Abstract
Cryptosporidium infection is commonly observed among children and immunocompromised individuals in developing countries, but large-scale outbreaks of disease among adults have not been reported. In contrast, outbreaks of cryptosporidiosis in the United States and Canada are increasingly common among patients of all ages. Thus, it seems likely that residents of regions where Cryptosporidium is highly endemic acquire some level of immunity, while residents of the developed world do not. A new immunodominant Cryptosporidium parvum antigen in the 15- to 17-kDa size range was identified as the Cryptosporidium parvum 60S acidic ribosomal protein P2 (CpP2). We developed a recombinant protein-based enzyme-linked immunosorbent assay for serologic population surveillance for antibodies that was 89% sensitive and 92% specific relative to the results of the large-format Western blot assay. The human IgG response is directed almost exclusively toward the highly conserved, carboxy-terminal 15 amino acids of the protein. Although IgG antibody cross-reactivity was documented with sera from patients with acute babesiosis, the development of an anti-CpP2 antibody response in our Peru study population correlated better with Cryptosporidium infection than with infection by any other parasitic protozoan. In Haiti, the prevalence of antibodies to CpP2 plateaus at 11 to 20 years of age. Because anti-CpP2 IgG antibodies were found only among residents of countries in the developing world where Cryptosporidium infection occurs early and often, we propose that this response may be a proxy for the intensity of infection and for acquired immunity.
Cryptosporidium parvum and C. hominis are enteric protozoan parasites that commonly cause outbreaks of diarrheal disease in the developed world (for reviews, see references 24 and 26). All age groups are affected, and the disease is usually self-limiting in immunocompetent individuals (5, 13). Outbreaks have been linked to public water system treatment failures, recreational exposure to contaminated water, contamination of unpasteurized fresh-squeezed juices, and contamination of food products by infected food handlers (14, 28, 35, 37, 39, 58). In the developing world, where potential sources of food and water contamination are widespread, acute cryptosporidiosis is usually limited to young children and to immunocompromised populations (4, 5, 48, 50, 59). In a longitudinal serologic study of enteric parasites in Peru, we reported that repeated infection was common among young children and that Cryptosporidium-specific IgG antibody levels increased with age and with experience of infection (54). Large-scale outbreaks of overt illness among immunocompetent adults in these regions where cryptosporidiosis is highly endemic have not been reported. These observations suggest that some level of immunity to disease (although not necessarily to infection) may eventually develop upon repeated exposure to the parasite (20).
In previous work (68), we noted that sera from individuals who live in Haiti often contain IgG antibodies to several C. parvum antigens, in addition to the immunodominant 27- and 17-kDa antigens. In the current work, we demonstrate that one of these novel antigens, located in the 17-kDa-molecular-mass range but distinct from the C. parvum 17-kDa antigen family (56), is the C. parvum acidic ribosomal protein P2 (CpP2). Several acidic ribosomal proteins (P0, P1, P2, or variants) have been described as prominent antigens in leishmaniasis (69, 70), Chagas' disease (32, 65, 67), malaria (10), Brucella abortus infection (6), Babesia bovis infection (12), and systemic lupus erythematosus (SLE) (16, 17, 62). In particular, ribosomal proteins P0 and P2 from Leishmania spp., Plasmodium falciparum, and Trypanosoma cruzi have been reported to be immunostimulatory, as sera from infected animals and humans recognize these antigens (10, 65, 66, 67,69, 70). Although the acidic ribosomal proteins are classically associated with the cytoplasmic ribosomes, they have also been localized to the cell surface of some parasites. Chatterjee et al. (9) used antibody fluorescence to demonstrate the presence of the P0 protein on the surface of P. falciparum merozoites, and Sehgal et al. (63) used transiently transfected Toxoplasma gondii cells to demonstrate the translocation of tagged P0 to the parasite surface.
Because of their surface localization and immunogenicity, it has been suggested that P proteins may be possible vaccine candidates. In recent reports, immunization with the P-domain peptide of ribosomal protein P0 provided protection against P. falciparum challenge (60), immunization with Babesia gibsoni P0 protein was cross-protective for infection with Babesia microti (73), and antibodies against Neospora caninum P0 inhibited infection with T. gondii in vitro (79). Furthermore, a Leishmania infantum ribosomal protein DNA vaccine conferred protective immunity against Leishmania major infection in mice (22). The strong anti-CpP2 antibody responses observed for most of the Haitians who were also antibody positive for the 27-kDa antigen suggest that the CpP2 antigen may play a role in the generation of immune responses against C. parvum in areas where it is highly endemic and, therefore, might be a potential vaccine target.
MATERIALS AND METHODS
Protein extraction and Western blot assay.
The Maine isolate of C. parvum was maintained by passage in Holstein calves, as described previously (3, 39). A crude antigen supernatant fraction was generated by sonication and freeze-thawing of purified Maine isolate oocysts, followed by centrifugation at 24,000 × g for 30 min (42). A membrane-associated protein fraction was isolated from the crude antigen by Triton X-114 detergent phase partitioning, as described previously, collected by acetone precipitation, and dissolved in buffer containing 0.5% SDS and 20 mM HEPES at pH 7.4 (55). Protein concentrations were determined by the bicinchoninic acid microassay (Pierce, Rockford, IL) with bovine serum albumin as the standard. Triton X-114-extracted proteins (140 μg) were resolved by preparative polyacrylamide gradient gel electrophoresis on a 3 to 25% discontinuous gel (44), and the proteins in the 17-kDa region were excised and recovered from the gel by electroelution (Elutrap; Schleicher & Schuell, Keene, NH).
Crude oocyst antigens were resolved on 10 to 22.5% SDS-polyacrylamide gels by using the buffer system of Laemmli (29) and electrotransferred onto a polyvinylidene difluoride (PVDF) membrane (Immobilon P; Millipore Corp., Bedford, MA). IgG Western blot assays were conducted on 2-mm-wide membrane strips with the biotinylated mouse monoclonal anti-human IgG and streptavidin-alkaline phosphatase system described previously (55). Rabbit and mouse IgG antibodies were detected by using biotinylated goat anti-rabbit IgG and monoclonal rat anti-mouse IgG, respectively (Zymed, South San Francisco, CA).
Rabbit immunization and screening of expression library.
A female New Zealand White rabbit was immunized three times at 4-week intervals with approximately 0.6 μg of the electroeluted 17-kDa region protein and an equal volume of TiterMax adjuvant per immunization (TiterMax USA, Inc., Norcross, GA). Serum was collected 2 weeks after the final immunization. No antibodies to C. parvum antigens were detected prior to immunization (data not shown). All animal work was approved by the Animal Use Committee of the Centers for Disease Control and Prevention (CDC).
An Iowa strain sporozoite cDNA library constructed from poly(A)+ mRNA in the bacteriophage λ UniZAP XR vector (CpLib3; kindly provided by N. J. Pieniazek, M. J. Arrowood, S. B. Slemenda, and J. R. Mead, CDC, Atlanta, GA, and Emory University, Atlanta, GA) was screened for IgG antibody-reactive clones by using the rabbit serum at a dilution of 1:50 in buffer containing 0.85% NaCl and 10 mM Na2HPO4 at pH 7.2 (phosphate-buffered saline [PBS]) with 0.3% Tween 20. Positive plaques were identified by using the biotinylated goat anti-rabbit IgG and streptavidin-alkaline phosphatase system. The plasmids were excised from the bacteriophage λ phage cultures in XL1-Blue Escherichia coli cells by using R408 helper phage (Stratagene, La Jolla, CA) and were plated on LB agar in the presence of ampicillin. The plasmids were isolated from the resulting clones by using a Wizard plasmid purification kit (Promega, Madison, WI) and were sequenced by using primers T3 and T7. Deduced protein sequences were aligned by use of the ClustalW (version 2) program (30) and were manually optimized in the carboxy-terminal region.
CpP2 cloning and expression.
The following deoxyoligonucleotide pair was used to amplify the CpP2-coding sequence (GenBank accession number AF099744) by PCR for directional cloning into the KpnI and HindIII restriction sites of vectors pQE-41 and pQE-81 (Qiagen, Valencia, CA): 5′-GGG GTA CCC CTG GTT CCG CGT GGA TCC ATG GGT ATG AAA TAC GTT GC-3′ and 5′-CGC CCA AGC TTA TTT AAT TAG TCA AAC AAT GAG AAA CC-3′. The forward primer was designed so that the 6× His tag (pQE-81) or the 6× His-dihydrofolate reductase (DHFR) fusion partner (pQE-41) could be removed with thrombin protease. In these primer sequences and those presented below, the restriction sites are underlined, and the thrombin site-coding sequences are italicized. AmpliTaq Gold DNA polymerase (Perkin-Elmer Cetus, Foster City, CA) was used as directed by the manufacturer to amplify the target sequence from genomic sporozoite DNA (55).
The 6× His fusion proteins were expressed in E. coli DH5α(pQE-81) (Invitrogen, Carlsbad, CA) or E. coli JM-109(pQE-41) (Promega). The recombinant proteins were purified by nickel-affinity chromatography (HiTrap Chelating HP 1-ml column; GE Healthcare, Piscataway, NJ) in PBS and cleaved with thrombin to remove the amino-terminal tag, as directed by the manufacturer. Uncleaved protein and 6× His-tagged DHFR were removed by reapplication to the nickel column.
Cloning and expression of other apicomplexan P proteins.
The C. parvum protein P1 (CpP1)-coding sequence (GenBank accession number XM_626174) was amplified from genomic C. parvum DNA by PCR with the following forward and reverse deoxyoligonucleotide pairs: 5′-GGG GTA CCC CTG GTT CCG CGT GGT TCC ATG GCA GCT GTT TCA ATG AAT G-3′ and 5′-CGC CCA AGC TTA TTT AAT TAG TCA AAC AAT GAG AAA CC-3′, respectively. The Plasmodium falciparum CpP2-coding sequence (GenBank accession number U78753) was amplified from genomic DNA (from strain FCR3F86, kindly provided by N. Lang-Unnash, University of Alabama at Birmingham, Birmingham, AL) by PCR with the following forward and reverse deoxyoligonucleotide pairs: 5′-GGG GTA CCC CTG GTT CCG CGT GGA TCC ATG GCT ATG AAA TCA GTT GC-3′ and 5′-CGC CCA AGC TTA ACC AAA TAA GGA AAA TCC-3′, respectively. The Toxoplasma gondii CpP2-coding sequence (GenBank accession number XM_002364187) was amplified from cDNA (from strain RH, kindly provided by J. Boothroyd, Stanford University, Stanford, CA) by PCR with the following forward and reverse deoxyoligonucleotide pairs: 5′-GGG GTA CCC CTG GTT CCG CGT GGA TCC ATG GCA ATG AAA TAC GTC GC-3′ and 5′-CGC CCA AGC TTA GTC GAA GAG CGA GAA GCC-3′ PCR, respectively. The PCR conditions were those described above for the amplification of CpP2. The products were cloned into the KpnI- and HindIII-digested pQE-81 plasmid vector and expressed in E. coli DH5α cells (Invitrogen). The C. parvum protein P0 (CpP0)-coding sequence (GenBank accession number XM_625816) was amplified from genomic C. parvum DNA by PCR with the following forward and reverse deoxyoligonucleotide pairs: 5′-GGG GTA CCC CTG GTT CCG CGT GGT TCC ATG CCA TCT CCA GAG AAA GC-3′ and 5′-CGC CCC TGC AGT CAG TCA AAT AAT GAA AAA CC-3′, respectively. This product was cloned into the KpnI- and PstI-digested pQE-81 plasmid vector for expression. Recombinant proteins were purified on a nickel-affinity chromatography, digested with thrombin, and dialyzed against 25 mM Tris at pH 7.5 (Spectra/Por3; 3,500-Da cutoff; Spectrum Laboratories, Rancho Dominguez, CA). The proteins were bound on a Mono Q HR 5/5 strong anion-exchange column (GE Healthcare) and eluted with a linear gradient of from 0 to 1 M NaCl in 25 mM Tris at pH 7.5. The protein-containing fractions were concentrated with Centricon-10 centrifugal filter devices (Millipore Corporation, Bedford, MA), and the 6× His-tagged, uncleaved proteins were removed by reapplication onto nickel columns in PBS buffer.
