Abstract
T cells are strongly affected by immune aging, a phenomenon that leads to increased susceptibility to infections and decreased vaccination efficacy in elderly individuals. Cytomegalovirus (CMV) infection induces vigorous T-cell immune responses in humans and is thought to be a driving force of immune aging. In the present study we analyzed CMV-induced quantitative and qualitative differences in the cytokine-expressing T-cell repertoire from individuals of different age groups after in vitro stimulation. The CMV pp65 peptide pool and the superantigen Staphylococcus enterotoxin B (SEB) induced higher proportions of CD8+ effector T cells expressing gamma interferon (IFN-γ), tumor necrosis factor alpha (TNF-α), and granulocyte-macrophage colony-stimulating factor in the oldest study group, while only SEB induced increased responses in the middle-aged study group. Notably, CMV-specific multiple cytokine expression patterns revealed higher proportions of IFN-γ- and TNF-α-coexpressing CD8+ T cells exclusively in the oldest study group. These qualitative differences were absent in SEB-induced CD8+ effector T cells, although quantitative differences were detected. We report age-dependent qualitative changes in CMV-specific CD8+ T-cell cytokine patterns which are biocandidate markers of immune exhaustion in elderly individuals.
The term immune aging describes a conglomerate of features that affect immune functions and phenotype at late stages of human life (reviewed in reference 24). Clinically, immune aging is associated with higher susceptibility to diseases caused by “new” respiratory infections with bacteria (e.g., pneumococci) and viruses (e.g., influenza virus) (6, 7). The phenotype of immune aging includes several features and affects both innate and adaptive immunity. In particular, marked changes in T-cell immunity have been well documented (18, 20). These differences include changes on the single-cell level (e.g., T-cell receptor [TCR]- and coreceptor-dependent signal transduction [reviewed in reference 21]), as well as changes on the T-cell population level, which show (i) a decline in naïve T-cell numbers, (ii) a reduction in TCR repertoire diversity, and (iii) increases in memory and effector T-cell proportions (reviewed in reference 24). Longitudinal studies in human populations indicate that a subset of immune aging features can be used as biomarkers of mortality (25, 33). These studies identified cytomegalovirus (CMV) as a biomarker in the so-called immune risk profile (IRP).
Human CMV infection is common in all parts of the world, with infection rates between 60% in developed countries and up to 100% in developing countries (11). The CMV infection rate steadily increases during an individual's life time, with the highest prevalence in the very old (9). CMV infection is usually asymptomatic and leads to life-long persistence of the pathogen. Reactivation and development of clinical symptoms are rare and largely restricted to immunocompromised and immunosuppressed patients (10, 29). CMV infection rapidly induces immunodominant T-cell responses, mainly against two proteins, the immediate-early protein 1 (IE-1) and the matrix 65-kDa phosphoprotein (pp65) (14, 19, 34). This T-cell response is like a double-edged sword, because studies in humans and animal models suggest a protective role against CMV reactivation on the one hand (1, 12, 31) and, on the other hand, the T-cell response leads to vigorous and persistent clonal expansion of CD8+ T cells (reviewed in reference 22). These T-cell clones reach high numbers and thus dominate the peripheral blood T-cell repertoire (8, 28). In contrast to other chronic infection- or vaccine-induced T-cell responses, CMV-specific T-cell numbers are stable or even increase over time and lead to extremely high proportions of clonally expanded T cells in very old individuals (26). CMV-specific CD8+ and CD4+ T cells show some phenotypic peculiarities and continuously become dysfunctional or exhausted (5, 26). It has been suggested that CMV-induced changes in T-cell repertoire and function, although crucial for protection against CMV reactivation, challenge the maintenance of a balanced T-cell repertoire and in this way compromise host immunity against infections and efficacies of new vaccines in elderly individuals (reviewed in reference 24). Furthermore, it has been hypothesized that CMV infection itself is the driving force of immune aging (reviewed in reference 16).
Quantitative assessment of T-cell responses (e.g., gamma interferon [IFN-γ] and tumor necrosis factor alpha [TNF-α] expression) is a common tool used to predict disease susceptibility and vaccination efficacy, but for some diseases (e.g., tuberculosis) these parameters are not sufficient for use as biomarkers of protection (13). Recent studies have strengthened the crucial role of qualitative differences in the T-cell response (i.e., T-cell-expressed cytokine patterns) for the prediction of disease susceptibility (3, 17, 36). Our own previous studies suggest a role of granulocyte-macrophage colony-stimulating factor (GM-CSF) as a T-cell biomarker in childhood tuberculosis (23).
Here we analyzed proportions of cytokine-expressing T-cell subpopulations (i.e., for IFN-γ, TNF-α, GM-CSF, and interleukin-2 [IL-2]) and determined the patterns of cytokine coexpression after in vitro stimulation with CMV pp65 and the superantigen staphylococcus enterotoxin B (SEB) in individuals from different age groups. In this way we characterized concomitantly age-dependent, CMV-specific and nonspecific, quantitative as well as qualitative differences in the T-cell response.
MATERIALS AND METHODS
CMV-infected healthy donors.
