Abstract
Treatment of latent Mycobacterium tuberculosis infection on the basis of the tuberculin skin test (TST) result is inaccurate due to the false-positive TST results that occur after Mycobacterium bovis BCG vaccination or exposure to nontuberculous mycobacteria (NTM). Gamma interferon release assays (IGRAs) are based on M. tuberculosis-specific antigens. In a previous study among BCG-naïve military employees, a positive TST result after deployment was mostly associated with a negative IGRA result, suggesting exposure to NTM. Data regarding the kinetics of IGRAs are limited and controversial. The present study aimed to reassess the rate of false-positive TST results and to evaluate the kinetics of the Quantiferon TB Gold In-Tube assay (QFT-Git) in military personnel with a positive TST result. QFT-Git was performed at the time of inclusion in the study and was repeated after 2, 6, 12, and 18 or 24 months. Of 192 participants, 17 were recruits and 175 were screened after deployment (n = 169) or because of travel or health care work. Baseline positive QFT-Git results were observed in 7/17 (41.2%) and 12/174 (6.9%) participants, respectively. During follow-up, a negative QFT-Git result remained negative in 163/165 (98.8%) participants. Of 18 subjects with an initial positive QFT-Git result, reversion to a negative result occurred in 1/6 (16%) recruits, whereas it occurred in 8/12 (66%) subjects after deployment or with other risk factors (P = 0.046). The quantitative result was significantly lower in subjects with reversion than in those with consistent positive results (P = 0.017). This study confirmed a low rate of positive QFT-Git results among military personnel with a positive TST result after deployment, supporting the hypothesis of exposure to NTM. Reversion of the majority of initially low-positive QFT-Git results indicates that QFT-Git may be useful for the diagnosis of later reinfections.
Each year, about 3,000 Dutch army personnel are deployed to regions where tuberculosis (TB) is highly endemic. Screening of military personnel for latent Mycobacterium tuberculosis infection (LTBI) has thus far been based on the tuberculin skin test (TST). The Netherlands is a country with a low prevalence of TB, with a yearly incidence of 5.9 cases/100,000 population in 2007, only one-third of which occurred among native Dutch persons (Tuberculosis in The Netherlands 2007 [www.kncvtbc.nl]). Personnel are screened by the TST upon initial recruitment into the army, after deployment, or in the presence of other risk factors for TB exposure. Military personnel with TST conversion are prescribed isoniazid for 6 months to prevent TB disease. The risk of progression from untreated LTBI to active TB is generally believed to be about 10%, with half of the cases occurring within 2 years after infection. However, the risks observed in different studies comparing subjects treated with isoniazid or placebo varied widely, depending on the setting and the characteristics of the study population (38). A major disadvantage of the current policy is that a substantial proportion of TST conversions in this setting are thought to be caused by exposure to nontuberculous mycobacteria (NTM), skewing the risk-benefit ratio of preventive treatment (8). In addition, increasing proportions of the Dutch population and Dutch military recruits originate from countries where M. bovis BCG vaccination is routinely used. In BCG-vaccinated Dutch military personnel or those with a previous positive TST result, TST is not performed, as a rule, and chest radiography is used as an alternative, but radiography lacks sensitivity for the detection of LTBI. Finally, a positive TST result often remains positive, thus precluding the detection of reinfection.
In order to overcome the disadvantages of the TST, gamma interferon (IFN-γ) release assays (IGRAs) that use M. tuberculosis-specific antigens and that are not affected by BCG and most NTM were developed (2-4, 32, 34). In contact investigations, the results of IGRAs had a better correlation with measures of exposure (5, 19, 21, 43). Since 2005, IGRAs have increasingly been used for the detection of LTBIs either as a replacement of or as adjunct to the TST (18, 26, 28). Of the two presently commercially available IGRAs, the Quantiferon TB Gold In-Tube assay (QFT-Git) is a robust whole-blood-based test suitable for use for large-scale testing (5, 7, 22). In a previous study, QFT-Git was positive for only a minority of military personnel with a positive TST result after deployment (13). Those results were considered to be related to NTM exposure, in accordance with the high proportion of false-positive TST responses assessed by dual skin testing of army recruits by the use of tuberculin and atypical sensitin (8). The destinations of deployment at the time of the earlier study were mainly Iraq and Bosnia (13), but the destination has changed to Afghanistan in the past few years. As the incidence of TB is higher in Afghanistan than in most of the countries where the military personnel were deployed, in order to justify a change in treatment policy, the previously observed low rate of positive IGRA results in association with a positive TST result needed to be studied in the current setting. In the previous study (13), QFT-Git was performed only once, and the subjects were not assessed for eventual later conversion or reversion. Previous studies of the kinetics of IGRAs gave variable and partly conflicting results (9, 12, 14, 15, 17, 20, 29, 30, 35-37, 40), although the main trend was for high-positive results to usually remain positive, and reversion can occur when the results are low or moderately positive (12, 15, 20, 29, 30, 33, 35-37, 42). In a setting of LTBIs, the relevance of follow-up testing by IGRAs may lie in the possibility of detecting later reinfection if reversion to a negative result has been documented.
The aims of this study were to study the kinetics of QFT-Git during at least 6 months of follow-up in order to evaluate the possibility of detection of later reinfection and to confirm the previously observed very low rate of positive QFT-Git results in military personnel with a positive TST result after deployment.
MATERIALS AND METHODS
Study design.
