Abstract
Different avian bornavirus (ABV) genotypes have recently been detected in psittacine birds with proventricular dilatation disease (PDD), an inflammatory fatal central and peripheral nervous system disorder. An indirect immunofluorescence assay (IIFA) for intra vitam demonstration of ABV-specific serum antibodies was established since reverse transcription-PCR (RT-PCR) assays may not detect all ABV variants.
Proventricular dilatation disease (PDD) is a fatal worldwide disease of psittacines characterized by lymphoplasmocytic infiltration of the ganglia of the central and peripheral nervous system, leading to neurological signs, gastrointestinal dysfunction, and wasting. A viral etiology has long been suspected. Recently, different genotypes of avian bornavirus (ABV), a new species of the family Bornaviridae, were identified in psittacines that died from PDD (4, 6, 7, 11, 13). The first experimental data support the etiological role of ABV (2). Intra vitam diagnosis of ABV infection is possible by reverse transcription-PCR (RT-PCR) using crop and cloacal swabs (1, 7). However, due to the heterogeneity of the ABV strains (1, 14), existing PCR protocols may not detect all ABV variants and enhance the need for suitable diagnostic completion. Therefore, the aim of this study was to establish a further reliable tool for intra vitam diagnosis of ABV infection in birds with and without clinical signs of PDD.
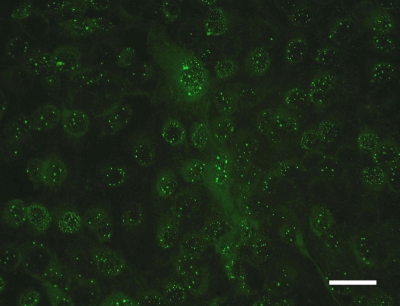
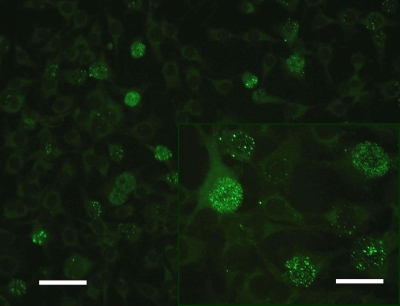
An indirect immunofluorescence assay (IIFA) was established and validated for the detection of ABV-specific serum antibodies. Methodological adequacy was confirmed by simultaneous isolation of infectious virus and detection of viral RNA, viral proteins, and typical histological lesions in six spontaneous PDD cases. The IIFA was adapted and modified using previously published protocols (8). Briefly, starting with a dilution of 1:10, doubling dilutions of sera were incubated on slides with acetone-fixed Madin-Darby canine kidney (MDCK) cells (CCL34; ATCC) persistently infected with Borna disease virus (BDV) H1766 (horse strain). After incubation for 30 min, cells were exposed for another 30 min with a fluorescein isothiocyanate (FITC)-conjugated goat anti-avian IgG (Bethyl Laboratories, Inc., Montgomery, TX) for visualization of binding of ABV-specific immunoglobulins to virus antigens. Sera containing ABV-specific antibodies caused a brilliant granular fluorescence in the nucleus of the BDV MDCK cells (Fig. 1). All six birds displayed antibodies against ABV, with titers ranging between 1:160 and 1:20,480 (Table 1 ). Specific-pathogen-free (SPF) chicken serum and 16 sera of an aviary without PDD history and of one Amazon parrot with intoxication (Ps21) (Table 1) served as negative controls. The specificity of the IIFA was confirmed by a lack of specific fluorescence with the use of the control sera. Besides, the quail cell line CEC32 (5, 15), which is persistently infected with the ABV isolate Ps22, was also used for the IIFA. About 90% of the cells were infected, leading to comparable levels of brilliant granular fluorescence of the nucleus with exposure to the sera from the six PDD cases (Fig. 2). The titers obtained with BDV MDCK cells and ABV CEC32 cells were comparable.
FIG. 1.
Indirect immunofluorescence assay for demonstration of ABV-specific antibodies, using BDV-infected MDCK cells. Note the brilliant granular fluorescence in the nucleus. Bar, 50 μm.
TABLE 1.
Demonstration of ABV-specific antibodies, infectious virus, ABV RNA, ABV antigen, and histopathological lesions characteristic of PDD
| Bird designation | Common name | Scientific name | Antibody titer | Result fora: |
Histopathology | ||
|---|---|---|---|---|---|---|---|
| Virus isolation | RT-PCR | IHC | |||||
| Ps7 | Blue-headed parrot | Pionus menstruus | 1:10,240 | + | + | + | PDD |
| Ps8 | African gray parrot | Psittacus erithacus | 1:20,480 | + | + | + | PDD |
| Ps19 | African gray parrot | Psittacus erithacus | 1:160 | + | + | + | PDD |
| Ps23 | Eclectus parrot | Eclectus roratus | 1:10,240 | + | + | + | PDD |
| Ps33 | African gray parrot | Psittacus erithacus | 1:2,560 | + | + | + | PDD |
| Ps35 | Greater sulfur- crested cockatoo | Cacatua galerita | 1:20,480 | + | + | + | PDD |
| Ps21 | Amazon parrot | Amazona sp. | <1:10 | − | − | NT | |
IHC, immunohistochemistry; NT, not tested.