Peripheral blood was obtained from 59 CMV serum IgG-positive healthy donors and 20 CMV serum IgG-negative donors recruited at the senior citizens residences of St. Pauli am Elbpark, the Wohnstift Augustinum, the University Hospital Hamburg-Eppendorf, and the Bernhard-Nocht-Institute for Tropical Medicine. Individuals were divided into three groups according to their age. Age and gender distributions of each study group of the CMV IgG-positive donors are summarized in Table 1. CMV-specific serum IgG levels were determined for a subgroup of 22 donors by the Department of Transfusion Medicine, University Hospital Hamburg-Eppendorf. All donors gave informed consent. The study was approved by the local ethics committee (Aerztekammer, Hamburg [WF-024/08]).
TABLE 1.
Donor characteristics
| Donor group | n | Age range (yrs) | Mean ± SD age (yrs) | No. of women/no. of men |
|---|---|---|---|---|
| Young | 19 | 25-50 | 40.3 ± 8.2 | 11/8 |
| Middle-aged | 21 | 52-64 | 57.4 ± 3.7 | 3/18 |
| Old | 19 | 66-90 | 72.0 ± 3.7 | 8/11 |
Isolation and stimulation of PBMC from peripheral blood.
Peripheral blood monocytes (PBMC) were isolated by density centrifugation (Biocoll; Gibco) following the manufacturer's instructions. PBMC (2 × 105 cells/well) were then stimulated with 1 μg/ml CMV pp65 overlapping peptide pool (Miltenyi Biotech) and SEB (1 μg/ml; Sigma), or incubated without stimulus, for 20 h in 200 μl of medium A containing 1% penicillin-streptomycin, 1% l-glutamine (both from Sigma), 5% human serum, and 1% HEPES buffer solution (0.01 M) and 92% RPMI (both from PAA Laboratories). During the final 15 h, brefeldin A was added to avoid release of cytokines from the Golgi apparatus.
T-cell phenotyping and intracellular cytokine staining by flow cytometry.
Cells were then fixed and permeabilized using cytofix/cytoperm and washed with cytoperm/wash (both from BD Biosciences) following the manufacturer's instructions before monoclonal antibody (MAb) mixtures were added. These MAb mixtures contained anti-IFN-γ MAb (allophycocyanin [APC] labeled), anti-IL-2 MAb (fluorescein isothiocyanate labeled), anti-GM-CSF MAb (phycoerythrin [PE] labeled), anti-TNF-α MAb (Alexa 700 labeled), anti-CD4 MAb (APC-Cy7 labeled), anti-CD8 MAb (peridinin chlorophyll protein-Cy5.5 labeled), anti-CD45RA MAb (Pacific Blue labeled), and anti-CCR7 MAb (PE-Cy7 labeled) (all from BD Biosciences). An LSRII flow cytometer (BD) was used for measurements.
Data analyses and statistics.
We used the FCS express software (De Novo) for flow cytometry data analyses. T-cell subpopulation analyses were based on two surface antigens, CD45RA and CCR7, which allow discrimination between naïve T cells (CD45RA+ CCR7+) and “nonnaïve T cells” (i.e., central memory [CD45RA− CCR7+], effector memory [CD45RA− CCR7−], and effector [CD45RA+ CCR7−] T cells) combined with CD4+ and CD8+ T-cell markers (for gating procedures see Fig. S1 in the supplemental material). Absolute numbers as well as proportions of naïve and nonnaïve CD4+ and CD8+ cells were determined and analyzed, but no standard (e.g., a fixed number of beads) for the measured sample volume was included. Single cytokine expression levels were determined as proportions of CD4+ and CD8+ T-cell subpopulations (i.e., CD45RA− CCR7+ [central memory T cells] or CCR7− [effector memory/effector T cells]) (see Fig. S1). For qualitative analyses of cytokine expression patterns we determined absolute numbers of cytokine-expressing subsets (i.e., quadruple positives, IFN-γ+/TNF-α+/IL-2+/GM-CSF+; triple positives, IFN-γ−/TNF-α+/IL-2+/GM-CSF+, IFN-γ+/TNF-α−/IL-2+/GM-CSF+, IFN-γ+/TNF-α+/IL-2−/GM-CSF+, and IFN-γ+/TNF-α+/IL-2+/GM-CSF−; double positives, IFN-γ+/TNF-α+/IL-2−/GM-CSF−, IFN-γ−/TNF-α+/IL-2+/GM-CSF−, IFN-γ−/TNF-α−/IL-2+/GM-CSF+, IFN-γ+/TNF-α−/IL-2+/GM-CSF−, IFN-γ+/TNF-α−/IL-2−/GM-CSF+, and IFN-γ−/TNF-α+/IL-2−/GM-CSF+; single positives, IFN-γ+/TNF-α−/IL-2−/GM-CSF−, IFN-γ−/TNF-α+/IL-2−/GM-CSF−, IFN-γ−/TNF-α−/IL-2+/GM-CSF−, and IFN-γ−/TNF-α−/IL-2−/GM-CSF+) and calculated the percentage of each subset relative to the sum of cytokine-expressing T cells. These responding cells were defined as T cells that produced a minimum of one cytokine. CMV-specific cytokine expression patterns were analyzed in subgroups of donors from the study groups with a minimal number of 20 detected CMV-specific T cells per sample. Individual samples with relatively low numbers of CMV-specific T cells were analyzed twice to avoid bias due to false-positive results. These analyses showed high reproducibility of results (data not shown). The Mann-Whitney U-test or Student's t test was used to determine significant differences of cytokine-expressing T-cell proportions between study groups. The appropriate test was selected on the basis of Kolmogorov-Smirnov normality testing (SigmaPlot; Systat Software).
