Abstract
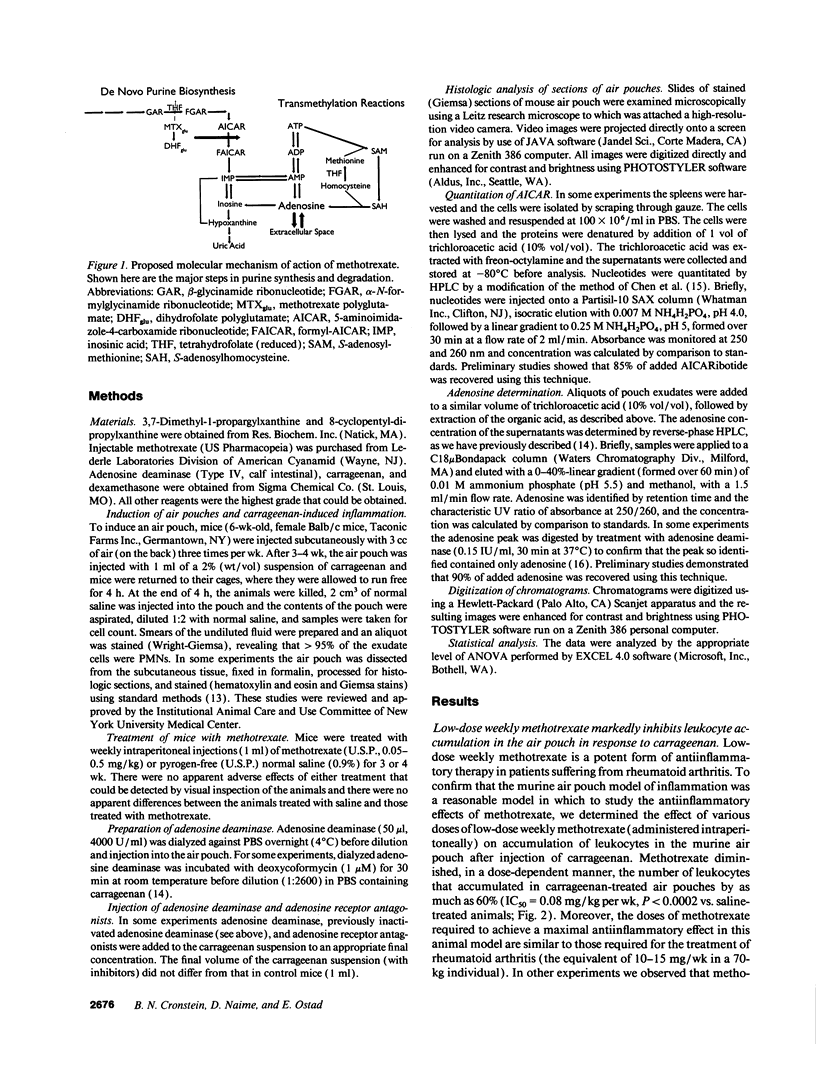
Methotrexate, a folate antagonist, is a potent antiinflammatory agent when used weekly in low concentrations. We examined the hypothesis that the antiphlogistic effects of methotrexate result from its capacity to promote intracellular accumulation of 5-aminoimidazole-4-carboxamide ribonucleotide (AICAR) that, under conditions of cell injury, increases local adenosine release. We now present the first evidence to establish this mechanism of action in an in vivo model of inflammation, the murine air pouch model. Mice were injected intraperitoneally with either methotrexate or saline for 3-4 wk during induction of air pouches. Pharmacologically relevant doses of methotrexate increased splenocyte AICAR content, raised adenosine concentrations in exudates from carrageenan-inflamed air pouches, and markedly inhibited leukocyte accumulation in inflamed air pouches. The methotrexate-mediated reduction in leukocyte accumulation was partially reversed by injection of adenosine deaminase (ADA) into the air pouch, completely reversed by a specific adenosine A2 receptor antagonist, 3,7-dimethyl-1-propargylxanthine (DMPX), but not affected by an adenosine A1 receptor antagonist, 8-cyclopentyl-dipropylxanthine. Neither ADA nor DMPX affected leukocyte accumulation in the inflamed pouches of animals treated with either saline or the potent antiinflammatory steroid dexamethasone. These results indicate that methotrexate is a nonsteroidal antiinflammatory agent, the antiphlogistic action of which is due to increased adenosine release at inflamed sites.
