Abstract
Although worldwide leprosy prevalence has been reduced considerably following multidrug therapy, new case detection rates remain relatively stable, suggesting that transmission of infection still continues. This calls for new efforts, among which is development of assays that can identify subclinical/early-stage Mycobacterium leprae-infected subjects, a likely source of transmission. Areas in which leprosy is endemic often lack sophisticated laboratories, necessitating development of field-friendly immunodiagnostic tests for leprosy, like short-term whole-blood assays (WBA). In classical, peripheral blood mononuclear cell (PBMC)-based gamma interferon (IFN-γ) release assays, M. leprae peptides have been shown to discriminate in a more specific fashion than M. leprae proteins between M. leprae-exposed contacts and patients as opposed to healthy controls from the same area of endemicity. However, peptides induced significantly lower levels of IFN-γ than did proteins, particularly when whole blood was used. Therefore, possibilities of specifically enhancing IFN-γ production in response to M. leprae peptides in 24-h WBA were sought by addition of various cytokines and antibodies or by mannosylation of peptides. In addition, other cytokines and chemokines were analyzed as potential biomarkers in WBA. We found that only interleukin 12 (IL-12), not other costimulants, increased IFN-γ production in WBA while maintaining M. leprae peptide specificity, as evidenced by lack of increase of IFN-γ in control samples stimulated with IL-12 alone. The IL-12-induced increase in IFN-γ was mainly mediated by CD4+ T cells that did not produce IL-2 or tumor necrosis factor (TNF). Mannosylation further allowed the use of 100-fold-less peptide. Although not statistically significantly, macrophage inflammatory protein 1β (MIP-1β) and macrophage c protein 1 (MCP-1) levels specific for M. leprae peptide tended to be increased by IL-12. IP-10 production was also found to be a useful marker of M. leprae peptide responses, but its production was enhanced by IL-12 nonspecifically. We conclude that IFN-γ-based WBA combined with IL-12 represents a more sensitive and robust assay for measuring reactivity to M. leprae peptides.
Leprosy is a disabling and stigmatizing disease caused by infection with Mycobacterium leprae. The characteristic immunological and clinical leprosy spectrum, classified by Ridley and Jopling in 1966 (25), ranges from tuberculoid (TT) or paucibacillary (PB) leprosy to lepromatous (LL) or multibacillary (MB) leprosy. In between these poles the borderline states borderline lepromatous (BL), borderline borderline (BB), and borderline tuberculoid (BT) leprosy are positioned. TT/BT patients in general show high cellular responses to M. leprae antigens injected in the skin as well as in in vitro T-cell assays; have low antibody titers to M. leprae antigens, including phenolic glycolipid I (PGL-I); and develop localized granulomatous disease with few, if any, detectable bacilli in their lesions. At the opposite end of the spectrum are LL/BL patients with a characteristic inability to generate M. leprae-specific Th1-cell responses and with disseminating progressive infection and high antibody titers to M. leprae antigens, including the M. leprae-specific cell surface antigen PGL-I.
Over the last 2 decades the WHO leprosy elimination program, partly in combination with wide coverage of Mycobacterium bovis BCG vaccination (28), has had a massive effect on the registered number of cases, which dropped from approximately 5.4 million in 1985 to 212,802 worldwide at the beginning of 2008. In addition, since 2003 the global number of new cases detected showed a drastic decrease at an average rate of nearly 20% per year, and a reported year-end prevalence below 1 per 10,000 was obtained in 2007 in all countries with a population of >1 million except for Brazil, Nepal, and East Timor (32). However, part of the decrease was achieved by changing leprosy control policies and does not necessarily reflect the reality of infection. Concomitantly, the elimination campaign has had a severe downside as it led to a discontinuation of leprosy control programs and a decrease in leprosy clinics, specialists, and research. Thus, leprosy patients have to be treated in integrated programs, where health workers lack the knowledge and time to diagnose and treat leprosy. This resulted in sustained transmission as evidenced by the hundreds of thousands of new cases of leprosy that keep being detected globally every year (254,525 in 2007) and a 3.1% increase between 2007 and 2008 of new case detection in children (32). In addition, countries that do not exceed this prevalence rate nationwide still harbor regions of high endemicity, where leprosy remains a public health problem (e.g., Angola, Central African Republic, India, and Tanzania). These figures demonstrate that M. leprae-infected contacts and persons with subclinical, undiagnosed leprosy, likely the major sources of unidentified transmission, are an incessant source of active transmission, making early detection of leprosy or M. leprae infection and prompt multidrug treatment (MDT) of utmost importance for control of the disabling effects of leprosy.
Diagnosis of leprosy is at present based only on clinical features and the number of skin lesions. Due to the loss of diagnostic skills and the decrease in skin smear services, the detection of M. leprae infection occurs in many cases only after significant and irreversible nerve damage has occurred. Since M. leprae is not cultivable in vitro, bacterial enumeration by microscopic examination is required for leprosy classification, monitoring chemotherapy regimens, and diagnosis of relapse, yielding, in inexperienced hands, data of limited specificity and sensitivity. There is no test available that can detect asymptomatic M. leprae infection or predict progression of infection to clinical disease. Assays that demonstrate the presence of IgM antibodies against PGL-I are useful for most MB patients but have limited value in identifying or predicting PB patients who typically develop cellular rather than humoral immunity (23).
In order to assess host immune responses after exposure to or infection with mycobacteria, the ex vivo whole-blood assay (WBA) is a helpful test. In the past, several variations of the WBA have been introduced in which unseparated heparinized blood is stimulated with antigen either overnight or for as long as 6 days, after which plasma or supernatant is analyzed for cytokines (8). Since WBAs are much simpler and faster than conventional assays using peripheral blood mononuclear cells (PBMC), they have been successfully applied in commercially available gamma interferon (IFN-γ) release assays (IGRAs) for diagnosis of tuberculosis (TB) (QuantiFERON-TB-Gold-In-Tube [QFT-G-IT]). These IGRAs have a number of advantages over skin tests (e.g., Mitsuda or Mantoux tests), as they can measure directly from whole blood within a single day, do not require repeated visits for reading of the test, and do not carry the inherent risk of boosting upon repeated testing. Furthermore, there are no exclusion criteria, as the test can also be performed for patients with skin disorders.