FIG. 2.
Indirect immunofluorescence assay for demonstration of ABV-specific antibodies, using ABV-infected CEC cells. Note the brilliant granular fluorescence in the nucleus. Bar, 100 μm; insert, 50 μm.
In the six ABV-seropositive psittacines, ABV infection was further confirmed by different approaches (Table 1). The infectivity assay was performed as described previously (8), using CEC32 cells as indicator cells. From all six psittacines, infectious ABV was isolated from the brain (infectivity titers of 103 to 107 50% infective doses [ID50]/ml). ABV RNA was detected in four of the six birds by real-time RT-PCR (1) and in the other two cases by applying a conventional RT-PCR protocol (1). Immunohistological analysis was performed by the avidin biotin complex (ABC) method using a rabbit antibody specific for BDV phosphoprotein (3). The presence of viral antigen was demonstrated in the brain, spinal cord, retina, myocard, proventriculus, and gizzard. Histopathologically, in all six psittacines characteristic PDD lesions consisted of nonpurulent meningoencephalitis, myelitis, neuritis, myocarditis, and/or ganglionitis in the gastrointestinal tract (9).
The IIFA was validated and applied for intra vitam detection of ABV-specific antibodies by using serum and swabs (crop and cloaca) from 77 psittacines from flocks with PDD history but no present clinical signs. Sera were tested by IIFA, and swabs were analyzed by real-time RT-PCR or by an additional standard RT-PCR (1). In total, 35/77 psittacines (45%) exhibited ABV-specific antibodies. The titers ranged from 1:10 to 1:40,960. ABV RNA was amplified in 28/77 psittacines (36%), and in 64% of them (18/28), ABV-specific antibodies were also detected. ABV-specific antibodies were present in 34% of the 49 ABV RNA-negative birds (17/49).
Due to the increasing impact of PDD in psittacines and probably also for other birds (12), reliable intra vitam diagnosis of ABV infection represents a challenging approach. To date, the precise time course of infection, the concurrent clinical disease, the role of the virulence of different ABV genotypes, the route of infection, and the susceptibility of psittacine species are unknown. Nevertheless, reliable tools for intra vitam diagnosis of ABV infection even in birds without clinical signs of PDD are urgently needed. Therefore, an IIFA for the detection of ABV-specific antibodies that based on the cross-reactivity of psittacine antibodies with mammalian BDV antigens and binding to ABV antigens was established. Its accuracy was proven by simultaneous detection of viral RNA and viral proteins and isolation of infectious virus in six spontaneous PDD cases. This is similar to what was observed for clinically overt Borna disease (BD) in horses where BDV-specific antibodies regularly coexist with viral proteins and RNA in the central nervous system (CNS) (3, 10). However, seroepidemiological data indicate that equine BDV infection mostly runs a nonobvious course (10). If this is similarly true for ABV infections, these infections have to be investigated by extensive serological surveys of psittacine flocks by use of a reliable test system applicable also for large numbers of serum samples. Therefore, our ABV-specific IIFA was initially tested under field conditions using sera from 77 psittacines from flocks with PDD history. ABV infection was confirmed by different RT-PCR approaches using crop and cloacal swabs. The results clearly indicate that the IIFA is an appropriate serological test, as shown by the presence of specific serum antibodies in 64% of ABV RNA-positive birds (18/28) and in 34% of ABV RNA-negative psittacines (17/49). Due to the genetic variability of ABV species, the currently available PCR assays might not be able to detect all ABV genotypes. ABV RNA might also not be present in swabs at all time points of sample collection. Thus, the IIFA represents a suitable alternative for diagnosis of ABV infection in live birds. In 13% of the psittacines (10/77), only ABV RNA was found in the swabs, probably due to sample collection at an early phase of the infection. Comparable results were obtained in a recent serological study using an ABV-specific Western blot assay (12). In general, many psittacines seem to be infected with ABV without clinical signs, which is comparable to what was observed for the prevalence of BDV-specific antibodies in healthy horses and to what was observed for preliminary avian studies (7, 10). In summary, analysis of ABV-specific serum antibodies combined with detection of ABV RNA in crop and cloacal swabs represents the current method of choice for reliable intra vitam diagnosis of ABV infection.
Acknowledgments
This study was supported by Loro Parque Fundacion (PP-65-2009-1). None of the authors has a conflict of interest that could inappropriately influence this study.
We thank Jürgen A. Richt for kindly providing the anti-BDV phosphoprotein antibody.
Footnotes
Published ahead of print on 14 April 2010.
REFERENCES
- 1.Enderlein, D., S. Herzog, C. Herden, D. Neumann, T. Briese, E. F. Kaleta, M. Lierz, U. Heffels-Redmann, and H. Müller. 2009. Aviäres Bornavirus: Antikörper- und Genomnachweis in PDD positiven Vögeln, p. 18-20. In M. E. Krautwald-Junghanns and E. F. Kaleta (ed.), Tagungsbericht 1. DVG-Tagung über Vogel- und Reptilienkrankheiten 25.-27.09.09. DVG-Verlag, Geissen, Germany.