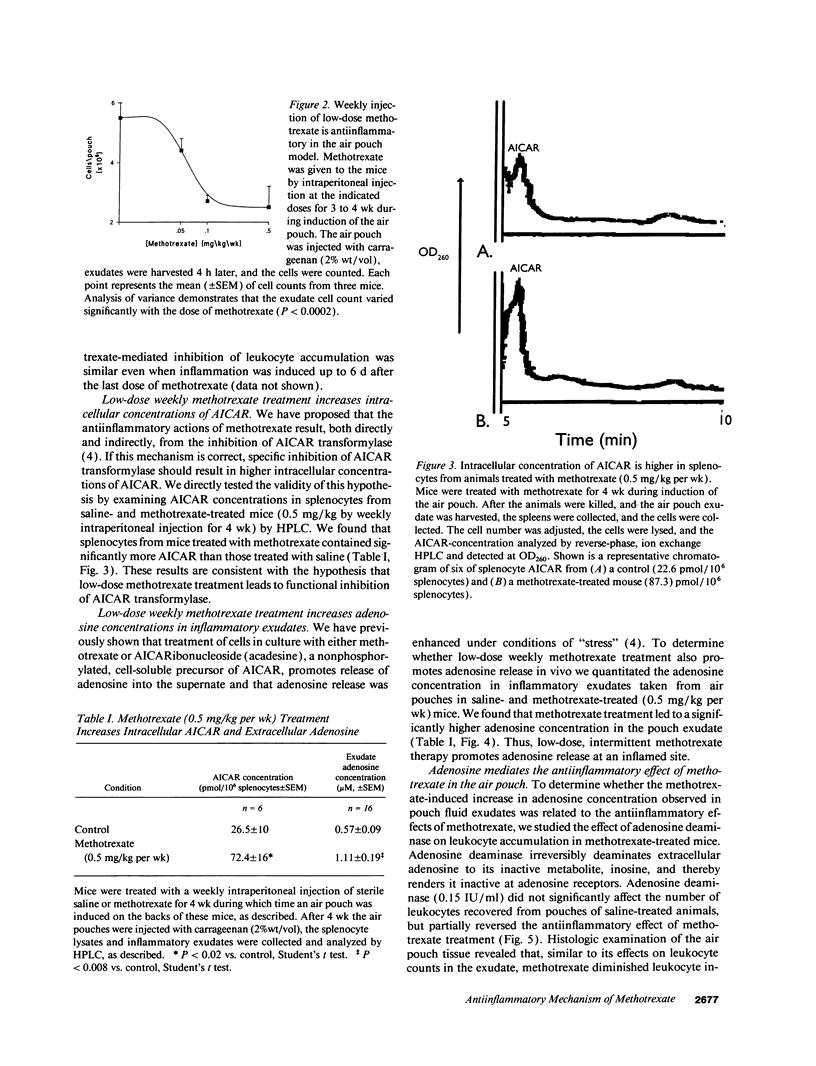
Full text