This prospective observational study included BCG-naïve military personnel with documented TST conversion during screening. TST screening is performed for all new recruits in order to obtain a baseline value upon entry into the army as well as in all previously TST-negative military personnel 6 to 8 weeks after they return from deployment to a region where TB is endemic. Finally, TST screening is performed on a yearly basis for nondeployed military personnel with risk factors, such as medical work or travel. Exclusion criteria were suspected or proven immune deficiency, BCG vaccination in the past, or active TB disease. Isoniazid treatment was offered according to protocol of the Dutch Defense Department. The QFT-Git results were not used for clinical decision making. Subjects who declined isoniazid treatment were monitored by the use of chest radiography every 6 months for 2 years. QFT-Git was performed at 0, 2, 6, and 12 months (the last time point of testing for subjects treated with isoniazid) and 18 or 24 months (when isoniazid was not used or was discontinued), whenever possible, considering the duties and deployment of the subjects. The subjects answered the questions on a written questionnaire regarding past TSTs, risk factors for TB infection, and, if applicable, the characteristics of the deployment abroad. The study protocol was approved by the Dutch Defense Department and the Ethical Board of the University Medical Center Utrecht (protocol number P06-217). Participation in the study required written informed consent.
TST.
TST placement and reading of the results were performed by trained personnel, according to existing guidelines described previously (13), and a single TST was used for screening. For deployed personnel, the interval between their return to the Netherlands and skin testing was always >6 weeks; thus, they were tested after a window period to allow detection of all recent conversions. TST conversion was defined as an induration of at least 10 mm and an increase in induration of ≥6 mm compared with that for a previous known TST, according to national guidelines. For recruits, isoniazid is usually offered to subjects with a TST result with an induration of ≥15 mm, as the time of infection is not known and could have been years earlier. TST results were analyzed as a value in mm; categorically as 10 to 14 mm, 15 to 19 mm, and ≥ 20 mm; and binary at a cutoff value of 15 mm.
Procedures.
For the Quantiferon-TB Gold In-Tube assay (Cellestis, Carnegie, Australia), two tubes of 1 ml whole blood were obtained by routine venous puncture and placed in antigen-precoated tubes containing saline as a negative control (NIL) or M. tuberculosis-specific antigens. A positive-control tube was not used, as has been approved by the European Commission for immunocompetent individuals. The tubes were incubated for 24 h at 37°C, followed by centrifugation and cold storage until the samples were tested. The concentration of gamma interferon in the samples was determined by a commercial enzyme-linked immunosorbent assay, according to the manufacturer's instructions. The test result was coded as negative or positive, according to the manufacturer's instructions. The QFT-Git result was considered positive if the gamma interferon level (M. tuberculosis-specific antigen minus NIL) was ≥0.35 IU/ml.
For subjects with at least two valid QFT-Git results, the response patterns were categorized as “consistent positive” if all results were positive or if positive results surrounded at most one negative result, “consistent negative” if all results were negative, “conversion” if one or more negative results were followed by one or more positive results, and “reversion” if one or more positive results were followed by one or more negative results.
Statistical analysis.
This observational prospective study used descriptive summary statistics. Differences between categorical parameters were analyzed by the Pearson chi-square test. Normally distributed values were compared by use of the t test, and the Mann-Whitney U test was used for analysis of nonparametric quantitative results. SPSS (version 16.0) software was used for statistical analysis. P values of <0.05 were considered statistically significant.
RESULTS
Patient characteristics.
Between February 2007 and October 2008, 200 subjects gave informed consent to participate in the study. Eight of these subjects were excluded due to BCG vaccination which had been overlooked at the time of inclusion in the study (n = 4), the subjects did not fulfill the criteria for TST conversion (n = 3), or the subject failed blood sampling (n = 1). Of the 192 subjects included, 17 (8.9%) were new recruits, 169 (88%) were screened after their return from deployment abroad, and 6 (3.1%) were army personnel with specific risk factors, such as travel to countries where TB is endemic or health care work. The 169 participants screened after deployment and the 6 who were screened for other reasons were similar in most respects, such as by age, duration of employment in the army (median of 7 years and range of 1 to 40 years for those screened after deployment and median of 7.2 years and range of 3 to 12 years for those screened for other reasons), reported TB contact, TST results, and the proportion of individuals with positive QFT-Git results (12/168 [7.1%] and 0/6, respectively; P was not significant). The only differences were a higher proportion of women in the group screened for other reasons (8/169 and 2/6, respectively; P = 0.04) and a more frequent history of travel to tropical areas for the group screened for other reasons (26/169 and 4/6, respectively; P = 0.001). Therefore, both groups were analyzed together as one group, and the group is referred to as the “after deployment/other risk factors group.” The recruits were younger than those in the after deployment/other risk factors group, and the proportion of women in the after deployment/other risk factors group was lower than that among the recruits. There were no statistically significant differences between the groups in the country of birth, reported TB contact, and visits to the tropics. The characteristics of the subjects are listed in Table 1.
TABLE 1.
