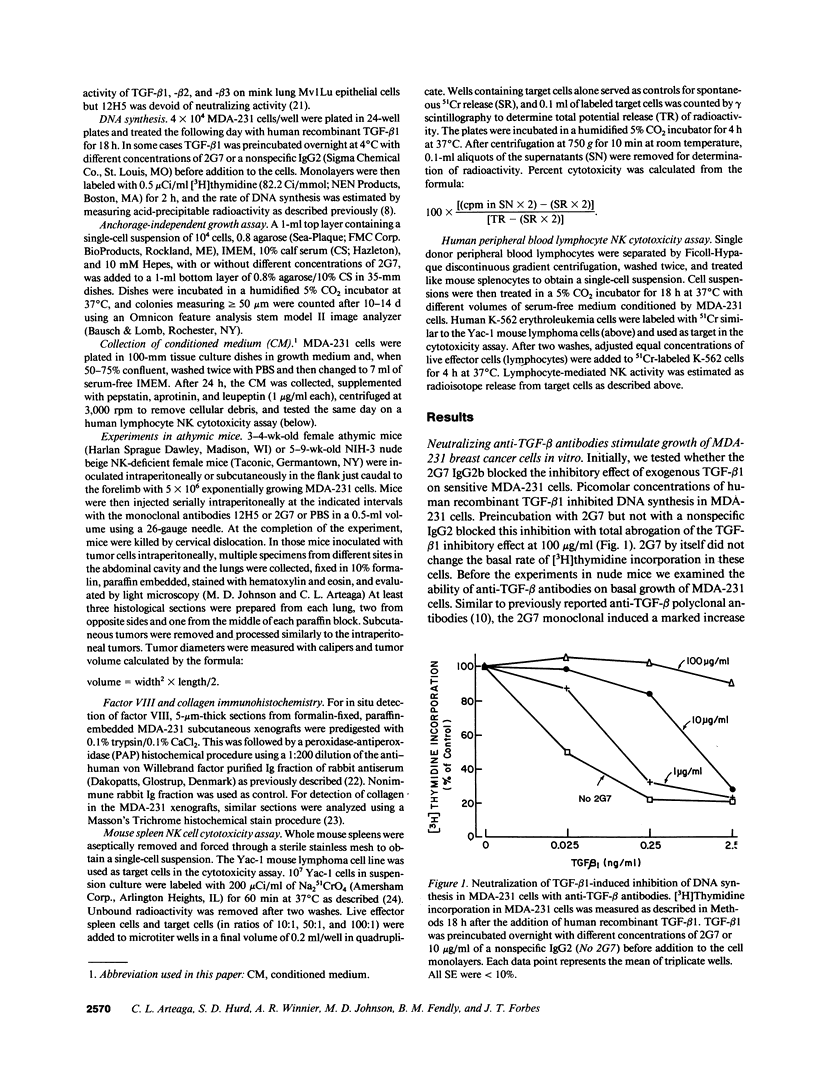
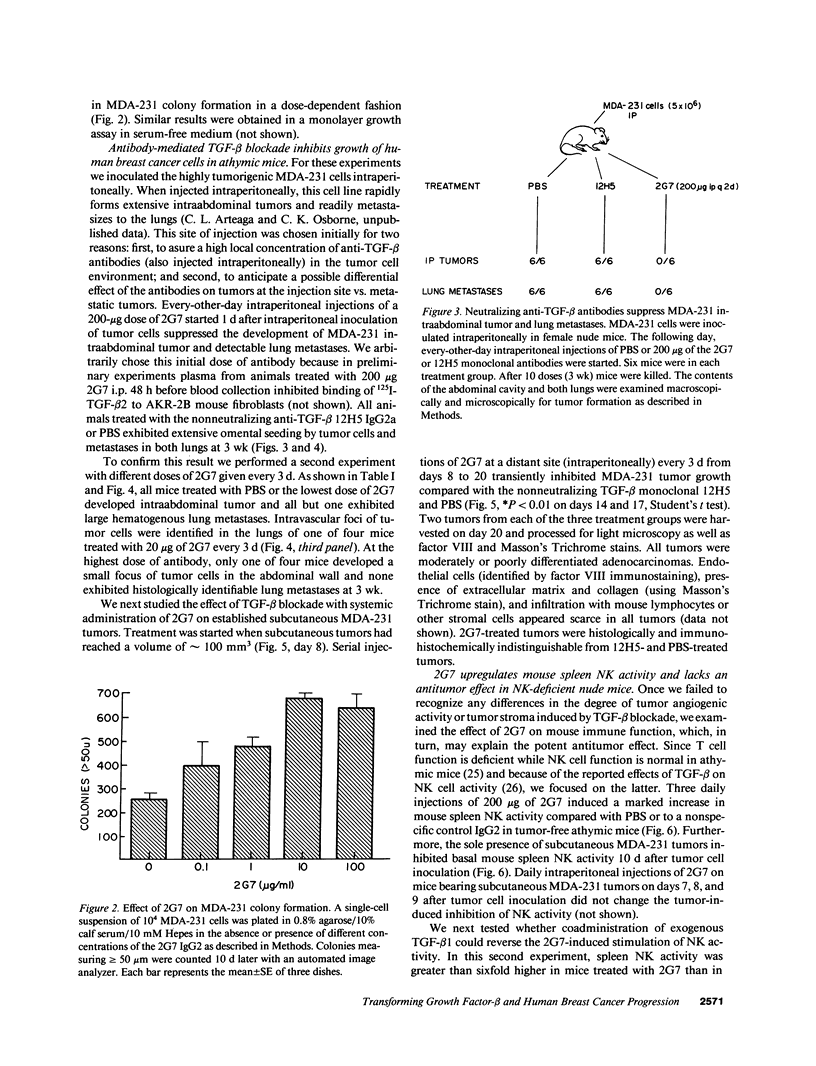
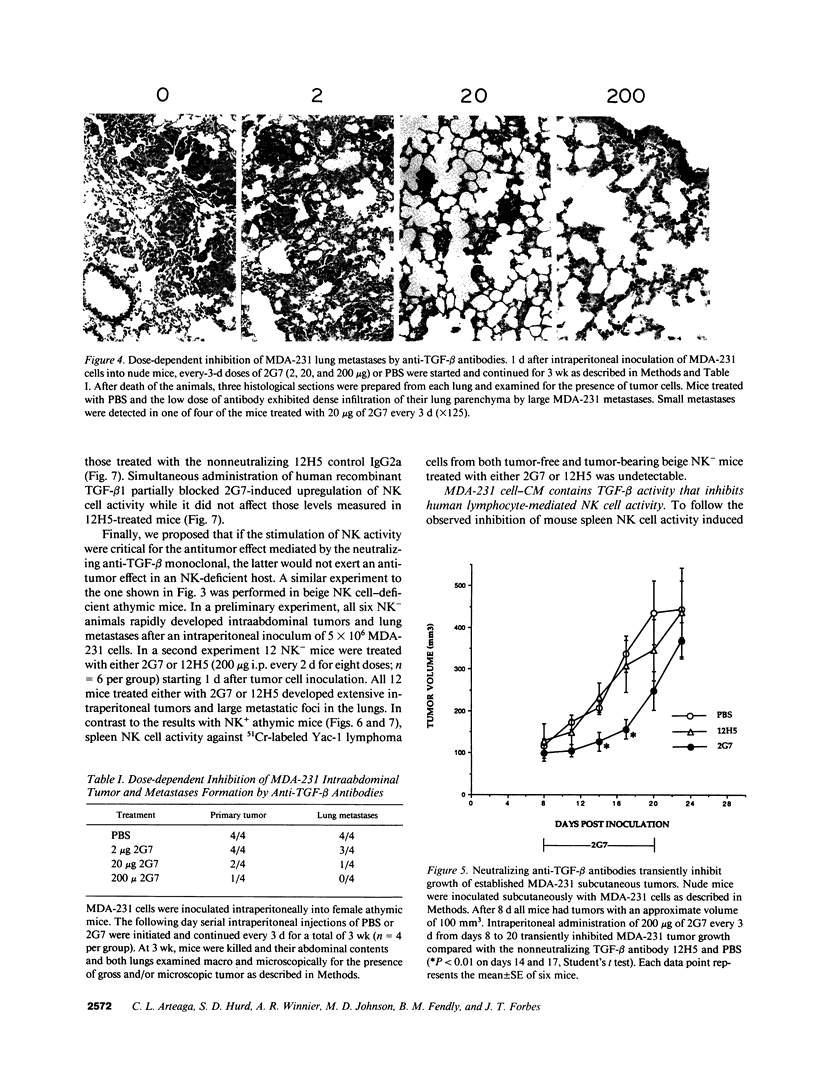
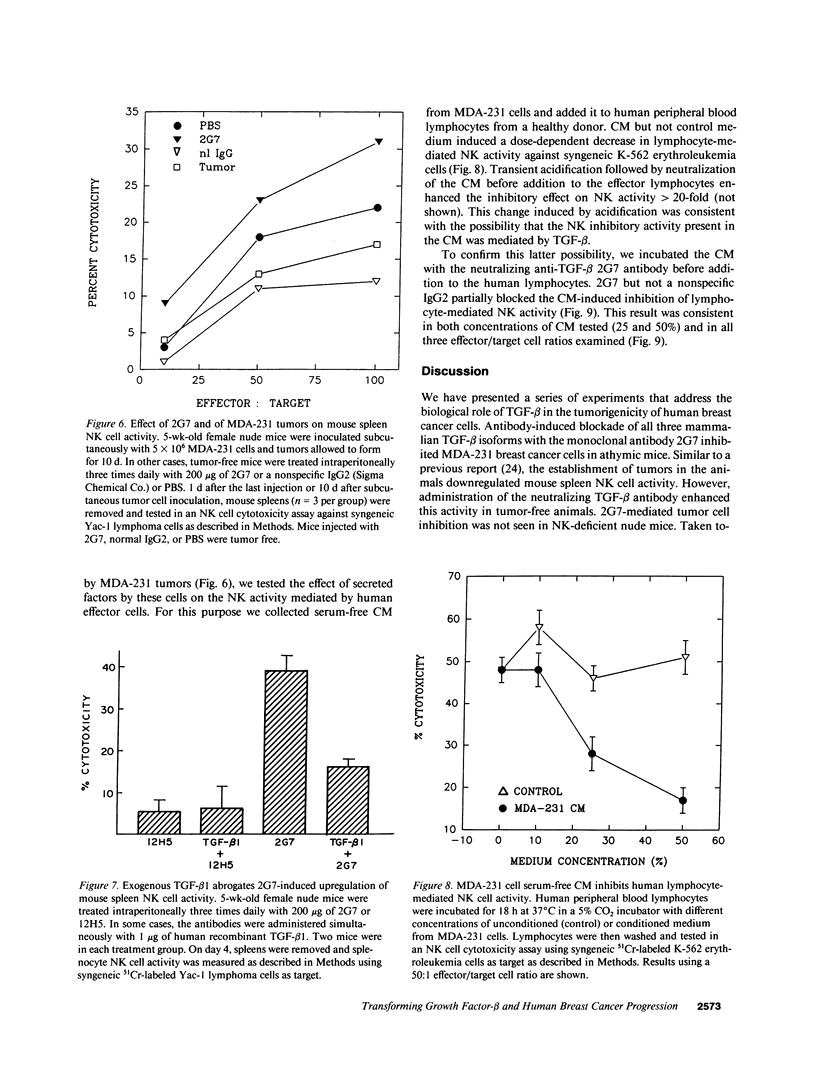
Abstract
TGF-beta effects on angiogenesis, stroma formation, and immune function suggest its possible involvement in tumor progression. This hypothesis was tested using the 2G7 IgG2b, which neutralizes TGF-beta 1, -beta 2, and -beta 3, and the MDA-231 human breast cancer cell line. Inoculation of these cells in athymic mice decreases mouse spleen natural killer (NK) cell activity. Intraperitoneal injections of 2G7 starting 1 d after intraperitoneal inoculation of tumor cells suppressed intraabdominal tumor and lung metastases, whereas the nonneutralizing anti-TGF-beta 12H5 IgG2a had no effect. 2G7 transiently inhibited growth of established MDA-231 subcutaneous tumors. Histologically, both 2G7-treated and control tumors were identical. Intraperitoneal administration of 2G7 resulted in a marked increase in mouse spleen NK cell activity. 2G7 did not inhibit MDA-231 primary tumor or metastases formation, nor did it stimulate NK cell-mediated cytotoxicity in beige NK-deficient nude mice. Finally, serum-free conditioned medium from MDA-231 cells inhibited the NK cell activity of human blood lymphocytes. This inhibition was blocked by the neutralizing anti-TGF-beta 2G7 antibody but not by a nonspecific IgG2. These data support a possible role for tumor cell TGF-beta in the progression of mammary carcinomas by suppressing host immune surveillance.
