. Earhart, L. Loomis-Price, S. Janetzki, G. Ferrari, D. L. Birx, and J. H. Cox. 2002. A panel of MHC class I restricted viral peptides for use as a quality control for vaccine trial ELISPOT assays. J. Immunol. Methods 260:157-172. [DOI] [PubMed] [Google Scholar]
- 15.Disis, M. L., C. dela Rosa, V. Goodell, L. Y. Kuan, J. C. Chang, K. Kuus-Reichel, T. M. Clay, H. Kim Lyerly, S. Bhatia, S. A. Ghanekar, V. C. Maino, and H. T. Maecker. 2006. Maximizing the retention of antigen specific lymphocyte function after cryopreservation. J. Immunol. Methods 308:13-18. [DOI] [PubMed] [Google Scholar]
- 16.Dyer, W. B., S. L. Pett, J. S. Sullivan, S. Emery, D. A. Cooper, A. D. Kelleher, A. Lloyd, and S. R. Lewin. 2007. Substantial improvements in performance indicators achieved in a peripheral blood mononuclear cell cryopreservation quality assurance program using single donor samples. Clin. Vaccine Immunol. 14:52-59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Eller, L. A., M. A. Eller, B. Ouma, P. Kataaha, D. Kyabaggu, R. Tumusiime, J. Wandege, R. Sanya, W. B. Sateren, F. Wabwire-Mangen, H. Kibuuka, M. L. Robb, N. L. Michael, and M. S. de Souza. 2008. Reference intervals in healthy adult Ugandan blood donors and their impact on conducting international vaccine trials. PLoS One 3:e3919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.GE Healthcare. 2007. Ficoll-Paque Plus intended use for in vitro isolation of lymphocytes. GE Healthcare, Uppsala, Sweden.
- 19.Guwatudde, D., F. Wabwire-Mangen, L. A. Eller, M. Eller, F. McCutchan, H. Kibuuka, M. Millard, N. Sewankambo, D. Serwadda, N. Michael, and M. Robb. 2009. Relatively low HIV infection rates in rural Uganda, but with high potential for a rise: a cohort study in Kayunga District, Uganda. PLoS One 4:e4145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Harris, M., D. Serwada, N. K. Sewankambo, B. Kim, G. Kigozi, N. Kiwanuka, J. B. Philips, F. Wabwire, M. Meehen, T. Lutalo, J. R. Lane, R. Merling, R. H. Gray, M. J. Wawer, D. L. Birx, M. D. Robb, and F. E. McCutchan. 2002. Among 46 near full-length HIV type 1 genome sequences from Rakai District, Uganda, subtype D and AD recombinants predominate. AIDS Res. Hum. Retroviruses 18:1281-1290. [DOI] [PubMed] [Google Scholar]
- 21.Hoelscher, M., W. E. Dowling, E. Sanders-Buell, J. K. Carr, M. E. Harris, A. Thomschke, M. L. Robb, D. L. Birx, and F. E. McCutchan. 2002. Detection of HIV-1 subtypes, recombinants, and dual infections in east Africa by a multi-region hybridization assay. AIDS 16:2055-2064. [DOI] [PubMed] [Google Scholar]
- 22.Horton, H., E. P. Thomas, J. A. Stucky, I. Frank, Z. Moodie, Y. Huang, Y. L. Chiu, M. J. McElrath, and S. C. De Rosa. 2007. Optimization and validation of an 8-color intracellular cytokine staining (ICS) assay to quantify antigen-specific T cells induced by vaccination. J. Immunol. Methods 323:39-54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jeurink, P. V., Y. M. Vissers, B. Rappard, and H. F. Savelkoul. 2008. T cell responses in fresh and cryopreserved peripheral blood mononuclear cells: kinetics of cell viability, cellular subsets, proliferation, and cytokine production. Cryobiology 57:91-103. [DOI] [PubMed] [Google Scholar]
- 24.Jones, N., M. Eggena, C. Baker, F. Nghania, D. Baliruno, P. Mugyenyi, F. Ssali, B. Barugahare, and H. Cao. 2006. Presence of distinct subsets of cytolytic CD8+ T cells in chronic HIV infection. AIDS Res. Hum. Retroviruses 22:1007-1013. [DOI] [PubMed] [Google Scholar]
- 25.Kanof, M. E., P. D. Smith, and H. Zola. 2003. Preparation of human mononuclear cell populations and subpopulations. Curr. Protoc. Immunol. 2003(Suppl. 19):Unit 7.1. [Google Scholar]