To measure responses to recombinant M. leprae proteins and whole-cell sonicate of M. leprae, 6-day WBAs measuring IFN-γ in diluted blood samples have proved successful at field sites in various countries such as Nepal (33) and Malawi (4, 34). Since 6-day WBAs require access to a CO2 incubator, our investigations focused on the development of a practical diagnostic assay on 24-h WBA. Furthermore, as (mixtures of) M. leprae peptides proved to induce more specific IFN-γ release by PBMC than did recombinant M. leprae proteins (16, 29), we previously used M. leprae peptides for the 24-h WBA (17) in analogy to the successful peptide-based QuantiFERON-TB-Gold-In-Tube (11). However, the shortened incubation period hampered the use of M. leprae peptides in WBA, as IFN-γ production induced was weak in comparison to that when M. leprae proteins were used (17, 29). Therefore, in the present study we analyzed other cytokines/chemokines as potential readout for WBA and tested possible enhancement of IFN-γ production in response to M. leprae peptides by addition of several cytokines, antibodies against factors that block the optimal release of IFN-γ, or antibodies that can trigger costimulation.
MATERIALS AND METHODS
Synthetic peptides.
Peptides were produced at the Leiden University Medical Center (LUMC) facility by simultaneous multiple-peptide synthesis as described previously (19). Homogeneity and purity were confirmed by analytical reversed-phase high-pressure liquid chromatography (HPLC), and matrix-assisted laser desorption ionization-time of flight (MALDI-TOF) mass spectrometry showed the expected masses. Purity of the peptides was ≥75%. Synthetic peptides overlapping the complete sequence of ML2531 were produced as nine 15-mers overlapping 10 amino acids. ML2531 peptide 1-15 (MTQIMYNYPAMLDHA) and ML2531 peptide 11-25 (MLDHAGNMSACAGAL) were also synthesized in an N-terminal mannosylated version as described previously (30a). In short, mannosylation was accomplished by N-terminal elongation of the peptide with a building block containing lysine coupled to two tetra-acetyl-protected mannose groups. ML2531 p1-15 and p11-25 were elongated with bisacetyl lysine only, and the acetyl-protecting groups on the mannose moieties were removed using Tesser's base.
Recombinant ML2531 protein.
The ML2531 gene was amplified by PCR from genomic DNA of M. leprae and cloned using the Gateway technology platform (Invitrogen, Carlsbad, CA) with the pDEST17 expression vector containing an N-terminal histidine tag (Invitrogen, Carlsbad, CA) (14). Sequencing was performed on selected clones to confirm the identity of all cloned DNA fragments. Recombinant proteins were overexpressed in Escherichia coli BL21(DE3) and purified as described to remove any traces of endotoxin. The purified protein was analyzed by 12% SDS-PAGE followed by Coomassie brilliant blue staining and Western blotting with an anti-His antibody (Invitrogen, Carlsbad, CA) to confirm size and purity. Endotoxin contents were below 50 IU per mg recombinant protein as tested using a Limulus amebocyte lysate (LAL) assay (Cambrex, East Rutherford, NJ). To exclude protein nonspecific IFN-γ release or cellular toxicity, ML2531 was tested in IFN-γ release assays using PBMC or whole blood of M. leprae-unexposed, BCG-negative, Mantoux skin test-negative healthy donors.
Study subjects.
Leprosy patients were recruited at the Leiden University Medical Center (LUMC; Leiden, Netherlands). Informed consent was obtained from all individuals before venepuncture. Ethical approval of the study protocol was obtained through the appropriate local ethics committees.
Whole-blood assays (WBAs).
Within 2 h of venepuncture, undiluted, heparinized blood (450 μl per well) was incubated in 48-well cell cluster plates (Costar; Corning Incorporated) at 37°C, 5% CO2, and 70% relative humidity with 50 μl of antigen solution at a final concentration of 10 μg/ml, in the presence or absence of 50 μl cytokines or the following antibodies: interleukin 2 (IL-2; 10 U/ml; Cetus, Emeryville, CA), IL-7 (0.1, 1.0, or 10 pg/ml; eBioscience Ltd., Hatfield, United Kingdom), IL-12 (40, 200, or 1,000 pg/ml; R&D Systems Europe, Abingdon, United Kingdom), IL-18 (15, 75, or 275 pg/ml; MBL International Corporation, Woburn, MA), IL-23 (10, 50, or 250 pg/ml; R&D Systems Europe, Abingdon, United Kingdom), purified rat anti-human IL-10 (1 μg/ml; BD Pharmingen, San Jose, CA), purified rat IgG2aκ anti-IL-10 isotype (1 μg/ml; BD Biosciences Pharmingen, San Jose, CA), mouse anti-human CD49d (1 μg/ml; BD Biosciences, Eerbodegem, Belgium), and mouse anti-human CD28 (1 μg/ml; Sanquin, Amsterdam, Netherlands). Recombinant human CD40L (rhCD40L; Sigma, St. Louis, MO) and antibody against CD40 (anti-CD40 monoclonal antibody [MAb]; R&D Systems Europe, Abingdon, United Kingdom) were both used at a final concentration of 1 μg/ml. Blood was added to each well within 2 h of collection. After 20 to 24 h of culture, 300 μl of supernatants was removed from each well and frozen in aliquots at −20°C until further analysis.
IFN-γ ELISA.
IFN-γ levels were determined by enzyme-linked immunosorbent assay (ELISA; U-CyTech, Utrecht, Netherlands) (16). Optical density (OD) values were converted into concentrations using Microplate Manager software, version 5.2.1 (Bio-Rad Laboratories, Veenendaal, Netherlands). The cutoff value to define positive responses was set beforehand at 100 pg/ml. The assay sensitivity level was 40 pg/ml. Values for unstimulated whole-blood cultures were typically <20 pg/ml.
Flow cytometry.
Undiluted whole-blood samples were incubated in 48-well plates with antigen, with or without IL-12 (200 pg/ml; R&D Systems Europe, Abingdon, United Kingdom). After 6 h of stimulation, 3 μg/ml brefeldin A (Sigma, St. Louis, MO) was added for the last 16 h. Red blood cells were lysed by addition of a 10-fold excess of fluorescence-activated cell sorting (FACS) lysing solution (BD Biosciences, Eerbodegem, Belgium). Surface staining was performed at 4°C with the labeled antibodies directed against CD4-PerCP (peridinin chlorophyll protein)-Cy5.5 and CD56-PE (phycoerythrin)-Cy5 (both from BD Biosciences, Eerbodegem, Belgium) and CD16 Pacific Blue (BD Biosciences, Eerbodegem, Belgium). After 30 min cells were washed and fixed (4% paraformaldehyde; Dako Diagnostics, Glostrup, Denmark) and intracellular staining was performed using the intrastain kit (Dako Diagnostics, Glostrup, Denmark) with labeled antibodies against CD3-APC (allophycocyanin)-Cy7, CD8-Amcyan, IFN-γ-Alexa 700, IP-10-PE, and IL-2-FITC (fluorescein isothiocyanate) (all from BD Biosciences, Eerbodegem, Belgium); TNF (tumor necrosis factor)-PE-Cy7 (eBioscience Ltd., Hatfield, United Kingdom); and IL-17-Alexa 647 (eBioscience Ltd., Hatfield, United Kingdom). Cells were acquired on a FACS LSRII cell sorter with Diva software (BD Biosciences, Netherlands) and analyzed with FlowJo version 8.7.3 (Tree Star, Ashland, OR) and Spice software (http://exon.niaid.nih.gov/spice/; provided free of charge by Mario Roederer, Vaccine Research Center, NIAID, NIH).