Monoclonal antibody production and purification of monospecific polyclonal antibody.
A mouse monoclonal antibody (monoclonal antibody 1D6) to recombinant CpP2 (rCpP2) was generated by Southern Biotech (Birmingham, AL). This IgG1 isotype antibody was shown to react specifically with a linear epitope located between CpP2 residues 43 and 60 (VLISNMSGKLSHEVIASG) (data not shown). Anti-CpP2 antibodies were purified from human serum by using Western-blotted rCpP2 antigen and the MgCl2 elution method of Tsang and Wilkins (75). The eluted antibodies were desalted on G-25 M size-exclusion columns (PD-10; GE Healthcare) that were preequilibrated with buffer containing PBS with 0.3% Tween 20.
Epitope mapping.
A series of 33 overlapping biotinylated peptides (15-mers, 12-residue overlap) were synthesized on the basis of the CpP2 gene sequence. The peptides were in the format of biotin-SerGlySerGly-peptide sequence-amide. All peptides were dissolved in 0.05% Tween 20-PBS at a concentration of 2 μg/ml. The diluted peptides (50 μl/well) were incubated overnight at 4°C in streptavidin-coated 96-well microtiter plates (200 ng protein/well; Immulon 2 HB; Thermo Electron Corp., Milford, MA). After four washes with 0.05% Tween 20-PBS, diluted serum (50 μl/well, diluted 1:100 in 0.3% Tween 20-PBS with 1% casein) was added and the plates were incubated for 2 h at room temperature. The plates were washed four times, and a peroxidase-labeled, goat anti-human IgG antibody was added (50 μl/well, diluted 1:2,000 in 0.3% Tween 20-PBS with 1% casein). After 1 h of incubation at room temperature, the plates were washed four times and 50 μl of 2,2′-azino-di-[3-ethylbenzthiazoline sulfonate (6)] (ABTS; Kirkegaard & Perry, Gaithersburg, MD) substrate was added to each well. The absorbances were read at 405 nm with a Molecular Dynamics UVmax kinetic microplate reader (Sunnyvale, CA). Positive- and negative-control peptides were included on each plate and were incubated with a strong positive-control serum. The absorbances of the CpP2 peptide wells were expressed as a percentage of the positive-control absorbance.
Measurement of antibody responses by ELISA.
Test sera were diluted 1:50 with buffer containing PBS with 0.05% Tween 20 and were assayed in duplicate. Assays for the detection of IgG antibodies to the recombinant C. parvum 27-kDa antigen have been described previously (11, 54). Antibody values of >116 arbitrary units were considered a positive result (11).
Assays for the detection of IgG antibodies to the recombinant CpP2 antigen were conducted by using the same blocking, assay, and development conditions used for the detection of the C. parvum 27-kDa antigen. Immulon 2HB flat-bottom microtiter plates (Thermo Electron Corp.) were sensitized overnight at 4°C with 50 μl of purified, recombinant CpP2 antigen per well at a concentration of 0.4 μg/ml in 0.1 M sodium bicarbonate buffer (pH 9.6). Each CpP2 enzyme-linked immunosorbent assay (ELISA) plate included three positive controls, three negative controls, as well as a 2-fold serial dilution series (9 dilutions, 1:50 to 1:12,800) of a strong CpP2-positive serum sample. The test sera were assigned a unit value on the basis of a four-parameter curve fit of the positive-control dilution series absorbance values (405 nm) in which the value for the 1:50 dilution was arbitrarily set equal to 6,400 units. Antibody values >136 were considered positive on the basis of a receiver operating characteristic curve by use of the Western blot results as the “gold standard” (194 samples; 89% sensitivity and 92% specificity with 91% correct results) (81).
The definition of a CpP2 antibody response applied to the analysis of longitudinal serum samples from a Peruvian birth cohort used the 136-arbitrary-unit cutoff and the interval criteria previously described for the 27- and 17-kDa C. parvum antigens (53, 54). Briefly, a P2 serologic response was identified when two consecutive serum samples were collected ≤180 days apart, the P2 response of the second serum sample was above the cutoff, and the P2 response of the second serum sample increased ≥50% relative to that for the first serum sample. If the interval between consecutive responses was >90 days, they were considered separate events. Other characteristics and analyses of the cohort have been described previously (5, 53, 54, 78).
The levels of antibodies to other P proteins (20 ng antigen/well) were assayed by ELISA under the conditions described above. Absorbance values (405 nm) were expressed as a percentage of the CpP2 positive-control value (1:50 dilution). The secondary antibody used for assays containing hamster sera was biotinylated rabbit anti-hamster IgG (Zymed).
Human serum specimens.
Expired plasma samples from 30 anonymous Haiti residents were obtained from a local hospital in Leogane, Haiti, in 1998. Banked sera were available for analysis from 30 U.S. citizens who had no history of foreign travel (55). Serum specimens from 30 individuals who were involved in a food-borne outbreak of cryptosporidiosis in 1997 were collected approximately 8 weeks after exposure (7, 45). Twenty-five of the serum specimens were from symptomatic individuals, and 6 of these were from individuals whose infections were confirmed by the analysis of stool samples. Written informed consent was obtained from the study participants, and the study was approved by the Centers for Disease Control and Prevention Institutional Review Board. Pools of sera (10 serum samples per pool) from patients with acute infections caused by Cryptosporidium spp., Cyclospora cayetanensis, T. gondii, P. falciparum, and B. microti were made. The Cryptosporidium pool contained high-titer sera from patients involved in two U.S. cryptosporidiosis outbreaks whose infections were confirmed by analysis of stool samples. Sera were also obtained from patients with cyclosporiasis involved in a single U.S. outbreak of cyclosporiasis whose infections were confirmed by analysis of stool samples (27). The titers of antibodies to C. cayetanensis antigens were not determined. Sera were also obtained from patients with acute toxoplasmosis involved in a U.S. toxoplasmosis outbreak and were positive for both IgM and IgG with titers of >1:4,096, determined by immunofluorescence assay (74). Sera were collected from patients with sporadic cases of malaria and babesiosis in the United States, but demographic information on the patients was not available. A pool of sera was made from 10 U.S. residents with chronic T. gondii infections who were IgM negative and who had IgG titers of >1:1,024.
Serum samples (n = 533) from a subset of 61 children from a longitudinal birth cohort study of diarrheal disease in Peru were tested for antibodies to the CpP2 antigen (5, 53, 54). Reports describing the detection of parasites by stool microscopy and analysis of the serologic responses to the 17- and 27-kDa C. parvum antigens in a larger subset of the cohort have been published previously (5, 53, 54). Written informed consent was obtained from the parent or guardian of each participating child. The original cohort study was approved by the Institutional Review Boards of the Johns Hopkins University School of Public Health and the Asociación Benéfica Proyectos en Informática, Salud, Medicina, y Agricultura. The Cryptosporidium serology study was approved by the Centers for Disease Control and Prevention Institutional Review Board.
Sera from 441 inhabitants of Miton, Haiti, were collected in 1998 as part of a community-wide study of lymphatic filariasis and other parasitic infections (19). The protocol for this study was approved by the Centers for Disease Control and Prevention Institutional Review Board and by the Ethics Committee of the Saint Croix Hospital, Leogane, Haiti. Written consent was required for participation in the study.
Data analysis.
Frequencies were compared by the chi-square test with Yates' correction for continuity. Antibody responses to the CpP2 and 27-kDa antigens among the different age groups were compared by the Kruskal-Wallis test. The distributions of positive results for the two antigens among Miton, Haiti, residents were compared by the chi-square goodness-of-fit test. Statistical analyses were conducted with the SigmaStat for Windows (version 2.03.0) program (SPSS, Inc., Chicago, IL) or the SAS (version 9.0) program. Statistical significance was set at an alpha level of 0.05.
RESULTS
Identification, cloning, and characterization of the CpP2 antigen.
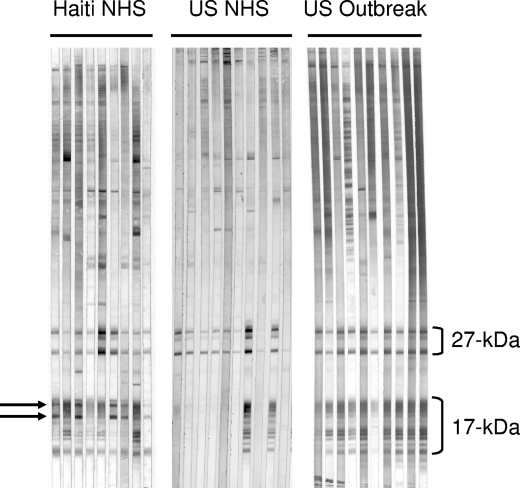
Infections with various species of Cryptosporidium have been shown to result in the development of characteristic IgG antibody responses to families of antigens in the 27- and 17-kDa regions (43, 46, 55). These families are composed of both soluble proteins that lack modifications and membrane-associated proteins that are posttranslationally modified by the addition of fatty acids or a glycosylphosphatidylinositol (GPI) anchor (52, 57; J. W. Priest et al., unpublished observations). The lower-molecular-mass, unmodified proteins remain in the aqueous phase, while the modified proteins readily partition into the detergent phase during Triton X-114 detergent extraction. Large-format Western blot studies of sera from healthy Haitian adults (normal human sera ([NHS]) by use of a total C. parvum antigen preparation showed that all serum samples had antibodies to the 27-kDa antigen, 93% had antibodies to the 17-kDa antigen, and 70% had antibodies to two novel antigens in the 17-kDa size range. Although our panels of NHS from healthy U. S. adults and sera from U.S. adults involved in a cryptosporidiosis outbreak also had high levels of antibodies to the 27- and 17-kDa antigens (93% and 57%, respectively, for NHS and 100% and 93%, respectively, for outbreak sera) the two new antigens in the 17-kDa region were not detected. Representative blots of NHS samples from 9 Haitian adults (Haiti NHS), 10 U.S. adults (U.S. NHS), and 10 individuals involved in a cryptosporidiosis outbreak in the United States (U.S. outbreak) are shown in Fig. 1. We wondered whether antibodies to these two antigens were a marker for Cryptosporidium infection or were a marker for infection with some other agent not commonly found in the United States.
