Abstract
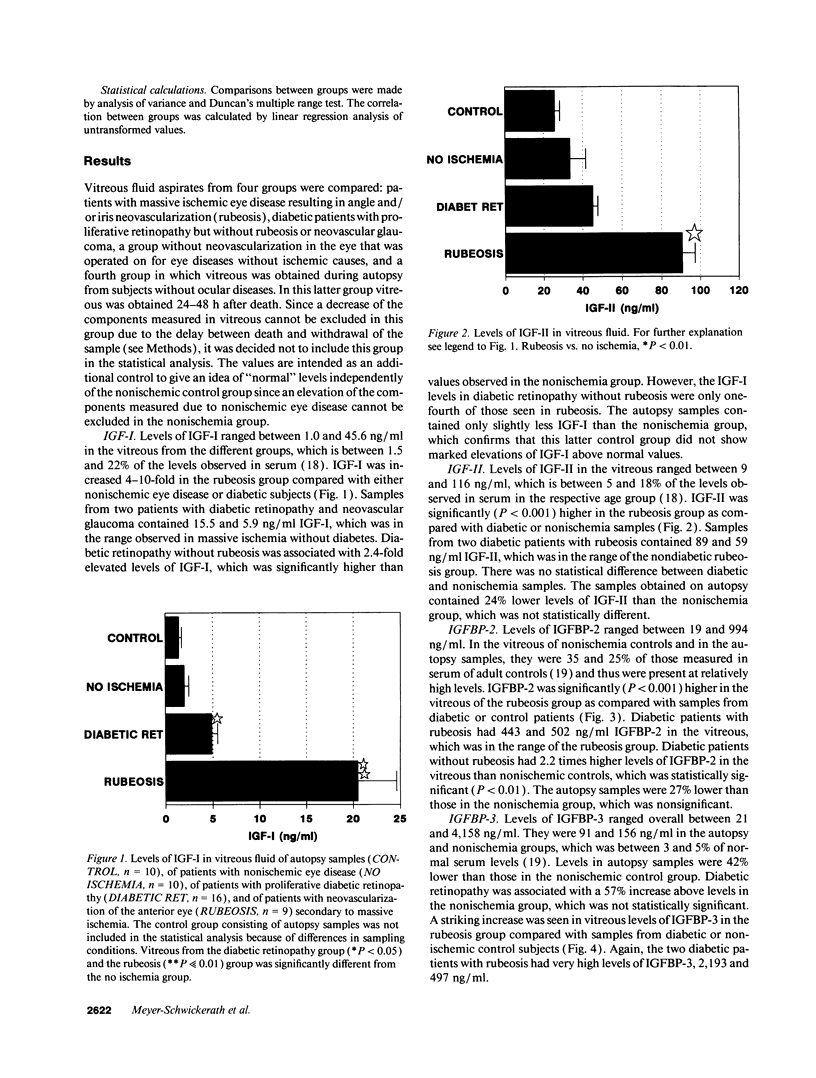
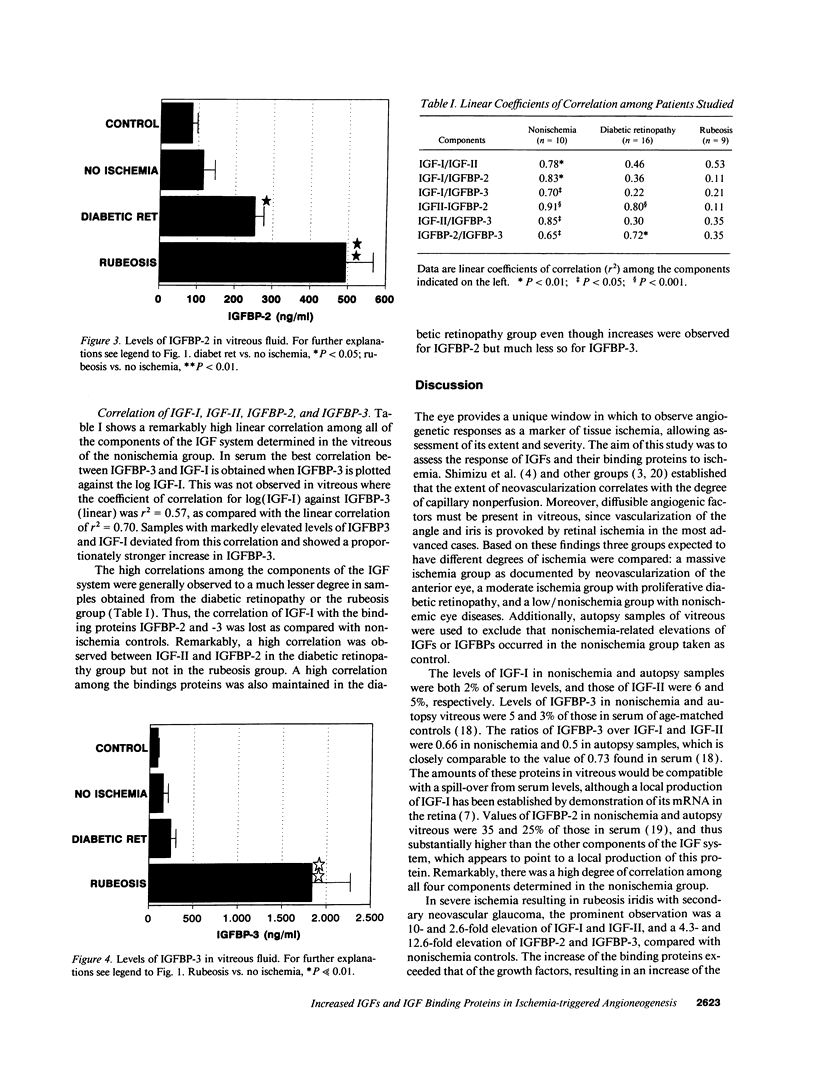
Retinal capillary nonperfusion results in neovascularization of the eye, which is restricted to the retina in less severe cases and progresses to the anterior chamber and the iris angle in the most advanced case, called rubeosis. This angioneogenesis may be induced by the release of retinal growth factors into the vitreous. This study compared levels of the IGF-I and IGF-II, and of the IGF binding protein-2 (IGFBP-2) and IGFBP-3 in vitreous from three groups with different degrees of retinal ischemia, as judged by the extent of neovascularization: a control group without new vessel formation, retinal neovascularization in patients with proliferative diabetic retinopathy, and massive ischemia of various causes resulting in rubeosis. IGF-I and IGFBP-3 were increased 10- and 13-fold in rubeosis (P << 0.01) compared with no ischemia (n = 10), while IGF-II and IGFBP-2 were elevated 2.7- and 4.3-fold (P < 0.01). Within the rubeosis group similar changes were observed independently of the cause of ischemia, which was central vein occlusion, ischemic ophthalmopathy, or intraocular tumor in seven cases and diabetic retinopathy in three samples from two patients. Vitreous from patients with proliferative diabetic retinopathy but without rubeosis (n = 16) contained 2.5- and 2.2-fold elevated levels of IGF-I and of IGFBP-2 (P < 0.05), while IGF-II and IGFBP-3 were increased 1.4- and 1.6-fold, which was not significant. We conclude that: (a) ischemia appears to be a strong stimulus for the local production of IGF-I and -II and of IGFBP-2 and -3 in the eye. (b) Changes in IGF-I and IGFBP-2 in proliferative diabetic retinopathy may be secondary to local ischemia rather than being specific for diabetic retinopathy. (c) IGF-I and IGFBP-3 may play a role in mediating angioneogenesis in the eye.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Blum W. F., Jenne E. W., Reppin F., Kietzmann K., Ranke M. B., Bierich J. R. Insulin-like growth factor I (IGF-I)-binding protein complex is a better mitogen than free IGF-I. Endocrinology. 1989 Aug;125(2):766–772. doi: 10.1210/endo-125-2-766. [DOI] [PubMed] [Google Scholar]
- Blum W. F., Ranke M. B., Bierich J. R. A specific radioimmunoassay for insulin-like growth factor II: the interference of IGF binding proteins can be blocked by excess IGF-I. Acta Endocrinol (Copenh) 1988 Jul;118(3):374–380. doi: 10.1530/acta.0.1180374. [DOI] [PubMed] [Google Scholar]
- Blum W. F., Ranke M. B., Bierich J. R. Isolation and partial characterization of six somatomedin-like peptides from human plasma Cohn fraction IV. Acta Endocrinol (Copenh) 1986 Feb;111(2):271–284. doi: 10.1530/acta.0.1110271. [DOI] [PubMed] [Google Scholar]
