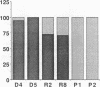
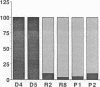
Abstract
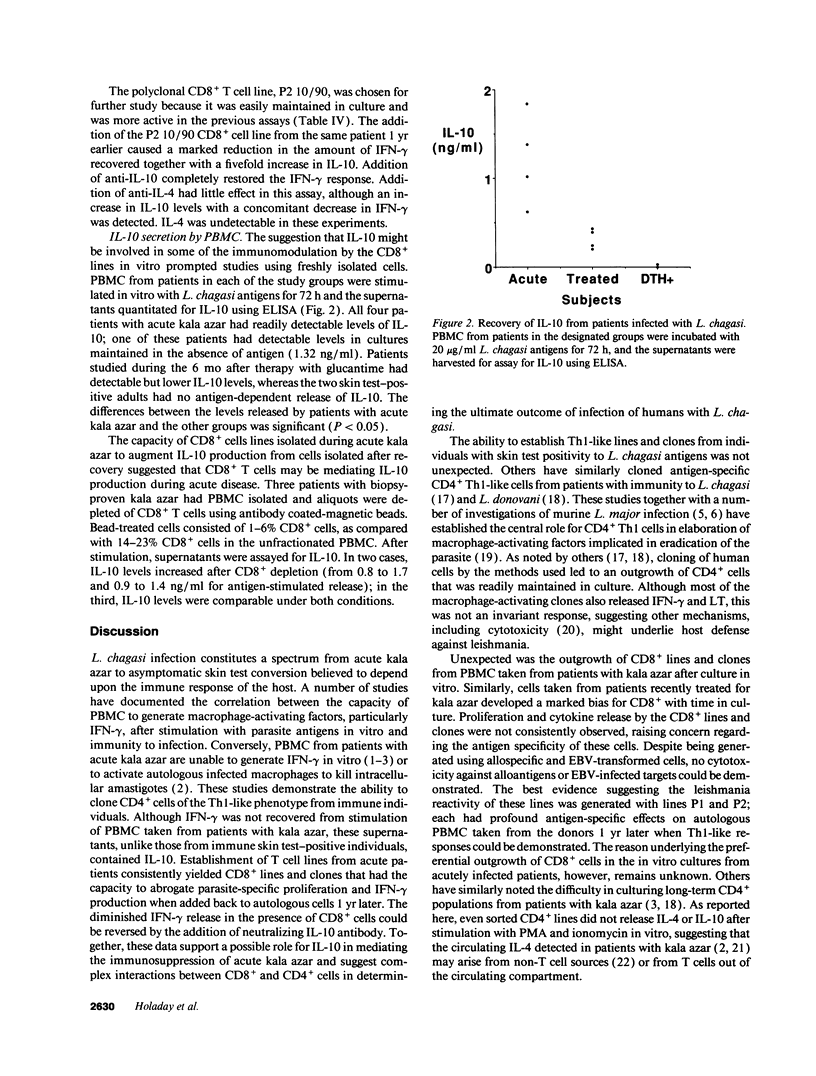
Patients with acute kala azar are generally nonreactive in a number of immunologic assays, including T cell proliferation and generation of macrophage-activating cytokines, principally IFN-gamma, in response to leishmania antigens in vitro. To test for potential immunosuppressive factors, a series of T cell lines and clones were established from patients with acute kala azar, from patients after chemotherapy for kala azar, and from skin test-positive adults from the same endemic region. Although CD4+ T cell lines and clones could be readily established from the skin test-positive adults, lines and clones from acute or treated patients were heavily biased in expression of CD8+. The CD8+ cells from acute patients did not themselves release cytokines in response to leishmania antigens in vitro, but markedly affected the cytokine profile of peripheral blood mononuclear cells isolated 1 yr later after recovery. Addition of the CD8+ cells caused inhibition of lymphoproliferation and IFN-gamma release, with augmentation of IL-6 and IL-10 release. The inhibitory effects of the CD8+ cells could be partially abrogated by antibodies to IL-10 but not by antibodies to IL-4. Analysis of four patients with acute kala azar demonstrated release of IL-10 that could not be demonstrated in supernatants from asymptomatic skin test-positive individuals. Generation of IL-10 may contribute to the profound suppression of IFN-gamma release that occurs during kala azar due to Leishmania chagasi.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abrams J. S., Roncarolo M. G., Yssel H., Andersson U., Gleich G. J., Silver J. E. Strategies of anti-cytokine monoclonal antibody development: immunoassay of IL-10 and IL-5 in clinical samples. Immunol Rev. 1992 Jun;127:5–24. doi: 10.1111/j.1600-065x.1992.tb01406.x. [DOI] [PubMed] [Google Scholar]
- Aderka D., Le J. M., Vilcek J. IL-6 inhibits lipopolysaccharide-induced tumor necrosis factor production in cultured human monocytes, U937 cells, and in mice. J Immunol. 1989 Dec 1;143(11):3517–3523. [PubMed] [Google Scholar]
- Badaro R., Falcoff E., Badaro F. S., Carvalho E. M., Pedral-Sampaio D., Barral A., Carvalho J. S., Barral-Netto M., Brandely M., Silva L. Treatment of visceral leishmaniasis with pentavalent antimony and interferon gamma. N Engl J Med. 1990 Jan 4;322(1):16–21. doi: 10.1056/NEJM199001043220104. [DOI] [PubMed] [Google Scholar]
- Belosevic M., Finbloom D. S., Van Der Meide P. H., Slayter M. V., Nacy C. A. Administration of monoclonal anti-IFN-gamma antibodies in vivo abrogates natural resistance of C3H/HeN mice to infection with Leishmania major. J Immunol. 1989 Jul 1;143(1):266–274. [PubMed] [Google Scholar]
- Berens R. L., Marr J. J. An easily prepared defined medium for cultivation of Leishmania donovani promastigotes. J Parasitol. 1978 Feb;64(1):160–160. [PubMed] [Google Scholar]
