Abstract
An automated hepatitis C virus (HCV) antigen (Ag) assay was evaluated with clinical samples. Determination of HCV Ag and RNA levels in 282 subjects using Abbott HCV Ag and Roche Cobas TaqMan assays revealed that these two tests were highly correlated (r = 0.9464). Thus, the HCV Ag assay could be an alternative test to quantitative reverse transcription-PCR.
Hepatitis C virus (HCV) infection usually causes a progressive disease that can result in chronic hepatitis, liver cirrhosis, and hepatocellular carcinoma (12). Diagnosis and monitoring of HCV infection are commonly based on anti-HCV assays, recombinant immunoblotting, and HCV RNA viral load in current clinical practices (13). However, anti-HCV assays have limitations, including a lack of detection sensitivity in the early window periods of 45 to 68 days after infection (5), although second- and third-generation anti-HCV assays have improved. Real-time quantitative reverse transcription-PCR (qRT-PCR) analyses for measuring viral loads also have some drawbacks. These techniques are not completely free from contamination and false-positive results. Furthermore, some PCR assays require technical skills and may also be labor intensive and expensive.
HCV core antigen (Ag) tests have been introduced to supplement anti-HCV tests or HCV qRT-PCR analyses over the last decade (1, 16), and these quantitative HCV Ag assays could be used for the monitoring of antiviral therapy as well as for diagnosis of HCV infection (3, 6). Furthermore, the HCV Ag assay could also be useful in monitoring immunocompromised patients and those undergoing hemodialysis (10). However, most of the past studies detecting HCV Ag utilized enzyme-linked immunosorbent assays (ELISAs) or enzyme immunoassays (EIAs) (4, 7, 14, 18), which may need considerable time and skills. Recently, a fully automated chemiluminescent immunoassay (CLIA) with higher sensitivity and throughput was developed to overcome shortcomings of the conventional core Ag assays (11). Thus, we evaluated the newly introduced HCV Ag assay and compared it with a quantitative RNA assay to verify the utility of this automated Ag assay as an alternative to HCV RNA qRT-PCR.
A total of 282 serum samples from the 268 patients who visited a university hospital in Korea with suspected HCV infection were collected from January to April 2009. HCV Ag and qRT-PCR assays were performed for all 282 samples, while anti-HCV antibodies and liver enzymes were measured in 279 and 250 subjects, respectively. HCV genotyping was also performed in 127 specimens. Serum HCV Ag was determined by an Abbott Architect i2000SR analyzer (Abbott Laboratories, Abbott Park, IL) with an HCV Ag assay kit (Abbott Japan Co., Ltd., Tokyo, Japan). The Architect HCV Ag assay is a two-step chemiluminescent microparticle immunoassay for the quantitation of HCV core Ag. The sample volume required is about 110 μl, and the total assay time is 36 min. The cutoff value is 3.0 fmol/liter; thus, HCV Ag levels below 3.0 fmol/liter were considered nonreactive. HCV RNA viral load was determined by Cobas AmpliPrep/Cobas TaqMan HCV assay (CAM/CTM) (Roche Molecular Systems, Inc., Pleasanton, CA). The lower detection limit of CAM/CTM is 15 IU/ml. Anti-HCV was tested using a Cobas e411 analyzer with Elecsys Anti-HCV assay kit (Roche Diagnostics, Mannheim, Germany). HCV genotyping was carried out using linear array HCV genotyping (Roche Molecular Systems).
Statistical analysis was performed using SPSS 15.0 for Windows (SPSS Inc., Chicago, IL). The correlation coefficients between serum levels of HCV Ag and RNA were calculated by Spearman's rank test, and a comparison between the groups was carried out using the Mann-Whitney U test. A P value of <0.05 was considered statistically significant.
Among the 282 specimens tested, all 108 cases in which HCV RNA was not detected were nonreactive for HCV Ag. Of the 161 cases in which HCV RNA levels exceeded 15 IU/ml, 157 (97.5%) were reactive for HCV Ag (Table 1). HCV RNA levels of the 4 RNA-positive and HCV Ag-nonreactive specimens were 73, 82, 261, and 814 IU/ml. Another group of 13 cases with HCV RNA levels below 15 IU/ml were all nonreactive for HCV Ag. The qualitative results determined by HCV Ag and HCV RNA assays showed an agreement of 94.0%. Sensitivity, specificity, and positive and negative predictive values (PPV and NPV) of the HCV Ag test for predicting HCV RNA detection were 90.2%, 100%, 100%, and 86.4%, respectively.