RESULTS
Age-dependent differences in T-cell subpopulation proportions.
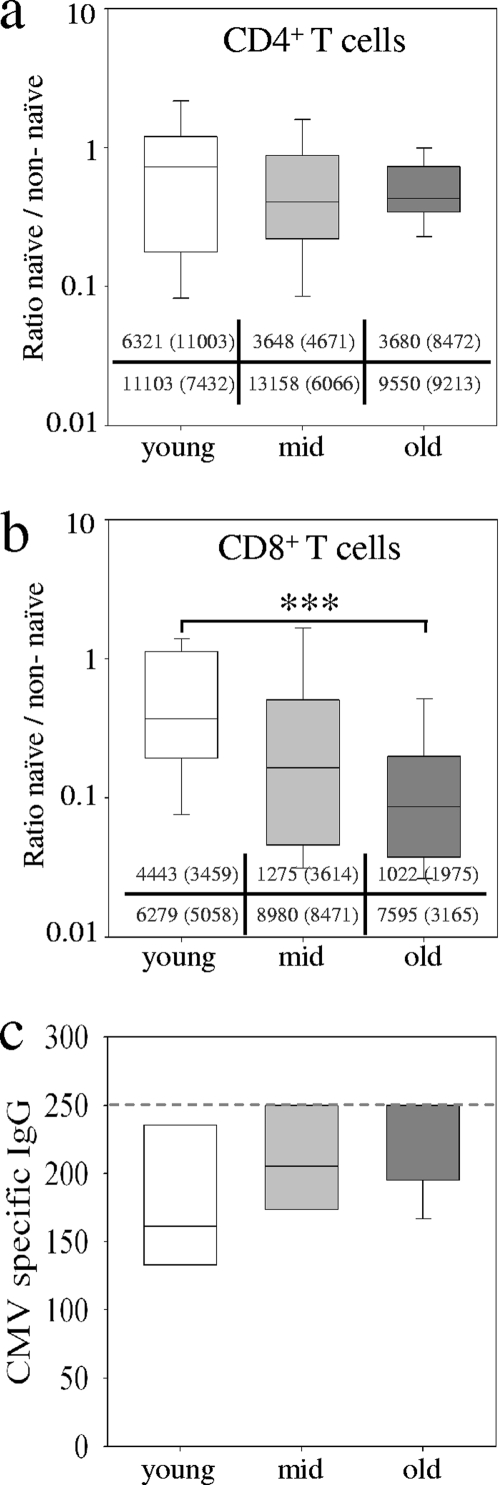
The overall phenotype and repertoire of T cells change with aging and lead to increased proportions of memory and effector T cells in elderly individuals. First we determined the proportions of naïve T cells and nonnaïve (memory or effector) T cells (for gating procedures, see Fig. S1 in the supplemental material) in three study groups of individuals between 25 and 50 years of age (for reasons of simplicity, this group was termed “young” in this paper), individuals between 52 and 64 years old (termed “middle-aged”), and individuals between 66 and 90 years old (termed “old”). Ratios of proportions (percent naïve/percent nonnaïve) for individual donors indicated a slight but not significant decrease of naïve T-cell proportions for CD4+ T cells in the middle-aged and old study group (Fig. 1a). For CD8+ T cells we detected ratio differences between young and old individuals (P = 0.001) and intermediate ratios for the middle-aged study group (Fig. 1b). This indicated a decrease in the proportion of naïve CD8+ T cells with age. We did not detect gender-specific differences, and the ratio of CD4+ and CD8+ T cells was similar between the study groups (data not shown). CMV-specific serum IgG levels from subgroups of donors were compared between the study groups, and slightly higher values were detected in the middle-aged and old study groups compared to the young study group (Fig. 1c).
FIG. 1.
T-cell population proportions and CMV-specific serum IgG levels from different age groups. The ratios of naïve (CD45RA+ CCR7+) versus nonnaïve (CD45RA− CCR7+, CD45RA− CCR7−, and CD45RA− CCR7−) cell proportions for CD4+ (a) and CD8+ (b) T cells are shown. Median values and standard deviations (in parentheses) of absolute numbers are given for naïve T cells (upper row) and nonnaïve T cells (lower row) for each study group. (c) CMV-specific serum IgG levels are shown. The dashed line indicates the upper limit of the serum test. For this analysis 20 donors (5 young, 8 middle-aged, and 9 old) were included. Box plots and error bars represent the 5, 25, 50, 75, and 95 percentiles. Different colors indicate the young study group (white), the middle-aged study group (light gray), and the old study group (dark gray). Nominal two-sided P values for the Mann-Whitney U-test are indicated (***, P < 0.001).
CD8+ effector T cells show age-dependent quantitative differences of SEB- and CMV-induced cytokine expression.
