Abstract
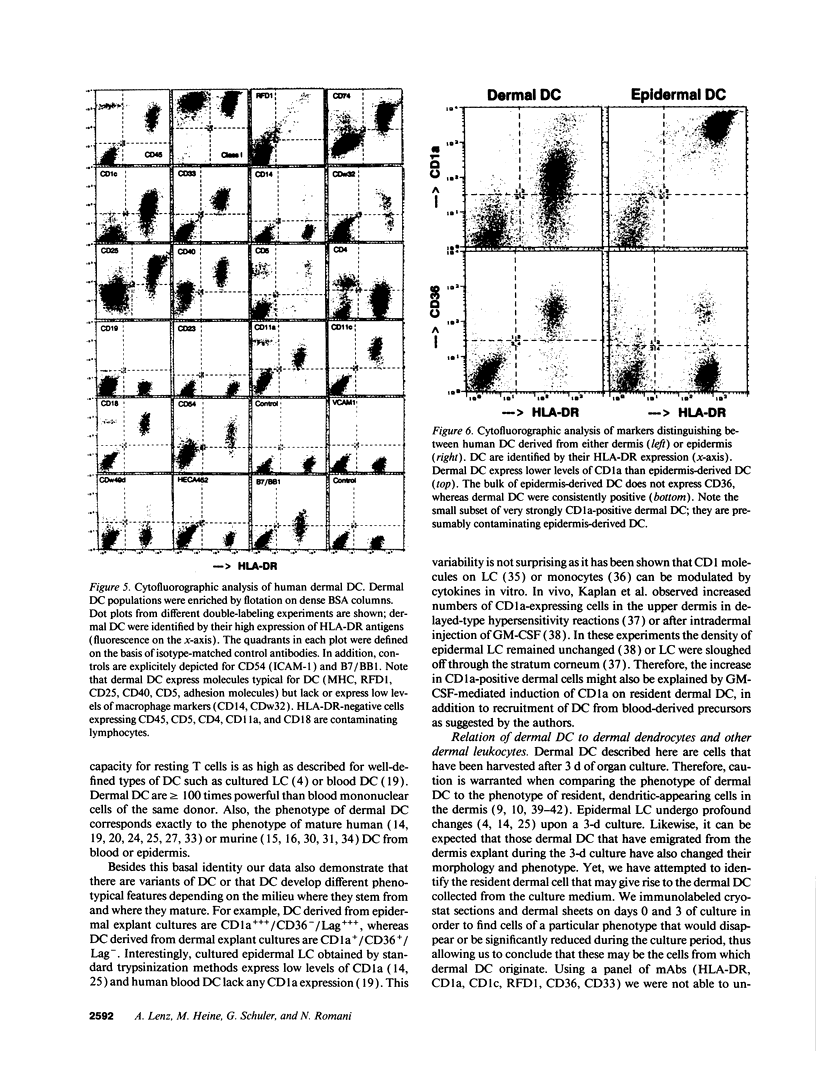
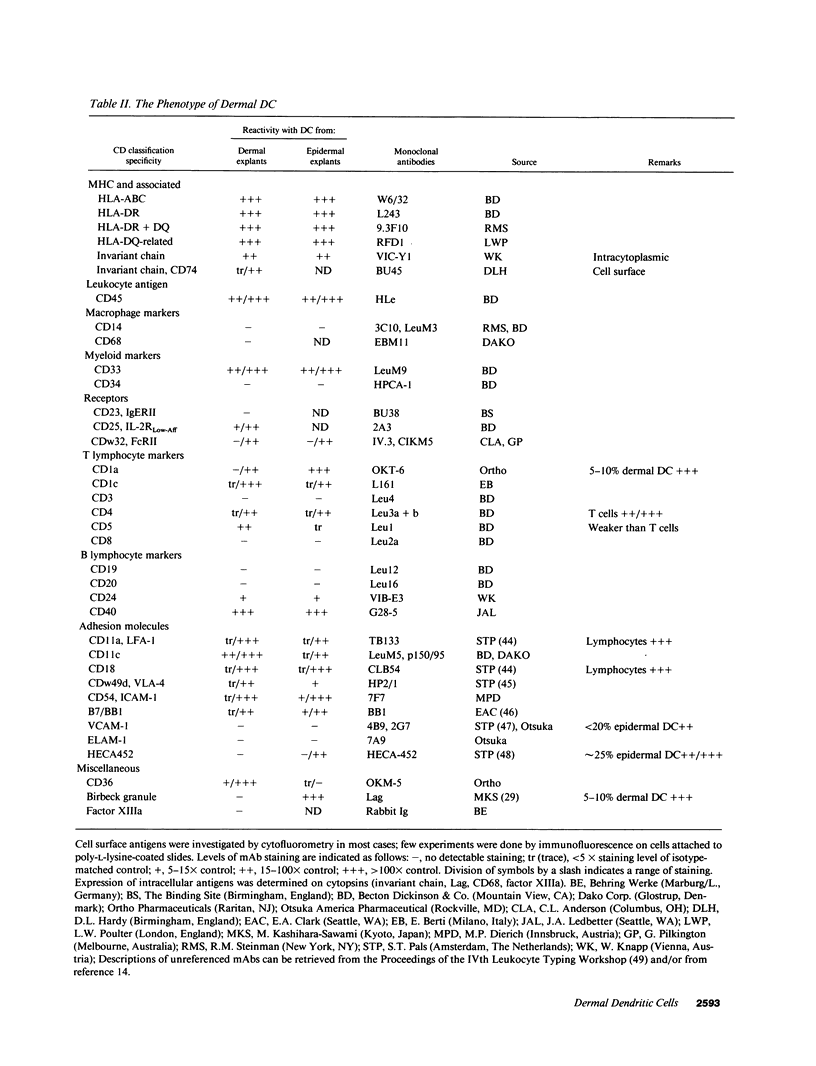
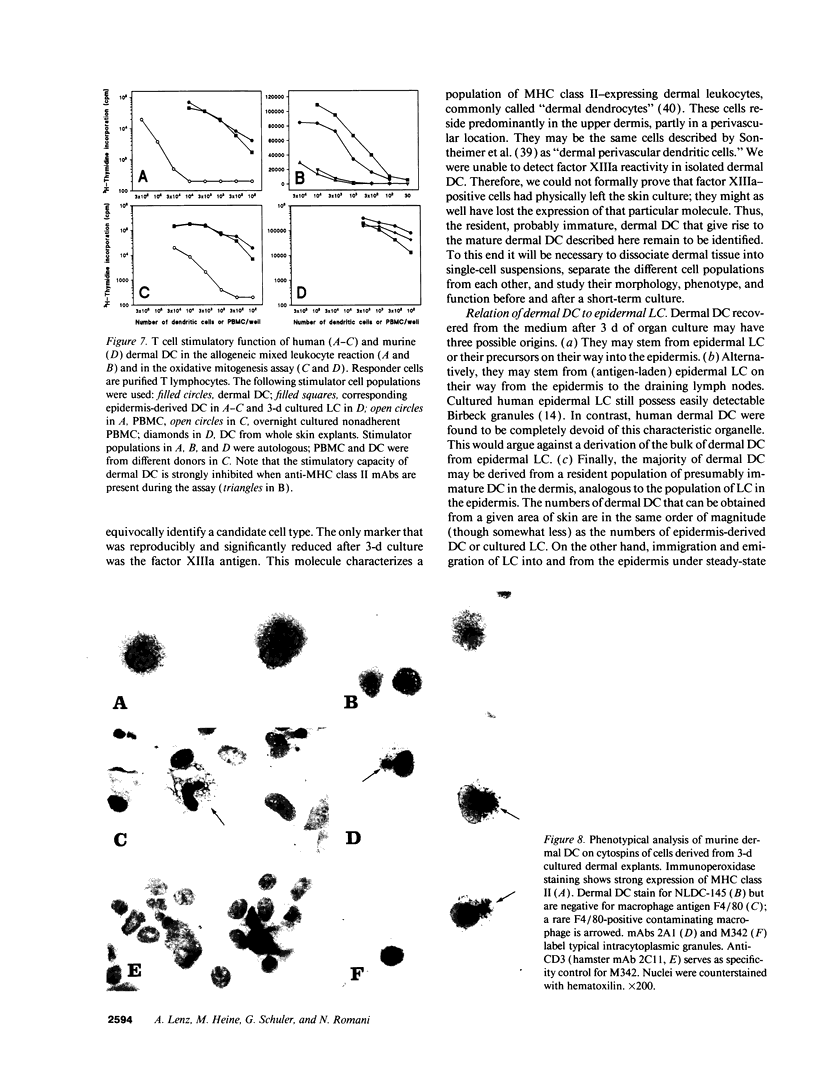
Dendritic cells (DC) comprise a system of cells in lymphoid and nonlymphoid organs that are specialized to present antigens and to initiate primary T cell responses. The Langerhans cell of the epidermis is used as a prototype for studies of DC in the skin. We have characterized a population of DC in human dermis, one of the first examples of these cells in nonlymphoid organs other than epidermis. To identify their distinct functions and phenotype, we relied upon the preparation of enriched populations that emigrate from organ explants of dermis. The dermal cells have the following key features of mature DC: (a) sheet-like processes, or veils, that are constantly moving; (b) very high levels of surface MHC products; (c) absence of markers for macrophages, lymphocytes, and endothelium; (d) substantial expression of adhesion/costimulatory molecules such as CD11/CD18, CD54 (ICAM-1), B7/BB1, CD40; and (e) powerful stimulatory function for resting T cells. Dermal DC are fully comparable to epidermis-derived DC, except for the lack of Birbeck granules, lower levels of CD1a, and higher levels of CD36. DC were also detected in explants of mouse dermis. We conclude that cutaneous DC include both epidermal and dermal components, and suggest that other human nonlymphoid tissues may also serve as sources of typical immunostimulatory DC.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Agger R., Witmer-Pack M., Romani N., Stossel H., Swiggard W. J., Metlay J. P., Storozynsky E., Freimuth P., Steinman R. M. Two populations of splenic dendritic cells detected with M342, a new monoclonal to an intracellular antigen of interdigitating dendritic cells and some B lymphocytes. J Leukoc Biol. 1992 Jul;52(1):34–42. doi: 10.1002/jlb.52.1.34. [DOI] [PubMed] [Google Scholar]
- Carlos T. M., Schwartz B. R., Kovach N. L., Yee E., Rosa M., Osborn L., Chi-Rosso G., Newman B., Lobb R., Rosso M. Vascular cell adhesion molecule-1 mediates lymphocyte adherence to cytokine-activated cultured human endothelial cells. Blood. 1990 Sep 1;76(5):965–970. [PubMed] [Google Scholar]
