Abstract
Intrahepatic hepatitis B virus (HBV) core antigen (HBcAg) is a hallmark of viral replication in hepatitis B virus e antigen (HBeAg)-positive chronic hepatitis B (CHB). The aim of this study was to evaluate the role of HBcAg in HBeAg-negative CHB. One hundred six HBeAg-negative CHB patients who underwent ultrasonographically guided liver biopsy were reviewed for their HBV DNA load and clinical and histological data. Factors associated with the expression of intrahepatic HBcAg were analyzed. Among the patients, 35 (33%) were positive for HBcAg by immunohistostaining. In patients whose HBV DNA loads were higher than 107 copies (cp)/ml, nearly one-half (52%) had detectable HBcAg. Compared with HBcAg-negative patients, HBcAg-positive patients had higher serum alanine transaminase (ALT) and HBV DNA levels and more-severe hepatic necroinflammation. High serum ALT level (>160 U/liter) and HBV viral load were the determinants of HBcAg expression in multivariate analysis. Large amounts of HBcAg expression were frequently detected in patients with high DNA loads, and the patterns of HBcAg distribution were not related to histological activity or HBV DNA levels. In patients with lower HBV DNA loads, the expression of HBcAg was the key factor associated with active hepatic necroinflammation (hazard ratio = 11.25; 95% confidence interval [CI], 1.42 to 89.26; P = 0.022). In conclusion, the expression of HBcAg is not frequent in HBeAg-negative CHB. The expression of intrahepatic HBcAg indicates active hepatic necroinflammation, even in patients with low HBV DNA load. Both HBV viral load and HBcAg expression have implications in the pathogenesis of HBeAg-negative CHB.
Hepatitis B virus (HBV) is a circular, partially double-stranded DNA virus (10, 32). HBV infection often leads to chronic hepatitis when it occurs in the neonatal period or early childhood. The natural history of chronic hepatitis B (CHB) has been divided into 4 phases: immune tolerance, immune clearance, immune control, and reactivation after HBV e antigen (HBeAg) seroconversion (19). HBV c antigen (HBcAg) is an intracellular antigen that is expressed in HBV-infected hepatocytes. HBcAg is an immunogenic protein and plays a role in serving as a target antigen for the host immune reaction. HLA class I- and II-restricted T-cell responses to the HBcAg are required for viral clearance (5). In the HBeAg-positive phase (including immune tolerance and immune clearance phases), HBV DNA levels in serum and liver are high (20, 23) and HBcAg is often present in hepatocytes (7, 14, 26, 30). Generally, after the process of HBeAg seroconversion, the level of HBV DNA declines and the HBcAg is undetectable in hepatocytes (immune control phase) (7). However, the active phase of HBeAg-negative CHB occurs in some patients (reactivation phase) (13, 15). HBeAg-negative CHB is prevalent in Asia and Mediterranean Europe and may lead to cirrhosis and hepatocellular carcinoma (HCC) (15). Mutations in precore (G1896A) and basic core promoter (A1762T and G1764A) regions that stop or decrease the production of HBeAg are the major variants in HBeAg-negative CHB (2, 22, 27, 31, 33). These mutations theoretically do not interfere with the initiation and production of HBcAg. The intrahepatic HBcAg will reappear in some, but not all, HBeAg-negative CHB cases. The clinical significance of HBcAg in HBeAg-negative CHB is unclear.
Previous reports have found that the localization and expression level of HBcAg are associated with active liver disease or viral replication in the HBeAg-positive stage (7, 14, 26). In the immune tolerance phase, patients usually have a higher level of HBV viremia and the expression of HBcAg is mainly localized in the nucleus, whereas in the immune clearance phase HBcAg can be expressed in the nucleus, cytoplasm, or both in infected hepatocytes (7, 14, 26). It has been suggested that cytoplasmic expression of HBcAg correlates with the severity of liver damage and that nuclear expression of HBcAg reflects the level of viral replication (8). HBcAg distribution might have a certain correlation with serum aminotransferase, HBV DNA, and HBeAg status (18). However, the study population in previous reports was mainly positive for HBeAg (immune tolerance and immune clearance phases), and only a few patients were in the HBeAg-negative phase. Factors associated with the expression and distribution of intrahepatic HBcAg after HBeAg seroconversion deserve study.
Measurement of HBV DNA levels is now a useful test for evaluating HBV replication and is considered a marker to determine not only the start and the endpoint of antiviral treatment but also the risk of HCC development (23, 28). The clinical significance of reappearance of intrahepatic HBcAg in the reactivation phase of CHB based on HBV viral loads had not been well evaluated. In the current study, we tried to clarify the relationship among the localization, degree of intrahepatic HBcAg expression, level of HBV viremia, and pathological findings in HBeAg-negative CHB.
MATERIALS AND METHODS
Patients.
