Abstract
We previously reported that ethanol-killed cells of a noncapsulated strain of Streptococcus pneumoniae, given intranasally with cholera toxin as an adjuvant, protect rats against pneumonia and mice against colonization of the nasopharynx and middle ear by capsulated pneumococci of various serotypes. The acceleration of pneumococcal clearance from the nasopharynx in mice is CD4+ T cell-dependent and interleukin 17A (IL-17A) mediated and can be antibody independent. Here, anticipating human studies, we have demonstrated protection with a new vaccine strain expressing a nonhemolytic derivative of pneumolysin and grown in bovine-free culture medium. Killing the cells with chloroform, trichloroethylene, or beta-propiolactone—all used without postinactivation washing—produced more-potent immunogens than ethanol, and retention of soluble components released from the cells contributed to protection. Two sequential intranasal administrations of as little as 1 μg of protein (total of cellular and soluble combined) protected mice against nasopharyngeal challenge with pneumococci. Nontoxic single and double mutants of Escherichia coli heat-labile toxin were effective as mucosal adjuvants. Protection was induced by the sublingual and buccal routes, albeit requiring larger doses than when given intranasally. Protection was likewise induced transdermally with sonicates of the killed-cell preparation. Thus, this whole-cell antigen can be made and administered in a variety of ways to suit the manufacturer and the vaccination program and is potentially a solution to the need for a low-cost vaccine to reduce the burden of childhood pneumococcal disease in low-income countries.
Streptococcus pneumoniae (pneumococcus) imparts a major disease burden among children in low-income countries (27). Capsular polysaccharide conjugate vaccine provides type-specific protection but has the disadvantages of limited serotype coverage, serotype replacement, and high cost of production, storage, and injection (12, 29). Therefore, potentially more economical serotype-independent vaccines based upon species-common protein antigens are being pursued (32). In one such approach, we have studied killed cells of noncapsulated pneumococci, with the intent of maximizing the exposure of species-common subcapsular antigens. This antigen preparation, designated “whole-cell vaccine” (WCV), when formulated with a suitable adjuvant, is intended for mucosal administration to reduce pneumococcal colonization. Killing the cells with 70% (vol/vol) ethanol at 4°C produces a more immunogenic antigen (WCE) than traditional methods of inactivation such as heat, formalin, or UV radiation (21) (unpublished data). Intranasal (i.n.) application had been examined initially, since this route is effective for inducing both systemic and mucosal immunity. Vaccination i.n. with WCE plus cholera toxin (CT) as a mucosal adjuvant prevents fatal serotype 3 pneumonia in rats and reduces nasopharygeal (NP) and middle ear colonization in mice by strains of serotype 6B or 23F (22, 23). Although the levels of serum antibodies are raised by the i.n. vaccination, protection against colonization is induced in mice in the absence of antibodies by a CD4+ T-cell-dependent, interleukin 17A (IL-17A)-mediated mechanism (20, 24). As few as 107 cells (ca. 10 μg of protein) of WCE, given thrice sequentially, are protective in the colonization model (34). This potency and the potential low cost of manufacture were motivation for a partnership with PATH and Instituto Butantan (São Paolo, Brazil) for further development of the WCV.
Previous studies used strain Rx1AL expressing native pneumolysin, a potent cytolysin. Here, anticipating human studies, a derivative of pneumolysin (PdT), with mutations W433F, D385N, and C428G (which render the molecule nonhemolytic and unable to activate complement [5] but maintain its TLR4 agonistic properties [21]), was used. Previously, cells were grown in Todd-Hewitt-yeast broth, which contains beef heart infusion. Here, to avoid any hazard of bovine components, cells grown in a soy-based medium (19) were examined for protection. The challenge of safe handling and disposal of large volumes of ethanol led to the evaluation of three alternative agents of inactivation that are bactericidal at low concentration: chloroform, trichloroethylene, and beta-propiolactone. Since chloroform and trichloroethylene are both highly volatile and beta-propiolactone is inactivated by warming, these agents may be removed without postinactivation washing, which permitted convenient examination of soluble components released from the killed cells. Soluble components produced by sonication also were examined.
The possible side effects of enterotoxins as adjuvants (25) and other problems with intranasal vaccination prompted the consideration of genetically detoxified enterotoxin derivatives and of the buccal and sublingual routes of administration. Transdermal immunization was also examined with sonicated antigen preparations. These varied immunization procedures were surveyed for protection against colonization, evident as acceleration of nasopharyngeal clearance after intranasal challenge with a strain of serotype 6B (20). The results indicate that the cells can be inactivated by several agents to generate a potent whole-cell antigen that could be given in a variety of ways to accommodate preferences of a particular vaccination program.
MATERIALS AND METHODS
Materials.
Cholera toxin (CT) was from List Biological Laboratories (Campbell, CA). Mutated derivatives of Escherichia coli heat-labile toxin LT—mLT (R192G) and dmLT (R192G/L211A)—were obtained as described previously (9). LT, cotton gauze patches, and sandpaper for transcutaneous immunization were provided by Intercell USA, Gaithersburg, MD.
Generation of vaccine strain RM200 (Rx1E PdT ΔlytA).