- Blum W. F., Ranke M. B., Kietzmann K., Gauggel E., Zeisel H. J., Bierich J. R. A specific radioimmunoassay for the growth hormone (GH)-dependent somatomedin-binding protein: its use for diagnosis of GH deficiency. J Clin Endocrinol Metab. 1990 May;70(5):1292–1298. doi: 10.1210/jcem-70-5-1292. [DOI] [PubMed] [Google Scholar]
- Breier B. H., Gallaher B. W., Gluckman P. D. Radioimmunoassay for insulin-like growth factor-I: solutions to some potential problems and pitfalls. J Endocrinol. 1991 Mar;128(3):347–357. doi: 10.1677/joe.0.1280347. [DOI] [PubMed] [Google Scholar]
- Clemmons D. R., Thissen J. P., Maes M., Ketelslegers J. M., Underwood L. E. Insulin-like growth factor-I (IGF-I) infusion into hypophysectomized or protein-deprived rats induces specific IGF-binding proteins in serum. Endocrinology. 1989 Dec;125(6):2967–2972. doi: 10.1210/endo-125-6-2967. [DOI] [PubMed] [Google Scholar]
- Flyvbjerg A. Growth factors and diabetic complications. Diabet Med. 1990 Jun;7(5):387–399. doi: 10.1111/j.1464-5491.1990.tb01413.x. [DOI] [PubMed] [Google Scholar]
- Gelato M. C., Alexander D., Marsh K. Differential tissue regulation of insulin-like growth factor binding proteins in experimental diabetes mellitus in the rat. Diabetes. 1992 Dec;41(12):1511–1519. doi: 10.2337/diab.41.12.1511. [DOI] [PubMed] [Google Scholar]
- Grant M. B., Mames R. N., Fitzgerald C., Ellis E. A., Aboufriekha M., Guy J. Insulin-like growth factor I acts as an angiogenic agent in rabbit cornea and retina: comparative studies with basic fibroblast growth factor. Diabetologia. 1993 Apr;36(4):282–291. doi: 10.1007/BF00400229. [DOI] [PubMed] [Google Scholar]
- Grant M., Russell B., Fitzgerald C., Merimee T. J. Insulin-like growth factors in vitreous. Studies in control and diabetic subjects with neovascularization. Diabetes. 1986 Apr;35(4):416–420. doi: 10.2337/diab.35.4.416. [DOI] [PubMed] [Google Scholar]
- Hyldahl L., Engström W., Schofield P. N. Stimulatory effects of insulin-like growth factors on DNA synthesis in the human embryonic cornea. J Embryol Exp Morphol. 1986 Nov;98:71–83. [PubMed] [Google Scholar]
- King G. L., Goodman A. D., Buzney S., Moses A., Kahn C. R. Receptors and growth-promoting effects of insulin and insulinlike growth factors on cells from bovine retinal capillaries and aorta. J Clin Invest. 