- Cerio R., Griffiths C. E., Cooper K. D., Nickoloff B. J., Headington J. T. Characterization of factor XIIIa positive dermal dendritic cells in normal and inflamed skin. Br J Dermatol. 1989 Oct;121(4):421–431. doi: 10.1111/j.1365-2133.1989.tb15509.x. [DOI] [PubMed] [Google Scholar]
- Crowley M., Inaba K., Witmer-Pack M., Steinman R. M. The cell surface of mouse dendritic cells: FACS analyses of dendritic cells from different tissues including thymus. Cell Immunol. 1989 Jan;118(1):108–125. doi: 10.1016/0008-8749(89)90361-4. [DOI] [PubMed] [Google Scholar]
- Duijvestijn A. M., Horst E., Pals S. T., Rouse B. N., Steere A. C., Picker L. J., Meijer C. J., Butcher E. C. High endothelial differentiation in human lymphoid and inflammatory tissues defined by monoclonal antibody HECA-452. Am J Pathol. 1988 Jan;130(1):147–155. [PMC free article] [PubMed] [Google Scholar]
- Fossum S. Dendritic leukocytes: features of their in vivo physiology. Res Immunol. 1989 Nov-Dec;140(9):883–926. doi: 10.1016/0923-2494(89)90048-5. [DOI] [PubMed] [Google Scholar]
- Freudenthal P. S., Steinman R. M. The distinct surface of human blood dendritic cells, as observed after an improved isolation method. Proc Natl Acad Sci U S A. 1990 Oct;87(19):7698–7702. doi: 10.1073/pnas.87.19.7698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inaba K., Inaba M., Romani N., Aya H., Deguchi M., Ikehara S., Muramatsu S., Steinman R. M. Generation of large numbers of dendritic cells from mouse bone marrow cultures supplemented with granulocyte/macrophage colony-stimulating factor. J Exp Med. 1992 Dec 1;176(6):1693–1702. doi: 10.1084/jem.176.6.1693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan G., Laal S., Sheftel G., Nusrat A., Nath I., Mathur N. K., Mishra R. S., Cohn Z. A. The nature and kinetics of a delayed immune response to purified protein derivative of tuberculin in the skin of lepromatous leprosy patients. J Exp Med. 1988 Nov 1;168(5):1811–1824. doi: 10.1084/jem.168.5.1811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan G., Walsh G., Guido L. S., Meyn P., Burkhardt R. A., Abalos R. M., Barker J., Frindt P. A., Fajardo T. T., Celona R. Novel responses of human skin to intradermal recombinant granulocyte/macrophage-colony-stimulating factor: Langerhans cell recruitment, keratinocyte growth, and enhanced wound healing. J Exp Med. 1992 Jun 1;175(6):1717–1728. doi: 10.1084/jem.175.6.1717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kashihara M., Ueda M., Horiguchi Y., Furukawa F., Hanaoka M., Imamura S. A monoclonal antibody specifically reactive to human Langerhans cells. J Invest Dermatol. 1986 Nov;87(5):602–607. doi: 10.1111/1523-1747.ep12455849. [DOI] [PubMed] [Google Scholar]