- 2.Gancz, A. Y., A. L. Kistler, A. L. Greninger, Y. Farnoushi, S. Mechani, S. Perl, A. Berkowitz, N. Perez, S. Clubb, J. L. DeRisi, D. Ganam, and A. Lublin. 2009. Experimental induction of proventricular dilatation disease in cockatiels (Nymphicus hollandicus) inoculated with brain homogenates containing avian bornavirus 4. Virol. J. 6:100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Herden, C., S. Herzog, T. Wehner, C. Zink, J. A. Richt, and K. Frese. 1999. Comparison of different methods of diagnosing Borna disease in horses post mortem, p. 286-290. In U. Wernery, J. Wade, J. A. Mumford, and O. R. Kaaden (ed.), Equine infectious diseases VIII. R&W Publications, Newmarket, United Kingdom.
- 4.Honkavuori, K. S., H. L. Shivaprasad, B. L. Williams, P. L. Quan, M. Hornig, C. Street, G. Palacios, S. K. Hutchison, M. Franca, M. Egholm, T. Briese, and W. I. Lipkin. 2008. Novel Borna virus in psittacine birds with proventricular dilatation disease. Emerg. Infect. Dis. 14:1883-1886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kaaden, O. R., S. Lange, and B. Stiburek. 1982. Establishment and characterization of chicken embryo fibroblast clone LSCC-H32. In Vitro 18:827-834. [DOI] [PubMed] [Google Scholar]
- 6.Kistler, A. L., A. Gancz, S. Clubb, P. Skewes-Cox, K. Fischer, K. Sorber, C. Y. Chiu, A. Lublin, S. Mechani, Y. Farnoushi, A. Greninger, C. C. Wen, S. B. Karlene, D. Ganem, and J. L. DeRisi. 2008. Recovery of divergent avian bornaviruses from cases of proventricular dilatation disease: identification of a candidate etiologic agent. Virol. J. 5:88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lierz, M., H. M. Hafez, K. S. Honkavuori, A. D. Gruber, P. Olias, E. M. Abdelwhab, A. Kohls, W. I. Lipkin, T. Briese, and R. Hauck. 2009. Anatomic distribution of avian Borna virus in parrots, its occurrence in clinically healthy birds and ABV-antibody detection. Avian Pathol. 38:491-496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Narayan, O., S. Herzog, K. Frese, H. Scheefers, and R. Rott. 1983. Pathogenesis of Borna disease in rats: immune-mediated viral ophthalmoencephalopathy causing blindness and behavioral abnormalities. J. Infect. Dis. 148:305-315. [DOI] [PubMed] [Google Scholar]
- 9.Ouyang, N., R. Storts, Y. Tian, W. Wigle, I. Villanueva, N. Mirhosseini, S. Payne, P. Gray, and I. Tizard. 2009. Histopathology and the detection of avian bornavirus in the nervous system of birds diagnosed with provenrticular dilatation disease. Avian Pathol. 38:393-401. [DOI] [PubMed] [Google Scholar]
- 10.Richt, J. A., A. Grabner, S. Herzog, W. Garten, and C. Herden. 2007. Borna disease in equines, p. 201-216. In D. C. Sellon and M. Long (ed.), Equine infectious diseases. Saunders-Elsevier, St. Louis, MO.
- 11.Rinder, M., A. Ackermann, H. Kempf, B. Kaspers, R. Korbel, and P. Staeheli. 2009. Broad tissue and cell tropism of avian bornavirus in parrots with proventricular dilatation disease. J. Virol. 83:5401-5407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Villanueva, I., P. Gray, N. Mirhosseini, S. Payne, S. Hoppes, K. S. Honkavuori, T. Briese, D. Turner, and I. Tizard. 2009.The diagnosis of proventricular dilatation disease: use of Western blot assay to detect antibodies against avian Borna virus. Vet. Microbiol. doi: 10.1016/j.vetmic.2009.11.041. [DOI] [PubMed]
- 13.Weissenböck, H., K. Sekulin, T. Bakonyi, S. Högler, and N. Nowotny. 2009. Novel avian bornavirus in a nonpsittacine species (Canary; Serinus canaria) with enteric ganglioneuritis and encephalitis. J. Virol. 83:11367-11371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Weissenböck, H., T. Bakonyi, K. Sekulin, F. Ehrensperger, R. J. T. Doneley, R. Dürrwald, R. Hoop, K. Erdélyi, J. Gál, J. Kolodziejek, and N. Nowotny. 2009. Avian bornaviruses in psittacine birds from Europe and Australia with proventricular dilatation disease. Emerg. Infect. Dis. 15:1453-1459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zöller, B., I. Redman-Müller, I. Nanda, M. Guttenbach, E. Dosch, M. Schmid, R. Zoorob, and C. Jungwirth. 2000. Sequence comparison of avian interferon regulatory factors and identification of the avian CEC-32 cell as a quail cell line. J. Interferon Cytokine Res. 20:711-717. [DOI] [PubMed] [Google Scholar]