Abstract
Glucocorticoids (GC) are potent drugs proven to effectively treat inflammatory diseases, although patients typically begin therapy after the onset of symptoms. Clinical studies with cytokine inhibitors prove that these mediators drive inflammatory responses in diseases such as rheumatoid arthritis and Crohn's disease. Despite the clear sequence of cytokine-induced inflammation followed by effective GC treatment, most basic science investigations have examined the ability of GC to prevent an inflammatory response rather than halt its progression. The current studies used the Toll-like receptor 2 (TLR2) agonist palmitoyl3-cysteine-serine-lysine4 (PAM) or the TLR4 agonist lipopolysaccharide (LPS) to stimulate human whole blood and determine whether postponing the addition of the GC dexamethasone (DEX) limits its ability to decrease cytokine production. Twenty-four hours after stimulation, tumor necrosis factor (TNF), interleukin-1β (IL-1β), IL-6, and IL-8 levels were measured, in addition to the cytokine inhibitors IL-1 soluble receptor II (SRII), IL-1 receptor antagonist, and TNF SRII. LPS rapidly induced all of the proinflammatory mediators over 24 h while failing to induce any of the cytokine inhibitors. PAM stimulation also induced IL-1β, IL-6, and IL-8. Concomitant addition of DEX plus LPS or PAM significantly suppressed all cytokine levels. Delaying the addition of DEX until 6 h after LPS stimulation failed to decrease TNF or IL-6. In contrast, delayed DEX addition significantly suppressed PAM-induced IL-1β, IL-6, or IL-8 and also suppressed LPS-induced IL-1β and IL-8. Our results show that cytokines which typically increase in concentration between 6 and 24 h after stimulation were significantly suppressed by the addition of DEX 6 h after stimulation.
Glucocorticoids (GC) are members of the corticosteroid family whose anti-inflammatory properties have been widely exploited both as a clinical therapy and as a tool for understanding the mechanisms of inflammation (20). GC are widely used to treat a number of inflammatory conditions such as rheumatoid arthritis (30), bacterial meningitis (11), and allergic asthma (29). GC have been shown to have a beneficial effect when administered after the onset of some inflammatory disorders. Inhaled corticosteroids, for example, are commonly used to treat acute allergic asthma. Early systemic administration of corticosteroids has significantly reduced the incidence of hospitalization and relapse and expedited recovery for severe asthma patients presenting in the clinic with an acute exacerbation (28). A small study showed that GC rescue in patients with established acute respiratory distress syndrome significantly improved sequential organ failure assessment scores in trauma patients (17). Cytokines have been implicated in the pathogenesis of several of these diseases (16), and cytokine inhibitors have revolutionized the treatment of diseases such as rheumatoid arthritis.
Multiple stimuli such as infections, trauma, autoimmune disorders, and allergies activate immune responses and initiate inflammation by stimulating the secretion of cytokines. These mediators drive both the innate and adaptive immune responses by perpetuating inflammatory responses via paracrine and autocrine mechanisms (15). Invading pathogens shed their outer membrane components, which, upon binding to cell surface receptors, initiate cytokine and chemokine secretion by inflammatory cells over several hours to days, and in some instances, chemokines are produced over several weeks (26). Published data report that in response to various stimuli, cytokines and chemokines often display distinct kinetic profiles (4, 9, 10). Cytokines such as tumor necrosis factor (TNF), interleukin-1β (IL-1β), and IL-6 are known to be rapidly induced and cleared, while chemokines such as IL-8 have been shown to be steadily, continuously produced over time (10).
Despite proven clinical efficacy when GC are given after the onset of inflammation, virtually no studies have examined the ability of GC to inhibit the production of cytokines if added several hours after the initial stimulus. The experiments described here were designed to determine if dexamethasone (DEX) could decrease cytokine production after inflammation had been initiated in the whole-blood model. While the whole-blood model does not provide the same complexity as in vivo studies, the model has been used extensively by numerous investigators to study the regulation of cytokine production (6) and has become a standardized method (18). These studies determined the mechanism by which delayed treatment with GC controls inflammation by determining which mediators could potentially be decreased.
MATERIALS AND METHODS
Reagents.
Heparin sodium derived from porcine intestinal mucosa was obtained from American Pharmaceutical Partners, Inc. (Schaumburg, IL). Lipopolysaccharide (LPS; from Escherichia coli serotype O111:B4) and water-soluble DEX were obtained from Sigma-Aldrich (St. Louis, MO; catalog no. D-2915). Pam3CSK4·3HCl (product no. ALX-165-066-M002) was purchased from Alexis Biochemicals (Farmingdale, NY). Capture and biotinylated detection antibodies for enzyme-linked immunosorbent assay (ELISA) measurement of TNF, IL-1β, IL-6, and IL-8 were purchased from R&D Systems (Minneapolis, MN). RPMI 1640 was obtained from Invitrogen Life Technologies (Carlsbad, CA). The water-soluble tetrazolium (WST) cell counting kit (CCK-8) was purchased from Dojindo Molecular Technologies, Inc. (Rockville, MD). The Chromo-LAL reagent was purchased from the Associates of Cape Cod, Inc. (Cape Cod, MA).