The chart records of HBeAg-negative CHB patients who underwent liver biopsy from July 2007 to April 2009 at the Division of Gastroenterology, Taipei Veterans General Hospital, were reviewed retrospectively. One hundred six consecutive patients were enrolled; enrollment was limited to patients who (i) were positive for both serum HBV surface antigen (HBsAg) and anti-HBe and negative for HBeAg for more than 6 months, (ii) were positive for quantitative HBV DNA as determined by a Cobas Amplicor HBV Monitor test (Roche Diagnostic Systems, Basel, Switzerland; the detection limit of this assay was 300 copies [cp]/ml), (iii) had had at least two episodes of serum alanine transaminase (ALT) levels ≥80 U/liter (2 times the upper limit of normal [ULN]) 1 month apart prior to liver biopsy, and (iv) were seronegative for autoantibodies (antinuclear, anti-smooth muscle, and antimitochondrial antibodies) and antibodies to hepatitis C virus (HCV), hepatitis D virus (HDV), or human immunodeficiency virus (HIV). None of the patients had HCC, and none received antiviral treatments (nucleoside/nucleotide analogues or interferon) before liver biopsy. The HBV DNA levels were obtained within 2 weeks of liver biopsy.
Immunohistochemistry and histological grading.
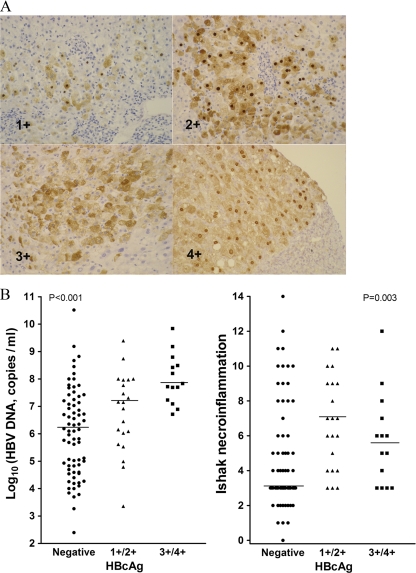
Expression of HBcAg in hepatocytes was studied by the avidin-biotin immunoperoxidase method with a paraffin section of a liver specimen (8). A rabbit polyclonal antibody against HBcAg (Novocastra, Newcastle, United Kingdom) was used for detection. The levels of intrahepatic HBcAg expression were scored from 0 to 4 according to the percentage of hepatocytes that expressed HBcAg: 0, none; 1, less than 10%; 2, 11 to 25%; 3, 26 to 50%; 4, more than 50% (Fig. 1A) (8). The locations of HBcAg were recorded as nucleus, cytoplasm, or mixed. The grade of hepatic necroinflammation and the stage of fibrosis were recorded according to the Ishak score (17). Moderate to severe hepatic necroinflammation (Ishak grading ≥ 7) was defined as significant necroinflammation. All the specimens were reviewed by the same pathologist to avoid interobserver bias.
FIG. 1.
(A) Immunostain for HBcAg. Magnification, ×20. The level of intrahepatic HBcAg expression was scored as 0 (no HBcAg), 1 (less than 10% of hepatocytes expressed HBcAg), 2 (11% to 25% of hepatocytes), 3 (26% to 50% of hepatocytes), or 4 (more than 50% of hepatocytes). (B) Correlation of the expression levels of intrahepatic HBcAg with serum HBV DNA loads (left) and Ishak necroinflammation (right). The Kruskal-Wallis ANOVA test was used for statistical analysis among the three groups.
Statistical methods.
The statistical analyses were performed by using the chi-square test with Yates's correction, Fisher's exact test, Mann-Whitney U test, or Kruskal-Wallis analysis of variance (ANOVA) where appropriate for the comparison of variables between groups or within members of a group. For continuous variables, the median value or the most discriminating one was chosen as the cutoff value. Variables that achieved statistical significance (P < 0.05) or were close to significance (P < 0.1) in univariate analysis were subjected to multivariate analysis using a forward stepwise logistic regression model. A two-tailed P value of less than 0.05 was considered to be statistically significant for all tests.
RESULTS
Clinical features of HBeAg-negative CHB cases with intrahepatic HBcAg.
Among the 106 HBeAg-negative CHB patients, 35 (33%) patients were positive for intrahepatic HBcAg (Table 1). Compared with HBcAg-negative patients, HBcAg-positive cases had higher ALT and aspartate aminotransferase (AST) levels, a lower platelet count, a more severe Ishak necroinflammatory grading, and a higher HBV DNA level. There was no statistical difference in sex, age, total bilirubin level, prothrombin time, and Ishak fibrosis stage between patients with and without HBcAg.
TABLE 1.