- Kasinrerk W., Baumruker T., Majdic O., Knapp W., Stockinger H. CD1 molecule expression on human monocytes induced by granulocyte-macrophage colony-stimulating factor. J Immunol. 1993 Jan 15;150(2):579–584. [PubMed] [Google Scholar]
- Kitano Y., Okada N. Separation of the epidermal sheet by dispase. Br J Dermatol. 1983 May;108(5):555–560. doi: 10.1111/j.1365-2133.1983.tb01056.x. [DOI] [PubMed] [Google Scholar]
- Kraal G., Breel M., Janse M., Bruin G. Langerhans' cells, veiled cells, and interdigitating cells in the mouse recognized by a monoclonal antibody. J Exp Med. 1986 Apr 1;163(4):981–997. doi: 10.1084/jem.163.4.981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larsen C. P., Ritchie S. C., Pearson T. C., Linsley P. S., Lowry R. P. Functional expression of the costimulatory molecule, B7/BB1, on murine dendritic cell populations. J Exp Med. 1992 Oct 1;176(4):1215–1220. doi: 10.1084/jem.176.4.1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larsen C. P., Steinman R. M., Witmer-Pack M., Hankins D. F., Morris P. J., Austyn J. M. Migration and maturation of Langerhans cells in skin transplants and explants. J Exp Med. 1990 Nov 1;172(5):1483–1493. doi: 10.1084/jem.172.5.1483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy G. F., Bronstein B. R., Knowles R. W., Bhan A. K. Ultrastructural documentation of M241 glycoprotein on dendritic and endothelial cells in normal human skin. Lab Invest. 1985 Mar;52(3):264–269. [PubMed] [Google Scholar]
- Murphy G. F., Messadi D., Fonferko E., Hancock W. W. Phenotypic transformation of macrophages to Langerhans cells in the skin. Am J Pathol. 1986 Jun;123(3):401–406. [PMC free article] [PubMed] [Google Scholar]
- Nickoloff B. J. The human progenitor cell antigen (CD34) is localized on endothelial cells, dermal dendritic cells, and perifollicular cells in formalin-fixed normal skin, and on proliferating endothelial cells and stromal spindle-shaped cells in Kaposi's sarcoma. Arch Dermatol. 1991 Apr;127(4):523–529. [PubMed] [Google Scholar]