Characteristics of study population
| Characteristic | Recruits (n = 17) | Individuals in after deployment/other risk factors group (n = 175) | P value |
|---|---|---|---|
| Mean ± SD age (yr) | 23.0 ± 4.1 | 29.7 ± 9.6 | 0.001 |
| No. (%) of participants | |||
| Female sex | 4 (23.5) | 10 (5.7) | 0.007 |
| Foreign born | 0 | 8 (4.6) | 0.37 |
| Travel to tropics | 5 (29.4) | 30 (17.1) | 0.21 |
| Reported contact with TB | 3 (17.6) | 19 (10.9) | 0.4 |
| Mean ± SD TST result (mm) | 15.9 ± 2.9 | 13.3 ± 2.8 | <0.001 |
| TST category | |||
| 10-14 mm | 4 (23.5) | 132 (75.4) | <0.001 |
| 15-19 mm | 11 (64.7) | 35 (20.0) | |
| ≥ 20 mm | 2 (11.8) | 8 (4.6) | |
| Mean ± SD no. of QFT-Git performed | 2.8 ± 1.1 | 3.3 ± 0.9 | 0.065 |
| Initial no. (%) of participants QFT-Git positive | 7 (41.2) | 12a (6.9) | <0.001 |
| No. (%) of participants with QFT-Git follow-up patternb: | <0.001 | ||
| Consistent negative | 8 (53.4) | 155 (92.3) | |
| Conversion | 1 (6.7) | 1 (0.6) | |
| Consistent positive | 5 (33.3) | 4 (2.4) | |
| Reversion | 1 (6.7) | 8 (4.8) |
Denominator was 174 due to one missing sample; all 12 positive results were in personnel returned from deployment abroad.
For definitions, see Materials and Methods.
TST results.
The average TST result was higher among the recruits (Table 1), with 75% having an induration of ≥15 mm, whereas 25% of military personnel in the after deployment/other risk factors group had a positive TST result comprising an induration of ≥15 mm, which could be explained by the preferential referral of recruits with TST indurations of ≥15 mm for isoniazid treatment. TST results were not affected by age, sex, birth outside the Netherlands, travel to tropical countries, or reported contact with a TB patient.
QFT-Git results.
Samples for testing by QFT-Git were collected between February 2007 and July 2009.
QFT-Git result at inclusion.
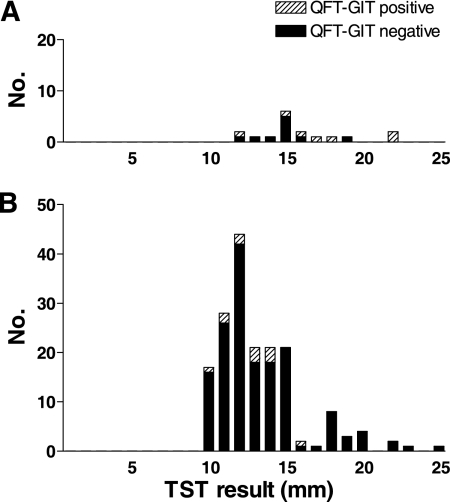
Valid QFT-Git results at inclusion were obtained for 191/192 participants, of which a positive result was observed for 7/17 (41.2%) recruits and 12/174 (6.9%) subjects in the after deployment/other risk factors group. Figure 1 shows the QFT-Git results in relation to TST results. The initial value was missing for one subject, while all three follow-up samples from that subject were negative.
FIG. 1.
Distribution of TST results in recruits (A) or military personnel in the after deployment/other risk factors group (B).
The results of QFT-Git at inclusion did not depend on age, sex, birth outside the Netherlands, travel to tropical countries, or reported contact with a TB patient. Surprisingly, the QFT-Git results were negative for 42/43 subjects with a TST induration of ≥15 mm after deployment (Fig. 1B), and 5 of these subjects had a previous TST result with indurations of between 4 and 9 mm. There was no significant association between the induration size by TST and positive QFT-Git results.
QFT-Git during follow-up.
The number of tests per individual varied, depending on the limitations imposed by redeployment and compliance with scheduled return visits, and consisted of 1 (n = 9), 2 (n = 23), 3 (n = 72), 4 (n = 83), and 5 (n = 5) follow-up tests for the indicated numbers of individuals. The results of tests performed at least at 0 and 2 months, 0 and 6 months, or 0 and 12 months were available for 162, 173, and 99 participants, respectively.
Almost all (163/165) initially negative QFT-Git results remained consistently negative during follow-up, with only two conversions being observed: one in a recruit who had a negative result at 0, 2, and 6 months, followed by a result of 0.39 IU/ml at 12 months, and another in a subject after deployment with IFN-γ values of 0.32 and 0.28 IU/ml, values just below the cutoff, at 0 and 2 months, respectively, followed by values of 0.59 and 0.56 IU/ml at 6 and 12 months, respectively.
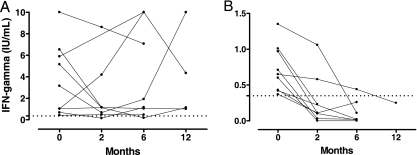
Of 18 subjects with an initial positive QFT-Git result, 7 were consistently positive during follow-up, 2 had one negative value between low-positive values, while 9 reverted to negative; 7 of the last 9 subjects had reverted at the second available time point of 2 months (n = 6) or 6 months (n = 1). Reversion occurred in 1/6 (16%) recruits with an initial positive test result, whereas reversion occurred in 8/12 (66%) subjects in the after deployment/other risk factors group (P = 0.046). Figure 2 shows that for subjects with an initial positive QFT-Git result, the quantitative test result was significantly lower for subjects with reversions than for those who demonstrated a consistent positive pattern (0.72 ± 0.33 and 3.76 ± 3.3 IU/ml IFN-γ, respectively, P = 0.017).
FIG. 2.
