Abstract
A matched 1:3 case-control study investigated factors predicting colistin-resistant versus colistin-susceptible KPC-producing Klebsiella pneumoniae acquisition and its impact on patient outcomes. Case patients were more often admitted from other institutions (P = 0.019) and had longer therapy with β-lactam/β-lactamase inhibitors (P = 0.002) and higher overall mortality (P = 0.05). All 52 study isolates were clonally related, suggesting horizontal dissemination. None of these parameters independently predicted colistin resistance, which probably occurred in a susceptible KPC-KP strain that was subsequently disseminated horizontally.
Klebsiella pneumoniae isolates producing KPC carbapenemases (KPC-KP) have been widely disseminated in several regions worldwide (15). In Greek hospitals, carbapenem resistance among K. pneumoniae isolates, mediated by verona imipenemase (VIM) or, more recently, KPC enzymes is a major problem (16, 17). Colistin represents one of the few antimicrobials active against KPC producers (15) and is frequently used for the treatment of the respective infections. The emergence of colistin resistance among KPC-KP (1, 2, 5, 11) poses a new threat that necessitates detailed investigation.
In our hospital, KPC-KP isolates are widespread, and since April 2008, colistin-resistant (CR) KPC-KP isolates have also emerged. Studies analyzing risk factors for CR KPC-KP acquisition have not been described in the literature. In this context, we conducted a case-control study to investigate the risk factors and epidemiological characteristics associated with the acquisition of CR KPC-KP and its impact on patient outcomes.
Hospital setting.
The Tzaneio General Hospital, Piraeus, Greece, is a 480-bed tertiary care hospital that has a 10-bed general intensive care unit (ICU) and a 10-bed cardiology ICU.
Selection of cases and controls and matching criteria.
The study population included adults hospitalized from April 2008 through June 2009, when a matched 1:3 case-control study was initiated. All patients from whom a CR KPC-KP (colistin MIC, ≥4 mg/liter) was isolated from a clinical sample (not surveillance culture) during the study period were defined as cases. For each case patient, three patients that yielded a colistin-susceptible (CS) KPC-KP clinical isolate during the same period were selected as controls. We did not include control patients harboring carbapenemase-negative K. pneumoniae isolates, as such isolates are rarely recovered from critically ill patients in our institution. Matching was performed according to the site of isolation, considering the patient's infection versus colonization status and ward of hospitalization. One isolate per patient, recovered from the most clinically significant site, was included in the study.
Phenotypic testing.
Identification and susceptibility testing of isolates were performed by using a Vitek 2 system (bioMérieux, France). Colistin and tigecycline MICs were determined by the CLSI reference broth microdilution method (4). For tigecycline, the U.S. Food and Drug Administration recommendation was used (≤2 μg/ml, susceptible; ≥8 μg/ml, resistant), and for colistin, the European Committee on Antimicrobial Susceptibility Testing (EUCAST) clinical breakpoints for Enterobacteriaceae were used (≤2 μg/ml, susceptible; >2 μg/ml, resistant) (7).
Phenotypic screening for carbapenemases was performed for all isolates exhibiting reduced susceptibility to carbapenems (MIC, >1 μg/ml) by using the modified Hodge test and the combined disk tests using carbapenems with and without EDTA or boronic acid (8, 18).
Molecular typing.
Pulsed-field gel electrophoresis (PFGE) of SpeI-digested genomic DNA of the KPC-producing K. pneumoniae strains was performed with a CHEF-DR III system (Bio-Rad, Hemel Hempstead, United Kingdom) (16). Three contemporary KPC-negative K. pneumoniae isolates were also genotyped in parallel for comparison.
PCR assays and DNA sequencing.
Broad-spectrum β-lactamase genes (KPC, plasmidic AmpC, OXA-48 carbapenemase, metallo-β-lactamase [MBL], and extended-spectrum β-lactamase [ESBL] genes) were detected by PCR and sequencing (16).
Abstraction of patient data.
Data regarding cases and controls were extracted from patients' medical charts. Parameters assessed included age and gender, site of isolation, infection versus colonization (9), ward and duration of hospitalization at isolation time, comorbidities, prior exposures to antibiotics for ≥3 days, invasive procedures, nosocomial environment exposures (admission from another institution, prior hospitalization and ICU stay, prior surgery), and outcomes (in-hospital mortality and attributable mortality).
Statistical analysis.