Multiplex determination of cytokines and chemokines.
According to the manufacturer's guidelines, 18 inflammatory and immunomodulatory cytokines or chemokines (IL-1β, IL-2, IL-4, IL-5, IL-6, IL-7, IL-8, IL-10, IL-12p70, IL-13, IL-17, granulocyte colony-stimulating factor [G-CSF], granulocyte-macrophage CSF [GM-CSF], IFN-γ, CXCL10/IP-10, CCL2/monocyte chemoattractant protein 1 [MCP-1], CCL4/macrophage inflammatory protein 1β [MIP-1β], and TNF) were measured in unstimulated, antigen-stimulated, or mitogen-stimulated samples by the Bio-Plex suspension array system powered by Luminex xMAP multiplex technology (Bio-Rad Laboratories, Veenendaal, Netherlands) and analyzed with the Bio-Plex Manager software 4.0 (Bio-Rad Laboratories, Veenendaal, Netherlands). After the filter was prewetted with assay solution, the beads were washed twice with washing solution using 96-well multiscreen filter plates (Millipore), an Aurum vacuum manifold, and a vacuum pump (Bio-Rad Laboratories, Veenendaal, Netherlands). Supernatant samples (50 μl) were added to the plates, and the plates were incubated for 45 min at room temperature in the dark at 300 rpm on a plate shaker. After three washes, 12.5 μl detection antibody cocktail was added per well and plates were incubated at room temperature in the dark for 30 min on a plate shaker. After three washes, 25 μl strepavidin-PE solution was added per well and incubated for 10 min. After three washes, 100 μl of assay solution was added to each well and the plates were placed in the Bio-Plex system. From each well, a minimum of 100 analyte-specific beads were analyzed for fluorescence. Cytokine measurements in plasma from unstimulated and antigen-stimulated whole blood were performed in duplicates; measurements in mitogen-stimulated whole blood were performed once. A curve fit was applied to each standard curve according to the manufacturer's manual. Sample concentrations were interpolated from these standard curves. Analyte concentrations outside the upper or lower limits of quantification were assigned the values of the limits of quantification of the cytokine or chemokine.
Statistical analysis.
Differences in cytokine/chemokine levels between test groups were analyzed with the two-tailed Mann-Whitney U test for nonparametric distribution using Graph Pad Prism (version 4). P values were corrected for multiple comparisons. The statistical significance level used was P < 0.05.
RESULTS
Effects of addition of cytokines or antibodies on IFN-γ release in 24-h undiluted WBAs.
In order to improve the sensitivity of IFN-γ release assays for detection of M. leprae peptide-specific T-cell responses in whole blood, we first determined whether candidate immunomodulators like cytokines or antibodies would increase nonspecific background IFN-γ production. Undiluted whole blood of BT leprosy patients was incubated for 24 h in the absence of specific antigen with (i) cytokines (IL-7, IL-12, IL-18, or IL-23) in concentration ranges described previously (20); (ii) antibodies directed against CD49d and CD28 (18), CD40, or IL-10 (7); or (iii) CD40 ligand (CD40L). Except for IL-18, none of the added compounds induced any increase in IFN-γ release compared to background release, although IL-7 (10 pg/ml) induced an increase in one individual (Fig. 1 a).
FIG. 1.
(a) IFN-γ production measured after 24-h culture of undiluted whole blood derived from BT leprosy patients (n = 8) in the presence of cytokines (IL-7, IL-12, IL-18, or IL-23 at indicated concentrations), CD40L, or antibodies directed against CD49d and CD28, CD40, or IL-10 (all at concentrations of 1 μg/ml). Each symbol represents one individual. The dotted line indicates 3× average medium value. (b) IFN-γ production in response to medium, ML2531 recombinant protein (10 μg/ml), ML2531 peptide 1-15 (10 μg/ml), or ML2531 peptide 11-25 (10 μg/ml) measured by ELISA after 24-h culture of undiluted whole blood of BT leprosy patients (n = 8) in the presence of cytokines (IL-7 [1 pg/ml], IL-12 [200 pg/ml], IL-23 [50 pg/ml], or IL-2 [10 U/ml] with IL-12 [200 pg/ml]) or antibodies directed against IL-10, CD49d and CD28, CD40, or CD40L (all at 1 μg/ml).
To analyze whether the added compounds (except for IL-18) could substantially increase IFN-γ release in response to M. leprae antigens, whole blood of BT leprosy patients was stimulated for 24 h with M. leprae recombinant protein or peptides (Fig. 1b). For optimization of WBA conditions, M. leprae peptides derived from ML2531 were selected. This homolog of M. tuberculosis TB10.4 (Rv0288; 70% identity) is one of the three members of the M. leprae ESAT-6 family (together with ML0049 and ML0363) that has been retained as a functional gene in M. leprae (5) and is recognized by T cells from leprosy patients (10) and healthy controls from areas of endemicity (A. Geluk et al., unpublished data), thereby providing a useful model antigen to study enhancement of responses to its peptides in WBA. By screening IFN-γ release in response to overlapping peptides of ML2531 using whole blood, ML2531 peptide 1-15 was identified as an immunodominant epitope, whereas ML2531 peptide 11-25 was recognized much less frequently (Fig. 1b). IFN-γ release in response to ML2531 or ML2531 p1-15 was significantly (P = 0.0002) increased only after addition of IL-12.
None of the other cytokines induced enhancement of IFN-γ production, and the combination of IL-12 with IL-2 did not significantly further enhance IFN-γ responses compared to IL-12 alone. The average optimal dose for IL-12 that enhanced IFN-γ release but did not increase background responses was determined to be 200 pg/ml (data not shown) and was used for all subsequent experiments. Attempts to decrease the incubation time of the WBA indicated that overnight incubation (20 to 24 h) was required for sensitive detection of IFN-γ after M. leprae peptide stimulation even in the presence of IL-12 (data not shown).