The mean QFT-Git result for subjects with consistently positive results or with one negative value between low-positive values (n = 9) was 3.76 ± 3.3 IU/ml IFN-γ (A), which was significantly higher than the mean of 0.72 ± 0.33 IU/ml for nine subjects with reversion to negativity (B) (P = 0.017). The corresponding median IFN-γ values were 2.3 and 0.66 IU/ml, respectively. Dotted line, cutoff value for a positive response (0.35 IU/ml). Note that the y axes in the two panels have different scales.
Among 18 subjects with a baseline positive QFT-Git result, consistently positive results were more frequent in subjects with a larger TST induration size, which was not a statistically significant difference, possibly due to the overall low number of positive test results. There was a trend toward higher quantitative test results in subjects with a TST induration of ≥15 mm than in those with an induration of <15 mm (medians, 2.22 and 0.91 IU/ml IFN-γ, respectively; P = 0.052).
The participants who had been deployed were rather homogeneous, with the main destination of deployment being Afghanistan for 163/169 (96.4%) participants and with the durations of deployment being <4 months for 47/168 (27.8%) participants and 4 to 6 months for 108/168 (63.9%) participants. There was no effect between the size of the TST induration, the duration or destination of the deployment, the level of contact with the local population, performance of a medical task, or visiting a local hospital during deployment and the QFT-Git result (data not shown).
Among 17 recruits, positive QFT-Git results were found for 1/4 (25%), 4/11 (36.4%), and 2/2 (100%) participants with TST indurations of 10 to 14, 15 to 19, and ≥20 mm, respectively, the differences of which were not statistically significant, possibly due to the low overall number of positive results.
Preventive treatment.
All TST-positive participants were offered isoniazid preventive treatment, and two of these individuals declined treatment. Liver function elevations of <3 times, 3 to 5 times, 5 to 10 times, and ≥10 times the upper limit of normal occurred in 40 (21%), 7 (4%), 4 (2%), and 2 (1%) subjects, respectively. Treatment was discontinued in 11 participants after a median of 2 months (range, 2 weeks to 4 month) because of abnormal liver function test results, according to guidelines (values greater than or equal to five times the upper limit of normal or greater than or equal to three times the upper limit of normal in association with complaints) or subjective complaints without objective signs. For the subjects who did or who did not complete 6 months of isoniazid treatment, there was no significant difference in the proportion with a positive QFT-Git result at the inclusion (18/178 [10.1%] and 1/13 [7.7%], respectively) or at 6 months (9/155 [5.8%] and 0/11 [0%], respectively). The number of subjects who did not complete 6 months of preventive treatment was, however, too small for a robust statistical analysis. No subject had or developed active TB during the follow-up period.
DISCUSSION
The results of this study showed a 6-fold lower rate of positive QFT-Git results among military personnel with TST conversion in the after deployment/other risk factors group (6.9%) than in recruits (41.2%), confirming and extending the findings of a previous study by Franken et al., who observed a positive QFT-Git result in 11.5% and 44.4% of the individuals in these groups, respectively (13). The very low rate of positive QFT-Git results after deployment would be consistent with the idea that most positive TST results in this group were false positive due to exposure to NTM, while the higher rate of positive QFT-Git responses in recruits reflected previous infection with M. tuberculosis.
Surprisingly, the QFT-Git results were negative for 42/43 subjects with a TST induration of ≥15 mm after deployment. In these BCG-naïve participants, this represented documented conversion, since all had had a negative TST result upon entry into the army. If the conversion observed would have been caused by actual infection with M. tuberculosis during deployment, most should have had a positive QFT-Git result, since this assay is highly sensitive for the detection of recent infection. However, most QFT-Git results were negative. False-positive TST results due to BCG mostly do not have indurations that exceed 15 mm (39). However, there are no solid data on the TST induration sizes due to NTM when the test is performed shortly after exposure, as would be the case in our participants. Therefore, our data raise the hypothesis that exposure to NTM can cause large TST induration responses immediately after a period of exposure.
Pseudoepidemics of TST conversion among deployed U.S. military personnel have been analyzed, and exposure to NTM was considered one of the possible explanations (25), but no tests were done to support that idea. More specific diagnostic tests such as IGRAs are therefore of potential value for the screening of army personnel. Only a few studies that used TB-specific IGRAs to test military personnel have been published, and apart from the present study and the previously published study by Franken et al. (13), none of these were done in a setting of TST screening after deployment. Two prior studies included military recruits. The study by Mazurek et al. compared the first- and second-generation Quantiferon assays, only the latter of which is based on M. tuberculosis-specific antigens, in with 856 U.S. navy recruits, 5.1% of whom had TST indurations of ≥10 mm, while only 0.6% had a positive QFT-Git result (27). The other study used a TB-specific in-house enzyme-linked immunospot (ELISPOT) assay to test 100 mostly BCG-vaccinated Chinese military recruits, and positive TST and positive ELISPOT assay results were obtained for 41% and 21% of the subjects, respectively, which reflects the higher prevalence of true LTBIs in China and shows that IGRAs may contribute to the more accurate diagnosis of LTBIs in a setting with a high level of endemicity of TB and routine BCG vaccination (41).