1985 Mar;75(3):1028–1036. doi: 10.1172/JCI111764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knauer D. J., Smith G. L. Inhibition of biological activity of multiplication-stimulating activity by binding to its carrier protein. Proc Natl Acad Sci U S A. 1980 Dec;77(12):7252–7256. doi: 10.1073/pnas.77.12.7252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laatikainen L., Blach R. K. Behaviour of the iris vasculature in central retinal vein occlusion: a fluorescein angiographic study of the vascular response of the retina and the iris. Br J Ophthalmol. 1977 Apr;61(4):272–277. doi: 10.1136/bjo.61.4.272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin D. M., Yee D., Feldman E. L. Gene expression of the insulin-like growth factors and their receptors in cultured human retinal pigment epithelial cells. Brain Res Mol Brain Res. 1992 Jan;12(1-3):181–186. doi: 10.1016/0169-328x(92)90082-m. [DOI] [PubMed] [Google Scholar]
- McAvoy J. W., Chamberlain C. G. Growth factors in the eye. Prog Growth Factor Res. 1990;2(1):29–43. doi: 10.1016/0955-2235(90)90008-8. [DOI] [PubMed] [Google Scholar]
- Merimee T. J. Diabetic retinopathy. A synthesis of perspectives. N Engl J Med. 1990 Apr 5;322(14):978–983. doi: 10.1056/NEJM199004053221406. [DOI] [PubMed] [Google Scholar]
- Meyer-Schwickerath R., Neuerburg-Heusler D., Schulte M. Ischämische Ophthalmopathie, Ursache und Differentialdiagnose. Fortschr Ophthalmol. 1988;85(6):789–792. [PubMed] [Google Scholar]
- Ocrant I., Valentino K. L., King M. G., Wimpy T. H., Rosenfeld R. G., Baskin D. G. Localization and structural characterization of insulin-like growth factor receptors in mammalian retina. Endocrinology. 1989 Nov;125(5):2407–2413. doi: 10.1210/endo-125-5-2407. [DOI] [PubMed] [Google Scholar]
- Patz A. Clinical and experimental studies on retinal neovascularization. XXXIX Edward Jackson Memorial Lecture. Am J Ophthalmol. 1982 Dec;94(6):715–743. doi: 10.1016/0002-9394(82)90297-5. [DOI] [PubMed] [Google Scholar]
- Piffaretti J. C., Kressebuch H., Aeschbacher M., Bille J., Bannerman E., Musser J. M., Selander R. K., Rocourt J. Genetic characterization of clones of the bacterium Listeria monocytogenes causing epidemic disease. Proc Natl Acad Sci U S A. 1989 May;86(10):3818–3822. doi: 10.1073/pnas.86.10.3818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross M., Francis G. L., Szabo L., Wallace J. C., Ballard F. J. Insulin-like growth factor (IGF)-binding proteins inhibit the biological activities of IGF-1 and IGF-2 but not des-(1-3)-IGF-1. Biochem J. 1989 Feb 15;258(1):267–272. doi: 10.1042/bj2580267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimizu K., Kobayashi Y., Muraoka K. Midperipheral fundus involvement in diabetic retinopathy. Ophthalmology. 1981 Jul;88(7):601–612. doi: 10.1016/s0161-6420(81)34983-5. [DOI] [PubMed] [Google Scholar]