Continuous variables were compared between groups by t test. Categorical variables were compared using Fisher's exact test. A logistic regression model that included parameters with P values of ≤0.1 between groups in the univariate analysis was fitted to identify independent risk factors associated with colistin resistance. A two-tailed P value of ≤0.05 was considered statistically significant. Statistical analyses were performed with SPSS (SPSS, Inc., Chicago, IL).
Of the 13 case patients, 8 were infected (7 with bloodstream infections) and 5 were colonized by CR KPC-KP isolates. Of note, 2 case patients carried CS before yielding CR KPC-KP and were receiving colistin in the meantime. Most of the case patients (9 of 13) were hospitalized at the general ICU, while 3 patients were hospitalized in medical wards and 1 in a surgical ward, respectively. The isolates did not cluster in time, as usually 1 new case patient was identified each month of the study period. The 39 control patients (24 infected and 15 colonized; 27 general ICU and 12 non-ICU patients) were selected using the matching criteria among 71 patients that yielded CS KPC-KP isolates during the same period.
All 13 CR KPC-KP isolates were susceptible to tigecycline, 12 to gentamicin, and 3 to meropenem. Colistin MICs ranged from 4 to 24 μg/ml. Among the 39 CS KPC-KP isolates, 38 were susceptible to gentamicin, 33 to tigecycline, 16 to meropenem, and 9 to imipenem. All isolates were phenotypically identified as possible KPC-KP by a positive modified Hodge test, a negative EDTA test, and a positive boronic acid disk test. All 52 isolates were found by PCR and sequencing to carry blaKPC-2, and 48 of them were found to carry the blaSHV-12 allele. Other broad-spectrum β-lactamase genes were not detected.
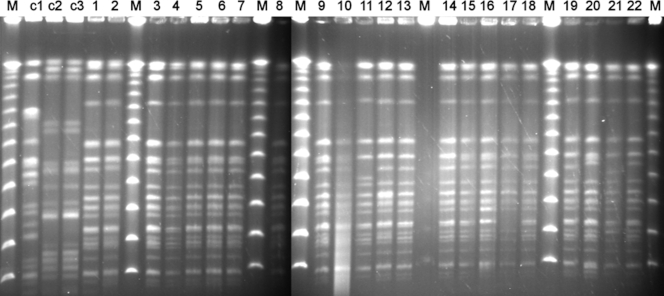
PFGE analysis showed that the 13 CR and 39 CS KPC-KP isolates belonged to a single pulsotype, with five subtypes that differed from each other by two bands (Fig. 1). Forty-five isolates (12 CR and 33 CS) belonged to the predominant subtype, Ia, while 1 CR isolate belonged to subtype Ib. Six CS isolates belonged to subtypes Ic to Ie; 3 to subtype Ic, 1 to subtype Id, and 2 to subtype Ie. The isolates of subtypes Ib and Id came from patients hospitalized in the medical department, while the isolates of subtypes Ic and Ie were recovered from ICU patients.
FIG. 1.
PFGE profiles of 12 CR KPC-KP and 10 CS KPC-KP isolates representing all subtypes of the single KPC-KP clone. Lanes M, lambda phage molecular size marker; lanes c1 to c3, three contemporary KPC-negative K. pneumoniae isolates recovered during the study period; lanes 1 to 10 and 12, CR KPC-KP of the subtype Ia; lane 11, CR KPC-KP of the subtype Ib; lanes 13 to 16, 18, 19, and 22, CS KPC-KP of the subtype Ia; lane 17, CS KPC-KP of the subtype Ic; lane 20, CS KPC-KP of the subtype Id; lane 21, CS KPC-KP of the subtype Ie.
The univariate analysis between patient groups is presented in Tables 1 and 2. Regarding nosocomial exposure data, the only factor with a significant correlation with CR KPC-KP acquisition was the admission from other hospitals (P = 0.019). Regarding the antibiotic exposure, the duration of β-lactam/β-lactamase inhibitor combination administration was significantly longer for case patients (P = 0.002). Multivariable analysis revealed that none of the variables entered into the model was an independent risk factor for colistin resistance (data not shown).
TABLE 1.