Two other studies of military personnel were done as part of contact investigations. Among mostly BCG-vaccinated Swiss military personnel who were contacts of a colleague with cavitating pulmonary TB, QFT-Git was used as the primary screening tool and resulted in 34/168 (20.2%) positive results and a good correlation between the QFT-Git result and the level of exposure (19). In a similar study among South Korean military camp contacts of soldiers with active pulmonary TB, the QFT-Git result was positive for 25/175 (14.3%) of the individuals (10). These studies show that the risk of LTBI can be substantial in a military setting with actual exposure to known smear-positive cases, albeit it is still lower than the 30 to 40% positive TST results found among close contacts in a civilian setting. This confirms that IGRAs can be valuable when the TST result is unreliable due to BCG vaccination.
The only published studies that used IGRAs to test military personnel following deployment to a region with a higher prevalence of TB are the present study and the study by Franken et al. (13) mentioned above. Both were limited to non-BCG-vaccinated Dutch military personnel, thereby excluding BCG as a cause of false-positive TST results. The main difference between the studies was the destination of deployment. As IGRAs are highly sensitive for the detection of a recent LTBI, it is reasonable to assume that only the observed 6.9% positive QFT-Git results are for most of those with true LTBIs. The low rate of positive QFT-Git results indicates that most positive TST results in this setting were probably not caused by recent LTBIs and could be related to exposure to NTM; yet all individuals with TST conversion were offered and mostly completed preventive treatment with isoniazid. Significant elevations in liver function test levels were frequent, and side effects were the cause of the discontinuation of treatment for 5.8% of the subjects. On the basis of the results of this and the previous study (13), presumably mostly unjustified treatment of most individuals, together with the considerable costs, risks, and disadvantages of treatment, we believe that it is justified to reconsider the cost-benefit ratio of preventive treatment in military personnel.
Although the negative and positive predictive values of IGRAs remain to be proven definitively, the findings presented in several recent publications show that progression to active TB occurred only in subjects with a positive IGRA result at the time of screening, while TB did not occur in those with negative test results (1, 11, 16). A study for which the association was less clear was conducted with exposed and mostly treated children in Turkey, where reinfections may be more common (6). Several national guidelines now advocate the results of IGRAs, either as a replacement of or as adjunct to the TST, for clinical decision making (26, 28). In the Netherlands, a preliminary guideline was issued in 2008. In brief, that guideline advocates the use of a two-step approach, in which a positive TST result can be followed by confirmation by an IGRA for clinical decision making (18).
Because the results of the present study showed even lower rates of positive QFT-Git results than the previous study, the results prompted a change in treatment policy at the Central Military Hospital in November 2008, with preventive treatment being offered exclusively to military personnel with documented TST conversion as well as a positive QFT-Git result, while those with a negative QFT-Git result are monitored for 2 years by the use of a visit for clinical examination and chest radiography every 6 months. The benefit of restricting preventive treatment to less than 10% of subjects with TST conversions represents considerable benefit in terms of avoided costs, inconvenience, and side effects. If the negative predictive value proves to be sufficiently high, the monitoring of subjects with negative QFT-Git results may not be necessary, resulting in additional logistical and financial savings. This policy could most likely be extrapolated to BCG-vaccinated subjects, since the risk of a false-positive TST result is even higher in that group.
Thus far, the role of IGRAs during follow-up is not clear, and it has been suggested that repeated IGRAs are not useful at all (12, 17, 36). Reports on the kinetics of IGRAs are limited in number, and the results were, in part, contradictory, which may be related to differences in the study populations, the assays used, and the endemicity of TB and the risk of reinfection. A list of studies that reported the results of repeat testing by IGRAs in a setting of active or latent TB infection is provided as supplemental material to this article. In patients treated for active TB, the quantitative results of follow-up IGRAs decreased, as a rule, but the rates of reversion to a negative result varied from 10% to 71% (20, 24, 35-37). Follow-up IGRAs for subjects with latent M. tuberculosis infection showed more variable results, but several studies reported an inverse association between the baseline IGRA response and the chance of reversion, which is in accordance with the results of the present study (9, 12, 15, 17, 24, 29, 30, 40). In our study, an initial negative QFT-Git result remained negative for 163/165 subjects. Half of the individuals with initially positive results reverted to negative, which was limited to baseline positive values comprising a moderate increase (<1.2 IU/ml IFN-γ in the present study) and which mainly occurred in subjects in the after deployment/other risk factors group. This pattern has been observed previously (12, 29-31), indicating that the result of a follow-up IGRA is unlikely to revert to negative when a positive result exceeds a certain value, even though a quantitative decrease even of high values was frequently observed during follow-up, but that subjects with low-positive results have a considerable chance of reverting to having a negative result if the exposure was recent. The participants who were screened after deployment and who had a positive QFT-Git result were probably recently infected, while for recruits with positive QFT-Git results, the interval between infection and testing was unknown but was presumably longer than that for individuals screened after deployment. The available data indicate that a positive result remains positive for longer periods for roughly 40% of subjects with LTBIs (5, 23, 24), which is in agreement with the findings of our study for recruits.
The reason why QFT-Git results revert to negative over time in over half of the subjects may be caused by the natural kinetics of the immune response, with a change from large numbers of circulating effector cells to low numbers of memory cells that cannot respond with IFN-γ production during the 24 h of incubation used for the commercial IGRA (23). In addition, there may be an effect of preventive treatment, but this has thus far not been demonstrated unequivocally. The present study included a limited number of positive results, and almost all individuals were treated, which did not allow a robust analysis of the effect of treatment. In the past, a documented positive TST result in an individual who thereafter needed to be screened repeatedly for LTBI implicated that future screening was possible only by chest radiography, which is sensitive for the detection of only active pulmonary TB and not LTBI. A documented negative QFT-Git result at the time of screening or reversion of a positive QFT-Git result to negative after 6 months strongly suggests that QFT-Git might be used to detect a later reinfection, although some variation around the threshold value was observed, and it cannot be concluded definitively that a negative result will remain negative thereafter. However, follow-up testing is probably not useful if the initial QFT-Git result exceeds a certain threshold value.