Strain Rx1E, in which the pneumolysin gene was replaced by a detoxified mutant PdT, was provided to us by James Paton (University of Adelaide, Australia). The entire lytA genomic coding region was replaced by the Janus cassette marked with kanamycin resistance gene rpsL, using the strategy described earlier (31, 33). Briefly, three PCR amplification products were created: (i) a 1-kb fragment upstream of the genomic region of lytA amplified with primers LAD1 (CAAGGTATCCATCATTCC) and LAD2 (CGCGGATCCACAGTAGAGCCAGATGGC; BamHI site underlined), (ii) an 800-bp fragment downstream of lytA amplified with primers LAD3 (TTTGGGCCCGTTGCACGCCGACTTGAGG; ApaI site underlined) and LAD4 (CTTTGCTTCTCAGAATCTAGG), and (iii) the Janus cassette amplified with primers DAM351 (with ApaI site) and DAM406 (with BamHI site) (31). The amplification products were digested at the sites introduced by PCR using the cognate restriction enzymes, gel purified, and then ligated overnight at 4°C. The ligation mixture was then used as a template for a final PCR using the outside primers LAD5 (CATAGCTTTATGACTGATACC) and LAD6 (AAGGTCTTCGAATCGGCAGTCG), yielding a 3.2-kb amplification product—a tripartite DNA molecule with the Janus cassette flanked by lytA upstream and downstream sequences. This molecule was then transformed into a kanamycin-sensitive and streptomycin-resistant strain of Rx1E (PdT) by selecting for kanamycin-resistant colonies wherein the wild-type lytA gene was now replaced by the lytA::Janus disruption fragment. The putative integrants were confirmed genotypically by PCR using a Janus-specific internal primer and the LAD5 primer, in comparison with the wild-type parental strains: the lytA::Janus strain yields that Janus-specific PCR product, but the wild-type strain does not. The lytA::Janus transformants were confirmed phenotypically by assessing susceptibility to lysis in the presence of 5% sodium deoxycholate: wild-type strains lyse, and strains lacking lytA are resistant to lysis. The lytA::Janus transformant of the Rx1E PdT strain was named RM200.
Antigen preparations.
Four different killed-cell preparations, in which inactivation was achieved with ethanol, chloroform, trichloroethylene, or beta-propriolactone (WCE, WCC, WCT, or WCB, respectively), have been used in this report. Generally, strain RM200 was grown to an A600 of 1.0, at which the viable count was approximately 6 × 108 CFU/ml. Further steps were performed at 4°C. The cells were collected by centrifugation and washed twice with lactated Ringer's solution (LR) (102 mM NaCl, 28 mM NaC3H5O3, 1.5 mM CaCl2, and 4 mM KCl). For WCE preparations, cells were resuspended to an A600 of 10 and ethanol was added to 70% (vol/vol) gradually within 15 min. The suspension was stirred for 55 min, and the cells were pelleted again, washed twice, resuspended to an A600 of 32 in LR containing 10% sucrose, cultured to ascertain sterility, and lyophilized in single-use aliquots. For WCC and WCT preparations, washed cells in LR with 10% sucrose at an A600 of 32 were mixed with chloroform or trichloroethylene (1/40 [vol/vol]) for 2 h. For WCB, washed cells in LR with 10% sucrose were mixed with beta-propiolactone (BPL) (1/4,000 [vol/vol]) for 24 h at 4°C followed by a 2-hour incubation at 37°C to inactivate BPL. For WCC and WCT, the killed cells were not washed but, rather, directly lyophilized (which eliminates residual organic solvent); WCB was similarly lyophilized after inactivation of BPL. Preparation of supernatants was done by vortexing the suspension for 1 min and then centrifuging at 16,000 × g for 5 min. Protein concentration was determined using the Total Protein Kit with bovine serum albumin as a standard (Sigma). SDS-PAGE was performed with precast 4 to 12% Bis-Tris SDS gels (Invitrogen, Carlsbad, CA). The WCC suspension was sonicated with a probe sonicator for at least 2 min at the highest intensity to prepare the WCC lysate.
Assay of IL-17A production in whole blood samples.
Fifty μl of heparinized blood was added to 450 μl Dulbecco modified Eagle medium (DMEM) (BioWhittaker, Walkersville, MD) containing 10% low-endotoxin defined fetal bovine serum (FBS) (HyClone, Logan, UT) and ciprofloxacin (10 μg/ml; Cellgro, Manassas, VA). Except for the nonstimulated control, the cultures were incubated at 37°C for 6 days with 107 cells of pneumococcal WCA. Supernatants were collected following centrifugation and stored at −80°C until analysis by enzyme-linked immunosorbent assay (ELISA) for IL-17A concentration (R&D Systems, Minneapolis, MN).
Immunization and challenge of mice.
C57BL/6J mice (Jackson Laboratories, Bar Harbor, ME) were used in all the experiments. The age at time of first immunization was between 4 and 6 weeks. Intranasal (i.n.) immunization was done by instilling 10 μl of saline, adjuvant only, or adjuvant mixed with antigen as specified atraumatically into nonanesthetized mice, a procedure that places no immunogen into the lungs; secondary immunizations were given after 1 week. Oral or sublingual immunization was carried out by placing 30 μl of vaccine mixed with 1% NaHCO3 and 30% sucrose on the oral surface or 5 μl of vaccine in the same diluent under the tongue, respectively. Oral or sublingual immunizations were carried out three times weekly, whereas only two doses were given with intranasal immunizations. WCC was used in the transcutaneous immunization (TCI) experiment. WCC was rehydrated in water with 0.1% Zwittergent 3-14 (Calbiochem, Gibbstown, NJ) and 1% arginine (Sigma) and then sonicated to an average size of 100 nm (WCA100) or 20 nm (WCA20). Mice were anesthetized with 2-2-2 tribromoethanol (Avertin; Sigma) and then shaved on the dorsum with a clipper. The shaved skin was hydrated by gentle touch with wet gauze, and excess water was removed by patting with dry, sterile gauze. After gentle abrasion with sandpaper, the immunizing solution (in a volume of 20 μl) was pipetted onto the patch, which was applied to the shaved area and left on the skin for 18 h. Immunization was carried out three times at 2-week intervals. Blood was drawn 2 weeks after the last immunization for all immunizations except in the case of intranasal immunization, in which blood was drawn 3 weeks later and assayed for IL-17A production after stimulation with WCA.