- Nicod L. P., Lipscomb M. F., Weissler J. C., Toews G. B. Mononuclear cells from human lung parenchyma support antigen-induced T lymphocyte proliferation. J Leukoc Biol. 1989 Apr;45(4):336–344. doi: 10.1002/jlb.45.4.336. [DOI] [PubMed] [Google Scholar]
- Pavli P., Hume D. A., Van De Pol E., Doe W. F. Dendritic cells, the major antigen-presenting cells of the human colonic lamina propria. Immunology. 1993 Jan;78(1):132–141. [PMC free article] [PubMed] [Google Scholar]
- Poulter L. W., Campbell D. A., Munro C., Janossy G. Discrimination of human macrophages and dendritic cells by means of monoclonal antibodies. Scand J Immunol. 1986 Sep;24(3):351–357. doi: 10.1111/j.1365-3083.1986.tb02104.x. [DOI] [PubMed] [Google Scholar]
- Romani N., Koide S., Crowley M., Witmer-Pack M., Livingstone A. M., Fathman C. G., Inaba K., Steinman R. M. Presentation of exogenous protein antigens by dendritic cells to T cell clones. Intact protein is presented best by immature, epidermal Langerhans cells. J Exp Med. 1989 Mar 1;169(3):1169–1178. doi: 10.1084/jem.169.3.1169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romani N., Lenz A., Glassel H., Stössel H., Stanzl U., Majdic O., Fritsch P., Schuler G. Cultured human Langerhans cells resemble lymphoid dendritic cells in phenotype and function. J Invest Dermatol. 1989 Nov;93(5):600–609. doi: 10.1111/1523-1747.ep12319727. [DOI] [PubMed] [Google Scholar]
- Romani N., Schuler G. The immunologic properties of epidermal Langerhans cells as a part of the dendritic cell system. Springer Semin Immunopathol. 1992;13(3-4):265–279. doi: 10.1007/BF00200527. [DOI] [PubMed] [Google Scholar]
- Scheeren R. A., Koopman G., Van der Baan S., Meijer C. J., Pals S. T. Adhesion receptors involved in clustering of blood dendritic cells and T lymphocytes. Eur J Immunol. 1991 May;21(5):1101–1105. doi: 10.1002/eji.1830210503. [DOI] [PubMed] [Google Scholar]
- Schuler G., Steinman R. M. Murine epidermal Langerhans cells mature into potent immunostimulatory dendritic cells in vitro. J Exp Med. 1985 Mar 1;161(3):526–546. doi: 10.1084/jem.161.3.526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sontheimer R. D., Matsubara T., Seelig L. L., Jr A macrophage phenotype for a constitutive, class II antigen-expressing, human dermal perivascular dendritic cell. J Invest Dermatol. 1989 Jul;93(1):154–159. doi: 10.1111/1523-1747.ep12277390. [DOI] [PubMed] [Google Scholar]