- 26.Karita, E., N. Ketter, M. A. Price, K. Kayitenkore, P. Kaleebu, A. Nanvubya, O. Anzala, W. Jaoko, G. Mutua, E. Ruzagira, J. Mulenga, E. J. Sanders, M. Mwangome, S. Allen, A. Bwanika, U. Bahemuka, K. Awuondo, G. Omosa, B. Farah, P. Amornkul, J. Birungi, S. Yates, L. Stoll-Johnson, J. Gilmour, G. Stevens, E. Shutes, O. Manigart, P. Hughes, L. Dally, J. Scott, W. Stevens, P. Fast, and A. Kamali. 2009. CLSI-derived hematology and biochemistry reference intervals for healthy adults in eastern and southern Africa. PLoS One 4:e4401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kibuuka, H., D. Guwatudde, R. Kimutai, L. Maganga, L. Maboko, C. Watyema, F. Sawe, D. Shaffer, D. Matsiko, M. Millard, N. Michael, F. Wabwire-Mangen, and M. Robb. 2009. Contraceptive use in women enrolled into preventive HIV vaccine trials: experience from a phase I/II trial in east Africa. PLoS One 4:e5164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kierstead, L. S., S. Dubey, B. Meyer, T. W. Tobery, R. Mogg, V. R. Fernandez, R. Long, L. Guan, C. Gaunt, K. Collins, K. J. Sykes, D. V. Mehrotra, N. Chirmule, J. W. Shiver, and D. R. Casimiro. 2007. Enhanced rates and magnitude of immune responses detected against an HIV vaccine: effect of using an optimized process for isolating PBMC. AIDS Res. Hum. Retroviruses 23:86-92. [DOI] [PubMed] [Google Scholar]
- 29.Kiwanuka, N., O. Laeyendecker, M. Robb, G. Kigozi, M. Arroyo, F. McCutchan, L. A. Eller, M. Eller, F. Makumbi, D. Birx, F. Wabwire-Mangen, D. Serwadda, N. K. Sewankambo, T. C. Quinn, M. Wawer, and R. Gray. 2008. Effect of human immunodeficiency virus type 1 (HIV-1) subtype on disease progression in persons from Rakai, Uganda, with incident HIV-1 infection. J. Infect. Dis. 197:707-713. [DOI] [PubMed] [Google Scholar]
- 30.Kreher, C. R., M. T. Dittrich, R. Guerkov, B. O. Boehm, and M. Tary-Lehmann. 2003. CD4+ and CD8+ cells in cryopreserved human PBMC maintain full functionality in cytokine ELISPOT assays. J. Immunol. Methods 278:79-93. [DOI] [PubMed] [Google Scholar]
- 31.Kutvirt, S. G., S. L. Lewis, and T. L. Simon. 1993. Lymphocyte phenotypes in infants are altered by separation of blood on density gradients. Br. J. Biomed. Sci. 50:321-328. [PubMed] [Google Scholar]
- 32.Kvarnström, M., M. C. Jenmalm, and C. Ekerfelt. 2004. Effect of cryopreservation on expression of Th1 and Th2 cytokines in blood mononuclear cells from patients with different cytokine profiles, analysed with three common assays: an overall decrease of interleukin-4. Cryobiology 49:157-168. [DOI] [PubMed] [Google Scholar]
- 33.Lugada, E. S., J. Mermin, F. Kaharuza, E. Ulvestad, W. Were, N. Langeland, B. Asjo, S. Malamba, and R. Downing. 2004. Population-based hematologic and immunologic reference values for a healthy Ugandan population. Clin. Diagn. Lab. Immunol. 11:29-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Macey, M. G., C. J. Hyam, and A. C. Newland. 1988. Enumeration of lymphocyte sub-populations: a comparative study of whole blood and gradient centrifugation methods. Med. Lab. Sci. 45:187-191. [PubMed] [Google Scholar]
- 35.McElrath, M. J., S. C. De Rosa, Z. Moodie, S. Dubey, L. Kierstead, H. Janes, O. D. Defawe, D. K. Carter, J. Hural, R. Akondy, S. P. Buchbinder, M. N. Robertson, D. V. Mehrotra, S. G. Self, L. Corey, J. W. Shiver, and D. R. Casimiro. 2008. HIV-1 vaccine-induced immunity in the test-of-concept Step Study: a case-cohort analysis. Lancet 372:1894-1905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.NCCLS. 2000. How to define and determine reference intervals in the clinical laboratory; approved guideline, 2nd ed. C28-A2, Vol. 20, No. 13. National Committee for Clinical Laboratory Standards, Wayne, PA.