To determine susceptibility to NP colonization, i.n. challenge with live encapsulated pneumococci was done as described previously (22): 4 weeks after the last immunization (or 2 weeks for mice immunized by TCI), mice were challenged with 107 CFU of serotype 6B strain 0603 in 10 μl of PBS applied as described. To determine NP colonization, an upper respiratory culture was done by instilling sterile saline retrograde through the transected trachea, collecting the first 6 drops (about 0.1 ml) from the nostrils, and plating neat or diluted samples on blood agar plates containing 2.5 μg gentamicin/ml. The figures show the numbers of CFU per nasal wash sample of individual mice; the geometric means (GM) are displayed as a horizontal bar. For ease of statistical analysis, a sterile sample was assigned half the lower limit of detection (1.6 CFU/nasal wash), or 0.8 CFU/nasal wash.
Statistical analysis.
NP colonization densities were compared by the Mann-Whitney U test using PRISM (version 4.0a; GraphPad Software, Inc.).
RESULTS
Characterization and killing of strain RM200 to make WC antigens.
In strain Rx1E, to improve yield by reducing autolysis, the entire lytA genomic coding region was replaced by the Janus cassette marked with kanamycin resistance gene rpsL (31, 33). An integrant that displayed the correct lytA::Janus genomic context, was resistant to deoxycholate lysis, and had growth kinetics similar to those of the wild-type strain was designated RM200. Rx1E expresses PdT, a nonhemolytic derivative of pneumolysin (5), which is both a toxin and protective antigen. To ascertain retention of PdT expression, RM200 was tested for hemolytic activity with sheep erythrocytes, standardized with the purified pneumolysin protein, and found to be nonhemolytic with as many as 5 × 108 cells, whereas 100-fold fewer pneumolysin-expressing whole cells induce full lysis in erythocytes (8 × 106 and 5 × 105 cells of pneumolysin-expressing WCE and WCC, respectively); the pneumolysin lower limit of detection of the assay is 0.05 ng/ml. Western blot analysis with anti-pneumolysin serum confirmed that the PdT protein was expressed (data not shown). RM200 thus was used for subsequent vaccine preparations.
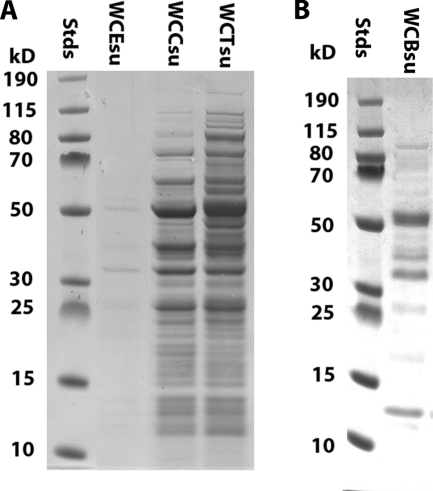
Killing to generate vaccine antigens was examined after growth to late log phase in a soy-based medium developed at Instituto Butantan (19), followed by washing and concentration to an A600 of 32 in lactated Ringer's solution (LR): the killing was done by stirring at 4°C with chloroform (C) (1/40 [vol/vol]) for 2 h, trichloroethylene (T) (1/40 [vol/vol]) for 2 h, or beta-propiolactone (B) (1/4,000 [vol/vol]) for 24 h. The cells were not further washed: C and T were removed by lyophilization, and B was decomposed by 2 h of incubation at 37°C before lyophilization. The resulting vaccine antigen preparations are referred to as WCC, WCT, and WCB, respectively. To examine for release of material from the cells, samples of lyophilized WCE, WCC, WCT, and WCB were suspended in LR at an A600 of 32, vortexed at 25°C for 1 min, and then centrifuged at 16,000 × g for 5 min. The total protein content of the supernatants was approximately 15% of the total protein of the noncentrifuged suspensions. SDS-PAGE (Fig. 1 A) showed a large number of soluble proteins in WCC and WCT, while WCE (which had been washed after killing to remove the large volume of 70% ethanol present) contained only a trace of such proteins. However, when a suspension of cells in 70% ethanol was examined without washing, a comparable mixture of proteins, which ordinarily would be lost in the preparation of WCE, was shown to have been solubilized (not illustrated). Figure 1B shows that a number of soluble proteins were likewise present in WCB. For vaccination experiments, killed-RM200 preparations, which were routinely lyophilized in single-use aliquots with sucrose as a stabilizer, were rehydrated just prior to the test.
FIG. 1.
SDS-PAGE of proteins released from cells of RM200. (A) Supernatants (16,000 × g, 5 min) of cell suspensions after killing by chloroform (WCCsu) or by trichloroethylene (WCTsu) (neither washed postkilling) compared with the supernatant of a suspension of ethanol-killed vaccine (WCEsu), which had been washed in the course of conventional vaccine preparation. (B) Supernatant of cells killed by beta-propiolactone (WCBsu), which were not washed postkilling. Stds, molecular size standards (in kilodaltons [kD]).
Protection against colonization and priming for IL-17A responses in vitro by variously prepared RM200 given intranasally.