- Sreilein J. W. Antigen-presenting cells in the induction of contact hypersensitivity in mice: evidence that Langerhans cells are sufficient but not required. J Invest Dermatol. 1989 Oct;93(4):443–448. doi: 10.1111/1523-1747.ep12284018. [DOI] [PubMed] [Google Scholar]
- Steinman R. M., Cohn Z. A. Identification of a novel cell type in peripheral lymphoid organs of mice. I. Morphology, quantitation, tissue distribution. J Exp Med. 1973 May 1;137(5):1142–1162. doi: 10.1084/jem.137.5.1142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinman R. M. The dendritic cell system and its role in immunogenicity. Annu Rev Immunol. 1991;9:271–296. doi: 10.1146/annurev.iy.09.040191.001415. [DOI] [PubMed] [Google Scholar]
- Steinman R. M., Young J. W. Signals arising from antigen-presenting cells. Curr Opin Immunol. 1991 Jun;3(3):361–372. doi: 10.1016/0952-7915(91)90039-4. [DOI] [PubMed] [Google Scholar]
- Stössel H., Koch F., Kämpgen E., Stöger P., Lenz A., Heufler C., Romani N., Schuler G. Disappearance of certain acidic organelles (endosomes and Langerhans cell granules) accompanies loss of antigen processing capacity upon culture of epidermal Langerhans cells. J Exp Med. 1990 Nov 1;172(5):1471–1482. doi: 10.1084/jem.172.5.1471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sánchez-Madrid F., De Landázuri M. O., Morago G., Cebrián M., Acevedo A., Bernabeu C. VLA-3: a novel polypeptide association within the VLA molecular complex: cell distribution and biochemical characterization. Eur J Immunol. 1986 Nov;16(11):1343–1349. doi: 10.1002/eji.1830161106. [DOI] [PubMed] [Google Scholar]
- Teunissen M. B., Wormmeester J., Krieg S. R., Peters P. J., Vogels I. M., Kapsenberg M. L., Bos J. D. Human epidermal Langerhans cells undergo profound morphologic and phenotypical changes during in vitro culture. J Invest Dermatol. 1990 Feb;94(2):166–173. doi: 10.1111/1523-1747.ep12874439. [DOI] [PubMed] [Google Scholar]
- Thomas R., Davis L. S., Lipsky P. E. Isolation and characterization of human peripheral blood dendritic cells. J Immunol. 1993 Feb 1;150(3):821–834. [PubMed] [Google Scholar]