Characteristics of 106 HBeAg-negative chronic hepatitis B patients
| Characteristica | Value for: |
Pb | ||
|---|---|---|---|---|
| All 106 patients | Patients who were HBcAg: |
|||
| Negative (n = 71) | Positive (n = 35) | |||
| Mean age (yr) ± SD | 51.3 ± 12.9 | 50.6 ± 12.9 | 52.7 ± 13.0 | 0.275 |
| Sex (male/female) | 73/33 | 48/23 | 25/10 | 0.86 |
| Median ALT (U/liter) (range) | 123.5 (22-2,390) | 108 (22-2,390) | 167 (43-1,821) | 0.035 |
| Median AST (U/liter) (range) | 77 (19-1,400) | 65 (19-1,400) | 99 (25-1,384) | 0.013 |
| Median total bilirubin (mg/dl) (range) | 0.7 (0.2-10) | 0.64 (0.2-8.8) | 0.79 (0.2-10) | 0.483 |
| Mean PT (INR) ± SD | 1.0 ± 0.08 | 1.0 ± 0.07 | 1.03 ± 0.08 | 0.066 |
| Mean platelets (×103/mm3) ± SD | 187.3 ± 50.1 | 193.6 ± 50.9 | 174.6 ± 46.4 | 0.034 |
| Median AFP (ng/ml) (range) | 6.07 (1-428) | 5.84 (1-428) | 7.82 (2-114) | 0.076 |
| Mean Ishak fibrosis stage ± SD | 1.94 ± 1.41 | 1.85 ± 1.45 | 2.14 ± 1.33 | 0.149 |
| Mean Ishak necroinflammation grading ± SD | 5.25 ± 3.0 | 4.7 ± 3.02 | 6.34 ± 2.68 | 0.001 |
| No. (%) HBcAg positive | 35 (33.0) | |||
| Median HBV DNA (log10 cp/ml) (range) | 6.650 (2.4-10.516) | 6.097 (2.4-10.516) | 7.465 (3.358-9.839) | <0.001 |
PT, prothrombin time; INR, international normalized ratio; AFP, alpha-fetoprotein.
The chi-square test with Yates's correction was used for the comparison of sex data, while the Mann-Whitney U test was used for the other variables.
Uni- and multivariate analysis of factors associated with the expression of intrahepatic HBcAg.
In univariate analysis, an ALT of >160 U/liter, an Ishak necroinflammatory grading of ≥7, and an HBV DNA level of >1 × 106 cp/ml were associated with the expression of intrahepatic HBcAg (Table 2). A serum AST of >80 U/liter also had a trend to associate with HBcAg expression. In multivariate analysis, a serum ALT of >160 U/liter and HBV DNA level higher than 1 × 106 cp/ml were the factors independently associated with the expression of HBcAg in HBeAg-negative CHB patients (Table 3).
TABLE 2.
Univariate analysis of factors associated with expression of intrahepatic HBcAg
| Factora | Fraction of patients (%) HBcAg positive | Pb |
|---|---|---|
| Age (yr), ≤50 vs >50 | 15/56 (26.8) vs 20/50 (40) | 0.216 |
| Sex, male vs female | 25/73 (34.2) vs 10/33 (30.3) | 0.86 |
| ALT (U/liter), ≤160 vs >160 | 15/63 (23.8) vs 20/43 (46.5) | 0.026 |
| AST (U/liter), ≤80 vs >80 | 13/54 (24.1) vs 22/51 (43.1) | 0.062 |
| Total bilirubin (mg/dl), ≤1.6 vs >1.6 | 32/99 (32.3) vs 2/5 (40) | 0.661 |
| PT (INR), ≤1.1 vs >1.1 | 28/90 (31.1) vs 7/15 (46.7) | 0.375 |
| Platelets, ≤ 150,000 vs >150,000 | 10/23 (43.5) vs 25/83 (30.1) | 0.34 |
| AFP (ng/ml), ≤20 vs >20 | 26/86 (30.2) vs 6/12 (50) | 0.198 |
| Ishak fibrosis stage, 0-1 vs ≥2 | 13/51 (25.5) vs 22/55 (40) | 0.167 |
| Ishak necroinflammation grading, <7 vs ≥7 | 20/75 (26.7) vs 15/31 (48.4) | 0.053 |
| HBV DNA (cp/ml), ≤1 × 105 vs >1 × 105 | 3/26 (11.5) vs 32/80 (40) | 0.015 |
| HBV DNA (cp/ml), ≤1 × 106 vs >1 × 106 | 5/39 (12.8) vs 30/67 (44.8) | 0.002 |
PT, INR, and AFP are as defined for Table 1.
Fisher's exact test was used for total bilirubin and AFP, while the chi-square test with Yates's correction was used for the other variables.
TABLE 3.