Blood collection and stimulation.
Venous blood was collected from healthy volunteers into heparinized (10 U/ml) syringes. For the kinetics of TNF, IL-1β, IL-6, and IL-8 expression, LPS (50 ng/ml, final concentration), palmitoyl3-cysteine-serine-lysine4 (PAM) at 10, 100, or 1,000 ng/ml, or RPMI 1640 vehicle was added to 1 ml of blood in 1.5-ml tubes and incubated in 5% CO2 and ambient air at 37°C on a rotating shaker using our previously described methods (8, 9, 33). The concentrations of LPS and PAM were selected based on pilot studies showing that these concentrations would induce robust cytokine production. Following stimulation, the blood was centrifuged at 1,000 × g for 5 min at the time points indicated in the graphs and plasma was collected and stored at −20°C for later cytokine analysis. For DEX experiments, DEX was added to blood at a final concentration of 10−6 M (diluted in RPMI 1640) simultaneously with LPS or PAM or 6 h after stimulation. These studies have been approved by the Institutional Review Board of Boston University and The University of Michigan.
Blood gas analysis.
At 0, 6, and 24 h after LPS, 500 μl of blood was analyzed for glucose and blood gases (pH, partial CO2 pressure [pCO2], and partial O2 pressure [pO2]) using the ABL 800 Flex (Sysmed Lab Inc., Chicago, IL).
Cell viability.
Leukocytes were collected after NH4Cl lysis from four different groups after 24 h: (i) blood incubated with the vehicle (RPMI 1640), (ii) blood stimulated with 50 ng/ml LPS alone, (iii) blood stimulated with LPS plus 10−6 M DEX, and (iv) unstimulated blood. Blood was centrifuged at 1,000 × g for 5 min, and the packed cell pellet was lysed twice with 14 ml of NH4Cl buffer (NH4Cl, NaHCO3, and tetra EDTA). Aliquots of cells were added to 96-well tissue culture plates in duplicate, 10 μl WST solution was added to each well, and absorbance at 450 nm was measured using a microplate reader (Bio-Tek Instruments, Inc., Winooski, VT).
Cytokine and cytokine inhibitor ELISAs.
Plasma levels of TNF-α, IL-1β, IL-6, IL-8, IL-1 receptor antagonist (IL-1ra), IL-1 soluble receptor II (SRII), and TNF SRII were determined by sandwich ELISA using matched antibody pairs according to our previously published methods (23).
Statistics.
Statistical analyses were performed using GraphPad Prism version 4.03 for Windows (GraphPad, San Diego, CA). Results were expressed as the mean ± the standard error of the mean (SEM). Statistical comparisons were made using a one-way analysis of variance (ANOVA), followed by the Newman-Keuls multiple-comparison post test. For data which were not normally distributed, we used the Kruskal-Wallis test with a Wilcoxon signed-rank post-hoc comparison. For direct comparisons between groups, the paired Student t test was used. The figure legends indicate which statistical tests were used.
RESULTS
Whole-blood glucose, pH, and blood gases.
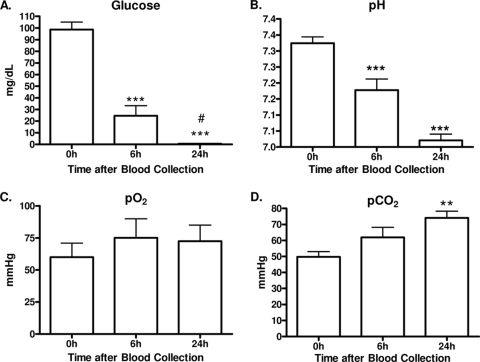
Heparinized human whole blood collected from healthy volunteers was analyzed for glucose and blood gas levels 0, 6, and 24 h after collection (Fig. 1). Glucose decreased by 75% (99 to 25 mg/dl) by 6 h and by 95% (0.5 mg/dl) after 24 h (Fig. 1A). The pH decreased from 7.30 ± 0.02 to 7.20 ± 0.03 by 6 h and to 7.00 ± 0.02 by 24 h (Fig. 1B). While the pO2 remained unaltered (Fig. 1C), the pCO2 significantly increased from 50 ± 3 to 74 ± 4 mm Hg after 24 h (Fig. 1D). Given the changes in glucose and blood gas parameters, cell viability was examined. Despite the decreases in glucose and pH, there was no reduction in cell viability (data not shown).