TABLE 1.
Summary of HCV Ag, HCV RNA, and anti-HCV test results
| HCV Ag | HCV RNA (no. of anti-HCV positive/total) |
|||
|---|---|---|---|---|
| Not detected | <15 IU/ml | >15 IU/ml | Total | |
| Nonreactive (<3 fmol/liter) | 108 (35/108) | 13 (9/13a) | 4 (4/4) | 125 (48/125) |
| Reactive (>3 fmol/liter) | 0 (0/0) | 0 (0/0) | 157 (154/154b) | 157 (154/154) |
| Total | 108 (35/108) | 13 (9/13) | 161 (158/158) | 282 (202/279c) |
The four anti-HCV-negative and HCV Ag-nonreactive cases were from patients undergoing hemodialysis multiple times.
Anti-HCV was positive in all 154 cases of which HCV Ag was reactive and the HCV RNA level was over 15 IU/ml.
Of the total 282 specimens tested for HCV RNA and Ag, 279 samples were assayed for anti-HCV.
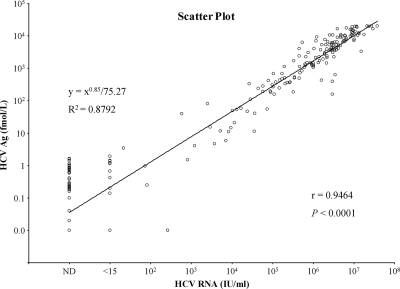
Figure 1 illustrates individual HCV Ag and RNA concentrations and it shows a good correlation between them (r = 0.9464; 95% confidence interval [CI], 0.9327 to 0.9574; P < 0.0001). Correlation coefficients were also calculated in 127 genotyped cases, and those of groups with genotypes 1, 2, and 3 were 0.9521 (n = 76; 95% CI, 0.9253 to 0.9695; P < 0.0001), 0.9513 (n = 45; 95% CI, 0.9126 to 0.9731; P < 0.0001), and 0.9429 (n = 6; 95% CI, 0.5591 to 0.9939; P = 0.0048), respectively.
FIG. 1.
Correlation between HCV Ag and HCV RNA concentrations. The levels of HCV Ag were highly correlated with those of HCV RNA by the Spearman's rank correlation test (r = 0.9464; P < 0.0001). x and y axes are log scaled. ND, not detected.
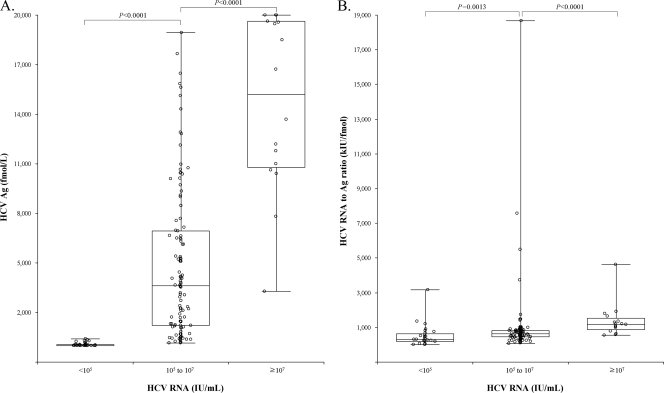
In the HCV RNA detected cases, the group classifications were made arbitrarily by HCV RNA levels of <105, 105 to 107, and ≥107 IU/ml (groups A, B, and C, respectively). Median HCV Ag levels of those groups were 11.1, 3,629.8, and 15,189.8 fmol/liter, respectively, and there were significant differences between them (P < 0.0001) (Fig. 2A ). However, the distributions of HCV Ag levels in groups B and C were considerably overlapped; therefore, the RNA-to-Ag ratio in each group was calculated. Median values of the RNA-to-Ag ratio in the groups A, B, and C were 300.5, 645.7, and 1,180.5 kIU/fmol (RNA/Ag), respectively, and significant differences between the ratios were also noted (P < 0.0013 for group A versus B, and P < 0.0001 for group B versus C) (Fig. 2B). Based on the linear regression analysis of cases with HCV RNA exceeding 15 IU/ml (n = 161), the following equation was established (r2 = 0.8679): HCV Ag (log10 fmol/liter) = 0.7881·HCV RNA (log10 IU/ml) − 1.4955.
FIG. 2.