FIG. 1.
Representative Western blots of NHS samples collected from 9 healthy Haitian blood donors (Haiti NHS), 10 healthy U.S. citizen blood donors (US NHS), and 10 U.S. cryptosporidiosis outbreak patients (US outbreak). A crude oocyst antigen preparation was resolved on 10 to 22.5% SDS-polyacrylamide gels and electrotransferred to a PVDF membrane. Individual serum samples were incubated with strips at a dilution of 1:100, and bound IgG antibodies were visualized by using the streptavidin-alkaline phosphatase system described in Materials and Methods. The locations of the 27- and 17-kDa antigens are indicated. Two new immunodominant antigens in the 15- to 17-kDa size range (indicated by arrows) were observed on some blots of the Haitian serum samples but were not evident on the blots of sera from the United States.
Triton X-114 detergent-soluble C. parvum proteins were eluted from the 17-kDa region of a 3 to 25% SDS-polyacrylamide gel and were used to immunize a rabbit. Instead of the expected pattern of five related 17-kDa antigen bands, the rabbit IgG antibodies recognized three new proteins in the 17-kDa region, and two of these had the same apparent molecular masses as the two new antigens observed by use of the Haitian sera. The antigens were present in the total crude C. parvum antigen preparation (Fig. 2, lane 1) as well as in the Triton X-114 extract (Fig. 2, lane 2). We used the rabbit sera to screen a C. parvum sporozoite cDNA bacteriophage lambda expression library and obtained two clones containing the C. parvum acidic ribosomal protein P2 sequence (Fig. 3). CpP2 shares between 48% and 55% identity with other reported apicomplexan P2 proteins and with the human P2 protein, but it is less similar to the kinetoplastid P2 proteins that have been used in diagnostic assays (40% identity with Leishmania infantum; 39% identity with Trypanosoma cruzi) (65, 69).
FIG. 2.
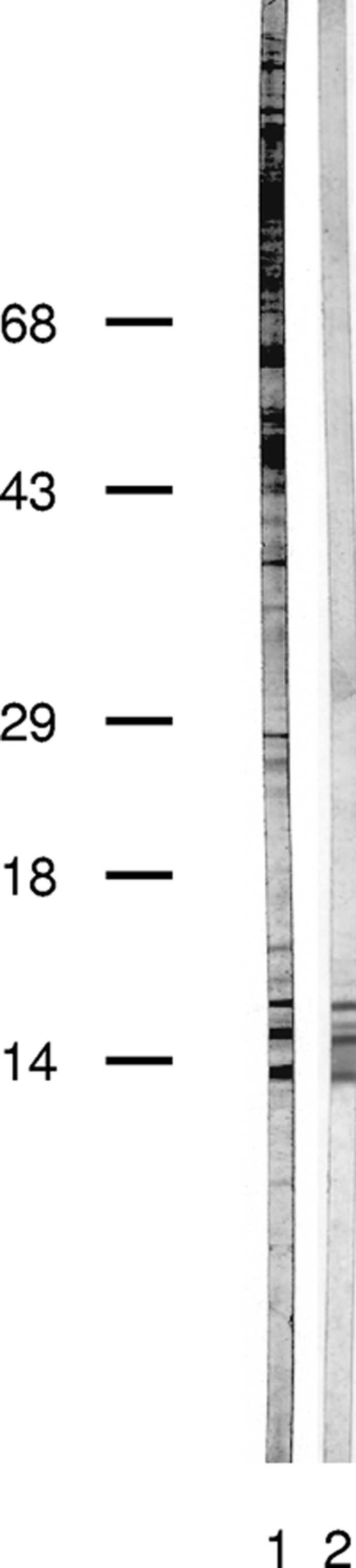
Generation of antibodies to the 17-kDa antigen fraction. A partially purified C. parvum 17-kDa antigen fraction, resolved and electroeluted as described in Materials and Methods, was used to immunize a New Zealand White rabbit. Rabbit serum was incubated with a total C. parvum antigen PVDF strip (lane 1) and with a Triton X-114-extracted C. parvum antigen PVDF strip (lane 2). Bound IgG antibodies were visualized by use of a biotinylated goat anti-rabbit IgG secondary antibody and developed as described in the legend to Fig. 1. The locations of the molecular mass markers are indicated on the left.
FIG. 3.
ClustalW sequence alignment of apicomplexan, kinetoplastid, and human acidic ribosomal P2 proteins. Predicted ribosomal protein P2 amino acid sequences from T. gondii (GenBank accession number XM_002364187), Sarcocystis neurona (GenBank accession number BQ784279), P. falciparum (GenBank accession number U78753), Eimeria tenella (GenBank accession number AAK38885), C. parvum (GenBank accession number AF099744), B. bovis (GenBank accession number XP_001611755), Theileria parva (GenBank accession number XP_764936), Homo sapiens (GenBank accession number AAA36472), L. infantum (GenBank accession number XP_001467169), and T. cruzi (GenBank accession number CAA52946) were aligned by using the ClustalW2 sequence alignment program (30). The human SLE P-protein consensus sequence is underlined in the human sequence (32). The carboxy-terminal 15-amino-acid peptide (EEEEEEGDLGFSLFD) recognized by IgG antibodies from P2-positive Haitian sera is indicated in boldface with underlining in the C. parvum P2 sequence. The linear epitope recognized by mouse monoclonal antibody 1D6 is underlined in italics in the C. parvum P2 sequence.
As with nearly all ribosomal P proteins, the CpP2 carboxy terminus contains elements of the human systemic lupus erythematosus P-protein consensus sequence (underlined in the human [Homo sapiens] sequence in Fig. 3) (32). In some SLE patients, this 11-amino-acid sequence (and especially the carboxy-terminal 6 residues of the sequence) elicits a strong autoimmune IgG antibody response (16, 32, 36). CpP2 contains 6 of the conserved-motif residues at Asp-104, Gly-106, Phe-108, Leu-109, Phe-110, and Asp-111, as well as 2 conserved substitutions at Glu-102 (for Asp) and Leu-105 (for Met). All of the apicomplexan P2 proteins reported thus far contain a Gly-to-Ser substitution at the third position of the critical GFGLFD hexapeptide sequence (Ser-108 in the CpP2 sequence). Amino acid replacement studies with anti-ribosomal protein P-positive sera from patients with SLE have suggested that a Gly-to-Ser substitution at the third position eliminates most of the IgG antibody binding to the hexapeptide (36).
From our expression library screening with the rabbit antibody, we also obtained two clones that encode CpP0. This probably occurred because the carboxy-terminal 16 amino acids of CpP0 are identical to the CpP2 terminal sequence (data not shown). Although the same is also true for CpP1, we did not obtain any clones containing the P1 sequence.
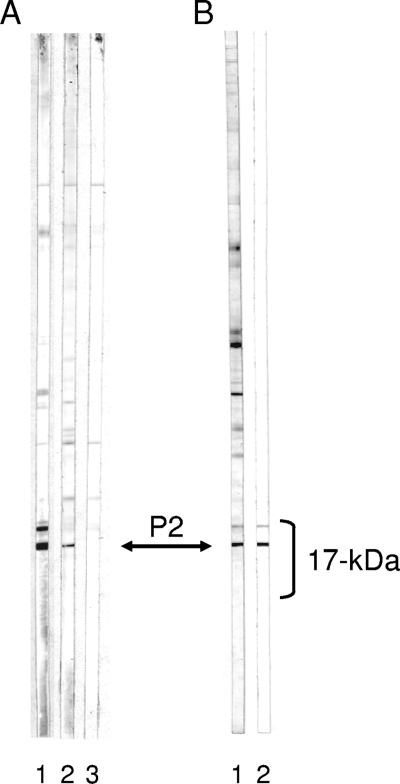
In order to identify which protein band on the Western blot was the CpP2 band, monoclonal and monospecific polyclonal antibodies were generated with rCpP2 as the antigen. Serum from a BALB/c mouse that was immunized with rCpP2 recognized two major bands in the 17-kDa region (Fig. 4 A, lane 1) in a pattern that was reminiscent of that observed with the Haitian sera in Fig. 1. However, monoclonal antibody 1D6, an antibody that recognized a CpP2 sequence that was not shared with either CpP0 or CpP1 (italicized and underlined in Fig. 3), recognized only the lower-molecular-mass band (Fig. 4A, lane 2). Polyclonal IgG antibodies affinity purified from a Haitian blood donor by using rCpP2 reacted with the same two bands as the mouse serum (Fig. 4B). Taken together, these results identify the lower band in the Western blots as CpP2 and strongly suggest that the higher-molecular-mass band that shares one or more antibody epitopes with CpP2 is CpP1. A protein band corresponding to CpP0 (predicted molecular mass, 33.5 kDa) was not conclusively identified on the blot. This protein may be underrepresented in our extract.
FIG. 4.
Identification of CpP2 band. (A) Total antigen C. parvum PVDF strips were incubated with serum from a mouse that was immunized with purified rCpP2 (lane 1), monoclonal antibody 1D6 raised against rCpP2 (lane 2), or buffer alone (lane 3). Bound IgG antibodies were visualized by use of a biotinylated rat anti-mouse IgG monoclonal antibody and developed as described in the legend to Fig. 1. (B) Total antigen C. parvum PVDF strips were incubated either with serum from a Haitian donor (lane 1) or with human serum antibodies that were eluted from purified rCpP2 (lane 2). Bound IgG antibodies were visualized as described in the legend to Fig. 1. The location of the CpP2 band is indicated by the double-headed arrow.
The apparent molecular masses of native CpP1 and CpP2 (approximately 14.5 and 13.8 kDa, respectively; Fig. 2) are greater than the predicted molecular masses of 12.1 and 11.5 kDa, respectively. Because recombinant CpP2 also migrated more slowly than expected, the size discrepancy may simply result from an unusual secondary structure induced by the alanine- and acidic residue-rich sequence of the carboxy-terminal one-third of the protein (61, 77).
Epitope mapping of CpP2.