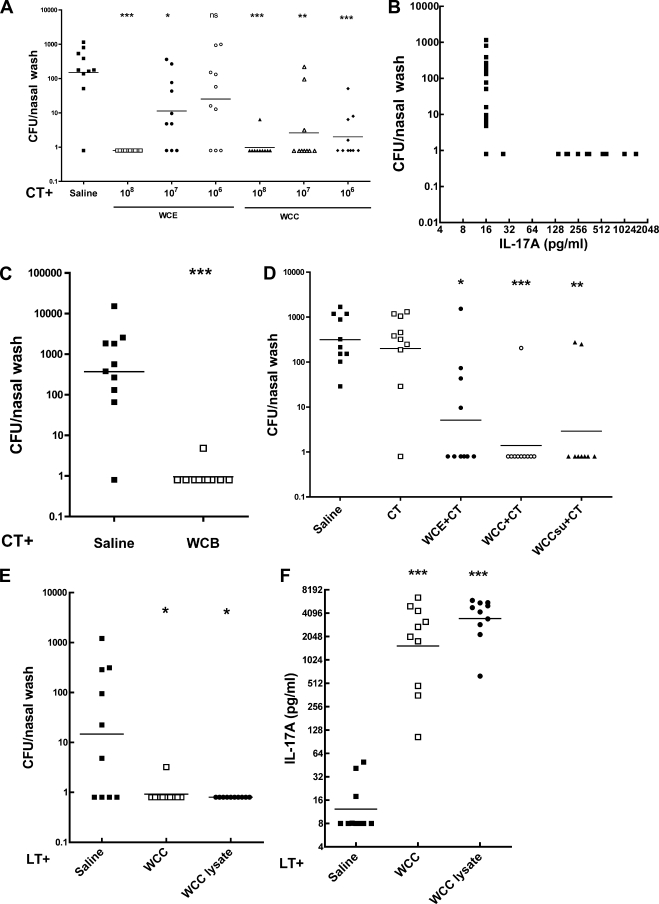
Whole-cell preparations killed by C, T, or B were compared with WCE, each given i.n. twice with CT adjuvant, for acceleration of clearance of an intranasal challenge with a pneumococcal strain of serotype 6B. Figure 2 A shows that WCE was protective (i.e., significantly reduced number of CFU recovered from the nasopharynx) at doses of 108 and 107 per immunization but not at 106, while WCC was protective at 106 as well (dosage expressed as number of CFU before killing). WCC, like WCE, primed for IL-17A expression by T cells in vitro: 1 week prior to challenge, the IL-17A expression of individual WCC-immunized mice was negatively correlated with the CFU recovered postchallenge (Fig. 2B; Spearman ρ = −0.54, P = 0.0007). In a separate experiment, WCE was protective at 108 but not significantly at 107, while WCT was significantly protective at both 108 and 107. Thus, WCC and WCT appeared about 10-fold more potent than WCE. WCB was likewise highly protective (Fig. 2C) and active in IL-17A priming (data not shown).
FIG. 2.
Protection against pneumococcal colonization and priming for IL-17A responses in vitro by intranasal vaccination with variously killed antigens prepared from RM200. The antigens were cell suspensions, 16,000 × g supernatant, and ultrasonic lysate from cells killed with ethanol (WCE), chloroform (WCC), or trichloroethylene (WCT), given to mice twice intranasally at weekly intervals at the dosages indicated, with 1 μg of cholera toxin (CT) or of E. coli heat-labile toxin (LT) as an adjuvant. The dosage here and in subsequent figures is based upon viable count before killing. Blood samples to determine WC antigen-stimulated IL-17A production in vitro were taken 3 weeks after the second vaccination, and intranasal challenge with serotype 6B was 1 week later. Determination of CFU per nasal wash was 1 week postchallenge. In this and subsequent figures, the significance of differences between adjuvant alone and with the antigen at indicated doses, calculated by the Mann-Whitney U test, is shown by asterisks: *, P < 0.05; **, P < 0.01; ***, P < 0.001. (A) Comparison of WCE and WCC at the doses indicated. (B) Correlation of IL-17A production in vitro and the CFU recovered from the nasopharynx in WCC-immunized mice. Spearman ρ = −0.54, P = 0.0007. (C) Protection by WCB at 108 dosage. (D) Comparison of WCE, WCC, and the supernatant fraction of WCC (WCCsu); the doses were 108 of WCE or WCC or the equivalent amount of supernatant of WCC. The indicated groups received CT; one control group received saline with no CT, to show the effect of CT alone in this model. (E) Comparison of WCC and an ultrasonic lysate of WCC, both at 108 cell equivalent, with 1 μg of LT as an adjuvant. (F) The IL-17A response in the mice used for the experiment in panel E, determined 3 weeks after the second immunization (1 week before the pneumococcal challenge).
However, since WCE consisted of cells washed to remove ethanol while the WCC, WCT, and WCB preparations contained released soluble components, including proteins, the supernatants after centrifugation were tested for protection. Figure 2D shows that the supernatant of WCC was as protective as or more so than the WCE cellular vaccine (dosage of both adjusted to represent what would be obtained from a prekilling dose of 108 cells). Similar results were found with the supernatants of WCT and WCB (not shown). Thus, apparently the retention of soluble components in WCC, WCT, and WCB contributes to their potency. The 106 dosage of WCC, WCT, or WCB corresponds to about 1.7 μg dry weight or 1 μg of total protein.
Protection by an ultrasonically solubilized preparation.
Extending the observation of protection by soluble components, a sample of WCC was sonicated to disrupt the cells, and this lysate was compared to the corresponding dosage of WCC. Figure 2E shows that the lysate was comparably protective, and Fig. 2F shows it to have comparably primed for IL-17A responses by blood cells in vitro, a correlate of protection in the colonization model (20).
Protection by WC antigen given intranasally with a nontoxic enterotoxin derivative.
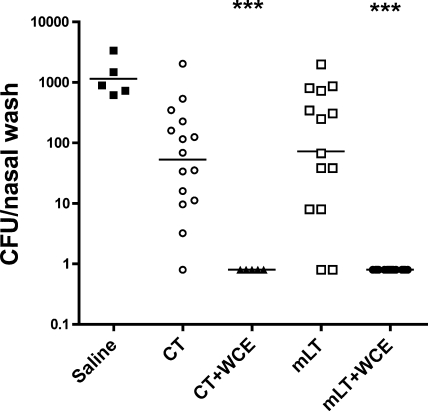
Neither CT nor LT is suitable for human use, which prompted the evaluation of nontoxic mutants of LT. A singly mutated derivative of Escherichia coli heat-labile toxin (mLT, R192G) was compared to CT. Figure 3 shows that, compared to saline, the adjuvants given alone gave a suggestion (albeit not statistically significant) of accelerated pneumococcal clearance, an expected result in this model where vaccination and challenge are by the same route. When given with 108 of WCE, however, mLT—like CT—promoted protection.