Multivariate analysis of factors associated with expression of intrahepatic HBcAga
| Variable, value | Hazard ratio (95% CI) | SE | P |
|---|---|---|---|
| ALT, >160 U/liter | 2.719 (1.119-6.609) | 0.453 | 0.027 |
| HBV DNA, >1 × 106 cp/ml | 5.659 (1.926-16.628) | 0.550 | 0.002 |
The variables, including age, sex, and ALT and HBV DNA levels, were used in the final stepwise logistic model.
Distribution patterns of HBcAg and their clinical and pathological features.
Some studies suggested that cytoplasmic HBcAg was a more reliable marker of HBV replication than nuclear core HBcAg (1, 11). Of the 35 intrahepatic-HBcAg-positive patients, 8 (22.9%) had a purely cytoplasmic distribution, 4 (11.4%) had a purely nuclear distribution, and 23 (65.7%) had a mixed cytoplasmic and nuclear distribution. There were no significant differences in age, sex, biochemical variables, Ishak fibrosis stage, and necroinflammatory grading among these subgroups of patients (Table 4). Of note, purely cytoplasmic expression of HBcAg had a trend to associate with a lower HBV DNA level (P = 0.061). Cytoplasmic expression of HBcAg did not represent more-severe hepatic necroinflammation.
TABLE 4.
Characteristics of patients with different patterns of HBcAg distribution
| Characteristica | Value for patients (n = 35) who were HBcAg positive in: |
Pb | ||
|---|---|---|---|---|
| Cytoplasm (n = 8) | Nucleus (n = 4) | Mixedc (n = 23) | ||
| Mean age (yr) ± SD | 54.5 ± 7.6 | 55.5 ± 5.2 | 51.7 ± 15.4 | 0.686 |
| Median ALT (U/liter) (range) | 228 (79-852) | 178 (138-569) | 116 (43-1,821) | 0.184 |
| Median AST (U/liter) (range) | 174.5 (39-563) | 118 (56-216) | 87 (25-1,384) | 0.221 |
| Median total bilirubin (mg/dl) (range) | 0.78 (0.4-1.2) | 0.91(0.6-1.3) | 0.82 (0.2-10) | 0.752 |
| Mean PT (INR) ± SD | 1.03 ± 0.09 | 1.03 ± 0.061 | 1.04 ± 0.08 | 0.991 |
| Mean platelets (×103/mm3) ± SD | 168.9 ± 68.7 | 174.8 ± 40.3 | 176.6 ± 39.9 | 0.553 |
| Median AFP (ng/ml) (range) | 6.3 (4-114) | 26 (10-44) | 5.9 (2-36) | 0.055 |
| Mean Ishak fibrosis stage ± SD | 2.13 ± 1.25 | 2.75 ± 2.22 | 2.04 ± 1.22 | 0.875 |
| Mean Ishak necroinflammation grading ± SD | 7.63 ± 3.42 | 7.75 ± 0.96 | 5.65 ± 2.41 | 0.101 |
| Median HBV DNA (log10 cp/ml) (range) | 6.30 (3.36-7.97) | 7.19 (4.79-8.49) | 7.72 (4.99-9.84) | 0.061 |
PT, INR, and AFP are as defined for Table 1.
The Kruskal-Wallis ANOVA test was used for statistical analysis of the three groups.
Mixed nuclear and cytoplasmic localization.
Level of HBcAg and its correlation with HBV DNA load and inflammation grading.
The level of intrahepatic HBcAg expression was graded by the pathologist and correlated with the amount of serum HBV DNA load. As shown in Fig. 1B, the patients with grade 3/4 expression of HBcAg had the highest HBV DNA viral loads, which are significantly higher than those of patients without HBcAg expression (P < 0.05). A high level of HBcAg expression significantly indicated high HBV viral load.
The degree of HBcAg expression was also compared with the grading of Ishak necroinflammation (Fig. 1B). The data showed that the presence of HBcAg, even at a low level, indicated significant hepatic necroinflammation. Patients with high-level HBcAg expression did not induce more-severe necroinflammation than those with low-level expression.
Correlation of HBV DNA load with the prevalence of HBcAg expression and the severity of necroinflammation.
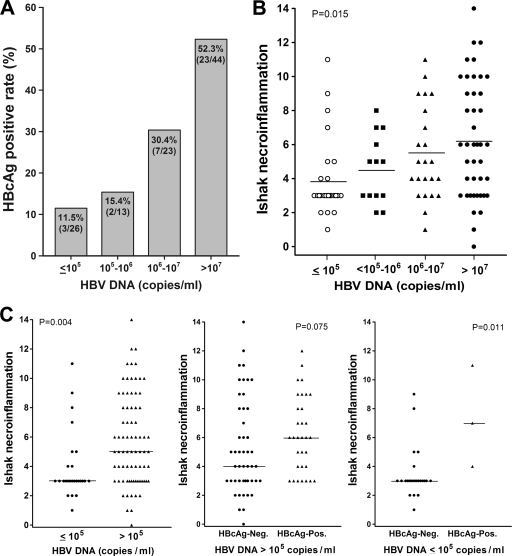
As shown in Fig. 2 A, there was a trend to have a higher HBcAg detection rate in patients with a higher HBV DNA load (P = 0.002). The HBcAg expression rate rose from 12% to 52% as the DNA load increased from under 105 cp/ml to over 107 cp/ml. Noteworthily, higher HBV DNA load was associated with more-severe hepatic necroinflammation grading (Fig. 2B). The median Ishak necroinflammatory gradings were 3.0, 5.0, and 6.0 in patients with HBV DNA loads under 105 cp/ml, between 105 and 107 cp/ml, and over 107 cp/ml, respectively (P = 0.015).