- Carvalho E. M., Bacellar O., Barral A., Badaro R., Johnson W. D., Jr Antigen-specific immunosuppression in visceral leishmaniasis is cell mediated. J Clin Invest. 1989 Mar;83(3):860–864. doi: 10.1172/JCI113969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carvalho E. M., Badaró R., Reed S. G., Jones T. C., Johnson W. D., Jr Absence of gamma interferon and interleukin 2 production during active visceral leishmaniasis. J Clin Invest. 1985 Dec;76(6):2066–2069. doi: 10.1172/JCI112209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chrétien I., Van Kimmenade A., Pearce M. K., Banchereau J., Abrams J. S. Development of polyclonal and monoclonal antibodies for immunoassay and neutralization of human interleukin-4. J Immunol Methods. 1989 Feb 8;117(1):67–81. doi: 10.1016/0022-1759(89)90120-8. [DOI] [PubMed] [Google Scholar]
- Del Prete G., De Carli M., Almerigogna F., Giudizi M. G., Biagiotti R., Romagnani S. Human IL-10 is produced by both type 1 helper (Th1) and type 2 helper (Th2) T cell clones and inhibits their antigen-specific proliferation and cytokine production. J Immunol. 1993 Jan 15;150(2):353–360. [PubMed] [Google Scholar]
- Enk A. H., Katz S. I. Identification and induction of keratinocyte-derived IL-10. J Immunol. 1992 Jul 1;149(1):92–95. [PubMed] [Google Scholar]
- Evans T. G., Teixeira M. J., McAuliffe I. T., Vasconcelos I., Vasconcelos A. W., Sousa A. de A., Lima J. W., Pearson R. D. Epidemiology of visceral leishmaniasis in northeast Brazil. J Infect Dis. 1992 Nov;166(5):1124–1132. doi: 10.1093/infdis/166.5.1124. [DOI] [PubMed] [Google Scholar]
- Ho J. L., Badaró R., Schwartz A., Dinarello C. A., Gelfand J. A., Sobel J., Barral A., Netto M. B., Carvalho E. M., Reed S. G. Diminished in vitro production of interleukin-1 and tumor necrosis factor-alpha during acute visceral leishmaniasis and recovery after therapy. J Infect Dis. 1992 Jun;165(6):1094–1102. doi: 10.1093/infdis/165.6.1094. [DOI] [PubMed] [Google Scholar]
- Holaday B. J., Pompeu M. M., Evans T., Braga D. N., Texeira M. J., Sousa A. de Q., Sadick M. D., Vasconcelos A. W., Abrams J. S., Pearson R. D. Correlates of Leishmania-specific immunity in the clinical spectrum of infection with Leishmania chagasi. J Infect Dis. 1993 Feb;167(2):411–417. doi: 10.1093/infdis/167.2.411. [DOI] [PubMed] [Google Scholar]
- Howard M., O'Garra A. Biological properties of interleukin 10. Immunol Today. 1992 Jun;13(6):198–200. doi: 10.1016/0167-5699(92)90153-X. [DOI] [PubMed] [Google Scholar]
- Karp C. L., el-Safi S. H., Wynn T. A., Satti M. M., Kordofani A. M., Hashim F. A., Hag-Ali M., Neva F. A., Nutman T. B., Sacks D. L. In vivo cytokine profiles in patients with kala-azar. Marked elevation of both interleukin-10 and interferon-gamma. J Clin Invest. 1993 Apr;91(4):1644–1648. doi: 10.1172/JCI116372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaye P. M., Curry A. J., Blackwell J. M. Differential production of Th1- and Th2-derived cytokines does not determine the genetically controlled or vaccine-induced rate of cure in murine visceral leishmaniasis. J Immunol. 1991 Apr 15;146(8):2763–2770. [PubMed] [Google Scholar]