Based on reports by others (3, 18), it was expected that the addition of anti-CD49d with anti-CD28 antibodies to whole-blood cultures would have a significant impact on IFN-γ release. However, anti-CD49d with anti-CD28 antibodies did not enhance IFN-γ release (Fig. 1b). Equally unexpected was that addition of anti-IL-10, which was previously reported to enhance IFN-γ release in ESAT-6/CFP-10-stimulated blood cultures in cattle (7), also failed to enhance IFN-γ release in response to ML2531 antigens. The combination of IL-12 with anti-CD49d and anti-CD28 antibodies did not further increase IFN-γ release compared to IL-12 alone.
CD40-mediated interactions play an important role in the response to infections by affecting the development, activation, proliferation, and differentiation of a variety of immune cells. This led us to examine the immunomodulatory effects of activation of CD40-mediated interactions on IFN-γ release in WBA using soluble human CD40L (rhCD40L) or anti-CD40 antibodies. Again no significant increase in the level of IFN-γ was detected (Fig. 1b).
Effect of mannosylation of M. leprae peptides.
Requirement of only low doses of antigens in commercial assays would be beneficial. Since the serum half-lives of peptides are relatively short, ranging for most peptides from 10 to 100 min (24), protection of the N or C terminus may prevent rapid degradation of peptides and thereby enhance responses to peptides in WBA, potentially allowing the use of lower peptide concentrations. Importantly, for N-terminal mannosylated peptides it was demonstrated that their in vitro uptake by mannose receptor-positive dendritic cells (DC) was facilitated and that similar effects could be obtained with 100- to 1,000-fold-reduced peptide concentrations (30a). Thus, ML2531 p1-15 was bis-mannosylated at the N terminus (designated ML2531 Man-p1-15), and induction of IFN-γ production was analyzed in 24-h WBA with or without addition of IL-12 (Fig. 2). Importantly, we observed that the optimal peptide concentration shifted from 10 μg/ml to 0.1 μg/ml, allowing the use of 100-fold less peptide while maintaining similar sensitivity, and this effect was maintained in the presence of IL-12. However, the increase between the mannosylated and unmannosylated peptides at each concentration was not significant.
FIG. 2.
IFN-γ production in response to different concentrations of ML2531 peptide 1-15 (p1-15) or N-terminal mannosylated peptide 1-15 (Manp1-15) measured by ELISA after 24-h culture of undiluted whole blood in the presence or absence of IL-12 (200 pg/ml). Error bars indicate variations in cytokine production for two experiments performed on two different bleeding dates of one BT leprosy patient.
In-tube IFN-γ assay.
Successful use of a diagnostic assay also depends on the assay format, especially for diagnosis of infectious diseases in remote areas. The QuantiFERON-TB-Gold-In-Tube (QFT-G-IT) assay for diagnosis of M. tuberculosis infection therefore uses tubes coated with antigen by lyophilization for collecting and subsequent incubation of heparinized blood samples. Thus, we next explored whether lyophilization of M. leprae peptide or protein with IL-12 prior to a 24-h in-tube incubation period influenced the production of IFN-γ. To this end, we lyophilized M. leprae antigens together with IL-12 in tubes before incubation with fresh heparinized blood samples. Simultaneously, identical blood samples were incubated with M. leprae antigens and IL-12 freshly prepared from stock solutions. Figure 3 shows that lyophilization of ML2531 or ML2531 p1-15 did not influence production of IFN-γ nor did it affect the additive effect of IL-12.
FIG. 3.
Effect on IFN-γ production of in-tube lyophilization of antigenic stimuli (10 μg/ml) in the absence (A) or presence (B) of IL-12 before 24-h culture of undiluted whole blood of BT leprosy patients (n = 8). Results are shown for fresh (•) or lyophilized (▴) stimuli. IFN-γ levels in response to ML2531 p1-15 with IL-12 were significantly different from IFN-γ levels in the absence of IL-12 for both fresh and lyophilized conditions (P ≤ 0.0003).
Flow cytometry-based analysis of IFN-γ responses to M. leprae antigens.
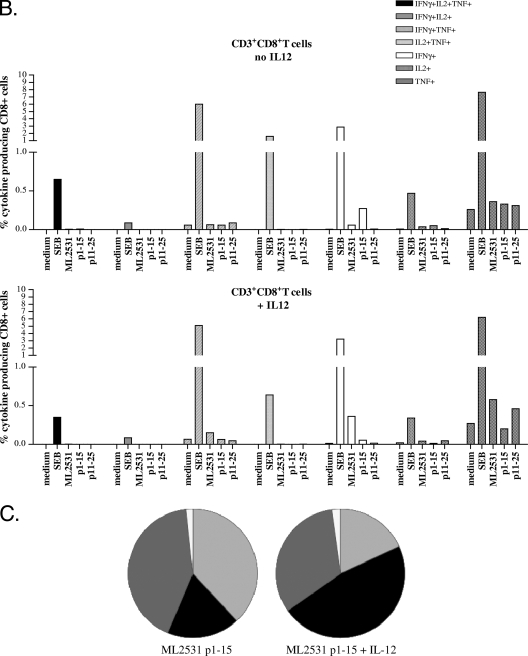
In order to estimate whether CD4+ and/or CD8+ T-cell populations might be responsible for the enhanced production of IFN-γ in the presence of IL-12, we used multiparameter flow cytometry to detect antigen-specific T cells (15). In this way, not only can the numbers of IFN-γ-producing cells be determined but also the immune phenotype of the cells responsible for its production, as well as the production of other cytokines like IL-2 and TNF. To this end, whole blood of ML2531-responsive individuals was incubated for 24 h with ML2531 p1-15 and their cells were subsequently analyzed for coexpression of CD3, CD4, CD8, CD56, CD16, IFN-γ, TNF, and IL-2 (Fig. 4) . Analysis of the cytokine production profiles after stimulation with ML2531 p1-15 showed that the increase in IFN-γ-producing cells after addition of IL-12 was mainly due to a higher percentage of CD3+ CD4+ IFN-γ+ T cells, which increased from 0.25% to 0.38% (Fig. 4A). Conversely, the percentage of CD3+ CD8+ IFN-γ+ cells decreased from 0.27% to 0.054% (Fig. 4B). There was no increase (but rather a decrease) in the percentage of IFN-γ+ NK cells after addition of IL-12 and ML2531 p1-15 as shown in Fig. 4C. This decrease corresponded to absolute numbers in the IFN-γ+ population of 1,863 IFN-γ+ NK cells (without IL-12) and 152 IFN-γ+ NK cells (with IL-12).
FIG. 4.