HCV Ag levels and RNA-to-Ag ratio according grouped by HCV RNA levels. (A) Median HCV Ag levels were 11, 3,630, and 15,190 fmol/liter for the groups of RNA levels <105, 105 to 107, and ≥107 IU/ml (groups A, B, and C), respectively. HCV Ag levels showed significant differences between those groups (P < 0.0001). (B) The RNA-to-Ag ratios of those groups were also significantly different from each other (median of 301, 646, and 1,181 kIU/fmol for groups A, B, and C, respectively) (P < 0.0013 for group A versus B, and P < 0.0001 for group B versus C). The upper and lower ends of boxes and box inner lines correspond to the upper and lower quartiles and median values, respectively. Whiskers denote minimum and maximum values, and circles indicate individual values.
Among 279 samples in which anti-HCV and HCV Ag were qualitatively determined, 48 of 125 (38.4%) HCV Ag-nonreactive cases were positive for anti-HCV. Among these, HCV RNA was not detected in 35 subjects, and HCV RNA levels of 9 cases were below 15 IU/ml (Table 1). The four HCV Ag-nonreactive cases in the group which showed negative results for anti-HCV as well as HCV RNA levels below 15 IU/ml were from patients undergoing hemodialysis multiple times. All the HCV Ag-reactive cases (n = 154) were also positive for anti-HCV (Table 1). The percent agreement between HCV Ag and anti-HCV was 82.8%. Although HCV Ag was highly correlated with HCV RNA, positive correlations of HCV Ag with anti-HCV antibodies (r = 0.5762; P < 0.0001) and liver enzymes (r = 0.5683 for aspartate transaminase [AST]; r = 0.5408 for alanine transaminase [ALT]; P < 0.0001) were also found.
In this study, HCV Ag concentrations were strongly correlated with HCV RNA levels (r = 0.9464). The newly developed automated HCV Ag assay could be used as an alternative test for HCV RNA assays, considering the correlation coefficient between them. In the previous report, which utilized the same Ag assay and a different RNA assay (Roche Cobas Amplicor HCV monitor 2.0), the correlation coefficient was 0.74 (n = 197) (11). Another HCV Ag assay also showed a good correlation (n = 213; r = 0.848) with the Amplicor HCV monitor 2.0 assay (18).
According to the manufacturer's declaration, the lower detection limit of the CAM/CTM is as low as 15 IU/ml, while that of the Cobas Amplicor HCV monitor 2.0 is 500 IU/ml. In addition, CLIA, which is used for the Architect HCV Ag assay, is known to be more sensitive than ELISA or EIA. The analytical sensitivity of the Architect HCV Ag assay was shown to be 3 fmol/liter (equivalent to 0.06 pg/ml of recombinant c11 Ag or approximately 440 IU/ml HCV RNA) (11), while that of a conventional HCV Ag assay by ELISA was 2.3 pg/ml; i.e., approximately 16,600 IU/ml RNA (2). Further evaluations with more specimens would be helpful to calculate the correlation coefficient between the Architect HCV Ag assay and the CAM/CTM, since this is the first study that demonstrated the correlation between them.
In addition, the HCV RNA-to-Ag ratio from our data was not constant but increased as the RNA level was increased. These data suggest that more HCV core Ag is released into the blood as more viral replication occurs, but not as much as the increment of viral load. This is plausible, since viral core Ag is not a direct marker of replication. Bouvier-Alias et al. (2) demonstrated that the variation of the RNA/Ag ratio resulted from the interindividual difference and suggested that 1 pg/ml Ag was equivalent to about 8,000 IU/ml RNA (5,000 to 12,000 IU/ml in the majority). However, based on the equation generated from our result, 1 pg/ml Ag corresponds roughly to 17,000 IU/ml RNA. This disagreement might be due to the difference between the assays adopted and would hinder the exact conversion between RNA and Ag concentrations. A more precise RNA-to-Ag ratio and the exact nature of its variation need to be assessed in further studies.
As expected, qRT-PCR was more sensitive than the HCV Ag assay. Of the 161 samples with HCV RNA exceeding 15 IU/ml, 157 cases were reactive for the HCV Ag assay, giving a detection rate of 97.5%. However, HCV RNA levels were low (<15 IU/ml) in 13 of 17 (76.5%) cases in which HCV RNA was detected but HCV Ag was nonreactive. HCV RNA levels of the remaining 4 HCV RNA-positive, HCV Ag-nonreactive samples were below the detection limit of the Architect HCV Ag assay (approximately 440 IU/ml RNA) except one with 814 IU/ml. These results suggest that the Architect HCV Ag assay is less sensitive than CAM/CTM because of its detection limit. However, the HCV Ag assay demonstrated 100% specificity and PPV from our data, although overall sensitivity for detecting HCV RNA-positive cases was 90.2% (157/174 subjects). In the previous study of HCV Ag performed in patients that have undergone liver transplantations, a similar detection rate of 92% (378/410 cases) was reported (9). Improving the lower detection limit would enhance the analytical power of the HCV Ag assay.