Univariate analysis for demographic and hospitalization data and comorbidities of patients with CR KPC-KP and CS KPC-KP isolates
| Variable | No. (%) of patients |
P value | Mean ± SDa |
P value | ||
|---|---|---|---|---|---|---|
| CR KPC-KPb | CS KPC-KPc | CR KPC-KP | CS KPC-KP | |||
| Demographics | ||||||
| Age (yr) | 68.7 ± 17.4 | 64.1 ± 21.0 | 0.48 | |||
| Male sex | 8 (61.5) | 27 (69.2) | 0.735 | |||
| Hospitalization data | ||||||
| Hospital stay (days) | 20.5 ± 20.8 | 16.4 ± 12.3 | 0.382 | |||
| ICU stay (days) | 26.4 ± 22.5 | 17.9 ± 11.4 | 0.14 | |||
| Admission from other hospital | 9 (69.2) | 11 (28.2) | 0.019 | |||
| Prior hospitalization | 9 (69.2) | 25 (64.1) | 1 | |||
| Duration (days) | 7.9 ± 7.3 | 14.5 ± 9.5 | 0.248 | |||
| Prior ICU stay | 4 (30.8) | 5 (12.8) | 0.203 | |||
| Duration (days) | 30.3 ± 26.8 | 32.8 ± 49 | 0.929 | |||
| Comorbidities | ||||||
| Heart and vascular disease | 7 (53.8) | 18 (46.2) | 0.752 | |||
| Cerebrovascular disease | 1 (7.7) | 9 (23.1) | 0.419 | |||
| Craniocerebral injury | 2 (15.4) | 9 (23.1) | 0.709 | |||
| Malignancy | 2 (15.4) | 7 (17.9) | 1 | |||
| Chronic obstructive pulmonary disease | 4 (30.8) | 6 (15.4) | 0.244 | |||
| Renal failure | 4 (30.8) | 13 (33.3) | 1 | |||
| Neurological disorders | 4 (30.8) | 7 (17.9) | 0.435 | |||
| Diabetes mellitus | 0 | 6 (15.4) | 0.317 | |||
| Prior surgery | 7 (53.8) | 21 (53.8) | 1 | |||
Units are given in the Variable column.
A total of 13 patients were tested.
A total of 39 patients were tested.
TABLE 2.
Univariate analysis for prior antibiotic exposure and invasive procedures among patients with CR KPC-KP and CS KPC-KP isolates
| Variable | No. (%) of patients |
P value | Mean duration of indicated exposure or procedure ± SD (days) |
P value | ||
|---|---|---|---|---|---|---|
| CR KPC-KP | CS KPC-KP | CR KPC-KP | CS KPC-KP | |||
| Prior antibiotic exposure | ||||||
| 3rd- and 4th-generation cephalosporins | 5 (38.5) | 14 (35.9) | 1 | 6.4 ± 3.4 | 9.7 ± 5 | 0.194 |
| β-Lactam/β-lactamase inhibitor combinations | 6 (46.2) | 27 (69.2) | 0.187 | 18.7 ± 6.5 | 10.5 ± 5.2 | 0.002 |
| Aminoglycosides | 5 (38.5) | 11 (28.2) | 0.506 | 12.2 ± 7 | 8.6 ± 4.8 | 0.243 |
| Quinolones | 7 (53.8) | 26 (66.7) | 0.51 | 13.7 ± 8.2 | 9.6 ± 5.3 | 0.116 |
| Carbapenems | 5 (38.5) | 9 (23.1) | 0.3 | 18.4 ± 13.4 | 12.2 ± 9.3 | 0.327 |
| Colistin | 4 (30.8) | 8 (20.5) | 0.466 | 20.5 ± 13 | 15.1 ± 7.2 | 0.369 |
| Tigecycline | 3 (23.1) | 6 (15.4) | 0.674 | 15.7 ± 11.5 | 15.0 ± 4.0 | 0.897 |
| Glycopeptides | 7 (53.8) | 13 (33.3) | 0.208 | 13.4 ± 11.1 | 14.5 ± 5.5 | 0.766 |
| Linezolid | 4 (30.8) | 6 (15.4) | 0.244 | 14.0 ± 8.3 | 11.0 ± 7.4 | 0.564 |
| Antifungals | 4 (30.8) | 9 (23.1) | 0.714 | 27.8 ± 22.0 | 11.6 ± 9.0 | 0.077 |
| Invasive procedures | ||||||
| Mechanical ventilation | 9 (69.2) | 26 (66.7) | 1 | 21 ± 15.6 | 14.4 ± 7.9 | 0.105 |
| Central line catheter | 13 (100) | 30 (76.9) | 0.091 | 20.2 ± 21.0 | 17.0 ± 11.4 | 0.517 |
| Urinary catheter | 13 (100) | 37 (94.9) | 1 | 20.5 ± 20.8 | 15.4 ± 11.8 | 0.282 |
| Nasogastric tube | 11 (84.6) | 30 (76.9) | 0.709 | |||
| Foreign materiala | 5 (38.5) | 9 (23.1) | 0.3 | |||
| Surgical operations after admission | 3 (23.1) | 10 (25.6) | 1 | |||
Foreign material included prosthetic valves, orthopedic devices, vascular and biliary stents, and pacemakers.