FIG. 2.
Correlation of HBV DNA load with (A) the seropositivity of HBcAg expression and (B) the grading of Ishak necroinflammation. The Kruskal-Wallis ANOVA test was used for statistical analysis among groups. (C) Association of HBV DNA levels with Ishak inflammatory grading (left) and the influence of HBcAg on the Ishak necroinflammation grading in patients with high (middle) or low (right) HBV DNA loads. The Mann-Whitney U test was used for statistical analysis.
Impact of HBV DNA level and HBcAg expression on hepatic necroinflammation.
Uni- and multivariate analyses of factors associated with significant hepatic necroinflammation in patients with lower levels of viremia (≤1 × 106 cp/ml) revealed that HBcAg expression was the only factor that contributed to active hepatic necroinflammation (Ishak necroinflammatory grading ≥ 7) in HBeAg-negative CHB (Table 5) (hazard ratio = 11.25; 95% confidence interval [CI], 1.42 to 89.26; P = 0.022).
TABLE 5.
Factors associated with active liver inflammation in 39 HBeAg-negative CHB patients with low viral load (DNA ≤ 1 × 106 cp/ml)
| Factora | Fraction (%) of patients with Ishak inflammation grading ≥7 | Pb |
|---|---|---|
| Age (yr), ≤50 vs >50 | 3/23 (13) vs 4/16 (25) | 0.415 |
| Sex, male vs female | 5/27 (18.5) vs 2/12 (16.7) | 1.0 |
| ALT (U/liter), ≤160 vs >160 | 3/25 (12) vs 4/14 (28.6) | 0.225 |
| AST (U/liter) ≤80 vs >80 | 4/23 (17.4) vs 3/16 (18.8) | 1.0 |
| Total bilirubin (mg/dl), ≤1.6 vs >1.6 | 6/38 (15.8) vs 1/1 (100) | 0.179 |
| PT (INR), ≤1.1 vs >1.1 | 7/36 (19.4) vs 0/3 (0) | 1.0 |
| Platelets, ≤150,000 vs >150,000 | 2/4 (50) vs 5/35 (14.3) | 0.141 |
| AFP (ng/ml), ≤20 vs >20 | 6/33 (18.2) vs 0/3 (0) | 1.0 |
| Intrahepatic HBcAg, negative vs positive | 4/34 (11.8) vs 3/5 (60) | 0.032 |
PT, INR, and AFP are as defined for Table 2.
Fisher's exact test was used for statistical analysis.
The 26 patients whose HBV DNA levels were lower than 1 × 105 cp/ml were analyzed. Among them, four (15.4%) had significant necroinflammation and HBcAg expression was still significantly associated with active hepatic necroinflammation (Fig. 2C).
DISCUSSION
HBcAg is an important viral antigen with regard to induction of the cellular immune response in the course of chronic HBV infection (5). After HBeAg seroconversion, HBcAg is undetectable in anti-HBe-positive HBV remission cases but may reappear in some HBeAg-negative CHB cases (6, 7). A great diversity in the prevalence of intrahepatic HBcAg in HBeAg-negative CHB was reported. A previous study suggested that a high proportion of anti-HBe-positive cases with chronic active hepatitis had detectable nuclear HBcAg, which was considered to be related to viral replication (12). A subsequent study showed that 46% of the anti-HBe-positive chronic active hepatitis cases had intrahepatic HBcAg expression (6), and cytoplasmic expression was found in all the cases. A recent study to evaluate the role of HBcAg in response to antiviral treatment showed that 36% of the CHB cases had intrahepatic HBcAg (34). In the current study, we found that 33% of the cases had intrahepatic HBcAg expression. In patients with higher HBV viral loads (>107 cp/ml), 52% had detectable HBcAg. Compatible with previous studies, HBcAg expression was infrequent in HBeAg-negative CHB.