We have evaluated the correlation between HCV Ag and HCV RNA with clinical specimens. Since there were no HCV Ag-reactive and anti-HCV-negative cases, we were not able to identify whether the early HCV infection without antibodies could be detected using the HCV Ag assay. However, Leary et al. (8) demonstrated that the HCV Ag was detected prior to the appearance of anti-HCV in the patients' sera and this phenomenon may have resulted in a reduction of the window period by 23 days or even longer. HCV Ag was also detected from more than 97% of HCV RNA-positive and antibody-negative samples in the same study.
There were 48 HCV Ag-nonreactive and anti-HCV-positive cases in this study. Among these cases, HCV RNA was not detected or was below 15 IU/ml in 44 (91.7%) cases, which may indicate stable infections (chronic infections without active viral replication). In addition, HCV RNA was not detected or was below 15 IU/ml in 121 (96.8%) of the 125 HCV Ag-nonreactive cases, irrespective of anti-HCV test results. Therefore, determination of the HCV Ag level would facilitate the distinction between active viral replication and stable infection and might be used to monitor the responses to antiviral therapies.
This study focused mainly on the evaluation of the correlation between HCV Ag and HCV RNA. Thus, we could not assess the effect of antiviral therapies on levels of HCV Ag and RNA. We also generated limited data regarding the effect of HCV genotypes on the correlation. Our results suggest that the HCV genotype does not influence the correlation. However, a previous study reported that the correlation coefficients were different from each other according to the genotype (17). In another study, HCV Ag and RNA levels were better correlated before therapy than after therapy (15). Further research on these subjects with the automated HCV Ag assay might be needed to clarify the relationship between HCV Ag and RNA, depending on the genotypes and therapies.
In conclusion, the automated HCV Ag assay showed results comparable to those of the HCV RNA viral load assay, and it has the advantages of easy testing and rapid reporting. This assay would be useful to monitor HCV infection and to discriminate active viral replication states from stable infections and could be an alternative to the quantitation of HCV RNA levels using qRT-PCR.
Footnotes
Published ahead of print on 29 March 2010.
The authors have paid a fee to allow immediate free access to this article.
REFERENCES
- 1.Aoyagi, K., C. Ohue, K. Iida, T. Kimura, E. Tanaka, K. Kiyosawa, and S. Yagi. 1999. Development of a simple and highly sensitive enzyme immunoassay for hepatitis C virus core antigen. J. Clin. Microbiol. 37:1802-1808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bouvier-Alias, M., K. Patel, H. Dahari, S. Beaucourt, P. Larderie, L. Blatt, C. Hezode, G. Picchio, D. Dhumeaux, A. U. Neumann, J. G. McHutchison, and J. M. Pawlotsky. 2002. Clinical utility of total HCV core antigen quantification: a new indirect marker of HCV replication. Hepatology 36:211-218. [DOI] [PubMed] [Google Scholar]
- 3.Enomoto, M., S. Nishiguchi, A. Tamori, M. Kohmoto, D. Habu, H. Sakaguchi, T. Takeda, N. Kawada, S. Seki, and S. Shiomi. 2005. Chemiluminescence enzyme immunoassay for monitoring hepatitis C virus core protein during interferon-alpha2b and ribavirin therapy in patients with genotype 1 and high viral loads. J. Med. Virol. 77:77-82. [DOI] [PubMed] [Google Scholar]
- 4.Gaudy, C., C. Thevenas, J. Tichet, N. Mariotte, A. Goudeau, and F. Dubois. 2005. Usefulness of the hepatitis C virus core antigen assay for screening of a population undergoing routine medical checkup. J. Clin. Microbiol. 43:1722-1726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Glynn, S. A., D. J. Wright, S. H. Kleinman, D. Hirschkorn, Y. Tu, C. Heldebrant, R. Smith, C. Giachetti, J. Gallarda, and M. P. Busch. 2005. Dynamics of viremia in early hepatitis C virus infection. Transfusion 45:994-1002. [DOI] [PubMed] [Google Scholar]