FIG. 3.
Comparative adjuvant activity of cholera toxin (CT) and a singly mutated E. coli heat-labile toxin, R192G (mLT) with WCE antigen by the intranasal route in protection against pneumococcal colonization. The experiment was conducted as described for Fig. 2. Dosages of the adjuvants were 1 μg of CT or 10 μg of mLT, given either alone or with 108 WCE.
Administration by the buccal and sublingual routes.
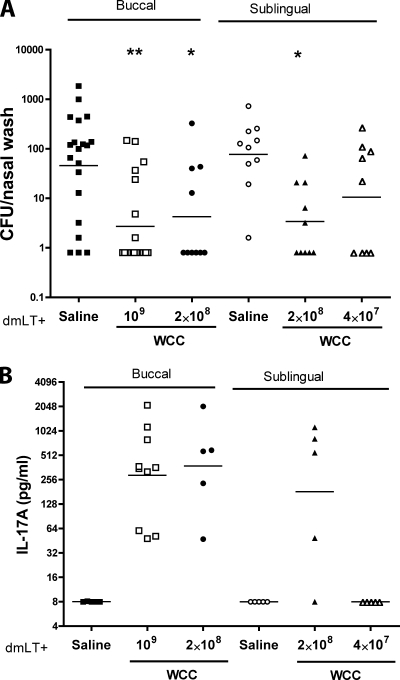
The studies described above were performed prior to the publication of a recent report, which raised concern over the safety of detoxified mutants of LT when given by the intranasal route and pointed to a possible association between their use and the development of Bell's palsy (16, 25). Thus, alternative routes were explored using WCC and a doubly mutated LT (dmLT, R192G/L211A) as an adjuvant. Dose-dependent protection was found with both routes of administration (Fig. 4A) but required about 10-fold more WCC than intranasal immunization and even at the higher doses resulted in fewer mice with no detectable CFU. Consistent with the protection, priming for IL-17A responses in vitro was observed (Fig. 4B).
FIG. 4.
Immunogenicity of WCC antigen at the indicated doses by the buccal and sublingual routes, tested with 10 μg of doubly mutated LT adjuvant, R192G/L211A (dmLT). These immunizations were given thrice with weekly intervals. Challenge was as described for Fig. 2. Three weeks post-third vaccination, blood samples were taken for assay of IL-17A priming. (A) Clearance of serotype 6B colonization. (B) Priming for IL-17A responses to WC antigen by blood cells in vitro.
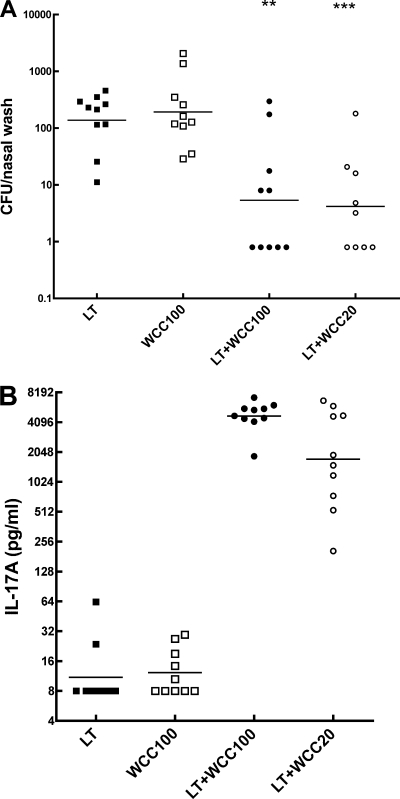
Transcutaneous immunization (TCI) with ultrasonically produced fragments of WCC.
The transcutaneous immunization route was tested by application of cotton gauze patches containing antigen and LT adjuvant to the dorsal skin lightly abraded to disrupt the stratum corneum as done previously for other vaccine preparations (38). Experience with other systems commended reduction of particle size, so fragments with mean diameters of 100 nm (WCC100) and 20 nm (WCC20) were tested. WCC100 and WCC20 were similarly protective when applied with LT, in comparison with LT or with WCC100 applied alone. Consistent with the protection, priming for IL-17A responses in vitro greatly exceeded levels associated with protection by i.n. immunization (Fig. 5B) (20).
FIG. 5.
Immunogenicity by the transcutaneous route of ultrasonically produced fragments of WCC. Fragments with a mean diameter of 100 or 20 nm in dosage equivalent to 108 cells were applied, along with 1 μg of LT adjuvant where indicated, in cotton gauze patches onto dorsal skin gently abraded to remove the stratum corneum. The patch was left in place for 18 h. This immunization was given thrice with a 2-week interval. Blood samples were taken 10 days after the third immunization for assays of IL-17A, and pneumococcal challenge was done 6 days later. (A) Clearance of serotype 6B colonization. (B) Priming for IL-17A responses to WC antigen by blood cells in vitro. For other details, see the legend to Fig. 2.
DISCUSSION
Pneumococcal capsular polysaccharide-protein conjugate vaccine has been effective against systemic disease in infancy for the included serotypes and has provided some herd immunity (13, 17, 36). However, the complexity of manufacture, relative high cost of production, and increasing serotype replacement disease (12) have led to efforts to develop a serotype-independent and more economical vaccine. These include purified protein antigens (4, 7, 10, 11) and vectored protein antigens (1, 15, 18, 26, 37), as well as the noncapsulated WCV studied here (20, 22, 23).