FIG. 1.
Glucose and blood gas analysis. Human whole blood was analyzed for glucose levels (mg/dl) (A), pH (B), pO2 (C), and pCO2 (D). Results are expressed as mean ± SEM (n = 8 donors) compared by ANOVA and the Newman-Keuls post test. **, P < 0.01; ***, P < 0.001 (compared to 0 h); #, P < 0.05 (compared to 6 h).
LPS-induced cytokine and chemokine kinetics.
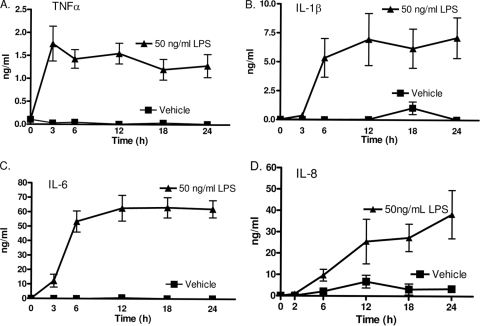
Our first studies closely examined LPS stimulation, since this has been frequently used in previous investigations using whole blood (8, 9, 37). A dose of 50 ng/ml LPS was used based on previous studies (35). To illustrate the kinetics of traditional proinflammatory cytokines/chemokines in this model, whole blood was stimulated with the classic Toll-like receptor 4 (TLR4) agonist LPS and protein levels were measured in plasma. Fig. 2 A indicates that TNF climaxed early, reaching maximal levels by 3 h and remaining essentially unchanged for 24 h. IL-1β and IL-6 displayed nearly identical protein kinetics over 24 h, both reaching maximum levels within 6 h of stimulation (Fig. 2B and C). LPS-induced IL-8 production, in contrast, yielded a kinetic profile different from that obtained with the cytokines. LPS stimulation resulted in a continuous increase in IL-8 protein levels over 24 h (Fig. 2D). IL-8 continued to increase over the entire 24 h of the study. These kinetics of cytokine production are similar to those described in multiple previous reports (7, 9, 10). Additionally, the continuing production of IL-8 indicates that cell viability was maintained over the 24 h of the study.
FIG. 2.
LPS-induced cytokine kinetics. Heparin-anticoagulated human whole blood was stimulated with 50 ng/ml LPS or the vehicle alone. TNF, IL-1β, and IL-6 plateaued within 6 h, while IL-8 levels continuously increased over 24 h. Plasma was collected from LPS-stimulated whole blood, and cytokine levels were determined by ELISA at the indicated time points. Results are expressed as mean ± SEM (n = 4 to 9 donors).
Since inflammation is such a well-orchestrated and tightly regulated process, it was important to evaluate a broad range of mediators and then carefully narrow the focus to those which were specifically affected. Thus, LPS-induced anti-inflammatory mediators were also measured. We examined the production of IL-1 receptor antagonist, IL-1 SRII, and TNF SRII. As depicted in Table 1, these anti-inflammatory cytokines were not significantly induced following LPS stimulation and were excluded from subsequent experiments.
TABLE 1.
LPS and PAM do not induce cytokine inhibitorsa
| Cytokine | Cytokine concn (ng/ml) |
||
|---|---|---|---|
| Vehicle | LPS | PAM | |
| IL-1 SRII | 9.0 ± 1.6 | 7.0 ± 0.5 | 4.2 ± 0.5 |
| IL-1 receptor antagonist | 4.1 ± 1.7 | 3.0 ± 1.2 | 4.7 ± 0.9 |
| TNF SRII | 2.0 ± 0.4 | 1.3 ± 0.1 | 3.2 ± 0.9 |
Plasma was collected from human whole blood stimulated with LPS (50 ng/ml), PAM (1000 ng/ml), or vehicle for 24 h. In contrast to the proinflammatory mediators, there was no significant increase in any of the cytokine inhibitors. Values represent the mean ± SEM (n = 5 to 9).
PAM-induced cytokine and chemokine kinetics.
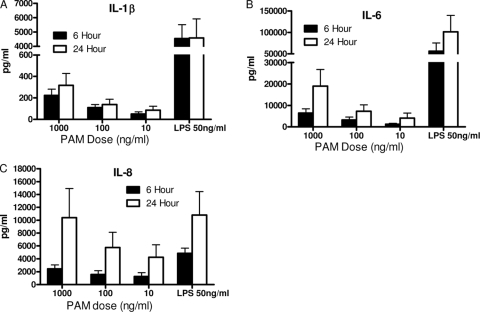
To determine the effects of the TLR2 agonist PAM on cytokine and chemokine kinetics in whole blood, a dose-response study with PAM was performed. PAM was added directly to whole blood, and cytokine levels were assayed at 6 and 24 h. PAM-induced TNF levels were barely above the vehicle control levels (data not shown); thus, PAM-induced TNF was not studied further. IL-1β (Fig. 3A) and IL-6 (Fig. 3B) were both induced in a dose-responsive manner by PAM at 6 and 24 h; however, the levels were less than those induced by LPS. In contrast, IL-8 was strongly upregulated by PAM and the 1,000-ng/ml dose induced concentrations nearly identical to those induced by LPS (Fig. 3C). PAM did not induce the production of any of the cytokine inhibitors, again similar to LPS (Table 1).