- 6.Hayashi, K., S. Hasuike, K. Kusumoto, A. Ido, H. Uto, N. Kenji, M. Kohara, S. O. Stuver, and H. Tsubouchi. 2005. Usefulness of a new immuno-radiometric assay to detect hepatitis C core antigen in a community-based population. J. Viral Hepat. 12:106-110. [DOI] [PubMed] [Google Scholar]
- 7.Laperche, S., N. Le Marrec, A. Girault, F. Bouchardeau, A. Servant-Delmas, M. Maniez-Montreuil, P. Gallian, T. Levayer, P. Morel, and N. Simon. 2005. Simultaneous detection of hepatitis C virus (HCV) core antigen and anti-HCV antibodies improves the early detection of HCV infection. J. Clin. Microbiol. 43:3877-3883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Leary, T. P., R. A. Gutierrez, A. S. Muerhoff, L. G. Birkenmeyer, S. M. Desai, and G. J. Dawson. 2006. A chemiluminescent, magnetic particle-based immunoassay for the detection of hepatitis C virus core antigen in human serum or plasma. J. Med. Virol. 78:1436-1440. [DOI] [PubMed] [Google Scholar]
- 9.Massaguer, A., X. Forns, J. Costa, A. Feliu, M. Garcia-Retortillo, M. Navasa, A. Rimola, J. C. Garcia-Valdecasas, and J. M. Sanchez-Tapias. 2005. Performance of hepatitis C virus core antigen immunoassay in monitoring viral load after liver transplantation. Transplantation 79:1441-1444. [DOI] [PubMed] [Google Scholar]
- 10.Medhi, S., S. K. Potukuchi, S. K. Polipalli, S. S. Swargiary, P. Deka, A. Chaudhary, N. Begum, Z. Hussain, R. S. Ahlawat, and P. Kar. 2008. Diagnostic utility of hepatitis C virus core antigen in hemodialysis patients. Clin. Biochem. 41:447-452. [DOI] [PubMed] [Google Scholar]
- 11.Morota, K., R. Fujinami, H. Kinukawa, T. Machida, K. Ohno, H. Saegusa, and K. Takeda. 2009. A new sensitive and automated chemiluminescent microparticle immunoassay for quantitative determination of hepatitis C virus core antigen. J. Virol. Methods 157:8-14. [DOI] [PubMed] [Google Scholar]
- 12.Niederau, C., S. Lange, T. Heintges, A. Erhardt, M. Buschkamp, D. Hurter, M. Nawrocki, L. Kruska, F. Hensel, W. Petry, and D. Haussinger. 1998. Prognosis of chronic hepatitis C: results of a large, prospective cohort study. Hepatology 28:1687-1695. [DOI] [PubMed] [Google Scholar]
- 13.Richter, S. S. 2002. Laboratory assays for diagnosis and management of hepatitis C virus infection. J. Clin. Microbiol. 40:4407-4412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Soffredini, R., M. G. Rumi, M. L. Parravicini, G. Ronchi, E. Del Ninno, A. Russo, and M. Colombo. 2004. Serum levels of hepatitis C virus core antigen as a marker of infection and response to therapy. Am. J. Gastroenterol. 99:1738-1743. [DOI] [PubMed] [Google Scholar]
- 15.Takahashi, M., H. Saito, M. Higashimoto, K. Atsukawa, and H. Ishii. 2005. Benefit of hepatitis C virus core antigen assay in prediction of therapeutic response to interferon and ribavirin combination therapy. J. Clin. Microbiol. 43:186-191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tanaka, E., C. Ohue, K. Aoyagi, K. Yamaguchi, S. Yagi, K. Kiyosawa, and H. J. Alter. 2000. Evaluation of a new enzyme immunoassay for hepatitis C virus (HCV) core antigen with clinical sensitivity approximating that of genomic amplification of HCV RNA. Hepatology 32:388-393. [DOI] [PubMed] [Google Scholar]
- 17.Veillon, P., C. Payan, G. Picchio, M. Maniez-Montreuil, P. Guntz, and F. Lunel. 2003. Comparative evaluation of the total hepatitis C virus core antigen, branched-DNA, and amplicor monitor assays in determining viremia for patients with chronic hepatitis C during interferon plus ribavirin combination therapy. J. Clin. Microbiol. 41:3212-3220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yokosuka, O., S. Kawai, Y. Suzuki, K. Fukai, F. Imazeki, T. Kanda, M. Tada, R. Mikata, A. Hata, and H. Saisho. 2005. Evaluation of clinical usefulness of second-generation HCV core antigen assay: comparison with COBAS AMPLICOR HCV MONITOR assay version 2.0. Liver Int. 25:1136-1141. [DOI] [PubMed] [Google Scholar]