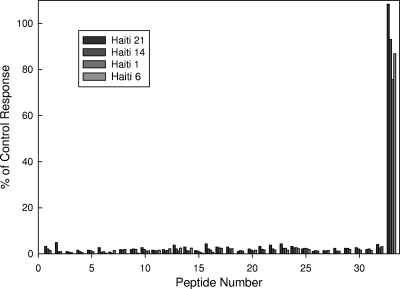
The antibody cross-reactivity noted above led us to make a closer examination of the antigenic characteristics of CpP2. Using a series of overlapping, biotinylated peptides, we determined that only the carboxy-terminal 15-amino-acid peptide (EEEEEEGDLGFSLFD) was recognized by IgG antibodies from P2-positive Haitian sera (Fig. 5). A 3-amino-acid upstream shift in the peptide sequence eliminated all antibody recognition in the human sera tested (Fig. 5 and data not shown). These results are similar to those reported for anti-ribosomal protein P sera from SLE patients by Mahler et al. (36) and for immune sera from patients with Chagas' disease by Skeiky et al. (67). Interestingly, sera from mice immunized with recombinant CpP2 strongly recognized two internal epitopes, in addition to the carboxy-terminal one (data not shown). One of these epitopes is recognized by our 1D6 mouse monoclonal antibody (underlined and italic sequence in Fig. 3).
FIG. 5.
Map of CpP2 epitopes recognized by human serum IgG antibodies. A series of 33 overlapping biotinylated peptides (15-mers, 12-residue overlap) were synthesized on the basis of the CpP2 cloned sequence. Peptides (100 ng/well) were incubated in streptavidin-coated 96-well microtiter plates (200 ng protein/well) overnight at 4°C. The plates were then washed and incubated with 1:100-diluted Haiti blood donor sera for 2 h at room temperature. The plates were washed and then developed by using peroxidase-labeled goat anti-human IgG and ABTS, as described in Materials and Methods. The CpP2 peptide responses are expressed as a percentage of the value for the positive-control peptide.
Anti-P2 antibody cross-reactivity.
The exclusive concentration of the human immune response on the highly conserved carboxy-terminal 15 amino acids of CpP2 raised the possibility that antibodies from patients with various apicomplexan parasitic infections might cross-react at this epitope. We expressed recombinant CpP0, CpP1, the P. falciparum P2 protein (PfP2), and the T. gondii P2 protein (TgP2) and used these along with CpP2 to screen high-titer serum pools from humans infected with Cryptosporidium, P. falciparum, T. gondii, and B. microti. As predicted from their identical carboxy-terminal sequences, all of the C. parvum P proteins reacted with the P2 positive-control serum sample (sample Haiti NHS 14) (Table 1). This serum sample also reacted with the T. gondii P2 protein but did not react significantly with the P. falciparum protein. In contrast to the Haiti NHS 14 sample, another P2-positive Haitian serum sample, Haiti NHS 1, did not discriminate between PfP2 and the other P proteins. This result suggests that there is some variability between individuals in the exact antibody recognition sequence within the conserved, carboxy-terminal 15-amino-acid domain.
TABLE 1.
Analysis of serum antibody responses to apicomplexan P proteins
| Serum sample | Relative ELISA responsea |
||||
|---|---|---|---|---|---|
| CpP2 | CpP0 | CpP1 | PfP2 | TgP2 | |
| Haiti NHS 14 | 100b | 107 | 106 | 6 | 111 |
| Haiti NHS 1 | 38 | 54 | 41 | 41 | 48 |
| Acute Cryptosporidium infectionc,d | 2 | 1 | 4 | 1 | 3 |
| Acute P. falciparum infectionc | 2 | 4 | 6 | 5 | 3 |
| Acute T. gondi infectionc | 2 | 3 | 2 | 2 | 3 |
| Chronic T. gondii infectione | 1 | 2 | 1 | 2 | 1 |
| Acute C. cayetanensis infectionf | 2 | 4 | 3 | 3 | 2 |
| Acute B. microti infectionc | 15 | 14 | 36 | 8 | 11 |
| Uninfected hamsterg | 2 | 4 | 3 | 1 | 2 |
| B. microti-infected hamsterg,h | 98 | 105 | 96 | 0 | 99 |
The absorbances recorded at 405 nm were divided by the absorbance observed for the CpP2 protein by use of the Haiti NHS 14 control serum sample, and the results are expressed in percent.
The value was arbitrarily set equal to 100%.
Pools of serum (n = 10 each) from patients with acute infection and high-titer IgG antibody responses to the organism of interest.
Sera from patients with acute cryptosporidiosis were demonstrated to be negative for antibodies to CpP2 and CpP1 by Western blotting.
Pooled sera from 10 patients with chronic toxoplasmosis who were IgM negative but who had IgG titers of >1:1,024.
Pooled sera from 10 U.S. patients confirmed to have cyclosporiasis by analysis of stool samples. The parasite-specific titers were not determined.
Hamster serum assays used a different secondary antibody than the human antibody assays: biotinylated rabbit anti-hamster IgG (Zymed).
Serum collected 28 days after parasite inoculation.
As demonstrated by Western blotting (Fig. 1), acute-phase, high-titer sera from patients involved in U.S. outbreaks of cryptosporidiosis did not react with the P proteins (Table 1). Similarly, significant levels of antibody to either the homologous or the heterologous P proteins were not evident in pools of 10 serum samples from U.S. patients with acute malaria, acute cyclosporiasis, acute toxoplasmosis, or chronic toxoplasmosis. In contrast, pooled sera from patients with acute babesiosis did react with most of the P proteins. Because clinical data were not available for the patients with babesiosis, we assayed a serum sample from an experimentally infected hamster to confirm that the antibody response was the result of B. microti infection rather than exposure to some other uncharacterized parasite. High levels of IgG antibodies to all of the P proteins except PfP2 were detected in a hamster 28 days after inoculation. These results confirm that CpP2 cross-reactive antibodies can develop during infection with another apicomplexan parasite, but because human babesiosis has not been reported in Haiti, the data do not definitively link a particular parasite to our observations.
Many P2 responses are temporally linked to Cryptosporidium sp. infection.
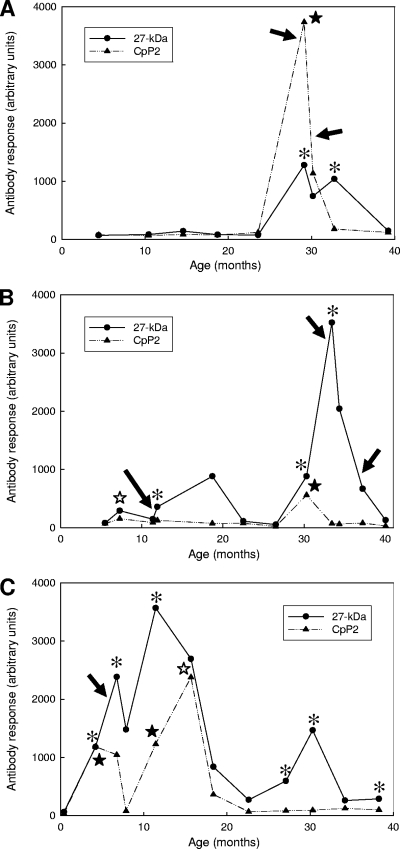
To determine whether the CpP2 antibody responses were coincident with Cryptosporidium infection, we evaluated 533 longitudinal serum specimens collected from 61 children who participated in a Peruvian birth cohort study of diarrheal disease. These 61 children, part of a larger cohort subset (n = 74) that was previously analyzed for antibody responses to the 17- and 27-kDa antigens by ELISA (53, 54), had 101 serologic responses to the 17- and 27-kDa antigens and had 90 discrete infections, detected by stool microscopy. However, only 71 of the oocyst detection events had appropriate paired serum samples for CpP2 antibody analysis. Representative profiles showing the antibody responses to rCpP2 and the 27-kDa antigen from three of the children are shown in Fig. 6. The child whose findings are represented in Fig. 6A had a very intense CpP2 antibody response (indicated by a closed star) during the first of two separate Cryptosporidium-specific antibody responses (indicated by asterisks). Oocysts were detected by microscopic stool assay at 29 and 30 months of age (indicated by arrows). Figure 6B represents the findings for a child who had two CpP2 responses, a weak response at 7 months of age (indicated by the open star) and a moderate response at 30 months of age, that correlated with a Cryptosporidium-specific antibody response. Interestingly, the P2 antibody levels decreased to the baseline level when the child was 33 months of age, even though subsequent Cryptosporidium infections were detected by both microscopy and serologic antibody assay. The child whose findings are represented in Fig. 6C had two CpP2 responses that coincided with Cryptosporidium-specific antibody responses at 4 and 11 months of age and one CpP2 response detected at 16 months of age, when the level of antibody to the 27-kDa antigen was in decline. None of the CpP2 responses were temporally associated with the single oocyst detection event at 5 months of age. Four additional Cryptosporidium-specific antibody responses were marked either by a declining CpP2 antibody level (at 7 months of age) or by CpP2 antibody levels below the positive cutoff value (at 27, 30, and 38 months of age).
FIG. 6.
Representative profiles showing longitudinal IgG responses to the 27-kDa antigen and CpP2 in three Peruvian children (A to C, respectively). Longitudinal serum samples collected from the children were assayed for IgG antibodies by using the recombinant 27-kDa protein and CpP2, as described in Materials and Methods. Responses are presented in arbitrary units on the basis of a standard curve with a maximum value of 6,400. Asterisks, intervals that had serologic antibody responses to both the 17- and 27-kDa antigens, according to our response definition; closed stars, intervals during which a CpP2 antibody response occurred in conjunction with a 17- and 27-kDa antibody response; open stars, CpP2 responses during intervals that did not meet our Cryptosporidium-specific antibody response definition for the 17- and 27-kDa antigens. Cryptosporidium oocyst detection events are shown by black arrows.
By using the antibody response definition described in Materials and Methods, 45 CpP2 antibody responses were identified among 428 serum intervals representing 115.1 child-years of surveillance (Table 2). All of the CpP2 responses were transient, and most (82%) occurred while the children were also positive for antibodies to both the 17- and 27-kDa antigens (54). Twenty-five (56%) of the responses occurred among individuals in whom a previous Cryptosporidium infection event was documented either by microscopy or by serologic antibody assay. A total of 8 CpP2 responses (18%) were coincident with Cryptosporidium-specific antibody responses to the 27- and 17-kDa antigens in the presence of oocyst shedding (as defined in reference 54), 19 CpP2 responses (42%) were associated with an antibody response in the absence of detectable oocyst shedding, 2 CpP2 responses (4%) were coincident with the detection of Cryptosporidium oocysts by stool microscopy in the absence of an antibody response to the 17- and 27-kDa antigens, and 16 responses (36%) could not be linked to Cryptosporidium-specific antibody responses (according to our definition) or to oocyst shedding (Table 2). Thus, 10 of 71 (14%) oocyst detection events obtained with serum samples appropriately spaced for analysis were associated with a CpP2 response and 27 of 101 (27%) serologic responses to the 17- and 27-kDa antigens were associated with a CpP2 response. The P2 antibody responses were significantly related to serologic antibody status (chi-square analysis of a two-by-two contingency table with Yates' correction, P < 0.001) but were not related to stool microscopy status (P = 0.435).