The expression of HBcAg in hepatocytes can attract HBV-specific T cells and recruit non-virus-specific T cells to induce liver inflammation if the infection cannot be controlled (24). Therefore, high serum ALT levels and liver necroinflammation grading were associated with HBcAg expression. In previous studies, different distribution patterns of HBcAg had different degrees of clinical significance (8, 9). Patients with predominantly nuclear HBcAg had higher levels of viral replication (8), whereas those with predominantly cytoplasmic HBcAg had significantly higher levels of biochemical and histological activities (9). However, some studies suggested that nuclear HBcAg might not be involved in HBV replication (1, 11). In those studies, the majority of the patients had HBeAg-positive CHB. Our data focusing on HBeAg-negative patients showed that nearly two-thirds of cases had the mixed-type HBcAg distribution. There is no predominant type of HBcAg distribution associated with hepatic necroinflammation. However, patients with isolated cytoplasmic HBcAg expression seemed to have lower HBV DNA levels, but the difference did not reach statistical significance. Only a high degree of expression of HBcAg (3+/4+) was associated with high HBV DNA load (Fig. 1B). The distribution and level of HBcAg expression may have different characteristics in HBeAg-negative CHB patients.
HBV DNA load is associated with the risk of cirrhosis and HCC (4, 16). Antiviral treatment to reduce the HBV DNA can improve the disease outcome (3). It is well accepted that high HBV DNA levels play an adverse role in liver histological grading. For HBeAg-negative CHB, an HBV DNA load higher than 20,000 IU/ml or 1 × 105 cp/ml is a common criterion for safety diagnosis; however, the finding of low HBV DNA load could not exclude the possibility of active hepatic necroinflammation (28). A previous study could not explain why significant necroinflammation existed in HBeAg-negative CHB patients with low HBV viral loads. The current findings show that HBcAg expression is an important factor associated with significant liver necroinflammation in such populations.
Sampling error is inevitable in liver biopsy. However, the finding that patients with HBcAg expression had significantly higher HBV DNA loads and severe hepatic necroinflammation suggested that sampling error could not completely explain the negativity of HBcAg in HBeAg-negative CHB. A previous study suggested that purely cytoplasmic HBcAg expression was more frequent in the presence of the precore 1896 mutation, which may block the translocation of HBcAg (29, 25). A recent study revealed that a basal core promoter (BCP) HBV mutant had lower intrahepatic HBcAg expression in HBeAg-positive CHB (21). In this retrospective study, the serum samples were not available for HBV genotyping and sequencing. Whether HBV genotype and mutations would influence the expression of intrahepatic HBcAg in HBeAg-negative CHB deserves further study in the future. Another limitation of the study is that the status of HBcAg in HBeAg-negative patients with normal ALT levels or low viral loads is still unknown. However, such cases usually represent inactive HBV carriers. Therefore, liver biopsy is not indicated in clinical practice.
In conclusion, the expression of HBcAg is not frequent in HBeAg-negative CHB. Only a high level of HBcAg expression is correlated with high HBV DNA loads. Antiviral treatment might be considered for patients with low levels of viremia but who are positive for intrahepatic HBcAg. The expression of intrahepatic HBcAg is associated with active hepatic necroinflammation, even in patients with low HBV DNA loads.
Acknowledgments
This work was supported by grants from Taipei Veterans General Hospital (VGH98A-026, V97C1-044, and V98C1-057), Taipei, Taiwan.
Footnotes
Published ahead of print on 28 April 2010.
REFERENCES
- 1.Burrell, C. J., E. J. Gowans, and B. P. Marmion. 1985. High levels of cytoplasmic hepatitis B core antigen as reliable marker of HBV DNA replication. Lancet i:454-455. [DOI] [PubMed] [Google Scholar]
- 2.Carman, W. F., M. R. Jacyna, S. Hadziyannis, P. Karayiannis, M. J. McGarvey, A. Makris, and H. C. Thomas. 1989. Mutation preventing formation of hepatitis B e antigen in patients with chronic hepatitis B infection. Lancet ii:588-591. [DOI] [PubMed] [Google Scholar]