- Melby P. C., Sacks D. L. Identification of antigens recognized by T cells in human leishmaniasis: analysis of T-cell clones by immunoblotting. Infect Immun. 1989 Oct;57(10):2971–2976. doi: 10.1128/iai.57.10.2971-2976.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paul N. L., Lenardo M. J., Novak K. D., Sarr T., Tang W. L., Ruddle N. H. Lymphotoxin activation by human T-cell leukemia virus type I-infected cell lines: role for NF-kappa B. J Virol. 1990 Nov;64(11):5412–5419. doi: 10.1128/jvi.64.11.5412-5419.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piccinni M. P., Macchia D., Parronchi P., Giudizi M. G., Bani D., Alterini R., Grossi A., Ricci M., Maggi E., Romagnani S. Human bone marrow non-B, non-T cells produce interleukin 4 in response to cross-linkage of Fc epsilon and Fc gamma receptors. Proc Natl Acad Sci U S A. 1991 Oct 1;88(19):8656–8660. doi: 10.1073/pnas.88.19.8656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reed S. G., Carvalho E. M., Sherbert C. H., Sampaio D. P., Russo D. M., Bacelar O., Pihl D. L., Scott J. M., Barral A., Grabstein K. H. In vitro responses to Leishmania antigens by lymphocytes from patients with leishmaniasis or Chagas' disease. J Clin Invest. 1990 Mar;85(3):690–696. doi: 10.1172/JCI114493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sacks D. L., Lal S. L., Shrivastava S. N., Blackwell J., Neva F. A. An analysis of T cell responsiveness in Indian kala-azar. J Immunol. 1987 Feb 1;138(3):908–913. [PubMed] [Google Scholar]
- Salgame P., Abrams J. S., Clayberger C., Goldstein H., Convit J., Modlin R. L., Bloom B. R. Differing lymphokine profiles of functional subsets of human CD4 and CD8 T cell clones. Science. 1991 Oct 11;254(5029):279–282. doi: 10.1126/science.254.5029.279. [DOI] [PubMed] [Google Scholar]
- Scott P., Natovitz P., Coffman R. L., Pearce E., Sher A. Immunoregulation of cutaneous leishmaniasis. T cell lines that transfer protective immunity or exacerbation belong to different T helper subsets and respond to distinct parasite antigens. J Exp Med. 1988 Nov 1;168(5):1675–1684. doi: 10.1084/jem.168.5.1675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith L. E., Rodrigues M., Russell D. G. The interaction between CD8+ cytotoxic T cells and Leishmania-infected macrophages. J Exp Med. 1991 Sep 1;174(3):499–505. doi: 10.1084/jem.174.3.499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yssel H., De Vries J. E., Koken M., Van Blitterswijk W., Spits H. Serum-free medium for generation and propagation of functional human cytotoxic and helper T cell clones. J Immunol Methods. 1984 Aug 3;72(1):219–227. doi: 10.1016/0022-1759(84)90450-2. [DOI] [PubMed] [Google Scholar]
- Yssel H., De Waal Malefyt R., Roncarolo M. G., Abrams J. S., Lahesmaa R., Spits H., de Vries J. E. IL-10 is produced by subsets of human CD4+ T cell clones and peripheral blood T cells. J Immunol. 1992 Oct 1;149(7):2378–2384. [PubMed] [Google Scholar]
- Zwingenberger K., Harms G., Pedrosa C., Omena S., Sandkamp B., Neifer S. Determinants of the immune response in visceral leishmaniasis: evidence for predominance of endogenous interleukin 4 over interferon-gamma production. Clin Immunol Immunopathol. 1990 Nov;57(2):242–249. doi: 10.1016/0090-1229(90)90038-r. [DOI] [PubMed] [Google Scholar]
- de Waal Malefyt R., Abrams J., Bennett B., Figdor C. G., de Vries J. E. Interleukin 10(IL-10) inhibits cytokine synthesis by human monocytes: an autoregulatory role of IL-10 produced by monocytes. J Exp Med. 1991 Nov 1;174(5):1209–1220. doi: 10.1084/jem.174.5.1209. [DOI] [PMC free article] [PubMed] [Google Scholar]