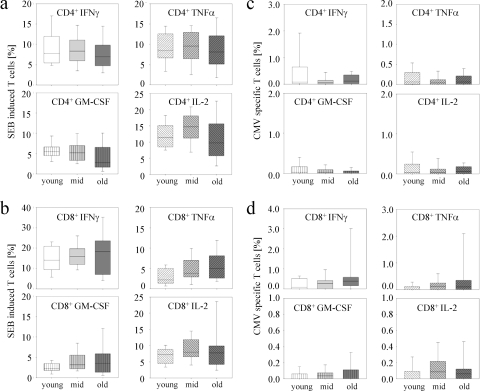
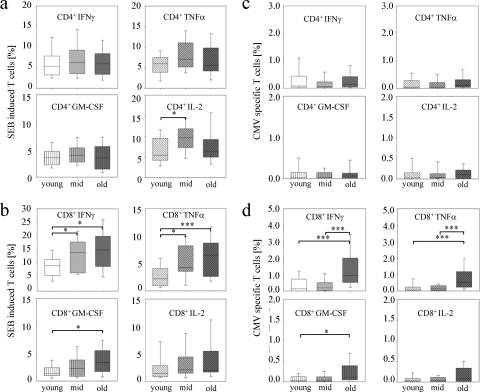
We investigated the T-cell cytokine responses after short-term in vitro stimulation of PBMC from each donor from the three age groups. CMV pp65 peptide pool or SEB, a superantigen that induces activation of a T-cell subset via TCR-major histocompatibility complex antigen cross-linking, were used, and expression levels of IFN-γ, TNF-α, IL-2, and GM-CSF were determined concomitantly. To avoid a possible bias due to differences in T-cell subpopulation proportions, we compared cytokines expressed in effector/effector memory T cells (CCR7− CD45RA+ or CCR7− CD45RA−; termed effector T cells hereafter in this paper) and central memory T cells (CCR7+ CD45RA−) for CD4+ as well as CD8+ T cells. CD4+ (Fig. 2 a and c) and CD8+ (Fig. 2b and d) central memory T cells from the different study groups showed only minor differences after stimulation with SEB (Fig. 2a and b) or CMV pp65 (Fig. 2c and d) for all cytokines measured. None of the CMV IgG-negative donors had detectable cytokine-expressing T cells after pp65 in vitro restimulation (data not shown). Proportions of CD4+ effector T cells that expressed IFN-γ, TNF-α, and GM-CSF were similar between the study groups after SEB or CMV stimulation (Fig. 3 a and c). Only the SEB-induced IL-2-expressing CD4+ effector T-cell proportion was slightly higher in the middle-aged and old study groups compared to the young study group (P = 0.048) (Fig. 3a).
FIG. 2.
SEB-induced and CMV-specific single-cytokine expression levels in CD4+ and CD8+ central memory T cells from different age groups. Box plots indicate SEB-induced (a and b) and CMV peptide pool-induced (c and d) proportions of IFN-γ (upper left graphs), TNF-α (upper right graphs), GM-CSF (lower left graphs), and IL-2 (lower right graphs) for CD4+ central memory T cells (a and c) as well as for CD8+ central memory T cells (b and d). Box plots and error bars represent the 5, 25, 50, 75, and 95 percentiles. Different hatchings mark each cytokine. Different colors indicate the young study group (white), the middle-aged study group (light gray), and the old study group (dark gray).
FIG. 3.
CD4+ and CD8+ effector T-cell single-cytokine expression levels after SEB-induced and CMV-specific in vitro stimulation for different age groups. Box plots indicate SEB-induced (a and b) and CMV peptide pool-induced (c and d) proportions of IFN-γ (upper left graphs), TNF-α (upper right graphs), GM-CSF (lower left graphs), and IL-2 (lower right graphs) for CD4+ effector T cells (a and c) as well as for CD8+ effector T cells (b and d). Box plots and error bars represent the 5, 25, 50, 75, and 95 percentiles. Different hatchings mark each cytokine. Different colors indicate the young study group (white), the middle-aged study group (light gray), and the old study group (dark gray). Nominal two-sided P values for the Mann-Whitney U-test are indicated (*, P < 0.05; ***, P < 0.001).
In contrast, we detected marked differences for CMV- or SEB-induced CD8+ effector T cells. SEB induced higher proportions of IFN-γ- and TNF-α-expressing CD8+ effector T cells in the middle-aged study group (IFN-γ, P = 0.02; TNF-α, P = 0.01) and in the old study group (IFN-γ, P = 0.02; TNF-α, P < 0.001) compared to the young study group (Fig. 3b). These results are in accordance with previous studies that detected increased levels of type I T-cell cytokines in elderly people (2, 4, 35). In addition, SEB induced higher CD8+ effector T-cell proportions expressing GM-CSF in the old study group (P = 0.01, compared to the young study group), whereas IL-2 expression was similar between the study groups (Fig. 3b). In contrast to SEB, CMV-specific changes were exclusively detected in the old study group, which had markedly higher proportions of IFN-γ+ and TNF-α+ CD8+ effector T cells than the young study group (IFN-γ, P < 0.001; TNF-α, P < 0.001) and the middle-aged study group (IFN-γ, P < 0.001; TNF-α, P < 0.001) (Fig. 3d). Proportions of GM-CSF- and IL-2-expressing T cells were also slightly higher in the old study group and reached significant values for GM-CSF (P = 0.02) in comparison to the young study group. Therefore, CMV-specific cytokine expression changes with age differed from SEB-induced age-dependent cytokine expression.
CMV-specific qualitative differences in cytokine expression patterns of CD8+ effector T cells.