The overall in-hospital mortality (mortality due to all causes of death) was significantly higher for case patients than for controls (69.2 versus 35.9%; P = 0.05). However, the overall mortality of the patients with clinically significant KPC-KP infection (75.0 versus 54.2%; P = 0.42) and the mortality attributed to KPC-KP infection (37.5% in both groups) did not differ between groups.
The global spread of KPC-KP represents an emerging phenomenon with clinical and public health implications. Treatment options for KPC infections are very limited, and colistin has been used either as monotherapy or in combination schemes (1, 6, 13, 14). Indeed, resistance to colistin has been only sporadically reported among KPC producers (1, 2, 3) and has been reported to occur during treatment for KPC-KP infections (5, 12). The emergence of CR KPC-KP in our hospital prompted this study to identify possible risk factors that contributed to its development.
Although several studies have focused on the description of outbreaks caused by KPC producers (1, 2, 6, 13, 14, 16), there is scarce data regarding risk factors for KPC-KP isolations (10). Risk factors associated with CR KPC-KP acquisition have not yet been described. The present case-control study investigates the characteristics of patients harboring CR compared with patients harboring CS KPC-KP. The study isolates belonged to a single PFGE type with five closely related subtypes. The predominant subtype included the vast majority of CR and CS KPC-KP isolates, possibly indicating that resistance was initially developed with a CS KPC-KP isolate under the pressure of colistin use and that the resistant strain was thereafter disseminated horizontally to other patients.
The proportion of patients that received colistin and the duration of its administration were not associated with CR KPC-KP isolation. However, the role of colistin usage may have been underestimated, as the molecular epidemiology findings support the clonal transmission of CR KPC-KP, independently of colistin administration.
The admission from other institutions was associated with CR KPC-KP isolation. Notably, two patients yielded CR KPC-KP upon admission to our hospital, indicating that the isolate was acquired elsewhere. It could be hypothesized that admission from other hospitals may implicate a longer exposure to potential risk factors, although detailed clinical data for the previous hospitalizations were not available.
It is of interest that many patients from both groups had β-lactam/β-lactamase inhibitor administrations that were significantly longer for case patients, prior to the KPC-KP isolation. This was probably due to the high MICs of β-lactam/β-lactamase inhibitors (usually >256 μg/ml), which might select for KPC-KP.
Considerable differences between the two groups, which included mainly severely ill patients, regarding their clinical characteristics and the mortality attributed to KPC-KP infections, were not identified. However, the significantly higher overall mortality of patients harboring CR KPC-KP may indicate a more critical clinical status.
Finally, none of the variables examined was independently associated with CR KPC-KP acquisition, supporting horizontal transmission as the main route of CR KPC-KP dissemination and underlining the need for more intensive infection control measures.
Footnotes
Published ahead of print on 7 April 2010.
REFERENCES
- 1.Bratu, S., D. Landman, R. Haag, R. Recco, A. Eramo, M. Alam, and J. Quale. 2005. Rapid spread of carbapenem-resistant Klebsiella pneumoniae in New York City. Arch. Intern. Med. 165:1430-1435. [DOI] [PubMed] [Google Scholar]
- 2.Bratu, S., P. Tolaney, U. Karumudi, J. Quale, M. Mooty, S. Nichani, and D. Landman. 2005. Carbapenemase-producing Klebsiella pneumoniae in Brooklyn, NY: molecular epidemiology and in vitro activity of polymyxin B and other agents. J. Antimicrob. Chemother. 56:128-132. [DOI] [PubMed] [Google Scholar]
- 3.Castanheira, M., H. S. Sader, L. M. Deshpande, T. R. Fritsche, and R. N. Jones. 2008. Antimicrobial activities of tigecycline and other broad-spectrum antimicrobials tested against serine carbapenemase- and metallo-beta-lactamase-producing Enterobacteriaceae: report from the SENTRY Antimicrobial Surveillance Program. Antimicrob. Agents Chemother. 52:570-573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Clinical and Laboratory Standards Institute. 2008. Performance standards for antimicrobial susceptibility testing, 18th informational supplement. M100-S18. Clinical and Laboratory Standards Institute, Wayne, PA.