Full text
PDF
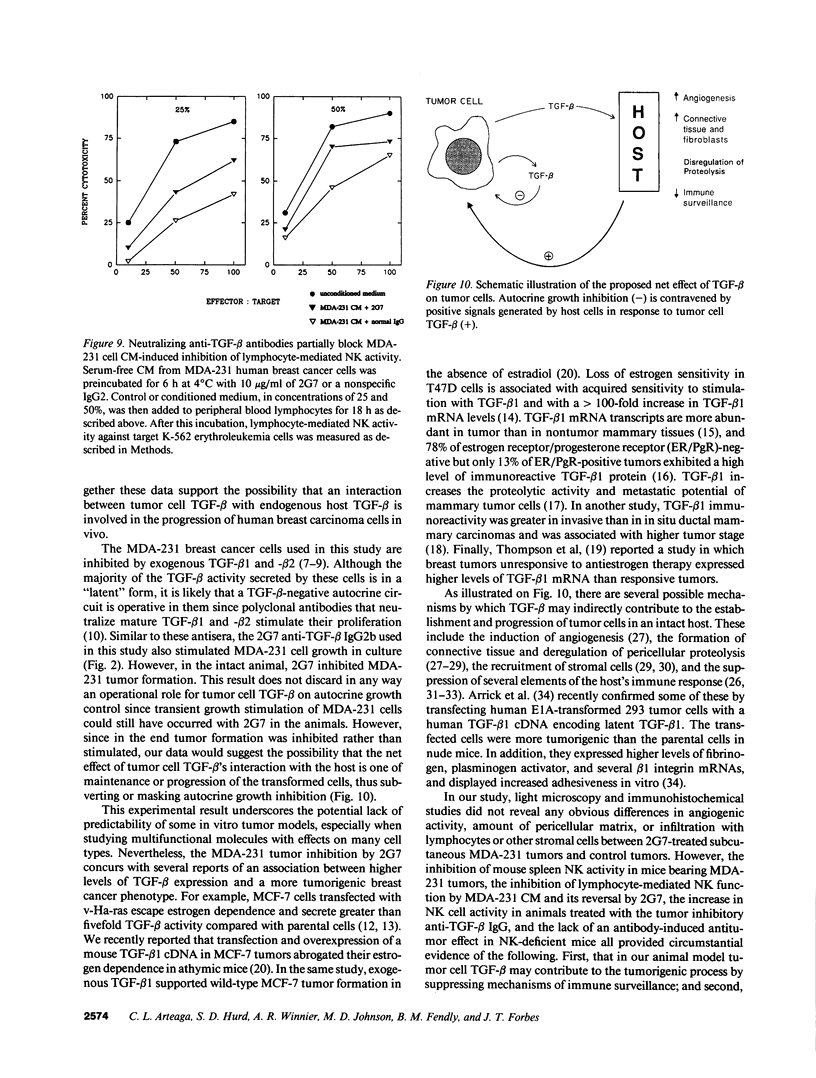


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arrick B. A., Korc M., Derynck R. Differential regulation of expression of three transforming growth factor beta species in human breast cancer cell lines by estradiol. Cancer Res. 1990 Jan 15;50(2):299–303. [PubMed] [Google Scholar]
- Arrick B. A., Lopez A. R., Elfman F., Ebner R., Damsky C. H., Derynck R. Altered metabolic and adhesive properties and increased tumorigenesis associated with increased expression of transforming growth factor beta 1. J Cell Biol. 1992 Aug;118(3):715–726. doi: 10.1083/jcb.118.3.715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arteaga C. L., Carty-Dugger T., Moses H. L., Hurd S. D., Pietenpol J. A. Transforming growth factor beta 1 can induce estrogen-independent tumorigenicity of human breast cancer cells in athymic mice. Cell Growth Differ. 1993 Mar;4(3):193–201. [PubMed] [Google Scholar]
- Arteaga C. L., Coffey R. J., Jr, Dugger T. C., McCutchen C. M., Moses H. L., Lyons R. M. Growth stimulation of human breast cancer cells with anti-transforming growth factor beta antibodies: evidence for negative autocrine regulation by transforming growth factor beta. Cell Growth Differ. 1990 Aug;1(8):367–374. [PubMed] [Google Scholar]
- Arteaga C. L., Tandon A. K., Von Hoff D. D., Osborne C. K. Transforming growth factor beta: potential autocrine growth inhibitor of estrogen receptor-negative human breast cancer cells. Cancer Res. 1988 Jul 15;48(14):3898–3904. [PubMed] [Google Scholar]
- Barrett-Lee P., Travers M., Luqmani Y., Coombes R. C. Transcripts for transforming growth factors in human breast cancer: clinical correlates. Br J Cancer. 1990 Apr;61(4):612–617. doi: 10.1038/bjc.1990.136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daly R. J., King R. J., Darbre P. D. Interaction of growth factors during progression towards steroid independence in T-47-D human breast cancer cells. J Cell Biochem. 1990 Jul;43(3):199–211. doi: 10.1002/jcb.240430302. [DOI] [PubMed] [Google Scholar]
- Dickson R. B., Kasid A., Huff K. K., Bates S. E., Knabbe C., Bronzert D., Gelmann E. P., Lippman M. E. Activation of growth factor secretion in tumorigenic states of breast cancer induced by 17 beta-estradiol or v-Ha-ras oncogene. Proc Natl Acad Sci U S A. 1987 Feb;84(3):837–841. doi: 10.1073/pnas.84.3.837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fraker L. D., Halter S. A., Forbes J. T. Effects of orally administered retinol on natural killer cell activity in wild type BALB/c and congenitally athymic BALB/c mice. Cancer Immunol Immunother. 1986;21(2):114–118. doi: 10.1007/BF00199858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasid A., Knabbe C., Lippman M. E. Effect of v-rasH oncogene transfection on estrogen-independent tumorigenicity of estrogen-dependent human breast cancer cells. Cancer Res. 1987 Nov 1;47(21):5733–5738. [PubMed] [Google Scholar]