TABLE 2.
Summary of stool microscopy and serologic antibody results for a subset of children from the Peruvian birth cohort study
| Stool microscopy status | Serologic antibody statusa | No. (%) of samples with the following result for P2 antibody: |
|
|---|---|---|---|
| Positiveb | Negative | ||
| Positive | Positive | 8 (18) | 40c |
| Positive | Negative | 2c (4) | 23 |
| Negative | Positive | 19d (42) | 35 |
| Negative | Negative | 16 (36) | 287 |
Serologic antibody responses to the 27- and 17-kDa C. parvum antigens determined as described by Priest et al. (53).
Percentage of the total number of P2 antibody responses (n = 45) that were present in each category.
Two oocyst detection events overlapped with separate antibody responses to the P2 protein and to the 27- and 17-kDa antigens.
Includes one consecutive antibody response to the 27- and 17-kDa antigens, as defined previously (53).
Given that the P2 protein from C. cayetanensis, a commonly reported enteric parasite in both Peru and Haiti (5, 15, 34), is likely to have the same conserved carboxy-terminal antibody recognition sequence as the other apicomplexan proteins whose sequences are shown in Fig. 3, we wanted to determine whether Cyclospora infection might be associated with the CpP2 antibody response. In our particular subset of 61 cohort children, 37 Cyclospora infections with durations of between 1 and 64 days (median, 11 days) were identified by stool microscopy, and for 27 of these infections serum sampling was done at appropriately spaced intervals for CpP2 antibody analysis. Thirty-six of the children (59%), including the three whose findings are shown in Fig. 6, had no evidence of Cyclospora infection at any time during the multiyear study. Only 5 of the 45 CpP2 responses (11%) were temporally associated with Cyclospora infection: three CpP2 responses occurred during intervals that also included Cryptosporidium-specific 17- and 27-kDa antibody responses, one occurred during an interval that included a Cryptosporidium oocyst excretion episode, and one occurred during an interval that did not have either a Cryptosporidium oocyst detection event or a Cryptosporidium-specific antibody response. Although the result is not definitive, it would appear that Cyclospora infection is not a major contributor to the CpP2 antibody response.
Age-specific distribution of CpP2 antibodies in a Haitian population.
Serum samples (n = 441; age range, 1 month to 90 years) collected from a convenience sample of the population of Miton, Haiti (population, approximately 2,000), as part of a 1998 trial of a drug used to control of lymphatic filariasis were tested by ELISA for the presence of IgG antibodies to Cryptosporidium antigens. The prevalence of Giardia was reported to be 11% in this community at the time of sample collection (19), but stool samples were not tested for the presence of Cryptosporidium or Cyclospora oocysts at the time of sample collection. In 2001, a sample of people between 1 and 86 years of age from a nearby community in Haiti was reported to have Giardia, Cyclospora, and Cryptosporidium prevalence rates, detected by stool microscopy, of 27.4%, 6.0%, and 0.7%, respectively (34).
As would be expected in a setting where Cryptosporidium is highly endemic, much of the population of Miton had IgG responses to the 27-kDa antigen (Table 3): 55% of individuals <21 years of age and 83% of adults were positive. Adults also had significantly higher median antibody levels than young children. The response to the CpP2 antigen showed a different profile from that of the 27-kDa antigen: young children (ages 0 to 5 years) were largely negative and had antibody levels significantly lower than those in individuals in all other age categories. Both the median level of antibody and the antibody prevalence increased sharply in individuals in the 6- to 10-year-old and 11- to 20-year old age categories but did not change significantly among older adults. The distributions of antibody-positive results across the age groups were significantly different for the two antigens by the chi-square goodness-of-fit test (P < 0.001).
TABLE 3.
Serum antibody responses to Cryptosporidium parvum antigens among residents of Miton, Haiti
| Age group (yr) | Response to 27-kDa antigen |
Response to CpP2 |
||||
|---|---|---|---|---|---|---|
| No. of samplesa | No. (%) of samples positive | Median no. of samplesb | No. of samples | No. (%) of samples positive | Median of samplesb | |
| 0-5 | 80 | 42 (52) | 128c | 82 | 18 (22) | 40.5f |
| 6-10 | 82 | 52 (63) | 154 | 83 | 41 (49) | 126g |
| 11-20 | 118 | 59 (49) | 117d | 119 | 83 (70) | 394 |
| 21-40 | 87 | 66 (76) | 238c,d | 87 | 67 (77) | 517g |
| >40 | 69 | 64 (93) | 347e | 70 | 50 (71) | 409 |
Some samples had insufficient volume for multiple antigen testing.
Arbitrary units.
Significantly different by the Kruskal-Wallis test (P < 0.05).
Significantly different by the Kruskal-Wallis test (P < 0.05).
Significantly different from all other age ranges by the Kruskal-Wallis test (P < 0.05).
Significantly different from all other age ranges by the Kruskal-Wallis test (P < 0.05).
Significantly different by the Kruskal-Wallis test (P < 0.05).
The ELISA results for the CpP2 antigen for adults are consistent with the Western blot results that we obtained for adult Haitian blood donors (Fig. 1). However, the results of the 27-kDa antigen ELISA are somewhat lower than expected and probably underestimate the true antibody prevalence, as reflected by the Western blot results. The sensitivity of the 27-kDa antigen ELISA is known to be lower in the region of the cutoff value (J. W. Priest, unpublished observation), and 44% of the samples from Miton fell within ±50% of the cutoff values. Even with this limitation, 77% of the individuals who had a CpP2 response also had antibodies to either the 27-kDa or the 17-kDa antigen by ELISA.
DISCUSSION
We have previously demonstrated that specific antibody responses to the 17- and 27-kDa sporozoite surface antigens usually increase in parallel following infection with Cryptosporidium (38, 43, 46, 51). Here we report that IgG antibodies to two additional low-molecular-mass antigens, the acidic ribosomal proteins P1 and P2, are frequently detected in sera from individuals who live in communities where potential environmental sources of oocyst contamination are widespread. Because detergent extraction of C. parvum sporozoites yielded a fraction that contained both CpP1 and CpP2 as well as the membrane-bound 17- and 27-kDa antigen species, we believe that CpP1 and CpP2, as with the P0 proteins of P. falciparum (2, 9, 10) and T. gondii (64), are likely to be associated with the cell surface. However, preliminary attempts at C. parvum sporozoite cell surface staining with our anti-CpP2 monoclonal antibody have so far been unsuccessful (data not shown).
Because antibodies to CpP2 are not found in sera from U.S. (55; this work) or Canadian (51) cryptosporidiosis outbreak patients and because the target of the human immune response is a highly conserved carboxy-terminal peptide epitope with a demonstrated potential for cross-reactivity (this work), the challenge has been to determine whether the observed CpP2 responses resulted from infections with Cryptosporidium or from infections with some as-yet-unidentified organism that is more prevalent in the developing world than in the North America. In support of Cryptosporidium being the causative agent, we observed that many Haitians who had IgG antibodies to CpP2 had concurrent evidence of past Cryptosporidium infection by serologic ELISA. Furthermore, the CpP2 responses in Peruvian children were often temporally associated with Cryptosporidium infection. We did, however, also note that 36% of the CpP2 responses did not correlate with serologic assay-defined Cryptosporidium infection. Four of these responses (9%), like the third response in Fig. 6C, occurred after a strong antibody response to both the 17- and 27-kDa antigens and in the presence of persistent and high levels of antibody to both antigens (more than four times the cutoff). Eight additional CpP2 responses (17.7%), like the first response in Fig. 6B, occurred during intervals when the titer of one of the antigens (usually the 27-kDa antigen) demonstrated an increase that met our serologic response definition but the titer of the other one (usually the 17-kDa antigen) did not. Thus, the correlation between the CpP2 and Cryptosporidium-specific 17- and 27-kDa antigen serologic responses that we reported is likely to be conservative and may be limited by our working definitions of antibody responses.
Even though the acidic ribosomal proteins are well conserved, we were not conclusively able to link any other infectious protozoan to the observed CpP2 antibody responses. Although Giardia intestinalis is ubiquitous in Haiti and other settings in the developing world (8, 19, 34, 47), it lacks the conserved carboxy-terminal epitope that is critical for P2 protein immune recognition (G. intestinalis P2; GenBank accession number XP_001707951) (41). Isospora belli infection was very rare in the Peruvian cohort study (V. Cama, CDC, personal communication). T. gondii is cosmopolitan in distribution and has a prevalence in the United States of approximately 16% (23), but we did not detect CpP2 antibodies among U.S. citizens, nor did we find CpP2 antibodies in sera from U.S. patients with acute or chronic toxoplasmosis. In addition, one of the four strongly CpP2 antibody-positive serum samples used in the epitope mapping study whose results are shown in Fig. 5 (sample Haiti NHS 1) was negative for antibodies to Toxoplasma (data not shown). From our study, it is clear that sera from patients with babesiosis in the United States react with most apicomplexan P proteins. In fact, the Babesia P0 protein has been reported to be an immunodominant antigen shared between Babesia species (72, 73). However, Babesia is not likely to be the agent responsible for the observed CpP2 responses, because no species capable of infecting humans has ever been reported in either of our study areas.
Similarly, we do not believe that P. falciparum infection can explain our CpP2 results, as we saw no evidence of P-protein antibodies in sera from patients with acute malaria who were U.S. residents. Malaria is not endemic in the region around Lima, Peru, where our child cohort study was conducted, nor is it common in the Leogane commune of Haiti, where Miton is located. However, antibodies to the P. falciparum P0 protein have been detected among clinically immune patients with malaria from India (33), and our results do not specifically address the potential impact of repeated or chronic infections with P. falciparum on the P-protein antibody response. We did test a serum sample from a laboratory-reared rhesus monkey that had been infected multiple times with various Plasmodium parasite species, but we were unable to detect IgG antibodies to any of the apicomplexan P proteins (data not shown). We would suggest that P-protein serologic results for patients with malaria should be interpreted with some caution, if the donors reside in regions of the developing world where infection with Cryptosporidium is frequent.
C. cayetanensis is the one parasite commonly found in our study areas that is difficult to rule out as a contributor to the CpP2 response. In a cross-sectional survey in Haiti, Cyclospora oocysts were detected by stool microscopy in 13% of children 0 to 10 years of age (34), and 33% of the children in the Peru cohort study had at least one episode of cyclosporiasis during follow-up (5). However, only one of the CpP2 responses found among our Peruvian study children was associated with a Cyclospora oocyst detection event in the absence of Cryptosporidium infection. Further work to conclusively rule out any contributions from Cyclospora infection would be greatly aided by the development of a Cyclospora-specific serologic antibody assay.