FIG. 3.
PAM dose response and kinetics. Human whole blood was stimulated with the indicated concentrations of PAM, while a separate aliquot was stimulated with 50 ng/ml LPS. Plasma was collected from separate tubes at either 6 h (filled bars) or 24 h (open bars). PAM induced lower levels of IL-1β and IL-6 than did LPS. Note the break in the y axis. In contrast, 1,000 ng/ml PAM induced levels of IL-8 nearly identical to those induced by LPS. Cytokine levels were determined by ELISA. Results are expressed as mean ± SEM (n = 10 donors).
Delayed DEX selectively suppresses LPS-induced cytokines.
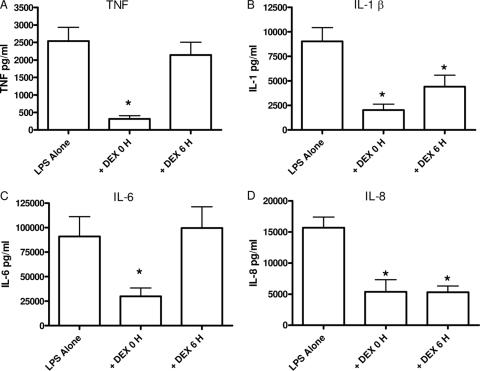
To determine if delaying the addition of anti-inflammatory therapy diminishes its capacity to regulate cytokines and chemokines, experiments compared the ability of 10−6 M DEX to suppress cytokine/chemokine levels when added to whole blood simultaneously with or after 6 h of stimulation. The data in Fig. 4 show that concomitant DEX treatment with LPS stimulation significantly suppressed TNF, IL-1β, and IL-6 protein levels measured at 24 h compared with LPS stimulation alone (Fig. 4A, B, and C, respectively). However, when DEX treatment was delayed by 6 h, there was a significant reduction in IL-1β.
FIG. 4.
Differential DEX regulation of LPS-induced cytokines. Cytokines were measured following the treatment of whole blood with LPS alone, simultaneous LPS plus DEX, or LPS plus 6-h-delayed DEX. Simultaneous addition of DEX inhibited all of the cytokines, while delayed DEX only decreased IL-1β and IL-8. Results are expressed as mean ± SEM (n = 13 to 22 donors). *, P < 0.05 versus LPS alone compared by ANOVA and Newman-Keuls post test.
The plasma levels of IL-8 continued to increase between 6 and 24 h (Fig. 3D); therefore, we determined the ability of DEX to suppress IL-8 levels when added 6 h after stimulation. Whole blood was collected and stimulated as previously described. Figure 4D shows that concomitant addition of LPS and DEX to whole blood significantly suppressed IL-8 protein compared with stimulant alone, similar to the results obtained with TNF, IL-1β, and IL-6. When administered 6 h after LPS stimulation, DEX retained its ability to significantly suppress LPS-induced IL-8 levels (Fig. 4D). Delayed DEX suppressed IL-8 protein by approximately 76%. These data show that DEX will suppress both IL-1β and IL-8 when added 6 h after the initial stimulant.
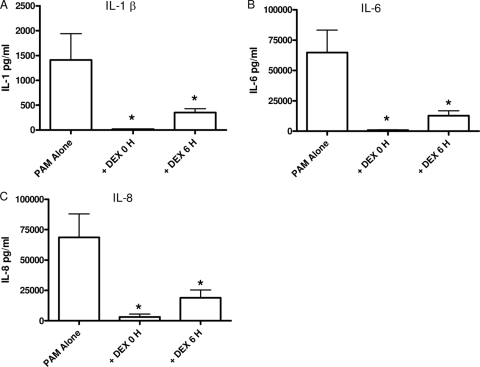
Delayed DEX suppression of PAM-induced cytokines.
Since PAM induced an increase in the cytokines IL-1β, IL-6, and IL-8 between 6 and 24 h (Fig. 3), it was possible that DEX would be able to exert a suppressive effect even when added after the initial stimulus. TNF showed little induction following PAM stimulation, so the effect of DEX treatment on TNF was not tested. As shown in Fig. 5 A, delaying the addition of DEX for 6 h after stimulation still significantly suppressed PAM-induced IL-1β compared with PAM stimulation alone. Delayed addition of DEX suppressed IL-1β by approximately 60%. IL-6 was also susceptible to DEX suppression when it was administered 6 h after PAM (Fig. 5B). Similar to LPS, even when the addition of DEX was postponed until 6 h after PAM-induced inflammation, IL-8 was significantly suppressed by approximately 70%. Taken together, these data demonstrate that anti-inflammatory GC selectively modulate inflammation even after the onset of inflammation.