- 3.Chen, C. J., H. I. Yang, and U. H. Iloeje. 2009. Hepatitis B virus DNA levels and outcomes in chronic hepatitis B. Hepatology 49:S72-S84. [DOI] [PubMed] [Google Scholar]
- 4.Chen, C. J., H. I. Yang, J. Su, C. L. Jen, S. L. You, S. N. Lu, G. T. Huang, and U. H. Iloeje. 2006. Risk of hepatocellular carcinoma across a biological gradient of serum hepatitis B virus DNA level. JAMA 295:65-73. [DOI] [PubMed] [Google Scholar]
- 5.Chisari, F. V., and C. Ferrari. 1995. Hepatitis B virus immunopathogenesis. Annu. Rev. Immunol. 13:29-60. [DOI] [PubMed] [Google Scholar]
- 6.Chu, C. M., and Y. F. Liaw. 1992. Immunohistological study of intrahepatic expression of hepatitis B core and E antigens in chronic type B hepatitis. J. Clin. Pathol. 45:791-795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chu, C. M., and Y. F. Liaw. 1987. Intrahepatic distribution of hepatitis B surface and core antigens in chronic hepatitis B virus infection. Hepatocyte with cytoplasmic/membranous hepatitis B core antigen as a possible target for immune hepatocytolysis. Gastroenterology 92:220-225. [DOI] [PubMed] [Google Scholar]
- 8.Chu, C. M., C. T. Yeh, R. N. Chien, I. S. Sheen, and Y. F. Liaw. 1997. The degrees of hepatocyte nuclear but not cytoplasmic expression of hepatitis B core antigen reflect the level of viral replication in chronic hepatitis B virus infection. J. Clin. Microbiol. 35:102-105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chu, C. M., C. T. Yeh, I. S. Sheen, and Y. F. Liaw. 1995. Subcellular localization of hepatitis B core antigen in relation to hepatocyte regeneration in chronic hepatitis B. Gastroenterology 109:1926-1932. [DOI] [PubMed] [Google Scholar]
- 10.Ganem, D., and H. E. Varmus. 1987. The molecular biology of the hepatitis B viruses. Annu. Rev. Biochem. 56:651-693. [DOI] [PubMed] [Google Scholar]
- 11.Gowans, E. J., C. J. Burrell, A. R. Jilbert, and B. P. Marmion. 1985. Cytoplasmic (but not nuclear) hepatitis B virus (HBV) core antigen reflects HBV DNA synthesis at the level of the infected hepatocyte. Intervirology 24:220-225. [DOI] [PubMed] [Google Scholar]
- 12.Hadziyannis, S. J., H. M. Lieberman, G. G. Karvountzis, and D. A. Shafritz. 1983. Analysis of liver disease, nuclear HBcAg, viral replication, and hepatitis B virus DNA in liver and serum of HBeAg Vs. anti-HBe positive carriers of hepatitis B virus. Hepatology 3:656-662. [DOI] [PubMed] [Google Scholar]
- 13.Hoofnagle, J. H., G. M. Dusheiko, L. B. Seeff, E. A. Jones, J. G. Waggoner, and Z. B. Bales. 1981. Seroconversion from hepatitis B e antigen to antibody in chronic type B hepatitis. Ann. Intern. Med. 94:744-748. [DOI] [PubMed] [Google Scholar]
- 14.Hsu, H. C., I. J. Su, M. Y. Lai, D. S. Chen, M. H. Chang, S. M. Chuang, and J. L. Sung. 1987. Biologic and prognostic significance of hepatocyte hepatitis B core antigen expressions in the natural course of chronic hepatitis B virus infection. J. Hepatol. 5:45-50. [DOI] [PubMed] [Google Scholar]
- 15.Hsu, Y. S., R. N. Chien, C. T. Yeh, I. S. Sheen, H. Y. Chiou, C. M. Chu, and Y. F. Liaw. 2002. Long-term outcome after spontaneous HBeAg seroconversion in patients with chronic hepatitis B. Hepatology 35:1522-1527. [DOI] [PubMed] [Google Scholar]
- 16.Iloeje, U. H., H. I. Yang, J. Su, C. L. Jen, S. L. You, and C. J. Chen. 2006. Predicting cirrhosis risk based on the level of circulating hepatitis B viral load. Gastroenterology 130:678-686. [DOI] [PubMed] [Google Scholar]
- 17.Ishak, K., A. Baptista, L. Bianchi, F. Callea, J. De Groote, F. Gudat, H. Denk, V. Desmet, G. Korb, R. N. MacSween, et al. 1995. Histological grading and staging of chronic hepatitis. J. Hepatol. 22:696-699. [DOI] [PubMed] [Google Scholar]
- 18.Kim, C. W., S. K. Yoon, E. S. Jung, C. K. Jung, J. W. Jang, M. S. Kim, S. Y. Lee, S. H. Bae, J. Y. Choi, S. W. Choi, N. I. Han, and C. D. Lee. 2007. Correlation of hepatitis B core antigen and beta-catenin expression on hepatocytes in chronic hepatitis B virus infection: relevance to the severity of liver damage and viral replication. J. Gastroenterol. Hepatol. 22:1534-1542. [DOI] [PubMed] [Google Scholar]