On the basis of the results presented here, we believe that Cryptosporidium infection is the most likely cause of the CpP2 antibodies observed in sera from Haiti and Peru, and we hypothesize that repeated, early infection may be required for the development of a persistent CpP2 antibody response. Populations in developing regions of the world, like Haiti and Peru, are certainly exposed to Cryptosporidium at a young age and suffer repeated infections. The prevalence of antibodies to the 27-kDa antigen was 2- to 3-fold higher for Haitian children ≤10 years of age (52 to 63%) (this work) than for U.S. children (21.3%) (21), and although the method of assay has varied between studies, similarly high seroprevalence values (40 to 100%) have been reported for children in other developing countries around the world (71, 76, 80). Incidence rates based on stool microscopy are also much higher among children in the developing world. Laupland and Church (31) reported an incidence of approximately 0.0003 infections per child-year among children 1 to 9 years of age in Calgary, Alberta, Canada, whereas the rate reported among Peruvian children ≤12 years of age was 0.22 infections per child-year (5). Repeated infections were seen in 11.7% of the Peruvian children during that study. In addition, the infection rate determined by stool microscopy may significantly underestimate the true rate in Peru: we previously reported that an infection rate of 0.34 infections per child-year was determined by serologic antibody assay among children who were consistently stool negative for oocyst excretion (53).
Two of our more intriguing observations are, first, that most Cryptosporidium infections in children do not elicit a CpP2 antibody response and, second, that the seroprevalence profile for CpP2 is different from that observed for the 27-kDa antigen. In Peru, we found that 86% of oocyst detection events with appropriately spaced serum samples and 73% of the serologic responses to the 17- and 27-kDa antigens were not associated with a CpP2 response. Whether the first observation is a reflection of some characteristic of the species or dose of Cryptosporidium, of the immune status (i.e., previous infection experience) or the nutritional status of the child, or of some other factor, such as the time interval between serum sample collections, is not known. We attempted to examine the effects of the strain or species of Cryptosporidium on the CpP2 response, but we had available too few infections for a meaningful analysis by DNA extraction.
There is evidence showing that acute cryptosporidiosis in children from developing countries is associated with intestinal inflammation and malnutrition. Kirkpatrick et al. (25) demonstrated that symptomatic children in Haiti <2 years of age had higher levels of fecal lactoferrin, interleukin-8, and tumor necrosis factor alpha receptor I than healthy control or noncryptosporidiosis diarrheal control children. Increased levels of lactoferrin, a proxy marker for leukocytes and intestinal inflammation, were also detected in a Brazilian study of cryptosporidiosis in children but were not apparent during experimental infections of healthy adults in the United States (1). Recent studies have shown that malnutrition significantly increases the risk for Cryptosporidium infection in children from Bangladesh (40). We do not yet know whether malnutrition can downregulate the immune response to Cryptosporidium as it does in the case of malaria (18) or promote inflammation and shift the Th1/Th2 dynamics of the immune response. Additional work is needed to determine the conditions necessary for the development of a CpP2 response in young children.
With respect to the seroprevalence profiles, we observed that the CpP2 responses in Peruvian children <4 years of age were invariably transient and that Haitian children <5 years old were mostly (78%) negative for anti-CpP2 antibodies. In contrast to the CpP2 response, persistent responses to the 27-kDa antigen were often observed among children, and >50% were positive by serologic ELISA by the age of 3 years (54). Age-related differences in the seroprevalence profiles for specific antigens from the same parasite have previously been reported for both Cryptosporidium and P. falciparum. We have shown that antibody responses to the 17-kDa antigen are more transient than those to the 27-kDa antigen, even though the half-lives of the responses to both antigens are similar (51). In fact, a significant proportion of the U.S. population is positive for antibodies to the 27-kDa antigen but negative for antibodies to the 17-kDa antigen, and the proportion of the population with detectable antibodies to both antigens increases with age (21). Similarly, the seroprevalence of antibodies to specific malarial antigens varies depending upon the age of the population and the intensity of transmission (49). In a stable and high-transmission area where the population has acquired some level of immunity to malaria, the prevalence of antibodies to apical membrane antigen 1 reached a plateau at 6 years of age, while the prevalence of antibodies to the circumsporozoite protein continued to increase with age to levels of >70%. However, in the unstable and low-transmission area where no immunity was acquired and epidemics occurred frequently, the seroprevalence of the circumsporozoite protein remained low (<10%) and the seroprevalence of apical membrane antigen 1 continued to increase with age. Thus, seroprevalence can vary between antigens from the same organism and can serve as a proxy for the development of immunity upon repeated infection. Given the Miton cross-section results, children and young adults between 6 and 20 years of age would probably be more appropriate for inclusion in a longitudinal study of the development of a CpP2 response than children under 4 years old. Further studies will be required to determine if the CpP2 antibody response is related to the acquisition of immunity to Cryptosporidium infection or disease. We are currently examining this antigen as a potential vaccine target in an animal model.
Acknowledgments
We thank J. B. Phu and D. Sara for their assistance with the Peru cohort study and Jean Marc Brissau for program support in Haiti. P. falciparum genomic DNA was kindly provided by N. Lang-Unnash, University of Alabama at Birmingham, Birmingham, AL. T. gondii cDNA was kindly provided by J. Boothroyd, Stanford University, Stanford, CA. N. J. Pieniazek and S. B. Slemenda (CDC) contributed to the creation of the Iowa strain C. parvum sporozoite cDNA library. We thank B. Collins and J. Sullivan (Malaria Branch, CDC) for access to sera from Plasmodium-infected monkeys and J. M. Roberts (DPD, CDC) for support with statistical analysis.
We acknowledge the support of NIH grant R21A1059661 (to R.H.G.).
The use of trade names is for identification only and does not imply endorsement by the Public Health Service or by the U.S. Department of Health and Human Services. The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention.
Footnotes
Published ahead of print on 21 April 2010.
REFERENCES
- 1.Alcantara, C. S., C.-H. Yang, T. S. Steiner, L. J. Barrett, A. A. M. Lima, C. L. Chappell, P. C. Okhuysen, A. C. White, Jr., and R. L. Guerrant. 2003. Interleukin-8, tumor necrosis factor-α, and lactoferrin in immunocompetent hosts with experimental and Brazilian children with acquired cryptosporidiosis. Am. J. Trop. Med. Hyg. 68:325-328. [PubMed] [Google Scholar]
- 2.Arevalo-Pinzon, G., H. Curtidor, C. Reyes, M. Pinto, C. Vizcaino, M. A. Patarroyo, and M. E. Patarroyo. 2010. Fine mapping of Plasmodium falciparum ribosomal phosphoproteins PfP0 revealed sequences with highly specific binding activity to human red blood cells. J. Mol. Med. 88:61-74. [DOI] [PubMed] [Google Scholar]
- 3.Arrowood, M. J., and C. R. Sterling. 1987. Isolation of Cryptosporidium oocysts and sporozoites using discontinuous sucrose and isopycnic Percoll gradients. J. Parasitol. 73:314-319. [PubMed] [Google Scholar]
- 4.Bern, C., B. Hernandez, M. B. Lopez, M. J. Arrowood, A. M. De Merida, and R. E. Klein. 2000. The contrasting epidemiology of Cyclospora and Cryptosporidium among outpatients in Guatemala. Am. J. Trop. Med. Hyg. 63:231-235. [PubMed] [Google Scholar]
- 5.Bern, C., Y. Ortega, W. Checkley, J. M. Roberts, A. G. Lescano, L. Cabrera, M. Verastegui, R. E. Black, C. Sterling, and R. H. Gilman. 2002. Epidemiologic differences between cyclosporiasis and cryptosporidiosis in Peruvian children. Emerg. Infect. Dis. 8:581-585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brooks-Worrell, B. M., and G. A. Splitter. 1992. Antigens of Brucella abortus S19 immunodominant for bovine lymphocytes as identified by one- and two-dimensional cellular immunoblotting. Infect. Immun. 60:2459-2464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.CDC. 1998. Foodborne outbreak of cryptosporidiosis—Spokane, Washington, 1997. MMWR Morb. Mortal. Wkly. Rep. 227:565-567. [PubMed] [Google Scholar]
- 8.Chacin-Bonilla, L., and Y. Sanchez-Chavez. 2000. Intestinal parasitic infections, with a special emphasis on cryptosporidiosis, in Amerindians from western Venezuela. Am. J. Trop. Med. Hyg. 62:347-352. [DOI] [PubMed] [Google Scholar]
- 9.Chatterjee, S., S. Singh, R. Sohoni, N. J. Singh, A. Vaidya, C. Long, and S. Sharma. 2000. Antibodies against ribosomal phosphoprotein P0 of Plasmodium falciparum protect mice against challenge with Plasmodium yoelii. Infect. Immun. 68:4312-4318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chatterjee, S., S. Singh, R. Sohoni, V. Kattige, C. Deshpande, S. Chiplunkar, N. Kumar, and S. Sharma. 2000. Characterization of domains of the phosphoprotein P0 of Plasmodium falciparum. Mol. Biochem. Parasitol. 107:143-154. [DOI] [PubMed] [Google Scholar]