Carriage always precedes pneumococcal disease (3), so the vaccine-induced enhanced clearance of carriage (20) may be protective against pneumonia and invasive disease. Our aim is to reduce the duration and intensity, not necessarily to eliminate carriage. In mice, WCV does not block colonization, but rather accelerates clearance from the nasopharynx (6, 20, 22-24).
Previously the WC antigen was made from cells expressing the cytolytic protein pneumolysin, which was hypothesized to contribute to immunogenicity due to its TLR4 agonist activity (21, 30). Here, anticipating possible side effects from this toxin, vaccine strain RM200 was constructed, which expresses a triply mutated nonlytic variant PdT (5) that nonetheless retains TLR4 activity (21). Previously the cells were grown in Todd-Hewitt-yeast medium (often used for pneumococcus), but to eliminate the possibility of prion disease from bovine components, a soy-based medium developed at Instituto Butantan (19) was used, and RM200 grown in the soy medium proved protective.
When studies of WCV began, among a number of traditional killing agents tried, 70% (vol/vol) ethanol gave the most protective preparations. However, because handling and disposal of large volumes of ethanol may be problematic in industrial settings, alternative methods of inactivation of the WCV were sought here: chloroform and trichloroethylene, which are bactericidal in low concentration, are easily separated by lyophilization, and have some record of pharmaceutical use, and the DNA-altering compound beta-propiolactone, which is readily decomposed into innocuous components and is used in the preparation of rabies vaccines (28). These three agents can be removed without a postkilling wash procedure, which would simplify the manufacturing process. WC antigen preparations killed with these agents without washing (WCC, WCT, and WCB, respectively) were tried and found to be more protective than ethanol-killed cells, which conventionally were washed to remove the ethanol. This result led to the realization that all four killing agents, as used here, solubilize about 15% of the total protein from the cells and that this complex mixture of proteins (and possibly nonprotein components) is protective per se in the mouse colonization model; their retention possibly contributes to the potency of the WCC, WCT, and WCB preparations. Chloroform and trichloroethylene generated equally immunogenic preparations with similar, complex bands of released proteins and thus probably act alike in damaging the bacterial membrane; they both are discussed here because they are viewed differently within the pharmaceutical industry. Practically, any of these killing agents could be used, with the choice being determined by the manufacturer's preference. This activity of soluble components prompted a cursory test of cells completely disrupted by sonication; this preparation was as protective and evocative of IL-17A as the original WCC preparation, a result which is reminiscent of prior studies of lysed pneumococcal preparations administered parenterally in mice providing protection against invasive disease challenge (2).
WCV was originally intended for intranasal administration. Study of intranasally applied WCV in mouse models revealed the induction of an antibody-independent, CD4+ T-cell-dependent mechanism requiring IL-17A (20) which was done with CT as the adjuvant. One drawback of the intranasal use of enterotoxin adjuvants is their penetration into the central nervous system via retrograde transport along the olfactory nerve, as seen in rodent models (35). The recent clinical experience wherein LT (25) and, very recently, a singly mutated derivative, LT-K63, (16) were suspected to have induced several cases of Bell's palsy when given intranasally further raises concern. Therefore, while we examined a recently developed doubly mutated, hyper-detoxified LT (dmLT [9]), its future study should probably involve mucosal approaches other than the intranasal route, while intranasal vaccination should be studied without the use of enterotoxin-related adjuvants.
Another drawback of intranasal vaccination in infancy is that in subjects presenting with copious nasal mucus—frequent in some clinical settings—effective contact of the vaccine with the mucosa would be compromised. Therefore, alternative mucosal routes were tested: application to the buccal mucosa alongside the lower molars (frequently used for live polio vaccination of children) and sublingual application, a route recently analyzed cytologically in detail (8). Both routes give access to the immunoresponsive tissues of Waldeyer's ring with less access to the central nervous system and circumvent the problem of nasal mucus. Both gave protection in the intranasal colonization model and priming for IL-17A responses. Either route could be clinically evaluated, but a higher dosage than that used for the i.n. route might be required. Transdermal vaccination, which has been previously investigated in several animal models, including Shiga toxin-producing Escherichia coli and anthrax (14, 38), was protective here with sonicates of the pneumococcal WC antigen and is another alternative to the intranasal route.
Although the killed WC antigen—obviously an array of many different antigens, present in both particulate and soluble forms—has potential challenges regarding manufacturing consistency and standardization, its potency, low cost of production, stability as a lyophile, and administration without syringes make it worthy of further evaluation.
Acknowledgments
This work was supported by PATH. R.M. is also supported by grants from the National Institutes of Health (AI067737-01 and AI51526-01).
We thank L. Leite for helpful discussions.
Footnotes
Published ahead of print on 28 April 2010.