FIG. 5.
Effect of DEX on PAM-induced cytokines. Cytokines were measured following the treatment of whole blood with PAM alone, simultaneous PAM plus DEX, or PAM plus 6-h-delayed DEX. Either simultaneous or delayed addition of DEX suppressed both cytokines. Results are expressed as mean ± SEM (n = 9 donors). *, P < 0.05 versus PAM alone compared by Kruskal-Wallis and Wilcoxon signed-rank tests.
DISCUSSION
Numerous clinical studies have demonstrated that treating patients with GC will actively suppress inflammation (2). While GC are effective, they are certainly not a panacea for the treatment of inflammatory disorders. Recent practice guidelines for the treatment of rheumatoid arthritis state that GC should be started within 3 months of the onset of symptoms (13). Inhaled GC are widely used by physicians for the treatment of asthma, including “rescue therapy” where treatment is started after the onset of symptoms (31). In certain settings, GC will effectively treat the inflammation of infectious diseases, such as improving outcomes for meningitis patients with culture-proven bacterial disease (25). Important unifying concepts in these diseases are the institution of therapy after the onset of symptoms and participation of cytokines in the pathogenesis of the disease. In a recent study using endotoxin infusion into normal human volunteers, long-term pretreatment with low-dose GC actually augmented plasma levels of IL-6 (39), although acute GC treatment reduced all cytokine levels in primates (40).
Inflammation is a rapid, efficient, and coordinated response to traumatic or infectious injury that is perpetuated in part by the secretion of cytokines such as TNF, IL-1β, IL-6, and IL-8. Previous studies have demonstrated that pretreatment or simultaneous treatment with anti-inflammatory reagents such as DEX, together with a stimulus such as LPS, suppresses the production of proinflammatory mediators in vitro (19, 22). Animal work has also shown that pretreatment with DEX will suppress the production of TNF, but waiting even 20 min markedly reduces the ability of DEX to stop production (27). In most clinical scenarios, patients present for medical care after the onset of inflammation, at a time when cytokines should have already been induced. The clinical question is whether treating a patient with a potent anti-inflammatory agent such as DEX will still be effective. In a study of patients with meningitis, adding DEX to the treatment regimen of antibiotics significantly improved the outcome for those patients with proven bacterial meningitis (25). This therapy was specifically initiated after the onset of symptoms, demonstrating that delayed GC therapy may still be clinically effective.
Our studies examined the mechanism of cytokine regulation using stimulated human whole blood. Cytokine regulation in whole blood has been used by a number of investigators as a bridge between in vivo and in vitro experiments. The value of the stimulated whole-blood model has been clearly demonstrated since it is the basis for an FDA-approved test for tuberculosis (1). Our studies show cytokine kinetics similar to those reported by other investigators (24, 34) and a similar profile of induced proinflammatory mediators (3). The whole-blood model has faster kinetics of cytokine production compared to isolated blood cells and higher concentrations (12). Furthermore, Damsgaard recently argued that whole blood is a “valid, low-cost method to measure cytokines” (6). We have also demonstrated that monocytes are primarily responsible for the synthesis of proinflammatory cytokines in the whole-blood model (36, 38).
The whole-blood model demonstrates remarkable stability of oxygenation over 24 h (Fig. 1). Since this is venous blood, the pO2 remained essentially unchanged, indicating that in the closed tubes oxygen is able to diffuse through the wall. There is also clear evidence of ongoing metabolism in this closed system since the glucose fell dramatically, as did the pH. These changes would be anticipated, as the white blood cells maintain their viability and undergo oxidative respiration.
We used the whole-blood model to address the mechanisms of how GC are still able to suppress inflammation after the initial stimulus, since most patients begin therapy after the onset of inflammation. Relatively few studies have examined delayed anti-inflammatory treatment. One such study undertaken by Mogensen et al. aimed to understand the mechanisms of action of GC by examining the effect of DEX prior to, concomitant with, or following Neisseria meningitidis or Streptococcus pneumoniae stimulation of peripheral blood mononuclear cells or THP-1 cells. They reported that, similar to our findings, postponing DEX partially suppressed IL-8 induction even when it was added at 7 h following infection (21).