- 11.Crump, J. A., C. E. Mendoza, J. W. Priest, R. I. Glass, S. S. Monroe, L. A. Dauphin, W. F. Bibb, M. B. Lopez, M. Alvarez, E. D. Mintz, and S. P. Luby. 2007. Comparing serologic response against enteric pathogens with reported diarrhea to assess the impact of improved household drinking water quality. Am. J. Trop. Med. Hyg. 77:136-141. [PubMed] [Google Scholar]
- 12.Dalrymple, B. P., and J. M. Peters. 1992. Identification of L10e/L12e ribosomal protein genes in Babesia bovis. Nucleic Acids Res. 20:2376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.DuPont, H. L., C. L. Chappell, C. R. Sterling, P. C. Okhuysen, J. B. Rose, and W. Jackubowski. 1995. The infectivity of Cryptosporidium parvum in healthy volunteers. N. Engl. J. Med. 332:855-859. [DOI] [PubMed] [Google Scholar]
- 14.Dworkin, M. S., D. P. Goldman, T. G. Wells, J. M. Kobayashi, and B. L. Herwaldt. 1996. Cryptosporidiosis in Washington State: an outbreak associated with well water. J. Infect. Dis. 174:1372-1376. [DOI] [PubMed] [Google Scholar]
- 15.Eberhard, M. L., E. K. Nace, A. R. Freeman, T. G. Streit, A. J. DaSilva, and P. J. Lammie. 1999. Cyclospora cayetanensis infections in Haiti: a common occurrence in the absence of watery diarrhea. Am. J. Trop. Med. Hyg. 60:584-586. [DOI] [PubMed] [Google Scholar]
- 16.Elkon, K., E. Bonfa, R. Llovet, W. Danho, and H. Weissbach. 1988. Properties of the ribosomal P2 protein autoantigen are similar to those of foreign protein antigens. Proc. Natl. Acad. Sci. U. S. A. 85:5186-5189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Elkon, K., S. Skelly, A. Parnassa, W. Moller, W. Danho, H. Weissbach, and N. Brot. 1986. Identification and chemical synthesis of a ribosomal protein antigenic determinant in systemic lupus erythematosus. Proc. Natl. Acad. Sci. U. S. A. 83:7419-7423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fillol, F., J. B. SArr, D. Boulanger, B. Cisse, C. Sokhna, G. Riveau, K. B. Simondon, and F. Remoue. 2009. Impact of child malnutrition on the specific anti-Plasmodium falciparum antibody response. Malar. J. 8:116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Freeman, A. R., P. J. Lammie, R. Houston, M. D. LaPointe, T. G. Streit, P. L. Jooste, J. M. Brissau, J. G. Lafontant, and D. G. Addiss. 2001. A community-based trial for the control of lymphatic filariasis and iodine deficiency using salt fortified with diethylcarbamazine and iodine. Am. J. Trop. Med. Hyg. 65:865-871. [DOI] [PubMed] [Google Scholar]
- 20.Frost, F. J., M. Roberts, T. W. Kunde, G. Craun, K. Tollestrup, L. Harter, and T. Muller. 2005. How clean must our drinking water be: the importance of protective immunity. J. Infect. Dis. 191:809-814. [DOI] [PubMed] [Google Scholar]
- 21.Frost, F. J., T. B. Muller, R. L. Caldreon, and G. F. Craun. 2004. Analysis of serological responses to Cryptosporidium antigen among NHANES III participants. Ann. Epidemiol. 14:473-478. [DOI] [PubMed] [Google Scholar]
- 22.Iborra, S., M. Soto, J. Carrion, A. Nieto, E. Fernandez, C. Alonso, and J. M. Requena. 2003. The Leishmania infantum acidic ribosomal protein P0 administered as a DNA vaccine confers protective immunity to Leishmania major infection in BALB/c mice. Infect. Immun. 71:6562-6572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jones, J. L., D. Kruszon-Moran, and M. Wilson. 2003. Toxoplasma gondii infection in the United States, 1999-2000. Emerg. Infect. Dis. 9:1371-1374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Karanis, P., C. Kourenti, and H. Smith. 2007. Waterborne transmission of protozoan parasites: a worldwide review of outbreaks and lessons learnt. J. Water Health 5:1-38. [DOI] [PubMed] [Google Scholar]
- 25.Kirkpatrick, B. D., M. M. Daniels, S. S. Jean, J. W. Pape, C. Karp, B. Littenberg, D. W. Fitzgerald, H. M. Ledermen, J. P. Nataro, and C. L. Sears. 2002. Cryptosporidiosis stimulates an inflammatory intestinal response in malnourished Haitian children. J. Infect. Dis. 186:94-101. [DOI] [PubMed] [Google Scholar]
- 26.Kosek, M., C. Alcantara, A. A. M. Lima, and R. L. Guerrant. 2001. Cryptosporidiosis: an update. Lancet Infect. Dis. 1:262-269. [DOI] [PubMed] [Google Scholar]
- 27.Koumans, E. H., D. J. Katz, J. M. Malecki, S. Kumar, S. P. Wahlquist, M. J. Arrowood, A. W. Hightower, and B. L. Herwaldt. 1998. An outbreak of cyclosporiasis in Florida in 1995: a harbinger of multistate outbreaks in 1996 and 1997. Am. J. Trop. Med. Hyg. 59:235-242. [DOI] [PubMed] [Google Scholar]
- 28.Kramer, M. H., F. E. Sorhage, S. T. Goldstein, E. Dalley, S. P. Wahlquist, and B. L. Herwaldt. 1998. First reported outbreak in the United States of cryptosporidiosis associated with a recreational lake. Clin. Infect. Dis. 26:27-33. [DOI] [PubMed] [Google Scholar]
- 29.Laemmli, U. K. 1970. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227:680-685. [DOI] [PubMed] [Google Scholar]
- 30.Larkin, M. A., G. Blackshields. N. P. Brown, R. Chenna, P. A. McGettigan, H. McWilliam, F. Valentin, I. M. Wallace, A. Wilm, R. Lopez, J. D. Thompson, T. J. Gibson, and D. G. Higgins. 2007. ClustalW and ClustalX version 2. Bioinformatics 23:2947-2948. [DOI] [PubMed] [Google Scholar]
- 31.Laupland, K. B., and D. L. Church. 2005. Population-based laboratory surveillance for Giardia sp. and Cryptosporidium sp. infections in a large Canadian health region. BMC Infect. Dis. 5:72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Levin, M. J., M. Vasquez, D. Kaplan, and A. G. Schijman. 1993. The Trypanosoma cruzi ribosomal P protein family: classification and antigenicity. Parasitol. Today 9:381-384. [DOI] [PubMed] [Google Scholar]
- 33.Lobo, C. A., S. K. Kar, B. Ravindran, L. Kabilan, and S. Sharma. 1994. Novel proteins of Plasmodium falciparum identified by differential immunoscreening using immune and patient sera. Infect. Immun. 62:651-656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lopez, A. S., J. M. Bendik, J. Y. Alliance, J. M. Roberts, A. J. daSilva, I. N. S. Moura, M. J. Arrowood, M. L. Eberhard, and B. L. Herwaldt. 2003. Epidemiology of Cyclospora cayetanensis and other intestinal parasites in a community in Haiti. J. Clin. Microbiol. 41:2047-2054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.MacKenzie, W. R., N. J. Hoxie, M. E. Proctor, M. S. Gradus, K. A. Blair, D. E. Peterson, J. J. Kazmierczak, D. G. Addiss, K. R. Fox, J. B. Rose, and J. P. Davis. 1994. A massive outbreak in Milwaukee of Cryptosporidium infection transmitted through the public water supply. N. Engl. J. Med. 331:161-167. [DOI] [PubMed] [Google Scholar]
- 36.Mahler, M., K. Kessenbrock, J. Raats, R. Williams, M. J. Fritzler, and M. Bluthner. 2003. Characterization of the human autoimmune response to the major C-terminal epitope of the ribosomal P proteins. J. Mol. Med. 81:194-204. [DOI] [PubMed] [Google Scholar]
- 37.McAnulty, J. M., D. W. Fleming, and A. H. Gonzalez. 1994. A community-wide outbreak of cryptosporidiosis associated with swimming at a wave pool. JAMA 272:1597-1600. [PubMed] [Google Scholar]
- 38.McDonald, A. C., W. R. MacKenzie, D. G. Addiss, M. S. Gradus, G. Linke, E. Zembrowski, M. R. Hurd, M. J. Arrowood, P. J. Lammie, and J. W. Priest. 2001. Cryptosporidium parvum-specific antibody responses among children residing in Milwaukee during the 1993 waterborne outbreak. J. Infect. Dis. 183:1373-1379. [DOI] [PubMed] [Google Scholar]
- 39.Millard, P. S., K. F. Gensheimer, D. G. Addiss, D. M. Sosin, G. A. Beckett, A. Houck-Jankoski, and A. Hudson. 1994. An outbreak of cryptosporidiosis from fresh-pressed apple cider. JAMA 272:1592-1596. [PubMed] [Google Scholar]
- 40.Mondal, D., R. Haque, R. B. Sack, B. D. Kirkpatrick, and W. A. Petri, Jr. 2009. Attribution of malnutrition to cause-specific diarrheal illness: evidence from a prospective study of preschool children in Mirpur, Dhaka, Bangladesh. Am. J. Trop. Med. Hyg. 80:824-826. [PMC free article] [PubMed] [Google Scholar]
- 41.Morrison, H. G., A. G. McArthur, F. D. Hillen, S. B. Aley, R. D. Adam, G. J. Olsen, A. A. Best, et al. 2007. Genomic minimalism in the early diverging intestinal parasite Giardia lamblia. Science 317:1921-1926. [DOI] [PubMed] [Google Scholar]
- 42.Moss, D. M., and P. J. Lammie. 1993. Proliferative responsiveness of lymphocytes from Cryptosporidium parvum-exposed mice to two separate antigen fractions from oocysts. Am. J. Trop. Med. Hyg. 49:393-401. [DOI] [PubMed] [Google Scholar]
- 43.Moss, D. M., C. L. Chappell, P. C. Okhuysen, H. L. DuPont, M. J. Arrowood, A. W. Hightower, and P. J. Lammie. 1998. The antibody response to 27-, 17-, and 15-kDa Cryptosporidium antigens following experimental infection in humans. J. Infect. Dis. 178:827-833. [DOI] [PubMed] [Google Scholar]
- 44.Moss, D. M., H. M. Mathews, G. S. Visvisvara, J. W. Dickerson, and E. M. Walker. 1991. Purification and characterization of Giardia lamblia antigens from the feces of Mongolian gerbils. J. Clin. Microbiol. 29:21-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Moss, D. M., J. M. Montgomery, S. V. Newland, J. W. Priest, and P. J. Lammie. 2004. Detection of Cryptosporidium antibodies in sera and oral fluids using multiplex bead assay. J. Parasitol. 90:397-404. [DOI] [PubMed] [Google Scholar]
- 46.Moss, D. M., S. N. Bennett, M. J. Arrowood, S. P. Wahlquist, and P. J. Lammie. 1998. Enzyme-linked immunoelectrotransfer blot analysis of a cryptosporidiosis outbreak on a United States Coast Guard cutter. Am. J. Trop. Med. Hyg. 58:110-118. [DOI] [PubMed] [Google Scholar]