- Kehrl J. H., Roberts A. B., Wakefield L. M., Jakowlew S., Sporn M. B., Fauci A. S. Transforming growth factor beta is an important immunomodulatory protein for human B lymphocytes. J Immunol. 1986 Dec 15;137(12):3855–3860. [PubMed] [Google Scholar]
- Kehrl J. H., Wakefield L. M., Roberts A. B., Jakowlew S., Alvarez-Mon M., Derynck R., Sporn M. B., Fauci A. S. Production of transforming growth factor beta by human T lymphocytes and its potential role in the regulation of T cell growth. J Exp Med. 1986 May 1;163(5):1037–1050. doi: 10.1084/jem.163.5.1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keski-Oja J., Blasi F., Leof E. B., Moses H. L. Regulation of the synthesis and activity of urokinase plasminogen activator in A549 human lung carcinoma cells by transforming growth factor-beta. J Cell Biol. 1988 Feb;106(2):451–459. doi: 10.1083/jcb.106.2.451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King R. J., Wang D. Y., Daly R. J., Darbre P. D. Approaches to studying the role of growth factors in the progression of breast tumours from the steroid sensitive to insensitive state. J Steroid Biochem. 1989;34(1-6):133–138. doi: 10.1016/0022-4731(89)90073-3. [DOI] [PubMed] [Google Scholar]
- Knabbe C., Lippman M. E., Wakefield L. M., Flanders K. C., Kasid A., Derynck R., Dickson R. B. Evidence that transforming growth factor-beta is a hormonally regulated negative growth factor in human breast cancer cells. Cell. 1987 Feb 13;48(3):417–428. doi: 10.1016/0092-8674(87)90193-0. [DOI] [PubMed] [Google Scholar]
- Kuppner M. C., Hamou M. F., Bodmer S., Fontana A., de Tribolet N. The glioblastoma-derived T-cell suppressor factor/transforming growth factor beta 2 inhibits the generation of lymphokine-activated killer (LAK) cells. Int J Cancer. 1988 Oct 15;42(4):562–567. doi: 10.1002/ijc.2910420416. [DOI] [PubMed] [Google Scholar]
- Laiho M., Keski-Oja J. Growth factors in the regulation of pericellular proteolysis: a review. Cancer Res. 1989 May 15;49(10):2533–2553. [PubMed] [Google Scholar]
- Lucas C., Bald L. N., Fendly B. M., Mora-Worms M., Figari I. S., Patzer E. J., Palladino M. A. The autocrine production of transforming growth factor-beta 1 during lymphocyte activation. A study with a monoclonal antibody-based ELISA. J Immunol. 1990 Sep 1;145(5):1415–1422. [PubMed] [Google Scholar]
- Moses H. L., Branum E. L., Proper J. A., Robinson R. A. Transforming growth factor production by chemically transformed cells. Cancer Res. 1981 Jul;41(7):2842–2848. [PubMed] [Google Scholar]