REFERENCES
- 1.Arevalo, M. T., Q. Xu, J. C. Paton, S. K. Hollingshead, M. E. Pichichero, D. E. Briles, N. Girgis, and M. Zeng. 2009. Mucosal vaccination with a multicomponent adenovirus-vectored vaccine protects against Streptococcus pneumoniae infection in the lung. FEMS Immunol. Med. Microbiol. 55:346-351. [DOI] [PubMed] [Google Scholar]
- 2.Au, C. C., and T. K. Eisenstein. 1981. Nature of the cross-protective antigen in subcellular vaccines of Streptococcus pneumoniae. Infect. Immun. 31:160-168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Austrian, R. 1986. Some aspects of the pneumococcal carrier state. J. Antimicrob. Chemother. 18(Suppl. A):35-45. [DOI] [PubMed] [Google Scholar]
- 4.Basset, A., C. M. Thompson, S. K. Hollingshead, D. E. Briles, E. W. Ades, M. Lipsitch, and R. Malley. 2007. Antibody-independent, CD4+ T-cell-dependent protection against pneumococcal colonization elicited by intranasal immunization with purified pneumococcal proteins. Infect. Immun. 75:5460-5464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Berry, A. M., J. E. Alexander, T. J. Mitchell, P. W. Andrew, D. Hansman, and J. C. Paton. 1995. Effect of defined point mutations in the pneumolysin gene on the virulence of Streptococcus pneumoniae. Infect. Immun. 63:1969-1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bogaert, D., D. Weinberger, C. Thompson, M. Lipsitch, and R. Malley. 2009. Impaired innate and adaptive immunity to Streptococcus pneumoniae and its effect on colonization in an infant mouse model. Infect. Immun. 77:1613-1622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Briles, D. E., S. Hollingshead, A. Brooks-Walter, G. S. Nabors, L. Ferguson, M. Schilling, S. Gravenstein, P. Braun, J. King, and A. Swift. 2000. The potential to use PspA and other pneumococcal proteins to elicit protection against pneumococcal infection. Vaccine 18:1707-1711. [DOI] [PubMed] [Google Scholar]
- 8.Cuburu, N., M. N. Kweon, C. Hervouet, H. R. Cha, Y. Y. Pang, J. Holmgren, K. Stadler, J. T. Schiller, F. Anjuere, and C. Czerkinsky. 2009. Sublingual immunization with nonreplicating antigens induces antibody-forming cells and cytotoxic T cells in the female genital tract mucosa and protects against genital papillomavirus infection. J. Immunol. 183:7851-7859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dickinson, B. L., and J. D. Clements. 1995. Dissociation of Escherichia coli heat-labile enterotoxin adjuvanticity from ADP-ribosyltransferase activity. Infect. Immun. 63:1617-1623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Giefing, C., A. L. Meinke, M. Hanner, T. Henics, M. D. Bui, D. Gelbmann, U. Lundberg, B. M. Senn, M. Schunn, A. Habel, B. Henriques-Normark, A. Ortqvist, M. Kalin, A. von Gabain, and E. Nagy. 2008. Discovery of a novel class of highly conserved vaccine antigens using genomic scale antigenic fingerprinting of pneumococcus with human antibodies. J. Exp. Med. 205:117-131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Glover, D. T., S. K. Hollingshead, and D. E. Briles. 2008. Streptococcus pneumoniae surface protein PcpA elicits protection against lung infection and fatal sepsis. Infect. Immun. 76:2767-2776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hanage, W. P. 2008. Serotype-specific problems associated with pneumococcal conjugate vaccination. Future Microbiol. 3:23-30. [DOI] [PubMed] [Google Scholar]
- 13.Hsu, H. E., K. A. Shutt, M. R. Moore, B. W. Beall, N. M. Bennett, A. S. Craig, M. M. Farley, J. H. Jorgensen, C. A. Lexau, S. Petit, A. Reingold, W. Schaffner, A. Thomas, C. G. Whitney, and L. H. Harrison. 2009. Effect of pneumococcal conjugate vaccine on pneumococcal meningitis. N. Engl. J. Med. 360:244-256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kenney, R. T., J. Yu, M. Guebre-Xabier, S. A. Frech, A. Lambert, B. A. Heller, L. R. Ellingsworth, J. E. Eyles, E. D. Williamson, and G. M. Glenn. 2004. Induction of protective immunity against lethal anthrax challenge with a patch. J. Infect. Dis. 190:774-782. [DOI] [PubMed] [Google Scholar]
- 15.Kong, W., S. Y. Wanda, X. Zhang, W. Bollen, S. A. Tinge, K. L. Roland, and R. Curtiss III. 2008. Regulated programmed lysis of recombinant Salmonella in host tissues to release protective antigens and confer biological containment. Proc. Natl. Acad. Sci. U. S. A. 105:9361-9366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lewis, D. J., Z. Huo, S. Barnett, I. Kromann, R. Giemza, E. Galiza, M. Woodrow, B. Thierry-Carstensen, P. Andersen, D. Novicki, G. Del Giudice, and R. Rappuoli. 2009. Transient facial nerve paralysis (Bell's palsy) following intranasal delivery of a genetically detoxified mutant of Escherichia coli heat labile toxin. PLoS One 4:e6999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lexau, C. A., R. Lynfield, R. Danila, T. Pilishvili, R. Facklam, M. M. Farley, L. H. Harrison, W. Schaffner, A. Reingold, N. M. Bennett, J. Hadler, P. R. Cieslak, and C. G. Whitney. 2005. Changing epidemiology of invasive pneumococcal disease among older adults in the era of pediatric pneumococcal conjugate vaccine. JAMA 294:2043-2051. [DOI] [PubMed] [Google Scholar]
- 18.Li, Y., S. Wang, G. Scarpellini, B. Gunn, W. Xin, S. Y. Wanda, K. L. Roland, and R. Curtiss III. 2009. Evaluation of new generation Salmonella enterica serovar Typhimurium vaccines with regulated delayed attenuation to induce immune responses against PspA. Proc. Natl. Acad. Sci. U. S. A. 106:593-598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liberman, C., M. Takagi, J. Cabrera-Crespo, M. E. Sbrogio-Almeida, W. O. Dias, L. C. Leite, and V. M. Goncalves. 2008. Pneumococcal whole-cell vaccine: optimization of cell growth of unencapsulated Streptococcus pneumoniae in bioreactor using animal-free medium. J. Ind. Microbiol. Biotechnol. 35:1441-1445. [DOI] [PubMed] [Google Scholar]