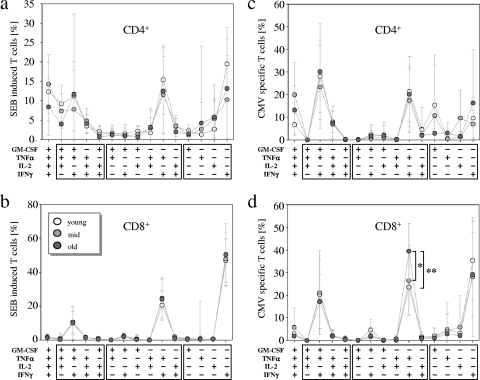
Next we analyzed the cytokine expression patterns in CD4+ and CD8+ effector T cells after stimulation with CMV peptide pools and SEB. CD4+ effector T cells mainly expressed all four cytokines, with triple-positive cells (i.e., IFN-γ+/TNF-α+/GM-CSF+ and IL-2+/TNF-α+/GM-CSF+) and double-positive cells (i.e., IFN-γ+/TNF-α+), as well as IFN-γ single-positive cells, after stimulation with SEB (Fig. 4a). CMV-specific CD4+ effector T cells were mainly triple positive (i.e., IFN-γ+/TNF-α+/GM-CSF+) or double positive (i.e., IFN-γ+/TNF-α+) (Fig. 4c). CMV- and SEB-induced CD4+ T-cell patterns were highly variable between individuals, and no significant differences between the study groups were detected (Fig. 4a and c).
FIG. 4.
Cytokine expression patterns induced by SEB or CMV in CD4+ and CD8+ effector T cells. Line and scatter plots indicate mean expression levels and standard deviations (y axes) of distinct cytokine coexpression pattern (x axes) for CD4+ effector T cells (a and c) and CD8+ effector T cells (b and d) stimulated with SEB (a and b) or CMV peptide pool (c and d). Lines connect mean values (circles) of the same study group (additionally indicated by the color: young study group [white], middle-aged study group [light gray], and old study group [dark gray]). For evaluation of the CMV-specific pattern 8 individuals from the young study group, 10 individuals from the middle-aged study group, and 9 individuals from the old study group who had more than 20 cytokine-positive effector T cells were included. Proportions of each cytokine coexpression subset were calculated relative to the number of cells expressing at least one of the analyzed cytokines: IFN-γ, TNF-α, GM-CSF, and IL-2. Boxes below the graphs enclose triple-, double-, or single-cytokine expression subsets. Nominal two-sided P values for the t test are indicated (*, P < 0.05; **, P < 0.01).
CD8+ effector T cells showed highly similar patterns after SEB stimulation, and no differences between the study groups were found (Fig. 4b). The variances between individuals were considerably smaller than for CD4+ effector T cells. SEB induced mainly CD8+ effector T cells that were single positive for IFN-γ, double positive (i.e., IFN-γ+/TNF-α+), or triple positive (i.e., IFN-γ+/TNF-α+/GM-CSF+) (Fig. 4b). Notably, CD8+ CMV-specific effector T cells had increased proportions of IFN-γ+/TNF-α+ double-positive cells exclusively in the old study group, in comparison to the middle-aged and the young study groups (P = 0.004 and 0.02, respectively) (Fig. 4d). While IL-2 was hardly expressed in CD8+ effector T cells, GM-CSF was coexpressed in double-positive (i.e., IFN-γ+/GM-CSF+) and triple-positive (i.e., IFN-γ+/TNF-α+/GM-CSF+) subsets, which were slightly increased in the young and the middle-aged study groups compared to the old study group. Therefore, CMV-specific responses indicated qualitative differences in CD8+ effector T-cell responses, including a bias toward TNF-α+/IFN-γ+ double-positive T cells in the old study group.
We conclude the following major points from this study: (i) the quantitative differences of CMV-specific T cells are accompanied by qualitative changes in the cytokine expression pattern, whereas quantitative, but not qualitative, differences are detected for SEB; (ii) nonspecific (SEB-induced) differences were detected in the middle-aged and old study groups (compared to the young group), whereas CMV-specific differences were detected solely in the old study group; (iii) quantitative and qualitative differences were exclusively detected for CD8+ T cells, whereas CD4+ T-cell cytokine expression levels varied markedly between individuals but not in an age-dependent manner. For future studies we suggest analysis of concomitant expression of IFN-γ, TNF-α, and GM-CSF to detect qualitative differences in cytokine expression profiles that cannot be deduced from a single-cytokine analysis.
DISCUSSION
Concomitant measurement of IFN-γ, TNF-α, and GM-CSF revealed age-dependent differences in CMV-specific CD8+ effector T cells. Single-cytokine analyses indicated increased proportions of IFN-γ+ and TNF-α+ as well as GM-CSF+ CD8+ effector T cells, whereas the cytokine expression patterns revealed a relative increase in IFN-γ- and TNF-α-coexpressing CD8+ effector T cells deficient for GM-CSF. These qualitative differences were CMV specific, since SEB-induced quantitative differences were not accompanied by a selective increase of IFN-γ- and TNF-α-coexpressing CD8+ effector T-cell proportions. This led to the conclusion that besides quantitative assessment of cytokines, the relative contribution of each cytokine-expressing subset should be determined. In addition, we propose to include GM-CSF as a candidate biomarker for CMV-induced immune exhaustion in elderly individuals.