- 5.Elemam, A., J. Rahimian, and W. Mandell. 2009. Infection with panresistant Klebsiella pneumoniae: a report of 2 cases and a brief review of the literature. Clin. Infect. Dis. 49:271-274. [DOI] [PubMed] [Google Scholar]
- 6.Endimiani, A., J. M. Depasquale, S. Forero, F. Perez, A. M. Hujer, D. Roberts-Pollack, P. D. Fiorella, N. Pickens, B. Kitchel, A. E. Casiano-Colón, F. C. Tenover, and R. A. Bonomo. 2009. Emergence of blaKPC-containing Klebsiella pneumoniae in a long-term acute care hospital: a new challenge to our healthcare system. J. Antimicrob. Chemother. 64:1102-1110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.European Committee on Antimicrobial Susceptibility Testing. 2009. Breakpoint tables for interpretation of MICs and zone diameters. Version 1.0, December 2009. European Committee on Antimicrobial Susceptibility Testing, Basel, Switzerland.
- 8.Franklin, C., L. Liolios, and A. Y. Peleg. 2006. Phenotypic detection of carbapenem-susceptible metallo-beta-lactamase-producing gram-negative bacilli in the clinical laboratory. J. Clin. Microbiol. 44:3139-3144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Garner, J. S., W. R. Jarvis, T. G. Emori, T. C. Horan, and J. M. Hughes. 1988. CDC definitions for nosocomial infections. Am. J. Infect. Control. 16:128-140. [DOI] [PubMed] [Google Scholar]
- 10.Gasink, L. B., P. H. Edelstein, E. Lautenbach, M. Synnestvedt, and N. O. Fishman. 2009. Risk factors and clinical impact of Klebsiella pneumoniae carbapenemase-producing K. pneumoniae. Infect. Control Hosp. Epidemiol. 30:1180-1185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Landman, D., C. Georgescu, D. A. Martin, and J. Quale. 2008. Polymyxins revisited. Clin. Microbiol. Rev. 21:449-465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lee, J., G. Patel, S. Huprikar, D. P. Calfee, and S. G. Jenkins. 2009. Decreased susceptibility of polymyxin B during treatment for carbapenem-resistant Klebsiella pneumoniae infection. J. Clin. Microbiol. 47:1611-1612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Maltezou, H. C., P. Giakkoupi, A. Maragos, M. Bolikas, V. Raftopoulos, H. Papahatzaki, G. Vrouhos, V. Liakou, and A. C. Vatopoulos. 2009. Outbreak of infections due to KPC-2-producing Klebsiella pneumoniae in a hospital in Crete (Greece). J. Infection 58:198-204. [DOI] [PubMed] [Google Scholar]
- 14.Nadkarni, A. S., T. Schliep, L. Khan, and C. B. Zeana. 2009. Cluster of bloodstream infections caused by KPC-2 carbapenemase-producing Klebsiella pneumoniae in Manhattan. Am. J. Infect. Control 37:121-126. [DOI] [PubMed] [Google Scholar]
- 15.Nordmann, P., G. Cuzon, and T. Naas. 2009. The real threat of Klebsiella pneumoniae carbapenemase producing bacteria. Lancet Infect. Dis. 9:228-236. [DOI] [PubMed] [Google Scholar]
- 16.Pournaras, S., E. Protonotariou, E. Voulgari, I. Kristo, E. Dimitroulia, D. Vitti, M. Tsalidou, A. N. Maniatis, A. Tsakris, and D. Sofianou. 2009. Clonal spread of KPC-2 carbapenemase-producing Klebsiella pneumoniae strains in Greece. J. Antimicrob. Chemother. 64:348-352. [DOI] [PubMed] [Google Scholar]
- 17.Psichogiou, M., P. T. Tassios, A. Avlamis, I. Stefanou, C. Kosmidis, E. Platsouka, O. Paniara, A. Xanthaki, M. Toutouza, G. L. Daikos, and L. S. Tzouvelekis. 2008. Ongoing epidemic of blaVIM-1-positive Klebsiella pneumoniae in Athens, Greece: a prospective survey. J. Antimicrob. Chemother. 61:59-63. [DOI] [PubMed] [Google Scholar]
- 18.Tsakris, A., I. Kristo, A. Poulou, K. Themeli-Digalaki, A. Ikonomidis, D. Petropoulou, S. Pournaras, and D. Sofianou. 2009. Evaluation of boronic acid disk tests for differentiating KPC-possessing Klebsiella pneumoniae isolates in the clinical laboratory. J. Clin. Microbiol. 47:362-367. [DOI] [PMC free article] [PubMed] [Google Scholar]