- 20.Lu, Y. J., J. Gross, D. Bogaert, A. Finn, L. Bagrade, Q. Zhang, J. K. Kolls, A. Srivastava, A. Lundgren, S. Forte, C. M. Thompson, K. F. Harney, P. W. Anderson, M. Lipsitch, and R. Malley. 2008. Interleukin-17A mediates acquired immunity to pneumococcal colonization. PLoS Pathog. 4:e1000159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Malley, R., P. Henneke, S. C. Morse, M. J. Cieslewicz, M. Lipsitch, C. M. Thompson, E. Kurt-Jones, J. C. Paton, M. R. Wessels, and D. T. Golenbock. 2003. Recognition of pneumolysin by Toll-like receptor 4 confers resistance to pneumococcal infection. Proc. Natl. Acad. Sci. U. S. A. 100:1966-1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Malley, R., M. Lipsitch, A. Stack, R. Saladino, G. Fleisher, S. Pelton, C. Thompson, D. Briles, and P. Anderson. 2001. Intranasal immunization with killed unencapsulated whole cells prevents colonization and invasive disease by capsulated pneumococci. Infect. Immun. 69:4870-4873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Malley, R., S. C. Morse, L. C. C. Leite, A. P. Mattos Areas, P. L. Ho, F. S. Kubrusly, I. C. Almeida, and P. Anderson. 2004. Multiserotype protection of mice against pneumococcal colonization of the nasopharynx and middle ear by killed nonencapsulated cells given intranasally with a nontoxic adjuvant. Infect. Immun. 72:4290-4292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Malley, R., K. Trzcinski, A. Srivastava, C. M. Thompson, P. W. Anderson, and M. Lipsitch. 2005. CD4+ T cells mediate antibody-independent acquired immunity to pneumococcal colonization. Proc. Natl. Acad. Sci. U. S. A. 102:4848-4853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mutsch, M., W. Zhou, P. Rhodes, M. Bopp, R. T. Chen, T. Linder, C. Spyr, and R. Steffen. 2004. Use of the inactivated intranasal influenza vaccine and the risk of Bell's palsy in Switzerland. N. Engl. J. Med. 350:896-903. [DOI] [PubMed] [Google Scholar]
- 26.Nayak, A. R., S. A. Tinge, R. C. Tart, L. S. McDaniel, D. E. Briles, and R. Curtiss. 1998. A live recombinant avirulent oral Salmonella vaccine expressing pneumococcal surface protein A induces protective responses against Streptococcus pneumoniae. Infect. Immun. 66:3744-3751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.O'Brien, K. L., L. J. Wolfson, J. P. Watt, E. Henkle, M. Deloria-Knoll, N. McCall, E. Lee, K. Mulholland, O. S. Levine, and T. Cherian. 2009. Burden of disease caused by Streptococcus pneumoniae in children younger than 5 years: global estimates. Lancet 374:893-902. [DOI] [PubMed] [Google Scholar]
- 28.Plotkin, S. A. 2000. Rabies. Clin. Infect. Dis. 30:4-12. [DOI] [PubMed] [Google Scholar]
- 29.Ray, G. T. 2002. Pneumococcal conjugate vaccine: economic issues of the introduction of a new childhood vaccine. Expert Rev. Vaccines 1:65-74. [DOI] [PubMed] [Google Scholar]
- 30.Srivastava, A., P. Henneke, A. Visintin, S. C. Morse, V. Martin, C. Watkins, J. C. Paton, M. R. Wessels, D. T. Golenbock, and R. Malley. 2005. The apoptotic response to pneumolysin is Toll-like receptor 4 dependent and protects against pneumococcal disease. Infect. Immun. 73:6479-6487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sung, C. K., H. Li, J. P. Claverys, and D. A. Morrison. 2001. An rpsL cassette, Janus, for gene replacement through negative selection in Streptococcus pneumoniae. Appl. Environ. Microbiol. 67:5190-5196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tai, S. S. 2006. Streptococcus pneumoniae protein vaccine candidates: properties, activities and animal studies. Crit. Rev. Microbiol. 32:139-153. [DOI] [PubMed] [Google Scholar]
- 33.Trzcinski, K., C. M. Thompson, and M. Lipsitch. 2003. Construction of otherwise isogenic serotype 6B, 7F, 14, and 19F capsular variants of Streptococcus pneumoniae strain TIGR4. Appl. Environ. Microbiol. 69:7364-7370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Trzcinski, K., C. M. Thompson, A. Srivastava, A. Basset, R. Malley, and M. Lipsitch. 2008. Protection against nasopharyngeal colonization by Streptococcus pneumoniae is mediated by antigen-specific CD4+ T cells. Infect. Immun. 76:2678-2684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.van Ginkel, F. W., R. J. Jackson, Y. Yuki, and J. R. McGhee. 2000. Cutting edge: the mucosal adjuvant cholera toxin redirects vaccine proteins into olfactory tissues. J. Immunol. 165:4778-4782. [DOI] [PubMed] [Google Scholar]
- 36.Whitney, C. G., M. M. Farley, J. Hadler, L. H. Harrison, N. M. Bennett, R. Lynfield, A. Reingold, P. R. Cieslak, T. Pilishvili, D. Jackson, R. R. Facklam, J. H. Jorgensen, and A. Schuchat. 2003. Decline in invasive pneumococcal disease after the introduction of protein-polysaccharide conjugate vaccine. N. Engl. J. Med. 348:1737-1746. [DOI] [PubMed] [Google Scholar]
- 37.Xin, W., Y. Li, H. Mo, K. L. Roland, and R. Curtiss III. 2009. PspA family fusion proteins delivered by attenuated Salmonella enterica serovar Typhimurium extend and enhance protection against Streptococcus pneumoniae. Infect. Immun. 77:4518-4528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhu, C., J. Yu, Z. Yang, K. Davis, H. Rios, B. Wang, G. Glenn, and E. C. Boedeker. 2008. Protection against Shiga toxin-producing Escherichia coli infection by transcutaneous immunization with Shiga toxin subunit B. Clin. Vaccine Immunol. 15:359-366. [DOI] [PMC free article] [PubMed] [Google Scholar]