GC, including DEX, act by altering signal transduction pathways within the cell (5). Under normal circumstances, ligand binding to TLRs on the cell surface initiates a cascade of intracellular signaling events. Nuclear factors (NF) exist in the cytoplasm in inactive forms due to their association with an inhibitory subunit, IκB, which must dissociate before translocating into the nucleus. These NF, including NF-κB and AP-1, bind to promoter elements to initiate the transcription of several proinflammatory cytokines, including those investigated in the current study. We have also reported that LPS rapidly induces NF-κB translocation and subsequent cytokine production (35). GC prevent gene transcription by blocking the dissociation of IκB from the NF complex, so that the NF cannot move to the nucleus. GC also induce a small protein, GC-inducible leucine zipper, which has broad anti-inflammatory properties, including regulation of chemokines (14).
A brief examination of the basic science literature would predict that GC would only reduce cytokine production if they were given before or concomitant with stimulation. The production of proinflammatory cytokines in vivo following endotoxin exposure is very rapid (32), and a delay of less than 1 h markedly reduces the ability of DEX to inhibit the production of TNF. Since TNF is a critical mediator of inflammatory conditions and GC are effective therapies, there is an apparent conflict between the basic science studies and the clinical reality. Our studies show that GC retain the capacity to selectively suppress cytokine production even when given after the inflammatory response has been initiated.
Acknowledgments
This study was supported in part by NIH grant GM50401.
We acknowledge the superb technical assistance of Elizabeth Schuller and Brian Japp.
Footnotes
Published ahead of print on 5 May 2010.
REFERENCES
- 1.Andersen, P., M. E. Munk, J. M. Pollock, and T. M. Doherty. 2000. Specific immune-based diagnosis of tuberculosis. Lancet 356:1099-1104. [DOI] [PubMed] [Google Scholar]
- 2.Barnes, P. J., and I. M. Adcock. 2009. Glucocorticoid resistance in inflammatory diseases. Lancet 373:1905-1917. [DOI] [PubMed] [Google Scholar]
- 3.Beitland, S., H. Opdahl, T. Aspelin, L. Saetre, and T. Lyberg. 2009. Blood leucocyte cytokine production after LPS stimulation at different concentrations of glucose and/or insulin. Acta Anaesthesiol. Scand. 53:183-189. [DOI] [PubMed] [Google Scholar]
- 4.Cassatella, M. A., L. Meda, S. Bonora, M. Ceska, and G. Constantin. 1993. Interleukin-10 (IL-10) inhibits the release of proinflammatory cytokines from human polymorphonuclear leukocytes—evidence for an autocrine role of tumor-necrosis-factor and IL-1-beta in mediating the production of IL-8 triggered by lipopolysaccharide. J. Exp. Med. 178:2207-2211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chinenov, Y., and I. Rogatsky. 2007. Glucocorticoids and the innate immune system: crosstalk with the Toll-like receptor signaling network. Mol. Cell. Endocrinol. 275:30-42. [DOI] [PubMed] [Google Scholar]
- 6.Damsgaard, C. T. 2009. Whole-blood culture is a valid low-cost method to measure monocytic cytokines—a comparison of cytokine production in cultures of human whole-blood, mononuclear cells and monocytes. J. Immunol. Methods 340:95-101. [DOI] [PubMed] [Google Scholar]
- 7.Dedrick, R. L., and P. J. Conlon. 1995. Prolonged expression of lipopolysaccharide (LPS)-induced inflammatory genes in whole blood requires continual exposure to LPS. Infect. Immun. 63:1362-1368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.DeForge, L. E., J. S. Kenney, M. L. Jones, J. S. Warren, and D. G. Remick. 1992. Biphasic production of IL-8 in lipopolysaccharide (LPS)-stimulated human whole blood. Separation of LPS- and cytokine-stimulated components using anti-tumor necrosis factor and anti-IL-1 antibodies. J. Immunol. 148:2133-2141. [PubMed] [Google Scholar]
- 9.DeForge, L. E., and D. G. Remick. 1991. Kinetics of TNF, IL-6, and IL-8 gene expression in LPS-stimulated human whole blood. Biochem. Biophys. Res. Commun. 174:18-24. [DOI] [PubMed] [Google Scholar]