Cytokine profiles of WBA in response to ML2531 protein (10 μg/ml), ML2531 p1-15 (10 μg/ml), ML2531 p11-25 (10 μg/ml), or staphylococcal enterotoxin B (SEB; 2 μg/ml) in the presence or absence of IL-12. Bars indicate the percentages of CD3+ CD4+ T cells (A) or CD3+ CD8+ T cells (B). Bars are coded according to the cytokine production profiles. Pies (C) indicate CD4+ cells (black), CD8+ cells (dark gray), or NK+ cells (white, CD56+; light gray, CD16+). Data shown are representative of independent experiments using blood samples of five different BT leprosy patients.
CD3+ IL-17+ cells were scarcely detected (0.014% of CD3+ cells), and CD3+ IP-10+ cells did not show a significant increase after addition of IL-12, remaining at 0.5% of the CD3+ cells (data not shown), in line with the fact that IP-10 is secreted by several cell types in response to IFN-γ.
Summarized, these data indicate that the increase in IFN-γ in WBA with ML2531 p1-15 after addition of IL-12 is mainly due to single IFN-γ+ CD4+ T cells and not to double (either IFN-γ+ TNF+ or IFN-γ+ IL-2+) cells, to triple TNF+ IL-2+ IFN-γ+ CD3+ cells, or to IFN-γ+ NK cells.
Multiplex analysis of whole-blood cultures with M. leprae antigens.
Immunological correlates of protection in leprosy are still lacking; even though antigen-specific IFN-γ production has often been used as a biomarker for M. leprae infection, it is possible that additional biomarkers exist that allow better and more sensitive detection of specific immune responses against M. leprae peptides. In order to start investigating this issue, 17 additional cytokines/chemokines were tested as candidate biomarkers in multiplex assays on supernatants of whole-blood cultures in the presence or absence of IL-12. As expected based on the ELISA data (Fig. 1b), ML2531 p1-15-induced IFN-γ production was confirmed to be specifically enhanced after addition of IL-12 (from 440 pg/ml to 930 pg/ml [Fig. 5 A]). Although this was also found for IP-10 (from 1,629 pg/ml to 6,762 pg/ml [Fig. 5B]), this enhancement was largely not specific, as IL-12 increased background IP-10 production in unstimulated control cultures. Consequently, the IL-12-induced increase in cytokine production was significant only for IFN-γ (P = 0.007; Fig. 6), not for IP-10 (P = 0.45; Fig. 6). However, IP-10 release in response to ML2531 p1-15 in the absence of IL-12 was high (1,629 pg/ml compared to 440 pg/ml for IFN-γ), indicating that IP-10 could be used as a sensitive readout for M. leprae peptide responses in WBA but not when adding IL-12.
FIG. 5.
Cytokine/chemokine production in response to ML2531 recombinant protein (10 μg/ml), ML2531 peptide 1-15 (10 μg/ml), or ML2531 peptide 11-25 (10 μg/ml) measured by multiplex analysis after 24-h culture of undiluted whole blood in the absence (white bars) or presence (black bars) of IL-12. Cytokines/chemokines used were IFN-γ (A), IP-10 (B), MIP-1β (C), MCP-1 (D), TNF (E), IL-17 (F), IL-6 (G), and IL-8 (H). Data shown are representative of independent experiments using blood samples of five different BT leprosy patients.
FIG. 6.
Differences in IFN-γ (left panel) and IP-10 (right panel) production in response to ML2531 peptide 11-25 (10 μg/ml) measured after 24-h culture of undiluted whole blood of 12 BT leprosy patients in the absence (left) or presence (right) of IL-12.
Production of MIP-1β (Fig. 5C) and MCP-1 (macrophage c protein 1) (Fig. 5D) in response to ML2531 p1-15 and IL-12 tended to be increased by IL-12, although this was not statistically significant (P = 0.2 and 0.4, respectively [data not shown]). Addition of IL-12 also increased peptide-specific production of TNF (Fig. 5E) but not in a significant manner (P = 0.22; data not shown), and the levels were much lower than those for the four cytokines described above. IL-17 production (Fig. 5F) in response to ML2531 and ML2531 p1-15 was low, and although ML2531 induced significant levels of IL-6 and IL-8, ML2531 p1-15 induced only low levels of these cytokines (Fig. 5G and H). No or hardly any effects of IL-12 on these low levels of IL-17, IL-6, and IL-8 in response to peptide were observed. For IL-1β, IL-2, IL-4, IL-5, IL-7, IL-10, IL-12p70, IL-13, GM-CSF, and G-CSF, no peptide-induced responses were detected after correction for medium values (data not shown).
DISCUSSION
The control of leprosy requires development of novel diagnostic tools in addition to wide and adequate application of multidrug therapy (MDT). The development of tests for early detection of preclinical M. leprae infection or subclinical disease, either of which is likely to be a significant source of unidentified transmission, has been an important topic in leprosy research in the last decade (2, 9, 10, 17, 29). In this respect, short assays requiring only direct incubation of blood are favored as diagnostic tests especially in areas with endemic leprosy, which usually lack sophisticated laboratories.
The successful use of T-cell-based IGRAs such as the QuantiFERON-TB-Gold-In-Tube WBA involving M. tuberculosis-specific peptides of ESAT-6 (Rv3875), CFP-10 (Rv3874), and TB7.7 (Rv2654) for tuberculosis (TB) diagnostics in humans (11) seemed to hold promise for the use of M. leprae peptides in diagnostic tests for leprosy based on cell-mediated immunity (CMI). Moreover, in combination with classical detection of anti-PGL-I IgM antibodies, similar CMI-based tests would allow detection of most forms (both MB and PB) of leprosy but may also detect preclinical forms of this disease, thereby enabling initiation of MDT at an early stage and reducing transmission of leprosy.
Previously, we have demonstrated the potential of M. leprae peptides in CMI-based diagnostic tests for leprosy: using IFN-γ production by purified peripheral blood mononuclear cells (PBMC) as a readout, significantly higher levels of IFN-γ were induced in response to these peptides by PBMC from M. leprae-exposed healthy household contacts and patients as opposed to healthy controls from the same area of endemicity (16, 29). Although at the cost of some sensitivity, the level of specificity reached in these assays using M. leprae peptides was higher than that when M. leprae proteins were used. Therefore, this study has focused on improving a 24-h WBA format in order to increase the sensitivity for low-frequency, M. leprae peptide-specific T cells.