- 47.Newman, R. D., S. R. Moore, A. A. M. Lima, J. P. Nataro, R. L. Guerrant, and C. L. Sears. 2001. A longitudinal study of Giardia lamblia infection in north-east Brazilian children. Trop. Med. Int. Health 6:624-634. [DOI] [PubMed] [Google Scholar]
- 48.Newman, R. D., T. Wuhib, A. A. M. Lima, R. L. Guerrant, and C. L. Sears. 1993. Environmental sources of Cryptosporidium in an urban slum in northeastern Brazil. Am. J. Trop. Med. Hyg. 49:270-275. [DOI] [PubMed] [Google Scholar]
- 49.Noland, G. S., B. Hendel-Paterson, X. M. Min, A. M. Moormann, J. M. Vulule, D. L. Narum, D. E. Lanar, J. W. Kazura, and C. C. John. 2008. Low prevalence of antibodies to preerythrocytic but not blood-stage Plasmodium falciparum antigens in an area of unstable malaria transmission compared to prevalence in an area of stable malaria transmission. Infect. Immun. 76:5721-5728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Pape, J. W., E. Levine, M. E. Beaulieu, F. Marshall, R. Verdier, and W. D. Johnson, Jr. 1987. Cryptosporidiosis in Haitian children. Am. J. Trop. Med. Hyg. 36:333-337. [DOI] [PubMed] [Google Scholar]
- 51.Priest, J. W., A. Li, M. D. Khan, M. J. Arrowood, P. J. Lammie, C. S. Ong, J. M. Roberts, and J. Isaac-Renton. 2001. Enzyme immunoassay detection of antigen-specific immunoglobulin G antibodies in longitudinal serum samples from patients with cryptosporidiosis. Clin. Diagn. Lab. Immunol. 8:415-423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Priest, J. W., A. Mehlert, D. M. Moss, M. J. Arrowood, and M. A. J. Ferguson. 2006. Characterization of the glycosylphosphatidylinositol anchor of the immunodominant Cryptosporidium parvum 17-kDa antigen. Mol. Biochem. Parasitol. 149:108-112. [DOI] [PubMed] [Google Scholar]
- 53.Priest, J. W., C. Bern, J. M. Roberts, J. P. Kwon, A. G. Lescano, W. Checkley, L. Cabrera, D. M. Moss, M. J. Arrowood, C. R. Sterling, R. H. Gilman, and P. J. Lammie. 2005. Changes in serum immunoglobulin G levels as a marker for Cryptosporidium sp. infection in Peruvian children. J. Clin. Microbiol. 43:5298-5300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Priest, J. W., C. Bern, L. Xiao, J. M. Roberts, J. P. Kwon, A. G. Lescano, W. Checkley, L. Cabrera, D. M. Moss, M. J. Arrowood, C. R. Sterling, R. H. Gilman, and P. J. Lammie. 2006. Longitudinal analysis of Cryptosporidium species-specific immunoglobulin G antibody responses in Peruvian children. Clin. Vaccine Immunol. 13:123-131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Priest, J. W., J. P. Kwon, D. M. Moss, J. M. Roberts, M. J. Arrowood, M. S. Dworkin, D. D. Juranek, and P. J. Lammie. 1999. Detection by enzyme immunoassay of serum immunoglobulin G antibodies that recognize specific Cryptosporidium parvum antigen. J. Clin. Microbiol. 37:1385-1392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Priest, J. W., J. P. Kwon, M. J. Arrowood, and P. J. Lammie. 2000. Cloning of the immunodominant 17-kDa antigen from Cryptosporidium parvum. Mol. Biochem. Parasitol. 106:261-271. [DOI] [PubMed] [Google Scholar]
- 57.Priest, J. W., L.-T. Xie, M. J. Arrowood, and P. J. Lammie. 2001. The immunodominant 17-kDa antigen from Cryptosporidium parvum is glycosylphosphatidylinositol-anchored. Mol. Biochem. Parasitol. 113:117-126. [DOI] [PubMed] [Google Scholar]
- 58.Quiroz, E. S., C. Bern, J. R. MacArthur, L. Xiao, M. Fletcher, M. J. Arrowood, D. K. Shay, M. E. Levy, R. I. Glass, and A. Lal. 2000. An outbreak of cryptosporidiosis linked to a foodhandler. J. Infect. Dis. 181:695-700. [DOI] [PubMed] [Google Scholar]
- 59.Raccurt, C. P., P. Brasseur, R. I. Verdier, X. Li, E. Eyma, C. P. Stockman, P. Agamey, K. Guyot, A. Totet, B. Liautaud, G. Navez, E. Dei-Cas, and J. W. Pape. 2006. Human cryptosporidiosis and Cryptosporidium spp. in Haiti. Trop. Med. Int. Health 11:929-934. [DOI] [PubMed] [Google Scholar]
- 60.Rajeshwari, K., K. Patel, S. Nambeesan, M. Mehta, A. Sehgal, T. Chakraborty, and S. Sharma. 2004. The P domain of the P0 protein of Plasmodium falciparum protects against challenge with malaria parasites. Infect. Immun. 72:5515-5521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Rich, B. E., and J. A. Steitz. 1987. Human acidic ribosomal phosphoproteins P0, P1, and P2: analysis of cDNA clones, in vitro synthesis, and assembly. Mol. Cell. Biol. 7:4065-4074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Sato, T., T. Uchiumi, T. Ozawa, M. Kikuchi, M. Nakano, R. Kominami, and M. Arakawa. 1991. Autoantibodies against ribosomal proteins found with high frequency in patients with systemic lupus erythematosus with active disease. J. Rheumatol. 18:1681-1684. [PubMed] [Google Scholar]
- 63.Sehgal, A., N. Kumar, V. B. Carruthers, and S. Sharma. 2003. Translocation of ribosomal protein P0 onto the Toxoplasma gondii tachyzoite surface. Int. J. Parasitol. 33:1589-1594. [DOI] [PubMed] [Google Scholar]
- 64.Singh, S., A. Sehgal, S. Waghmare, T. Chakraborty, A. Goswami, and S. Sharma. 2002. Surface expression of the conserved ribosomal protein P0 on parasite and other cells. Mol. Biochem. Parasitol. 119:121-124. [DOI] [PubMed] [Google Scholar]
- 65.Skeiky, Y. A. W., D. R. Benson, J. A. Guderian, P. R. Sleath, M. Parsons, and S. G. Reed. 1993. Trypanosoma cruzi acidic ribosomal P protein gene family. Novel P proteins encoding unusual cross-reactive epitopes. J. Immunol. 151:5504-5515. [PubMed] [Google Scholar]
- 66.Skeiky, Y. A. W., D. R. Benson, M. Elwasila, R. Badaro, J. M. Burns, and S. G. Reed. 1994. Antigens shared by Leishmania species and Trypanosoma cruzi: immunological comparison of the acidic ribosomal P0 proteins. Infect. Immun. 62:1643-1651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Skeiky, Y. A. W., D. R. Benson, M. Parsons, K. B. Elkon, and S. G. Reed. 1992. Cloning and expression of Trypanosoma cruzi ribosomal protein P0 and epitope analysis of anti-P0 autoantibodies in Chagas' disease patients. J. Exp. Med. 176:201-211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Smith, L. M., J. W. Priest, P. J. Lammie, and J. R. Mead. 2001. Human T and B cell immunoreactivity to a recombinant 23-kDa Cryptosporidium parvum antigen. J. Parasitol. 87:704-707. [DOI] [PubMed] [Google Scholar]
- 69.Soto, M., J. M. Requena, L. Quijada, and C. Alonso. 1996. Specific serodiagnosis of human leishmaniasis with recombinant Leishmania P2 acidic ribosomal proteins. Clin. Diagn. Lab. Immunol. 3:387-391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Soto, M., J. M. Requena, L. Quijada, S. O. Angel, L. C. Gomez, F. Guzman, M. E. Patarroyo, and C. Alonso. 1995. During active viscerocutaneous leishmaniasis the anti-P2 humoral response is specifically triggered by the parasite P proteins. Clin. Exp. Immunol. 100:246-252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Steinberg, E. B., C. E. Mendoza, R. Glass, B. Arana, M. B. Lopez, M. Mejia, B. D. Gold, J. W. Priest, W. Bibb, S. S. Monroe, C. Bern, B. P. Bell, R. M. Hoekstra, R. Klein, E. D. Mintz, and S. Luby. 2004. Prevalence of infection with waterborne pathogens: a seroepidemiologic study in children 6-36 months old in San Juan Sacatepequez, Guatemala. Am. J. Trop. Med. Hyg. 70:83-88. [PubMed] [Google Scholar]
- 72.Terkawi, M. A., H. Jia, A. Gabriel, Y.-K. Goo, Y. Nishikawa, N. Yokoyama, I. Igarashi, K. Fujisaki, and X. Xuan. 2007. A shared antigen among Babesia species: ribosomal phosphoprotein P0 as a universal babesial vaccine candidate. Parasitol. Res. 102:35-40. [DOI] [PubMed] [Google Scholar]
- 73.Terkawi, M. A., H. Jia, J. Zhou, E. Lee, I. Igarashi, K. Fujisaki, Y. Nishikawa, and X. Xuan. 2007. Babesia gibsoni ribosomal phosphoprotein P0 induces cross-protective immunity against B. microti infection in mice. Vaccine 25:2027-2035. [DOI] [PubMed] [Google Scholar]
- 74.Teutsch, S. M., D. D. Juranek, A. Sulzer, J. P. Dubey, and R. K. Sikes. 1979. Epidemic toxoplasmosis associated with infected cats. N. Engl. J. Med. 300:695-699. [DOI] [PubMed] [Google Scholar]
- 75.Tsang, V. C. W., and P. P. Wilkins. 1991. Optimum dissociating condition for immunoaffinity and preferential isolation of antibodies with high specific activity. J. Immunol. Methods 138:291-299. [DOI] [PubMed] [Google Scholar]
- 76.Ungar, B. L. P., R. H. Gilman, C. F. Lanata, and I. Perez-Schael. 1988. Seroepidemiology of Cryptosporidium infection in two Latin American populations. J. Infect. Dis. 157:551-556. [DOI] [PubMed] [Google Scholar]
- 77.Vard, C., D. Guillot, P. Bargis, J.-P. Lavergne, and J.-P. Reboud. 1997. A specific role for the phosphorylation of mammalian acidic ribosomal protein P2. J. Biol. Chem. 272:20259-20262. [DOI] [PubMed] [Google Scholar]
- 78.Xiao, L., C. Bern, J. Limor, I. Sulaiman, J. Roberts, W. Checkley, L. Cabrera, R. H. Gilman, and A. A. Lal. 2001. Identification of 5 types of Cryptosporidium parasites in children in Lima, Peru. J. Infect. Dis. 183:492-497. [DOI] [PubMed] [Google Scholar]
- 79.Zhang, H., E. Lee, M. Liao, M. K. A. Compaore, G. Zhang, O. Kawase, K. Fujisaki, C. Sugimoto, Y. Nishikawa, and X. Xuan. 2007. Identification of ribosomal phosphoprotein P0 of Neospora caninum as a potential common vaccine candidate for control of both neosporosis and toxoplasmosis. Mol. Biochem. Parasitol. 153:141-148. [DOI] [PubMed] [Google Scholar]
- 80.Zu, S.-X., J.-F. Li, L. J. Barrett, R. Fayer, S.-Y. Shu, J. F. McAuliffe, J. K. Roche, and R. L. Guerrant. 1994. Seroepidemiologic study of Cryptosporidium infection in children from rural communities of Anhui, China and Fortaleza, Brazil. Am. J. Trop. Med. Hyg. 51:1-10. [DOI] [PubMed] [Google Scholar]
- 81.Zweig, M. H., and G. Campbell. 1993. Receiver-operating characteristic (ROC) plots: a fundamental evaluation tool in clinical medicine. Clin. Chem. 39:561-577. [PubMed] [Google Scholar]