In conclusion, this study confirms that the majority of TST conversions after deployment are probably not caused by actual infection with M. tuberculosis but could be related to exposure to NTM. This supports a change in policy in which only subjects with TST conversion in association with a positive IGRA result should be treated, on the condition that they have adequate follow-up. This change in policy is under investigation. In the presence of a positive IGRA result, a follow-up IGRA was not useful when the initial test result was in the higher range, while initially low or moderately positive test results frequently reverted to negative with potential use of eventual later screening and detection of reinfection.
Supplementary Material
Acknowledgments
No funding was obtained for this study.
Footnotes
Published ahead of print on 7 April 2010.
Supplemental material for this article may be found at http://cvi.asm.org/.
REFERENCES
- 1.Aichelburg, M. C., A. Rieger, F. Breitenecker, K. Pfistershammer, J. Tittes, S. Eltz, A. C. Aichelburg, G. Stingl, A. Makristathis, and N. Kohrgruber. 2009. Detection and prediction of active tuberculosis disease by a whole-blood interferon-gamma release assay in HIV-1-infected individuals. Clin. Infect. Dis. 48:954-962. [DOI] [PubMed] [Google Scholar]
- 2.Andersen, P., M. E. Munk, J. M. Pollock, and T. M. Doherty. 2000. Specific immune-based diagnosis of tuberculosis. Lancet 356:1099-1104. [DOI] [PubMed] [Google Scholar]
- 3.Arend, S. M., P. Andersen, K. E. Van Meijgaarden, R. L. V. Skjøt, Y. W. Subronto, J. T. van Dissel, and T. H. M. Ottenhoff. 2000. Detection of active tuberculosis infection by T cell responses to early-secreted antigenic target 6-kDa protein and culture filtrate protein 10. J. Infect. Dis. 181:1850-1854. [DOI] [PubMed] [Google Scholar]
- 4.Arend, S. M., A. Geluk, K. E. Van Meijgaarden, J. T. van Dissel, M. Theisen, P. Andersen, and T. H. M. Ottenhoff. 2000. Antigenic equivalence of human T-cell responses to Mycobacterium tuberculosis-specific RD1-encoded protein antigens ESAT-6 and culture filtrate protein 10, and those to mixtures of synthetic peptides. Infect. Immun. 68:3314-3321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Arend, S. M., S. F. Thijsen, E. M. Leyten, J. J. Bouwman, W. P. Franken, B. F. Koster, F. G. Cobelens, A. J. van Houte, and A. W. Bossink. 2007. Comparison of two interferon-γ assays and tuberculin skin test for tracing tuberculosis contacts. Am. J. Respir. Crit. Care Med. 175:618-627. [DOI] [PubMed] [Google Scholar]
- 6.Bakir, M., D. P. Dosanjh, J. J. Deeks, A. Soysal, K. A. Millington, S. Efe, Y. Aslan, D. Polat, N. Kodalli, A. Yagci, I. Barlan, N. Bahceciler, E. E. Demiralp, and A. Lalvani. 2009. Use of T cell-based diagnosis of tuberculosis infection to optimize interpretation of tuberculin skin testing for child tuberculosis contacts. Clin. Infect. Dis. 48:302-312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Barnes, P. F. 2006. Weighing gold or counting spots: which is more sensitive to diagnose latent tuberculosis infection? Am. J. Respir. Crit. Care Med. 174:731-732. [DOI] [PubMed] [Google Scholar]
- 8.Bruins, J., J. H. Gribnau, and R. Bwire. 1995. Investigation into typical and atypical tuberculin sensitivity in the Royal Netherlands Army, resulting in a more rational indication for isoniazid prophylaxis. Tuber. Lung Dis. 76:540-544. [DOI] [PubMed] [Google Scholar]
- 9.Chee, C. B., K. W. Khinmar, S. H. Gan, T. M. Barkham, M. Pushparani, and Y. T. Wang. 2006. Latent tuberculosis infection treatment and T-cell responses to M. tuberculosis-specific antigens. Am. J. Respir. Crit. Care Med. 175:282-287. [DOI] [PubMed] [Google Scholar]
- 10.Choi, C. M., S. S. Hwang, C. H. Lee, H. W. Lee, C. I. Kang, C. H. Kim, S. K. Han, Y. S. Shim, and J. J. Yim. 2007. Latent tuberculosis infection in a military setting diagnosed by whole-blood interferon-gamma assay. Respirology 12:898-901. [DOI] [PubMed] [Google Scholar]
- 11.Diel, R., R. Loddenkemper, K. Meywald-Walter, S. Niemann, and A. Nienhaus. 2008. Predictive value of a whole blood IFN-gamma assay for the development of active tuberculosis disease after recent infection with Mycobacterium tuberculosis. Am. J. Respir. Crit. Care Med. 177:1164-1170. [DOI] [PubMed] [Google Scholar]
- 12.Franken, W. P., S. M. Arend, S. F. Thijsen, J. J. Bouwman, B. F. Koster, J. T. van Dissel, and A. W. Bossink. 2008. Interferon-gamma release assays during follow-up of tuberculin skin test-positive contacts. Int. J. Tuberc. Lung Dis. 12:1286-1294. [PubMed] [Google Scholar]