IL-12 is naturally produced by dendritic cells (21) and macrophages in response to antigenic stimulation and is a key cytokine in the development of type 1 response (13), which is crucial for protection against mycobacteria. IL-12 stimulates the production of IFN-γ and TNF from T cells and NK cells and reduces IL-4-mediated suppression of IFN-γ. IL-18 works together with IL-12 to induce cell-mediated immunity following infection with microbial products like lipopolysaccharide (LPS) and to induce T cells to produce IFN-γ. It has been reported that the lack of IFN-γ responses in lepromatous leprosy patients could be recovered by addition of IL-12 and of IL-12 with IL-2 (6) or IL-18 (22). Other studies showed that either the addition of the T-cell survival cytokine IL-7 (12) or inhibition of the anti-inflammatory cytokine IL-10 (7) significantly augmented antigen-specific IFN-γ production and thus increased test sensitivity. Since both IL-12 and IL-23 can stimulate the production of IFN-γ by activation of the transcription activator STAT4 (31) and since IL-23 specifically stimulates memory T cells, we analyzed the effect on IFN-γ production of exogenously added IL-7, anti-IL-10, IL-12, IL-18, and IL-23 in a 24-h WBA using ML2531 recombinant protein and peptides as antigenic stimuli. In contrast to these previous reports, no effects were observed using IL-7, IL-18, or antibodies directed against IL-10, nor did IL-23 increase the IFN-γ production in response to peptides significantly. Addition of IL-12, however, enhanced this readout by at least 50%.
Optimal T-cell responses require not only specific recognition of antigen but also costimulatory signals provided by the interaction of molecules on T cells and antigen-presenting cells. Thus, we next analyzed agents that are known to affect costimulation. Combined addition of antibodies directed against the CD49d and CD28 coreceptors has been reported to be beneficial for IFN-γ production in WBA mixtures stimulated with purified protein derivative (PPD) (18). CD28 is expressed on T cells, and stimulation through CD28 (normally by binding to B7.1 or B7.2 on the antigen-presenting cells) simultaneously with T-cell receptor (TCR) stimulation provides a potent costimulatory signal to T cells for the production of cytokines. The adhesion receptor CD49d, which shows increased expression on activated T cells, provides costimulatory signals as well. Finally, another possibility to stimulate antigen-presenting cells was investigated by activating CD40, a costimulatory protein found on antigen-presenting cells, through addition of anti-CD40 or CD40 ligand (CD40L). In summary, out of all (combinations of) agents tested only IL-12 was found to exert a clear and specific effect by increasing IFN-γ production in ML2531 antigen-stimulated blood samples. This effect was even fully maintained by using tubes precoated with lyophilized antigen and IL-12 on the inner walls, allowing an assay setup similar to the QuantiFERON-TB-Gold-In-Tube.
In order to further improve the WBA format, the use of mannosylated peptides (30a) was addressed next. Lectin-binding receptors such as the mannose receptor are required for efficient endocytosis of glycoproteins. Thus, mannosylation will facilitate peptide uptake by professional antigen-presenting cells, such as mannose receptor-positive dendritic cells (DC), leading to more efficient peptide presentation to T cells. For this purpose ML2531 p1-15 was bis-mannosylated and used as stimulus in 24-h whole-blood cultures. The optimum concentration for ML2531 p1-15 was observed at 10 μg/ml, whereas this was 0.1 μg/ml for its mannosylated version, indicating either improved uptake and presentation or increased peptide stability. However, when IL-12 was added as well, only slight increases in IFN-γ production were observed for mannosylated ML2531 p1-15 compared to the wild-type peptide. Thus, the use of mannosylated peptides in WBA does not lead to drastic improvement of sensitivity of the assay, although less peptide can be used, which could decrease the overall cost of the assay.
In order to determine whether indeed CD4+ or CD8+ T cells were responsible for IFN-γ production in this IL-12-enhanced WBA with M. leprae peptide, flow cytometry-based detection of T cells was used (15). Flow cytometry offers the advantage of determining not only the frequency of IFN-γ-producing cells but also the immunophenotype of responding cells, which is important in view of the fact that NK cells represent a substantial percentage of IFN-γ-producing cells in PBMC. Analysis of the cytokine production profiles of whole-blood cultures of ML2531 p1-15-responsive donors stimulated with ML2531 p1-15 showed that after addition of IL-12, the percentage of single-positive IFN-γ-producing CD4+ T cells increased at the expense of the double-positive IFN-γ+ TNF+ and IFN-γ+ IL-2+ CD4+ T cells.
IFN-γ is a critical component of the proinflammatory immune response that provides protection against mycobacteria. In the absence of an immunological correlate of protection, IFN-γ is often used as a biomarker to detect protective immune responses against M. leprae. In order to find alternative biomarkers for M. leprae peptide responses, 18 different cytokines/chemokines were analyzed in each sample. Besides the significant IL-12-dependent increase in IFN-γ, we found that also MIP-1β and MCP-1 levels specific for M. leprae peptide tended to be increased by IL-12, although this did not reach statistical significance.
Furthermore, IP-10 was found to be a useful additional marker—next to IFN-γ—as a (combined) readout to detect M. leprae-induced peptide responses in short-term WBA. The addition of IL-12, however, was found to enhance background IP-10 production in a nonspecific manner. In line with this, IP-10 was recently described as a suitable biomarker for active TB (26, 27) despite high IP-10 background levels compared to IFN-γ in unstimulated control samples of latently infected TB cases. The latter finding could be due to a chronic state of inflammation as the immune system attempts to control infection, which may also explain the elevations of plasma IP-10 observed for leprosy patients with reversal reactions compared to controls without reaction (30). Still, the high levels of IP-10 in response to mycobacterial antigens make it an attractive cytokine that requires further investigation as a possible biomarker in leprosy diagnostics.
Thus, this study shows that IFN-γ-based WBA using (mannosylated) M. leprae peptides combined with IL-12 may offer new possibilities for a reliable whole-blood assay which can also be used in an in-tube-lyophilized format. Importantly, besides IFN-γ, M. leprae peptides also induced high levels of IP-10, indicating its biomarker potential in WBA. However, more extensive studies involving leprosy patients, their contacts, and healthy controls in addition to selection of specific M. leprae peptides covering a variety of HLA restrictions will have to be performed in order to determine whether these constitute a biomarker profile which discriminates M. leprae-infected individuals from controls in the same area of leprosy endemicity.
Acknowledgments
This study was supported by the Netherlands Leprosy Relief Foundation (NLR) (ILEP no. 7.02.02.65) and the Q. M. Gastmann-Wichers Foundation. Additional support for this study was received from NLR (ILEP no. 7.01.02.48) and the Turing Foundation as part of the IDEAL (Initiative for Diagnostic and Epidemiological Assays for Leprosy) Consortium.
We thank E. van de Vosse and S. Arend for critically reading the manuscript and C. Prins for drawing donor blood.
Footnotes
Published ahead of print on 28 April 2010.
REFERENCES
- 1.Reference deleted.