- 37.Nilsson, C., S. Aboud, K. Karlén, B. Hejdeman, W. Urassa, and G. Biberfeld. 2008. Optimal blood mononuclear cell isolation procedures for gamma interferon enzyme-linked immunospot testing of healthy Swedish and Tanzanian subjects. Clin. Vaccine Immunol. 15:585-589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Owen, R. E., E. Sinclair, B. Emu, J. W. Heitman, D. F. Hirschkorn, C. L. Epling, Q. X. Tan, B. Custer, J. M. Harris, M. A. Jacobson, J. M. McCune, J. N. Martin, F. M. Hecht, S. G. Deeks, and P. J. Norris. 2007. Loss of T cell responses following long-term cryopreservation. J. Immunol. Methods 326:93-115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Reimann, K. A., M. Chernoff, C. L. Wilkening, C. E. Nickerson, and A. L. Landay. 2000. Preservation of lymphocyte immunophenotype and proliferative responses in cryopreserved peripheral blood mononuclear cells from human immunodeficiency virus type 1-infected donors: implications for multicenter clinical trials. The ACTG Immunology Advanced Technology Laboratories. Clin. Diagn. Lab. Immunol. 7:352-359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Riccio, E. K., I. Neves, D. M. Banic, S. Corte-Real, M. G. Alecrim, M. Morgado, C. T. Daniel-Ribeiro, and M. D. E. F. Ferreira-da-Cruz. 2002. Cryopreservation of peripheral blood mononuclear cells does not significantly affect the levels of spontaneous apoptosis after 24-h culture. Cryobiology 45:127-134. [DOI] [PubMed] [Google Scholar]
- 41.Romeu, M. A., M. Mestre, L. Gonzalez, A. Valls, J. Verdaguer, M. Corominas, J. Bas, E. Massip, and E. Buendia. 1992. Lymphocyte immunophenotyping by flow cytometry in normal adults. Comparison of fresh whole blood lysis technique, Ficoll-Paque separation and cryopreservation. J. Immunol. Methods 154:7-10. [DOI] [PubMed] [Google Scholar]
- 42.Sigma-Aldrich. 2003. Accuspin tubes procedure no. AST-1. Sigma-Aldrich, St. Louis, MO.
- 43.Tobery, T. W., S. A. Dubey, K. Anderson, D. C. Freed, K. S. Cox, J. Lin, M. T. Prokop, K. J. Sykes, R. Mogg, D. V. Mehrotra, T. M. Fu, D. R. Casimiro, and J. W. Shiver. 2006. A comparison of standard immunogenicity assays for monitoring HIV type 1 gag-specific T cell responses in Ad5 HIV type 1 gag vaccinated human subjects. AIDS Res. Hum. Retroviruses 22:1081-1090. [DOI] [PubMed] [Google Scholar]
- 44.Tugume, S. B., E. M. Piwowar, T. Lutalo, P. N. Mugyenyi, R. M. Grant, F. W. Mangeni, K. Pattishall, and E. Katongole-Mbidde. 1995. Hematological reference ranges among healthy Ugandans. Clin. Diagn. Lab. Immunol. 2:233-235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Weinberg, A., R. A. Betensky, L. Zhang, and G. Ray. 1998. Effect of shipment, storage, anticoagulant, and cell separation on lymphocyte proliferation assays for human immunodeficiency virus-infected patients. Clin. Diagn. Lab. Immunol. 5:804-807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Weinberg, A., L. Y. Song, C. Wilkening, A. Sevin, B. Blais, R. Louzao, D. Stein, P. Defechereux, D. Durand, E. Riedel, N. Raftery, R. Jesser, B. Brown, M. F. Keller, R. Dickover, E. McFarland, and T. Fenton. 2009. Optimization and limitations of use of cryopreserved peripheral blood mononuclear cells for functional and phenotypic T-cell characterization. Clin. Vaccine Immunol. 16:1176-1186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Weinberg, A., L. Zhang, D. Brown, A. Erice, B. Polsky, M. S. Hirsch, S. Owens, and K. Lamb. 2000. Viability and functional activity of cryopreserved mononuclear cells. Clin. Diagn. Lab. Immunol. 7:714-716. [DOI] [PMC free article] [PubMed] [Google Scholar]