- Postlethwaite A. E., Keski-Oja J., Moses H. L., Kang A. H. Stimulation of the chemotactic migration of human fibroblasts by transforming growth factor beta. J Exp Med. 1987 Jan 1;165(1):251–256. doi: 10.1084/jem.165.1.251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts A. B., Anzano M. A., Lamb L. C., Smith J. M., Sporn M. B. New class of transforming growth factors potentiated by epidermal growth factor: isolation from non-neoplastic tissues. Proc Natl Acad Sci U S A. 1981 Sep;78(9):5339–5343. doi: 10.1073/pnas.78.9.5339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts A. B., Sporn M. B., Assoian R. K., Smith J. M., Roche N. S., Wakefield L. M., Heine U. I., Liotta L. A., Falanga V., Kehrl J. H. Transforming growth factor type beta: rapid induction of fibrosis and angiogenesis in vivo and stimulation of collagen formation in vitro. Proc Natl Acad Sci U S A. 1986 Jun;83(12):4167–4171. doi: 10.1073/pnas.83.12.4167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts A. B., Thompson N. L., Heine U., Flanders C., Sporn M. B. Transforming growth factor-beta: possible roles in carcinogenesis. Br J Cancer. 1988 Jun;57(6):594–600. doi: 10.1038/bjc.1988.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rook A. H., Kehrl J. H., Wakefield L. M., Roberts A. B., Sporn M. B., Burlington D. B., Lane H. C., Fauci A. S. Effects of transforming growth factor beta on the functions of natural killer cells: depressed cytolytic activity and blunting of interferon responsiveness. J Immunol. 1986 May 15;136(10):3916–3920. [PubMed] [Google Scholar]
- Sehested M., Hou-Jensen K. Factor VII related antigen as an endothelial cell marker in benign and malignant diseases. Virchows Arch A Pathol Anat Histol. 1981;391(2):217–225. doi: 10.1007/BF00437598. [DOI] [PubMed] [Google Scholar]
- Steiner M. S., Barrack E. R. Transforming growth factor-beta 1 overproduction in prostate cancer: effects on growth in vivo and in vitro. Mol Endocrinol. 1992 Jan;6(1):15–25. doi: 10.1210/mend.6.1.1738367. [DOI] [PubMed] [Google Scholar]
- Torre-Amione G., Beauchamp R. D., Koeppen H., Park B. H., Schreiber H., Moses H. L., Rowley D. A. A highly immunogenic tumor transfected with a murine transforming growth factor type beta 1 cDNA escapes immune surveillance. Proc Natl Acad Sci U S A. 1990 Feb;87(4):1486–1490. doi: 10.1073/pnas.87.4.1486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsunawaki S., Sporn M., Ding A., Nathan C. Deactivation of macrophages by transforming growth factor-beta. Nature. 1988 Jul 21;334(6179):260–262. doi: 10.1038/334260a0. [DOI] [PubMed] [Google Scholar]
- Walker R. A., Dearing S. J. Transforming growth factor beta 1 in ductal carcinoma in situ and invasive carcinomas of the breast. Eur J Cancer. 1992;28(2-3):641–644. doi: 10.1016/s0959-8049(05)80116-9. [DOI] [PubMed] [Google Scholar]