- 2.Araoz, R., N. Honore, S. Banu, C. Demangel, Y. Cissoko, C. Arama, M. K. Uddin, S. K. Hadi, M. Monot, S. N. Cho, B. Ji, P. J. Brennan, S. Sow, and S. T. Cole. 2006. Towards an immunodiagnostic test for leprosy. Microbes Infect. 8:2270-2276. [DOI] [PubMed] [Google Scholar]
- 3.Beveridge, N. E., H. A. Fletcher, J. Hughes, A. A. Pathan, T. J. Scriba, A. Minassian, C. R. Sander, K. T. Whelan, H. M. Dockrell, A. V. Hill, W. A. Hanekom, and H. McShane. 2008. A comparison of IFNgamma detection methods used in tuberculosis vaccine trials. Tuberculosis (Edinburgh) 88:631-640. [DOI] [PubMed] [Google Scholar]
- 4.Black, G. F., R. E. Weir, S. Floyd, L. Bliss, D. K. Warndorff, A. C. Crampin, B. Ngwira, L. Sichali, B. Nazareth, J. M. Blackwell, K. Branson, S. D. Chaguluka, L. Donovan, E. Jarman, E. King, P. E. Fine, and H. M. Dockrell. 2002. BCG-induced increase in interferon-gamma response to mycobacterial antigens and efficacy of BCG vaccination in Malawi and the UK: two randomised controlled studies. Lancet 359:1393-1401. [DOI] [PubMed] [Google Scholar]
- 5.Cole, S. T., K. Eiglmeier, J. Parkhill, K. D. James, N. R. Thomson, P. R. Wheeler, N. Honore, T. Garnier, C. Churcher, D. Harris, K. Mungall, D. Basham, D. Brown, T. Chillingworth, R. Connor, R. M. Davies, K. Devlin, S. Duthoy, T. Feltwell, A. Fraser, N. Hamlin, S. Holroyd, T. Hornsby, K. Jagels, C. Lacroix, J. Maclean, S. Moule, L. Murphy, K. Oliver, M. A. Quail, M. A. Rajandream, K. M. Rutherford, S. Rutter, K. Seeger, S. Simon, M. Simmonds, J. Skelton, R. Squares, S. Squares, K. Stevens, K. Taylor, S. Whitehead, J. R. Woodward, and B. G. Barrell. 2001. Massive gene decay in the leprosy bacillus. Nature 409:1007-1011. [DOI] [PubMed] [Google Scholar]
- 6.de Jong, R., A. A. Janson, W. R. Faber, B. Naafs, and T. H. Ottenhoff. 1997. IL-2 and IL-12 act in synergy to overcome antigen-specific T cell unresponsiveness in mycobacterial disease. J. Immunol. 159:786-793. [PubMed] [Google Scholar]
- 7.Denis, M., D. N. Wedlock, A. R. McCarthy, N. A. Parlane, P. J. Cockle, H. M. Vordermeier, R. G. Hewinson, and B. M. Buddle. 2007. Enhancement of the sensitivity of the whole blood interferon-gamma assay for increasing detection of Mycobacterium bovis-infected cattle. Clin. Vaccine Immunol. 14:1483-1489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dockrell, H. M., G. F. Black, R. E. Weir, and P. E. Fine. 2000. Whole blood assays for interferon-gamma: practicalities and potential for use as diagnostic tests in the field. Lepr. Rev. 71(Suppl.):S60-S62. [DOI] [PubMed] [Google Scholar]
- 9.Dockrell, H. M., S. Brahmbhatt, B. D. Robertson, S. Britton, U. Fruth, N. Gebre, M. Hunegnaw, R. Hussain, R. Manandhar, L. Murillo, M. C. Pessolani, P. Roche, J. L. Salgado, E. Sampaio, F. Shahid, J. E. Thole, and D. B. Young. 2000. A postgenomic approach to identification of Mycobacterium leprae-specific peptides as T-cell reagents. Infect. Immun. 68:5846-5855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Duthie, M. S., W. Goto, G. C. Ireton, S. T. Reece, L. H. Sampaio, A. B. Grassi, A. L. Sousa, C. M. Martelli, M. M. Stefani, and S. G. Reed. 2008. Antigen-specific T-cell responses of leprosy patients. Clin. Vaccine Immunol. 15:1659-1665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ferrara, G., M. Losi, R. D'Amico, P. Roversi, R. Piro, M. Meacci, B. Meccugni, I. M. Dori, A. Andreani, B. M. Bergamini, C. Mussini, F. Rumpianesi, L. M. Fabbri, and L. Richeldi. 2006. Use in routine clinical practice of two commercial blood tests for diagnosis of infection with Mycobacterium tuberculosis: a prospective study. Lancet 367:1328-1334. [DOI] [PubMed] [Google Scholar]
- 12.Feske, M., R. J. Nudelman, M. Medina, J. Lew, M. Singh, J. Couturier, E. A. Graviss, and D. E. Lewis. 2008. Enhancement of human antigen-specific memory T-cell responses by interleukin-7 may improve accuracy in diagnosing tuberculosis. Clin. Vaccine Immunol. 15:1616-1622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Flynn, J. L., M. M. Goldstein, K. J. Triebold, J. Sypek, S. Wolf, and B. R. Bloom. 1995. IL-12 increases resistance of BALB/c mice to Mycobacterium tuberculosis infection. J. Immunol. 155:2515-2524. [PubMed] [Google Scholar]
- 14.Franken, K. L., H. S. Hiemstra, K. E. van Meijgaarden, Y. Subronto, J. den Hartigh, T. H. Ottenhoff, and J. W. Drijfhout. 2000. Purification of his-tagged proteins by immobilized chelate affinity chromatography: the benefits from the use of organic solvent. Protein Expr. Purif. 18:95-99. [DOI] [PubMed] [Google Scholar]
- 15.Fuhrmann, S., M. Streitz, and F. Kern. 2008. How flow cytometry is changing the study of TB immunology and clinical diagnosis. Cytometry A 73:1100-1106. [DOI] [PubMed] [Google Scholar]