- 10.DeForge, L. E., D. E. Tracey, J. S. Kenney, and D. G. Remick. 1992. Interleukin-1 receptor antagonist protein inhibits interleukin-8 expression in lipopolysaccharide-stimulated human whole blood. Am. J. Pathol. 140:1045-1054. [PMC free article] [PubMed] [Google Scholar]
- 11.de Gans, J., and D. van de Beek for the European Dexamethasone in Adulthood Bacterial Meningitis Study Investigators. 2002. Dexamethasone in adults with bacterial meningitis. N. Engl. J. Med. 347:1549-1556. [DOI] [PubMed] [Google Scholar]
- 12.De Groote, D., P. F. Zangerle, Y. Gevaert, M. F. Fassotte, Y. Beguin, F. Noizatpirenne, J. Pirenne, R. Gathy, M. Lopez, I. Dehart, D. Igot, M. Baudrihaye, D. Delacroix, and P. Franchimont. 1992. Direct stimulation of cytokines (IL-1-beta, TNF-alpha, IL-6, IL-2, IFN-gamma and GM-CSFf) in whole-blood in comparison with isolated PBMC stimulation. Cytokine 4:239-248. [DOI] [PubMed] [Google Scholar]
- 13.Deighton, C., R. O'Mahony, J. Tosh, C. Turner, and M. Rudolf. 2009. Management of rheumatoid arthritis: summary of NICE guidance. BMJ 338:b702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Eddleston, J., J. Herschbach, A. L. Wagelie-Steffen, S. C. Christiansen, and B. L. Zuraw. 2007. The anti-inflammatory effect of glucocorticoids is mediated by glucocorticoid-induced leucine zipper in epithelial cells. J. Allergy Clin. Immunol. 119:115-122. [DOI] [PubMed] [Google Scholar]
- 15.Holloway, A. F., S. Rao, and M. F. Shannon. 2002. Regulation of cytokine gene transcription in the immune system. Mol. Immunol. 38:567-580. [DOI] [PubMed] [Google Scholar]
- 16.Kim, J., L. McKinley, S. Natarajan, G. L. Bolgos, J. Siddiqui, S. Copeland, and D. G. Remick. 2006. Anti-tumor necrosis factor-alpha antibody treatment reduces pulmonary inflammation and methacholine hyper-responsiveness in a murine asthma model induced by house dust. Clin. Exp. Allergy 36:122-132. [DOI] [PubMed] [Google Scholar]
- 17.Koontz, C. S., K. K. Higdon, T. L. Ploger, B. W. t. Dart, C. M. Richart, and R. A. Maxwell. 2006. Glucocorticoid rescue for late-phase acute respiratory distress syndrome in trauma/surgical critical care patients. Am. Surg. 72:644-648. [PubMed] [Google Scholar]
- 18.Liebers, V., H. Stubel, M. Duser, T. Bruning, and M. Raulf-Heimsoth. 2009. Standardization of whole blood assay for determination of pyrogenic activity in organic dust samples. Int. J. Hyg. Environ. Health 212:547-556. [DOI] [PubMed] [Google Scholar]
- 19.Ma, W., K. Gee, W. Lim, K. Chambers, J. B. Angel, M. Kozlowski, and A. Kumar. 2004. Dexamethasone inhibits IL-12p40 production in lipopolysaccharide-stimulated human monocytic cells by down-regulating the activity of c-Jun N-terminal kinase, the activation protein-1, and NF-kappa B transcription factors. J. Immunol. 172:318-330. [DOI] [PubMed] [Google Scholar]
- 20.McGee, S., and J. Hirschmann. 2008. Use of corticosteroids in treating infectious diseases. Arch. Intern. Med. 168:1034-1046. [DOI] [PubMed] [Google Scholar]
- 21.Mogensen, T. H., R. S. Berg, S. R. Paludan, and L. Ostergaard. 2008. Mechanisms of dexamethasone-mediated inhibition of Toll-like receptor signaling induced by Neisseria meningitidis and Streptococcus pneumoniae. Infect. Immun. 76:189-197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mukaida, N., M. Morita, Y. Ishikawa, N. Rice, S. Okamoto, T. Kasahara, and K. Matsushima. 1994. Novel mechanism of glucocorticoid-mediated gene repression. Nuclear factor-kappa B is target for glucocorticoid-mediated interleukin 8 gene repression. J. Biol. Chem. 269:13289-13295. [PubMed] [Google Scholar]
- 23.Nemzek, J. A., J. Siddiqui, and D. G. Remick. 2001. Development and optimization of cytokine ELISAs using commercial antibody pairs. J. Immunol. Methods 255:149-157. [DOI] [PubMed] [Google Scholar]
- 24.Nerad, J. L., J. K. Griffiths, J. W. Van der Meer, S. Endres, D. D. Poutsiaka, G. T. Keusch, M. Bennish, M. A. Salam, C. A. Dinarello, and J. G. Cannon. 1992. Interleukin-1 beta (IL-1 beta), IL-1 receptor antagonist, and TNF alpha production in whole blood. J. Leukoc. Biol. 52:687-692. [DOI] [PubMed] [Google Scholar]