- 13.Franken, W. P., J. F. Timmermans, C. Prins, E. J. Slootman, J. Dreverman, H. Bruins, J. T. van Dissel, and S. M. Arend. 2007. Comparison of Mantoux and QuantiFERON TB Gold tests for diagnosis of latent tuberculosis infection in Army personnel. Clin. Vaccine Immunol. 14:477-480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Furst, D. E. 2009. The risk of infections with biologic therapies for rheumatoid arthritis. Semin. Arthritis Rheum. 39:327-346. [DOI] [PubMed] [Google Scholar]
- 15.Goletti, D., M. P. Parracino, O. Butera, F. Bizzoni, R. Casetti, D. Dainotto, G. Anzidei, C. Nisii, G. Ippolito, F. Poccia, and E. Girardi. 2007. Isoniazid prophylaxis differently modulates T-cell responses to RD1-epitopes in contacts recently exposed to Mycobacterium tuberculosis: a pilot study. Respir. Res. 8:5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Higuchi, K., S. Kondo, M. Wada, S. Hayashi, G. Ootsuka, N. Sakamoto, and N. Harada. 2009. Contact investigation in a primary school using a whole blood interferon-gamma assay. J. Infect. 58:352-357. [DOI] [PubMed] [Google Scholar]
- 17.Hill, P. C., R. H. Brookes, A. Fox, D. Jackson-Sillah, D. J. Jeffries, M. D. Lugos, S. A. Donkor, I. M. Adetifa, B. C. de Jong, A. M. Aiken, R. A. Adegbola, and K. P. McAdam. 2007. Longitudinal assessment of an ELISPOT test for Mycobacterium tuberculosis infection. PLoS Med. 4:e192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.IGRA-Werkgroep Commissie voor Praktische Tuberculosebestrijding. 2007. Plaatsbepaling van de Interferon Gamma Release Assays bij de diagnostiek van tuberculose. www.kncvtbc.nl. Voor professionals → Regelgeving → 25.000 → 25.101 Plaatsbepaling IGRA bij de Diagnostiek van Tuberculose. Accessed 29 March 2010.
- 19.Kipfer, B., M. Reichmuth, M. Buchler, C. Meisels, and T. Bodmer. 2008. Tuberculosis in a Swiss army training camp: contact investigation using an interferon gamma release assay. Swiss Med. Wkly. 138:267-272. [DOI] [PubMed] [Google Scholar]
- 20.Kobashi, Y., K. Mouri, S. Yagi, Y. Obase, N. Miyashita, and M. Oka. 2009. Transitional changes in T-cell responses to Mycobacterium tuberculosis-specific antigens during treatment. J. Infect. 58:197-204. [DOI] [PubMed] [Google Scholar]
- 21.Lalvani, A., A. A. Pathan, H. Durkan, K. A. Wilkinson, A. Whelan, J. J. Deeks, W. H. Reece, M. Latif, G. Pasvol, and A. V. Hill. 2001. Enhanced contact tracing and spatial tracking of Mycobacterium tuberculosis infection by enumeration of antigen-specific T cells. Lancet 357:2017-2021. [DOI] [PubMed] [Google Scholar]
- 22.Lee, J. Y., H. J. Choi, I. N. Park, S. B. Hong, Y. M. Oh, C. M. Lim, S. D. Lee, Y. Koh, W. S. Kim, D. S. Kim, W. D. Kim, and T. S. Shim. 2006. Comparison of two commercial interferon gamma assays for diagnosing Mycobacterium tuberculosis infection. Eur. Respir. J. 28:24-30. [DOI] [PubMed] [Google Scholar]
- 23.Leyten, E. M., S. M. Arend, C. Prins, F. G. Cobelens, T. H. Ottenhoff, and J. T. van Dissel. 2007. Discrepancy between Mycobacterium tuberculosis-specific gamma interferon release assays using short versus prolonged in vitro incubation. Clin. Vaccine Immunol. 14:880-885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Leyten, E. M., C. Prins, A. W. Bossink, S. Thijsen, T. H. Ottenhoff, J. T. van Dissel, and S. M. Arend. 2007. Effect of tuberculin skin testing on a Mycobacterium tuberculosis-specific interferon-γ assay. Eur. Respir. J. 29:1212-1216. [DOI] [PubMed] [Google Scholar]
- 25.Mancuso, J. D., S. K. Tobler, and L. W. Keep. 2008. Pseudoepidemics of tuberculin skin test conversions in the U.S. Army after recent deployments. Am. J. Respir. Crit. Care Med. 177:1285-1289. [DOI] [PubMed] [Google Scholar]
- 26.Mazurek, G. H., J. Jereb, P. Lobue, M. F. Iademarco, B. Metchock, and A. Vernon. 2005. Guidelines for using the QuantiFERON-TB Gold test for detecting Mycobacterium tuberculosis infection, United States. MMWR Recommend. Rep. 54(RR-15):49-55. [PubMed] [Google Scholar]
- 27.Mazurek, G. H., M. J. Zajdowicz, A. L. Hankinson, D. J. Costigan, S. R. Toney, J. S. Rothel, L. J. Daniels, F. B. Pascual, N. Shang, L. W. Keep, and P. A. LoBue. 2007. Detection of Mycobacterium tuberculosis infection in United States Navy recruits using the tuberculin skin test or whole-blood interferon-gamma release assays. Clin. Infect. Dis. 45:826-836. [DOI] [PubMed] [Google Scholar]
- 28.National Collaborating Centre for Chronic Conditions. 2010. Tuberculosis: clinical diagnosis and management of tuberculosis, and measures for its prevention and control. Royal College of Physicians, London, United Kingdom. http://www.nice.org.uk/page.aspx?o=CG033. Accessed 29 March 2010.