- 16.Geluk, A., J. Ploeg, R. O. Teles, K. L. Franken, C. Prins, J. W. Drijfhout, E. N. Sarno, E. P. Sampaio, and T. H. Ottenhoff. 2008. Rational combination of peptides derived from different Mycobacterium leprae proteins improves sensitivity for immunodiagnosis of M. leprae infection. Clin. Vaccine Immunol. 15:522-533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Geluk, A., J. S. Spencer, K. Bobosha, M. C. Pessolani, G. M. Pereira, S. Banu, N. Honore, S. T. Reece, M. Macdonald, B. R. Sapkota, C. Ranjit, K. L. Franken, M. Zewdie, A. Aseffa, R. Hussain, M. M. Stefani, S. N. Cho, L. Oskam, P. J. Brennan, and H. M. Dockrell. 2009. From genome-based in silico predictions to ex vivo verification of leprosy diagnosis. Clin. Vaccine Immunol. 16:352-359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hanekom, W. A., J. Hughes, M. Mavinkurve, M. Mendillo, M. Watkins, H. Gamieldien, S. J. Gelderbloem, M. Sidibana, N. Mansoor, V. Davids, R. A. Murray, A. Hawkridge, P. A. Haslett, S. Ress, G. D. Hussey, and G. Kaplan. 2004. Novel application of a whole blood intracellular cytokine detection assay to quantitate specific T-cell frequency in field studies. J. Immunol. Methods 291:185-195. [DOI] [PubMed] [Google Scholar]
- 19.Hiemstra, H. S., G. Duinkerken, W. E. Benckhuijsen, R. Amons, R. R. de Vries, B. O. Roep, and J. W. Drijfhout. 1997. The identification of CD4+ T cell epitopes with dedicated synthetic peptide libraries. Proc. Natl. Acad. Sci. U. S. A. 94:10313-10318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hoeve, M. A., N. D. Savage, T. de Boer, D. M. Langenberg, R. de Waal-Malefyt, T. H. Ottenhoff, and F. A. Verreck. 2006. Divergent effects of IL-12 and IL-23 on the production of IL-17 by human T cells. Eur. J. Immunol. 36:661-670. [DOI] [PubMed] [Google Scholar]
- 21.Kalinski, P., C. M. Hilkens, A. Snijders, F. G. Snijdewint, and M. L. Kapsenberg. 1997. IL-12-deficient dendritic cells, generated in the presence of prostaglandin E2, promote type 2 cytokine production in maturing human naive T helper cells. J. Immunol. 159:28-35. [PubMed] [Google Scholar]
- 22.Lopez Roa, R. I., V. C. Guerrero, N. A. Alvarado, B. M. Montoya, N. C. Garcia, and M. M. Fafutis. 2008. Recovery of IFN-gamma levels in PBMCs from lepromatous leprosy patients through the synergistic actions of the cytokines IL-12 and IL-18. Int. Immunopharmacol. 8:1715-1720. [DOI] [PubMed] [Google Scholar]
- 23.Oskam, L., E. Slim, and S. Buhrer-Sekula. 2003. Serology: recent developments, strengths, limitations and prospects: a state of the art overview. Lepr. Rev. 74:196-205. [PubMed] [Google Scholar]
- 24.Powell, M. F., H. Grey, F. Gaeta, A. Sette, and S. Colon. 1992. Peptide stability in drug development: a comparison of peptide reactivity in different biological media. J. Pharm. Sci. 81:731-735. [DOI] [PubMed] [Google Scholar]
- 25.Ridley, D. S., and W. H. Jopling. 1966. Classification of leprosy according to immunity. A five-group system. Int. J. Lepr. Other Mycobact. Dis. 34:255-273. [PubMed] [Google Scholar]
- 26.Ruhwald, M., M. Bjerregaard-Andersen, P. Rabna, K. Kofoed, J. Eugen-Olsen, and P. Ravn. 2007. CXCL10/IP-10 release is induced by incubation of whole blood from tuberculosis patients with ESAT-6, CFP10 and TB7.7. Microbes Infect. 9:806-812. [DOI] [PubMed] [Google Scholar]
- 27.Ruhwald, M., T. Bodmer, C. Maier, M. Jepsen, M. B. Haaland, J. Eugen-Olsen, and P. Ravn. 2008. Evaluating the potential of IP-10 and MCP-2 as biomarkers for the diagnosis of tuberculosis. Eur. Respir. J. 32:1607-1615. [DOI] [PubMed] [Google Scholar]
- 28.Setia, M. S., C. Steinmaus, C. S. Ho, and G. W. Rutherford. 2006. The role of BCG in prevention of leprosy: a meta-analysis. Lancet Infect. Dis. 6:162-170. [DOI] [PubMed] [Google Scholar]
- 29.Spencer, J. S., H. M. Dockrell, H. J. Kim, M. A. Marques, D. L. Williams, M. V. Martins, M. L. Martins, M. C. Lima, E. N. Sarno, G. M. Pereira, H. Matos, L. S. Fonseca, E. P. Sampaio, T. H. Ottenhoff, A. Geluk, S. N. Cho, N. G. Stoker, S. T. Cole, P. J. Brennan, and M. C. Pessolani. 2005. Identification of specific proteins and peptides in mycobacterium leprae suitable for the selective diagnosis of leprosy. J. Immunol. 175:7930-7938. [DOI] [PubMed] [Google Scholar]
- 30.Stefani, M. M., J. G. Guerra, A. L. Sousa, M. B. Costa, M. L. Oliveira, C. T. Martelli, and D. M. Scollard. 2009. Potential plasma markers of type 1 and type 2 leprosy reactions: a preliminary report. BMC Infect. Dis. 9:75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30a.Tan, A. M. C., R. Jordens, A. Geluk, B. O. Roep, T. Ottenhoff, J. W. Drijfhout, and F. Koning. 1998. Strongly increased efficiency of altered peptide ligands by mannosylation. Int. Immunol. 10:1299-1304. [DOI] [PubMed] [Google Scholar]
- 31.van de Vosse, E., G. Lichtenauer-Kaligis, J. T. van Dissel, and T. H. Ottenhoff. 2003. Genetic variations in the interleukin-12/interleukin-23 receptor (beta1) chain, and implications for IL-12 and IL-23 receptor structure and function. Immunogenetics 54:817-829. [DOI] [PubMed] [Google Scholar]
- 32.Weekly Epidemiological Record. 2008. Global leprosy situation, beginning of 2008. Wkly. Epidemiol. Rec. 83:293-300. [PubMed] [Google Scholar]
- 33.Weir, R. E., C. R. Butlin, K. D. Neupane, S. S. Failbus, and H. M. Dockrell. 1998. Use of a whole blood assay to monitor the immune response to mycobacterial antigens in leprosy patients: a predictor for type 1 reaction onset? Lepr. Rev. 69:279-293. [DOI] [PubMed] [Google Scholar]
- 34.Weir, R. E., A. R. Morgan, W. J. Britton, C. R. Butlin, and H. M. Dockrell. 1994. Development of a whole blood assay to measure T cell responses to leprosy: a new tool for immuno-epidemiological field studies of leprosy immunity. J. Immunol. Methods 176:93-101. [DOI] [PubMed] [Google Scholar]