The prediction of functionally intact and, hence, protective T-cell responses against infections is of great value for the development of novel vaccines and evaluation of immune aging as the cause for increased disease susceptibility in elderly individuals. Recent studies have indicated an important role for multicytokine-expressing CD4+ T cells for protection in animal models (3, 17). Here we did not detect significant differences for CD4+ T cells in either the ratios of naïve versus nonnaïve T cells or in the proportions of CMV-specific T cells between the study groups (besides an increase of IL-2-expressing CMV-specific CD4+ effector T cells, which occurred only in the middle-aged group but not in the old study group). These results suggest that the proportions of cytokine-expressing CD4+ T cells are less affected by immune aging and possible confounding effects of CMV infection. Previous studies demonstrated that CMV infection leads to an increased differentiation level of CMV-specific CD4+ T cells and decreased proliferative responses of CMV-specific CD4+ T cells in elderly individuals (5). Furthermore, and in contrast to the present study, increased proportions of CMV-specific CD4+ T cells with aging have been described (27, 30). The study by Vescovini et al. detected differences for CD4+ T cells solely in very old individuals (>85 years) but not in younger individuals (30). The study by Pourgheysari et al. used whole viral lysate from CMV cultures, which may explain the reported differential T-cell responses due to distinct immunodominant antigens expressed by CMV (1). Therefore, the influence of CMV infection on CD4+ T cells during aging may be restricted to certain antigens and likely occurs later in life than for CD8+ T cells.
For CD8+ effector T cells we identified distinguishable age-dependent effects on CMV- and SEB-induced responses. SEB induced gradually higher proportions of IFN-γ- and TNF-α-expressing CD8+ effector T cells with age, whereas CMV-specific changes were exclusively detected in the old study group. As in most studies performed, so far we have no information about the time point of individual CMV infection and therefore we cannot exclude a bias due to a more recent CMV infection in the old study group, although we have no evidence for this, and a previous study described a steady CMV infection rate in different age groups (9). Increased CMV-specific T-cell responses in old individuals have been described (15, 27, 30), and these changes likely contribute to immune aging (reviewed in reference 16). The similarities between CMV-induced changes of the CD8+ T-cell repertoire and immune aging led to the thesis that CMV infection may be the main driving force of immune aging (16). Since CMV- and SEB-induced differences of the CD8+ T-cell responses were not correlated in the present study, we hypothesize that CMV-independent processes contribute to alterations of CD8+ effector T cells during immune aging. CMV-independent processes, measured by SEB-induced cytokine expression, revealed higher proportions of IFN-γ- and TNF-α-expressing CD8+ effector T cells in the middle-aged and the old study groups and, furthermore, there were higher proportions of GM-CSF-expressing CD8+ effector T cells in the old study group. In contrast to CMV-specific responses, cytokine expression pattern analyses revealed highly similar relative contributions of different subsets. This qualitatively unchanged response distinguished CMV-independent and age-related processes from CMV-specific responses.
CMV-specific qualitative differences are characterized by the expansion of IFN-γ- and TNF-α-expressing CD8+ effector T cells lacking GM-CSF and IL-2. Decreased expression of IL-2 and a reduced proliferation capacity of T cells in elderly individuals have been described elsewhere (32), whereas the role of GM-CSF, as a T-cell factor, remains elusive. Recently, we described coexpression of GM-CSF in IFN-γ+/TNF-α+ double-positive CD4+ effector T cells from children with Mycobacterium tuberculosis infection (23). Those results suggested that GM-CSF is a marker of multifunctional CD4+ T cells (3). Future experiments will address the question of whether qualitative differences of GM-CSF expression in CD8+ effector T cells are associated with CMV reactivation, a cause for severe diseases, especially in immunocompromised and immunodeficient individuals.
Taken together, these results suggest a need for the analysis of cytokine expression patterns in addition to quantitative measurements of cytokines to improve the characterization of T-cell responses in chronic infections.
Supplementary Material
Acknowledgments
We thank Stefanie Schulz, Kerrin Heesch, and Katja Kleinsteuber for helpful comments on the manuscript. We thank Annemarie Peters-Behrmann and Frank Bentzien for their support in IgG serum level measurement and donor recruitment.
Footnotes
Published ahead of print on 28 April 2010.
Supplemental material for this article may be found at http://cvi.asm.org/.