- 19.Liaw, Y. F., and C. M. Chu. 2009. Hepatitis B virus infection. Lancet 373:582-592. [DOI] [PubMed] [Google Scholar]
- 20.Liaw, Y. F., C. M. Chu, I. J. Su, M. J. Huang, D. Y. Lin, and C. S. Chang-Chien. 1983. Clinical and histological events preceding hepatitis B e antigen seroconversion in chronic type B hepatitis. Gastroenterology 84:216-219. [PubMed] [Google Scholar]
- 21.Liu, C. J., Y. M. Jeng, C. L. Chen, H. R. Cheng, P. J. Chen, T. C. Chen, C. H. Liu, M. Y. Lai, D. S. Chen, and J. H. Kao. 2009. Hepatitis B virus Basal core promoter mutation and DNA load correlate with expression of hepatitis B core antigen in patients with chronic hepatitis B. J. Infect. Dis. 199:742-749. [DOI] [PubMed] [Google Scholar]
- 22.Lok, A. S., U. Akarca, and S. Greene. 1994. Mutations in the pre-core region of hepatitis B virus serve to enhance the stability of the secondary structure of the pre-genome encapsidation signal. Proc. Natl. Acad. Sci. U. S. A. 91:4077-4081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lok, A. S., and B. J. McMahon. 2007. Chronic hepatitis B. Hepatology 45:507-539. [DOI] [PubMed] [Google Scholar]
- 24.Maini, M. K., C. Boni, C. K. Lee, J. R. Larrubia, S. Reignat, G. S. Ogg, A. S. King, J. Herberg, R. Gilson, A. Alisa, R. Williams, D. Vergani, N. V. Naoumov, C. Ferrari, and A. Bertoletti. 2000. The role of virus-specific CD8(+) cells in liver damage and viral control during persistent hepatitis B virus infection. J. Exp. Med. 191:1269-1280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mani, H., and D. E. Kleiner. 2009. Liver biopsy findings in chronic hepatitis B. Hepatology 49:S61-S71. [DOI] [PubMed] [Google Scholar]
- 26.Naoumov, N. V., B. C. Portmann, R. S. Tedder, B. Ferns, A. L. Eddleston, G. J. Alexander, and R. Williams. 1990. Detection of hepatitis B virus antigens in liver tissue. A relation to viral replication and histology in chronic hepatitis B infection. Gastroenterology 99:1248-1253. [DOI] [PubMed] [Google Scholar]
- 27.Okamoto, H., F. Tsuda, Y. Akahane, Y. Sugai, M. Yoshiba, K. Moriyama, T. Tanaka, Y. Miyakawa, and M. Mayumi. 1994. Hepatitis B virus with mutations in the core promoter for an e antigen-negative phenotype in carriers with antibody to e antigen. J. Virol. 68:8102-8110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Papatheodoridis, G. V., E. K. Manesis, S. Manolakopoulos, I. S. Elefsiniotis, J. Goulis, J. Giannousis, A. Bilalis, G. Kafiri, D. Tzourmakliotis, and A. J. Archimandritis. 2008. Is there a meaningful serum hepatitis B virus DNA cutoff level for therapeutic decisions in hepatitis B e antigen-negative chronic hepatitis B virus infection? Hepatology 48:1451-1459. [DOI] [PubMed] [Google Scholar]
- 29.Park, Y. N., K. H. Han, K. S. Kim, J. P. Chung, S. Kim, and C. Park. 1999. Cytoplasmic expression of hepatitis B core antigen in chronic hepatitis B virus infection: role of precore stop mutants. Liver 19:199-205. [DOI] [PubMed] [Google Scholar]
- 30.Ramalho, F., M. R. Brunetto, G. Rocca, G. G. Piccari, A. Batista, E. Chiaberge, C. Lavarini, R. P. Dal Monte, M. Carneiro de Moura, and F. Bonino. 1988. Serum markers of hepatitis B virus replication, liver histology and intrahepatic expression of hepatitis B core antigen. J. Hepatol 7:14-20. [DOI] [PubMed] [Google Scholar]
- 31.Sato, S., K. Suzuki, Y. Akahane, K. Akamatsu, K. Akiyama, K. Yunomura, F. Tsuda, T. Tanaka, H. Okamoto, Y. Miyakawa, and M. Mayumi. 1995. Hepatitis B virus strains with mutations in the core promoter in patients with fulminant hepatitis. Ann. Intern. Med. 122:241-248. [DOI] [PubMed] [Google Scholar]
- 32.Seeger, C., and W. S. Mason. 2000. Hepatitis B virus biology. Microbiol. Mol. Biol. Rev. 64:51-68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Takahashi, K., K. Aoyama, N. Ohno, K. Iwata, Y. Akahane, K. Baba, H. Yoshizawa, and S. Mishiro. 1995. The precore/core promoter mutant (T1762A1764) of hepatitis B virus: clinical significance and an easy method for detection. J. Gen. Virol. 76:3159-3164. [DOI] [PubMed] [Google Scholar]
- 34.Uzun, Y., H. Bozkaya, E. Erden, K. Cinar, R. Idilman, C. Yurdaydin, and O. Uzunalimoglu. 2006. Hepatitis B core antigen expression pattern reflects the response to anti-viral treatment. J. Gastroenterol. Hepatol. 21:977-981. [DOI] [PubMed] [Google Scholar]