- Wallick S. C., Figari I. S., Morris R. E., Levinson A. D., Palladino M. A. Immunoregulatory role of transforming growth factor beta (TGF-beta) in development of killer cells: comparison of active and latent TGF-beta 1. J Exp Med. 1990 Dec 1;172(6):1777–1784. doi: 10.1084/jem.172.6.1777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welch D. R., Fabra A., Nakajima M. Transforming growth factor beta stimulates mammary adenocarcinoma cell invasion and metastatic potential. Proc Natl Acad Sci U S A. 1990 Oct;87(19):7678–7682. doi: 10.1073/pnas.87.19.7678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wrann M., Bodmer S., de Martin R., Siepl C., Hofer-Warbinek R., Frei K., Hofer E., Fontana A. T cell suppressor factor from human glioblastoma cells is a 12.5-kd protein closely related to transforming growth factor-beta. EMBO J. 1987 Jun;6(6):1633–1636. doi: 10.1002/j.1460-2075.1987.tb02411.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu S. P., Theodorescu D., Kerbel R. S., Willson J. K., Mulder K. M., Humphrey L. E., Brattain M. G. TGF-beta 1 is an autocrine-negative growth regulator of human colon carcinoma FET cells in vivo as revealed by transfection of an antisense expression vector. J Cell Biol. 1992 Jan;116(1):187–196. doi: 10.1083/jcb.116.1.187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zajchowski D., Band V., Pauzie N., Tager A., Stampfer M., Sager R. Expression of growth factors and oncogenes in normal and tumor-derived human mammary epithelial cells. Cancer Res. 1988 Dec 15;48(24 Pt 1):7041–7047. [PubMed] [Google Scholar]
- Zugmaier G., Ennis B. W., Deschauer B., Katz D., Knabbe C., Wilding G., Daly P., Lippman M. E., Dickson R. B. Transforming growth factors type beta 1 and beta 2 are equipotent growth inhibitors of human breast cancer cell lines. J Cell Physiol. 1989 Nov;141(2):353–361. doi: 10.1002/jcp.1041410217. [DOI] [PubMed] [Google Scholar]
- Zugmaier G., Paik S., Wilding G., Knabbe C., Bano M., Lupu R., Deschauer B., Simpson S., Dickson R. B., Lippman M. Transforming growth factor beta 1 induces cachexia and systemic fibrosis without an antitumor effect in nude mice. Cancer Res. 1991 Jul 1;51(13):3590–3594. [PubMed] [Google Scholar]
- de Martin R., Haendler B., Hofer-Warbinek R., Gaugitsch H., Wrann M., Schlüsener H., Seifert J. M., Bodmer S., Fontana A., Hofer E. Complementary DNA for human glioblastoma-derived T cell suppressor factor, a novel member of the transforming growth factor-beta gene family. EMBO J. 1987 Dec 1;6(12):3673–3677. doi: 10.1002/j.1460-2075.1987.tb02700.x. [DOI] [PMC free article] [PubMed] [Google Scholar]