REFERENCES
- 1.Bunde, T., A. Kirchner, B. Hoffmeister, D. Habedank, R. Hetzer, G. Cherepnev, S. Proesch, P. Reinke, H. D. Volk, H. Lehmkuhl, and F. Kern. 2005. Protection from cytomegalovirus after transplantation is correlated with immediate early 1-specific CD8 T cells. J. Exp. Med. 201:1031-1036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Candore, G., G. Di Lorenzo, M. Melluso, D. Cigna, A. T. Colucci, M. A. Modica, and C. Caruso. 1993. γ-Interferon, interleukin-4 and interleukin-6 in vitro production in old subjects. Autoimmunity 16:275-280. [DOI] [PubMed] [Google Scholar]
- 3.Darrah, P. A., D. T. Patel, P. M. De Luca, R. W. Lindsay, D. F. Davey, B. J. Flynn, S. T. Hoff, P. Andersen, S. G. Reed, S. L. Morris, M. Roederer, and R. A. Seder. 2007. Multifunctional TH1 cells define a correlate of vaccine-mediated protection against Leishmania major. Nat. Med. 13:843-850. [DOI] [PubMed] [Google Scholar]
- 4.Fagiolo, U., A. Cossarizza, E. Scala, E. Fanales-Belasio, C. Ortolani, E. Cozzi, D. Monti, C. Franceschi, and R. Paganelli. 1993. Increased cytokine production in mononuclear cells of healthy elderly people. Eur. J. Immunol. 23:2375-2378. [DOI] [PubMed] [Google Scholar]
- 5.Fletcher, J. M., M. Vukmanovic-Stejic, P. J. Dunne, K. E. Birch, J. E. Cook, S. E. Jackson, M. Salmon, M. H. Rustin, and A. N. Akbar. 2005. Cytomegalovirus-specific CD4+ T cells in healthy carriers are continuously driven to replicative exhaustion. J. Immunol. 175:8218-8225. [DOI] [PubMed] [Google Scholar]
- 6.Gavazzi, G., F. Herrmann, and K. H. Krause. 2004. Aging and infectious diseases in the developing world. Clin. Infect. Dis. 39:83-91. [DOI] [PubMed] [Google Scholar]
- 7.Gavazzi, G., and K. H. Krause. 2002. Ageing and infection. Lancet Infect. Dis. 2:659-666. [DOI] [PubMed] [Google Scholar]
- 8.Gillespie, G. M., M. R. Wills, V. Appay, C. O'Callaghan, M. Murphy, N. Smith, P. Sissons, S. Rowland-Jones, J. I. Bell, and P. A. Moss. 2000. Functional heterogeneity and high frequencies of cytomegalovirus-specific CD8(+) T lymphocytes in healthy seropositive donors. J. Virol. 74:8140-8150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hecker, M., D. Qiu, K. Marquardt, G. Bein, and H. Hackstein. 2004. Continuous cytomegalovirus seroconversion in a large group of healthy blood donors. Vox Sang. 86:41-44. [DOI] [PubMed] [Google Scholar]
- 10.Hillyer, C. D., D. R. Snydman, and E. M. Berkman. 1990. The risk of cytomegalovirus infection in solid organ and bone marrow transplant recipients: transfusion of blood products. Transfusion 30:659-666. [DOI] [PubMed] [Google Scholar]
- 11.Ho, M. 1990. Epidemiology of cytomegalovirus infections. Rev. Infect. Dis. 12(Suppl. 7):S701-S710. [DOI] [PubMed] [Google Scholar]
- 12.Holtappels, R., C. O. Simon, M. W. Munks, D. Thomas, P. Deegen, B. Kuhnapfel, T. Daubner, S. F. Emde, J. Podlech, N. K. Grzimek, S. A. Oehrlein-Karpi, A. B. Hill, and M. J. Reddehase. 2008. Subdominant CD8 T-cell epitopes account for protection against cytomegalovirus independent of immunodomination. J. Virol. 82:5781-5796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kaufmann, S. H. 2001. How can immunology contribute to the control of tuberculosis? Nat. Rev. 1:20-30. [DOI] [PubMed] [Google Scholar]
- 14.Kern, F., I. P. Surel, N. Faulhaber, C. Frommel, J. Schneider-Mergener, C. Schonemann, P. Reinke, and H. D. Volk. 1999. Target structures of the CD8(+)-T-cell response to human cytomegalovirus: the 72-kilodalton major immediate-early protein revisited. J. Virol. 73:8179-8184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Khan, N., N. Shariff, M. Cobbold, R. Bruton, J. A. Ainsworth, A. J. Sinclair, L. Nayak, and P. A. Moss. 2002. Cytomegalovirus seropositivity drives the CD8 T cell repertoire toward greater clonality in healthy elderly individuals. J. Immunol. 169:1984-1992. [DOI] [PubMed] [Google Scholar]
- 16.Koch, S., A. Larbi, D. Ozcelik, R. Solana, C. Gouttefangeas, S. Attig, A. Wikby, J. Strindhall, C. Franceschi, and G. Pawelec. 2007. Cytomegalovirus infection: a driving force in human T cell immunosenescence. Ann. N. Y. Acad. Sci. 1114:23-35. [DOI] [PubMed] [Google Scholar]
- 17.Lindenstrom, T., E. M. Agger, K. S. Korsholm, P. A. Darrah, C. Aagaard, R. A. Seder, I. Rosenkrands, and P. Andersen. 2009. Tuberculosis subunit vaccination provides long-term protective immunity characterized by multifunctional CD4 memory T cells. J. Immunol. 182:8047-8055. [DOI] [PubMed] [Google Scholar]
- 18.Linton, P. J., and K. Dorshkind. 2004. Age-related changes in lymphocyte development and function. Nat. Immunol. 5:133-139. [DOI] [PubMed] [Google Scholar]
- 19.McLaughlin-Taylor, E., H. Pande, S. J. Forman, B. Tanamachi, C. R. Li, J. A. Zaia, P. D. Greenberg, and S. R. Riddell. 1994. Identification of the major late human cytomegalovirus matrix protein pp65 as a target antigen for CD8+ virus-specific cytotoxic T lymphocytes. J. Med. Virol. 43:103-110. [DOI] [PubMed] [Google Scholar]