- Tse Y., Cooper K. D. Cutaneous dermal Ia+ cells are capable of initiating delayed type hypersensitivity responses. J Invest Dermatol. 1990 Mar;94(3):267–272. doi: 10.1111/1523-1747.ep12874114. [DOI] [PubMed] [Google Scholar]
- Van Voorhis W. C., Hair L. S., Steinman R. M., Kaplan G. Human dendritic cells. Enrichment and characterization from peripheral blood. J Exp Med. 1982 Apr 1;155(4):1172–1187. doi: 10.1084/jem.155.4.1172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsh L. J., Seymour G. J., Powell R. N. Modulation of gingival Langerhans cell T6 antigen expression in vitro by interleukin 1 and an interleukin 1 inhibitor. Clin Exp Immunol. 1986 May;64(2):334–341. [PMC free article] [PubMed] [Google Scholar]
- Wang B., Rieger A., Kilgus O., Ochiai K., Maurer D., Födinger D., Kinet J. P., Stingl G. Epidermal Langerhans cells from normal human skin bind monomeric IgE via Fc epsilon RI. J Exp Med. 1992 May 1;175(5):1353–1365. doi: 10.1084/jem.175.5.1353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber-Matthiesen K., Sterry W. Organization of the monocyte/macrophage system of normal human skin. J Invest Dermatol. 1990 Jul;95(1):83–89. doi: 10.1111/1523-1747.ep12874002. [DOI] [PubMed] [Google Scholar]
- Wood G. S., Freudenthal P. S. CD5 monoclonal antibodies react with human peripheral blood dendritic cells. Am J Pathol. 1992 Oct;141(4):789–795. [PMC free article] [PubMed] [Google Scholar]
- Yokochi T., Holly R. D., Clark E. A. B lymphoblast antigen (BB-1) expressed on Epstein-Barr virus-activated B cell blasts, B lymphoblastoid cell lines, and Burkitt's lymphomas. J Immunol. 1982 Feb;128(2):823–827. [PubMed] [Google Scholar]
- Young J. W., Koulova L., Soergel S. A., Clark E. A., Steinman R. M., Dupont B. The B7/BB1 antigen provides one of several costimulatory signals for the activation of CD4+ T lymphocytes by human blood dendritic cells in vitro. J Clin Invest. 1992 Jul;90(1):229–237. doi: 10.1172/JCI115840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young J. W., Steinman R. M. Accessory cell requirements for the mixed-leukocyte reaction and polyclonal mitogens, as studied with a new technique for enriching blood dendritic cells. Cell Immunol. 1988 Jan;111(1):167–182. doi: 10.1016/0008-8749(88)90061-5. [DOI] [PubMed] [Google Scholar]