- 25.Nguyen, T. H., T. H. Tran, G. Thwaites, V. C. Ly, X. S. Dinh, T. N. Ho Dang, Q. T. Dang, D. P. Nguyen, H. P. Nguyen, S. D. To, V. C. Nguyen, M. D. Nguyen, J. Campbell, C. Schultsz, C. Parry, M. E. Torok, N. White, T. C. Nguyen, K. Stepniewska, and J. J. Farrar. 2007. Dexamethasone in Vietnamese adolescents and adults with bacterial meningitis. N. Engl. J. Med. 357:2431-2440. [DOI] [PubMed] [Google Scholar]
- 26.Osuchowski, M. F., K. Welch, J. Siddiqui, and D. G. Remick. 2006. Circulating cytokine/inhibitor profiles reshape the understanding of the SIRS/CARS continuum in sepsis and predict mortality. J. Immunol. 177:1967-1974. [DOI] [PubMed] [Google Scholar]
- 27.Remick, D. G., R. M. Strieter, J. P. Lynch, D. Nguyen, M. Eskandari, and S. L. Kunkel. 1989. In vivo dynamics of murine tumor necrosis factor-alpha gene expression: kinetics of dexamethasone-induced suppression. Lab. Invest. 60:766-771. [PubMed] [Google Scholar]
- 28.Rowe, B. H., C. H. Spooner, F. M. Ducharme, J. A. Bretzlaff, and G. W. Bota. 2007. Corticosteroids for preventing relapse following acute exacerbations of asthma. Cochrane Database Syst. Rev. 2007:CD000195. doi: 10.1002/14651858.CD000195.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schwiebert, L. A., L. A. Beck, C. Stellato, C. A. Bickel, B. S. Bochner, and R. P. Schleimer. 1996. Glucocorticosteroid inhibition of cytokine production: relevance to antiallergic actions. J. Allergy Clin. Immunol. 97:143-152. [DOI] [PubMed] [Google Scholar]
- 30.Snow, M. H., and T. R. Mikuls. 2005. Rheumatoid arthritis and cardiovascular disease: the role of systemic inflammation and evolving strategies of prevention. Curr. Opin. Rheumatol. 17:234-241. [DOI] [PubMed] [Google Scholar]
- 31.Su, K. C., C. C. Tsai, L. C. Kuo, S. H. Kuo, and D. W. Perng. 2009. Budesonide/formoterol combination as a maintenance and rescue therapy: physicians' perspectives. J. Asthma 46:647-651. [DOI] [PubMed] [Google Scholar]
- 32.Ulich, T. R., K. Z. Guo, B. Irwin, D. G. Remick, and G. N. Davatelis. 1990. Endotoxin-induced cytokine gene expression in vivo. II. Regulation of tumor necrosis factor and interleukin-1 alpha/beta expression and suppression. Am. J. Pathol. 137:1173-1185. [PMC free article] [PubMed] [Google Scholar]
- 33.Villarete, L. H., and D. G. Remick. 1996. Transcriptional and post-transcriptional regulation of interleukin-8. Am. J. Pathol. 149:1685-1693. [PMC free article] [PubMed] [Google Scholar]
- 34.Wang, J. E., P. F. Jorgensen, M. Almlof, C. Thiemermann, S. J. Foster, A. O. Aasen, and R. Solberg. 2000. Peptidoglycan and lipoteichoic acid from Staphylococcus aureus induce tumor necrosis factor alpha, interleukin 6 (IL-6), and IL-10 production in both T cells and monocytes in a human whole-blood model. Infect. Immun. 68:3965-3970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Xing, L., and D. G. Remick. 2005. Mechanisms of dimethyl sulfoxide augmentation of IL-1 beta production. J. Immunol. 174:6195-6202. [DOI] [PubMed] [Google Scholar]
- 36.Xing, L., and D. G. Remick. 2003. Relative cytokine and cytokine inhibitor production by mononuclear cells and neutrophils. Shock 20:10-16. [DOI] [PubMed] [Google Scholar]
- 37.Xing, L. Y., and D. G. Remick. 2007. Mechanisms of oxidant regulation of monocyte chemotactic protein 1 production in human whole blood and isolated mononuclear cells. Shock 28:178-185. [DOI] [PubMed] [Google Scholar]
- 38.Xing, L. Y., and D. G. Remick. 2004. Neutrophils as firemen, production of anti-inflammatory mediators by neutrophils in a mixed cell environment. Cell. Immunol. 231:126-132. [DOI] [PubMed] [Google Scholar]
- 39.Yeager, M. P., A. J. Rassias, P. A. Pioli, M. L. Beach, K. Wardwell, J. E. Collins, H. K. Lee, and P. M. Guyre. 2009. Pretreatment with stress cortisol enhances the human systemic inflammatory response to bacterial endotoxin. Crit. Care Med. 37:2727-2732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zheng, Y. J., M. Hawk, H. Yuan, H. R. Hope, M. Baratta, and A. Zutshi. 2009. Pharmacokinetics and anti-inflammatory pharmacodynamics of prednisolone in cynomolgus monkey. Xenobiotica 39:862-870. [DOI] [PubMed] [Google Scholar]