- 29.Pai, M., R. Joshi, S. Dogra, D. K. Mendiratta, P. Narang, K. Dheda, and S. Kalantri. 2006. Persistently elevated T cell interferon-gamma responses after treatment for latent tuberculosis infection among health care workers in India: a preliminary report. J. Occup. Med. Toxicol. 1:7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pai, M., R. Joshi, S. Dogra, D. K. Mendiratta, P. Narang, S. Kalantri, A. L. Reingold, J. M. Colford, Jr., L. W. Riley, and D. Menzies. 2006. Serial testing of health care workers for tuberculosis using interferon-γ assay. Am. J. Respir. Crit. Care Med. 174:349-355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pai, M., R. Joshi, S. Dogra, A. A. Zwerling, D. Gajalakshmi, K. Goswami, M. V. Reddy, A. Kalantri, P. C. Hill, D. Menzies, and P. C. Hopewell. 2009. T-cell assay conversions and reversions among household contacts of tuberculosis patients in rural India. Int. J. Tuberc. Lung Dis. 13:84-92. [PMC free article] [PubMed] [Google Scholar]
- 32.Pai, M., S. Kalantri, and K. Dheda. 2006. New tools and emerging technologies for the diagnosis of tuberculosis. Part I. Latent tuberculosis. Expert Rev. Mol. Diagn. 6:413-422. [DOI] [PubMed] [Google Scholar]
- 33.Pai, M., and R. O'Brien. 2007. Serial testing for tuberculosis: can we make sense of T cell assay conversions and reversions? PLoS Med. 4:e208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pai, M., A. Zwerling, and D. Menzies. 2008. Systematic review: T-cell-based assays for the diagnosis of latent tuberculosis infection: an update. Ann. Intern. Med. 149:177-184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pathan, A. A., K. A. Wilkinson, P. Klenerman, H. McShane, R. N. Davidson, G. Pasvol, A. V. Hill, and A. Lalvani. 2001. Direct ex vivo analysis of antigen-specific IFN-gamma-secreting CD4 T cells in Mycobacterium tuberculosis-infected individuals: associations with clinical disease state and effect of treatment. J. Immunol. 167:5217-5225. [DOI] [PubMed] [Google Scholar]
- 36.Ribeiro, S., K. Dooley, J. Hackman, C. Loredo, A. Efron, R. E. Chaisson, M. B. Conde, N. Boechat, and S. E. Dorman. 2009. T-SPOT.TB responses during treatment of pulmonary tuberculosis. BMC Infect. Dis. 9:23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sauzullo, I., F. Mengoni, M. Lichtner, A. P. Massetti, R. Rossi, M. Iannetta, R. Marocco, C. Del Borgo, F. Soscia, V. Vullo, and C. M. Mastroianni. 2009. In vivo and in vitro effects of antituberculosis treatment on mycobacterial Interferon-gamma T cell response. PLoS One 4:e5187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Smieja, M. J., C. A. Marchetti, D. J. Cook, and F. M. Smaill. 2000. Isoniazid for preventing tuberculosis in non-HIV infected persons. Cochrane Database Syst. Rev. 2:CD001363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wang, L., M. O. Turner, R. K. Elwood, M. Schulzer, and J. M. FitzGerald. 2002. A meta-analysis of the effect of bacille Calmette Guérin vaccination on tuberculin skin test measurements. Thorax 57:804-809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wilkinson, K. A., O. M. Kon, S. M. Newton, G. Meintjes, R. N. Davidson, G. Pasvol, and R. J. Wilkinson. 2006. Effect of treatment of latent tuberculosis infection on the T cell response to Mycobacterium tuberculosis antigens. J. Infect. Dis. 193:354-359. [DOI] [PubMed] [Google Scholar]
- 41.Wu, X., Q. Li, Y. Yang, C. Zhang, J. Li, J. Zhang, Y. Liang, H. Cheng, J. Zhang, L. Zhu, G. Zhang, and L. Wang. 2009. Latent tuberculosis infection amongst new recruits to the Chinese army: comparison of ELISPOT assay and tuberculin skin test. Clin. Chim. Acta 405:110-113. [DOI] [PubMed] [Google Scholar]
- 42.Wu-Hsieh, B. A., C. K. Chen, J. H. Chang, S. Y. Lai, C. H. Wu, W. C. Cheng, P. Andersen, and T. M. Doherty. 2001. Long-lived immune response to early secretory antigenic target 6 in individuals who had recovered from tuberculosis. Clin. Infect. Dis. 33:1336-1340. [DOI] [PubMed] [Google Scholar]
- 43.Zellweger, J. P., A. Zellweger, S. Ansermet, B. de Senarclens, and P. Wrighton-Smith. 2005. Contact tracing using a new T-cell-based test: better correlation with tuberculosis exposure than the tuberculin skin test. Int. J. Tuberc. Lung Dis. 9:1242-1247. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.