- 20.Miller, R. A. 1996. The aging immune system: primer and prospectus. Science 273:70-74. [DOI] [PubMed] [Google Scholar]
- 21.Miller, R. A., G. Garcia, C. J. Kirk, and J. M. Witkowski. 1997. Early activation defects in T lymphocytes from aged mice. Immunol. Rev. 160:79-90. [DOI] [PubMed] [Google Scholar]
- 22.Moss, P., and N. Khan. 2004. CD8(+) T-cell immunity to cytomegalovirus. Hum. Immunol. 65:456-464. [DOI] [PubMed] [Google Scholar]
- 23.Mueller, H., A. K. Detjen, S. D. Schuck, A. Gutschmidt, U. Wahn, K. Magdorf, S. H. Kaufmann, and M. Jacobsen. 2008. Mycobacterium tuberculosis-specific CD4+, IFNγ+, and TNFα+ multifunctional memory T cells coexpress GM-CSF. Cytokine 43:143-148. [DOI] [PubMed] [Google Scholar]
- 24.Nikolich-Zugich, J. 2008. Ageing and life-long maintenance of T-cell subsets in the face of latent persistent infections. Nat. Rev. 8:512-522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Olsson, J., A. Wikby, B. Johansson, S. Lofgren, B. O. Nilsson, and F. G. Ferguson. 2000. Age-related change in peripheral blood T-lymphocyte subpopulations and cytomegalovirus infection in the very old: the Swedish longitudinal OCTO immune study. Mech. Ageing Dev. 121:187-201. [DOI] [PubMed] [Google Scholar]
- 26.Ouyang, Q., W. M. Wagner, A. Wikby, S. Walter, G. Aubert, A. I. Dodi, P. Travers, and G. Pawelec. 2003. Large numbers of dysfunctional CD8+ T lymphocytes bearing receptors for a single dominant CMV epitope in the very old. J. Clin. Immunol. 23:247-257. [DOI] [PubMed] [Google Scholar]
- 27.Pourgheysari, B., N. Khan, D. Best, R. Bruton, L. Nayak, and P. A. Moss. 2007. The cytomegalovirus-specific CD4+ T-cell response expands with age and markedly alters the CD4+ T-cell repertoire. J. Virol. 81:7759-7765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sylwester, A. W., B. L. Mitchell, J. B. Edgar, C. Taormina, C. Pelte, F. Ruchti, P. R. Sleath, K. H. Grabstein, N. A. Hosken, F. Kern, J. A. Nelson, and L. J. Picker. 2005. Broadly targeted human cytomegalovirus-specific CD4+ and CD8+ T cells dominate the memory compartments of exposed subjects. J. Exp. Med. 202:673-685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tyms, A. S., D. L. Taylor, and J. M. Parkin. 1989. Cytomegalovirus and the acquired immunodeficiency syndrome. J. Antimicrob. Chemother. 23(Suppl. A):89-105. [DOI] [PubMed] [Google Scholar]
- 30.Vescovini, R., C. Biasini, F. F. Fagnoni, A. R. Telera, L. Zanlari, M. Pedrazzoni, L. Bucci, D. Monti, M. C. Medici, C. Chezzi, C. Franceschi, and P. Sansoni. 2007. Massive load of functional effector CD4+ and CD8+ T cells against cytomegalovirus in very old subjects. J. Immunol. 179:4283-4291. [DOI] [PubMed] [Google Scholar]
- 31.Walter, E. A., P. D. Greenberg, M. J. Gilbert, R. J. Finch, K. S. Watanabe, E. D. Thomas, and S. R. Riddell. 1995. Reconstitution of cellular immunity against cytomegalovirus in recipients of allogeneic bone marrow by transfer of T-cell clones from the donor. N. Engl. J. Med. 333:1038-1044. [DOI] [PubMed] [Google Scholar]
- 32.Whisler, R. L., L. Beiqing, and M. Chen. 1996. Age-related decreases in IL-2 production by human T cells are associated with impaired activation of nuclear transcriptional factors AP-1 and NF-AT. Cell. Immunol. 169:185-195. [DOI] [PubMed] [Google Scholar]
- 33.Wikby, A., B. Johansson, J. Olsson, S. Lofgren, B. O. Nilsson, and F. Ferguson. 2002. Expansions of peripheral blood CD8 T-lymphocyte subpopulations and an association with cytomegalovirus seropositivity in the elderly: the Swedish NONA Immune Study. Exp. Gerontol. 37:445-453. [DOI] [PubMed] [Google Scholar]
- 34.Wills, M. R., A. J. Carmichael, K. Mynard, X. Jin, M. P. Weekes, B. Plachter, and J. G. Sissons. 1996. The human cytotoxic T-lymphocyte (CTL) response to cytomegalovirus is dominated by structural protein pp65: frequency, specificity, and T-cell receptor usage of pp65-specific CTL. J. Virol. 70:7569-7579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zanni, F., R. Vescovini, C. Biasini, F. Fagnoni, L. Zanlari, A. Telera, P. Di Pede, G. Passeri, M. Pedrazzoni, M. Passeri, C. Franceschi, and P. Sansoni. 2003. Marked increase with age of type 1 cytokines within memory and effector/cytotoxic CD8+ T cells in humans: a contribution to understand the relationship between inflammation and immunosenescence. Exp. Gerontol. 38:981-987. [DOI] [PubMed] [Google Scholar]
- 36.Zhou, W., J. Longmate, S. F. Lacey, J. M. Palmer, G. Gallez-Hawkins, L. Thao, R. Spielberger, R. Nakamura, S. J. Forman, J. A. Zaia, and D. J. Diamond. 2009. Impact of donor CMV status on viral infection and reconstitution of multifunction CMV-specific T cells in CMV-positive transplant recipients. Blood 113:6465-6476. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.