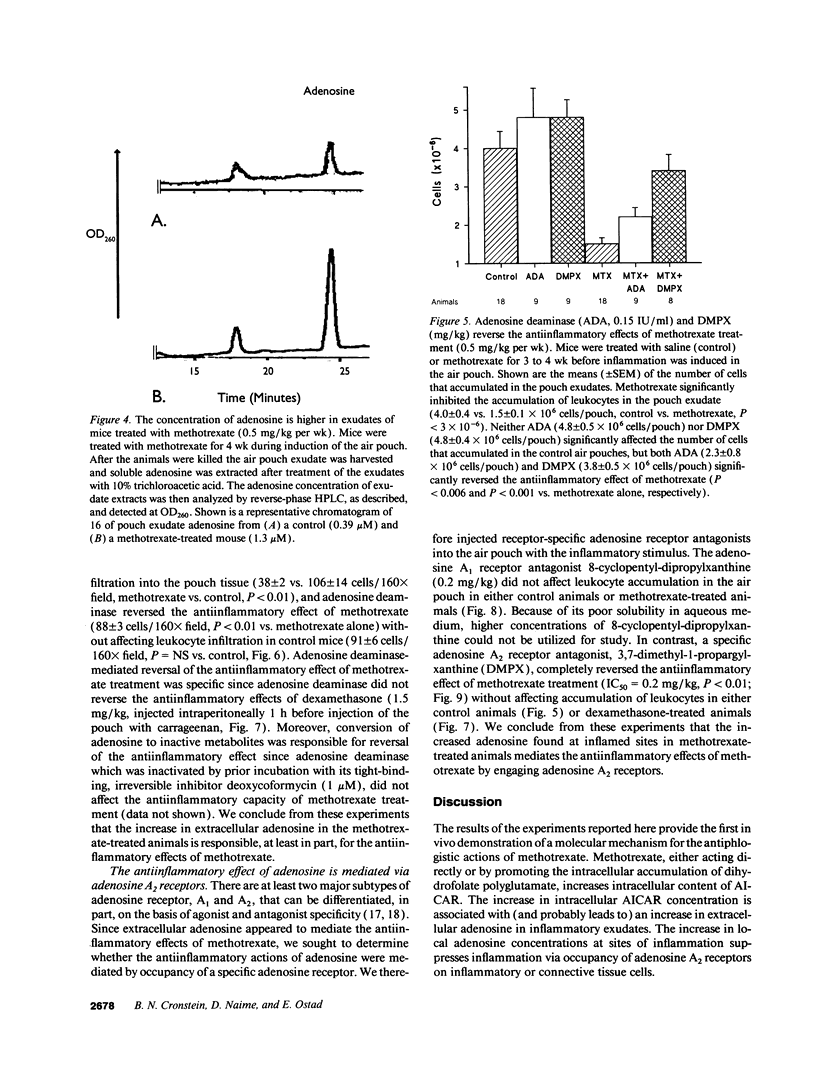
PDF
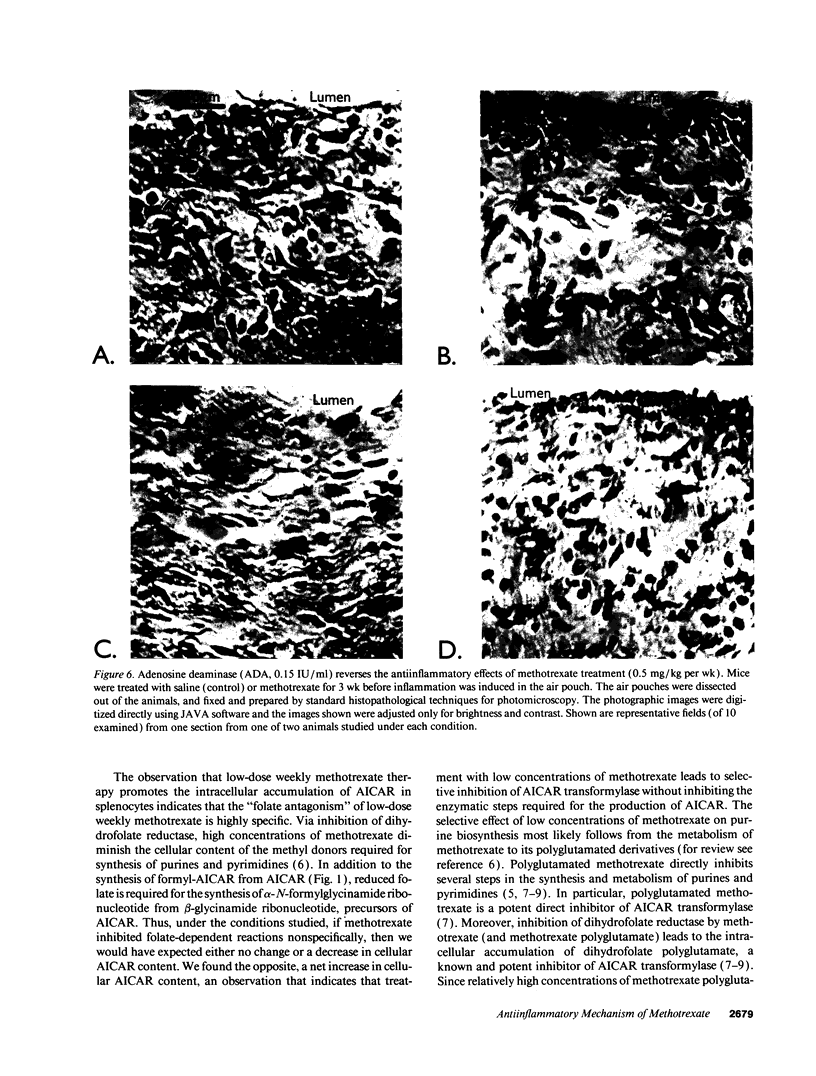
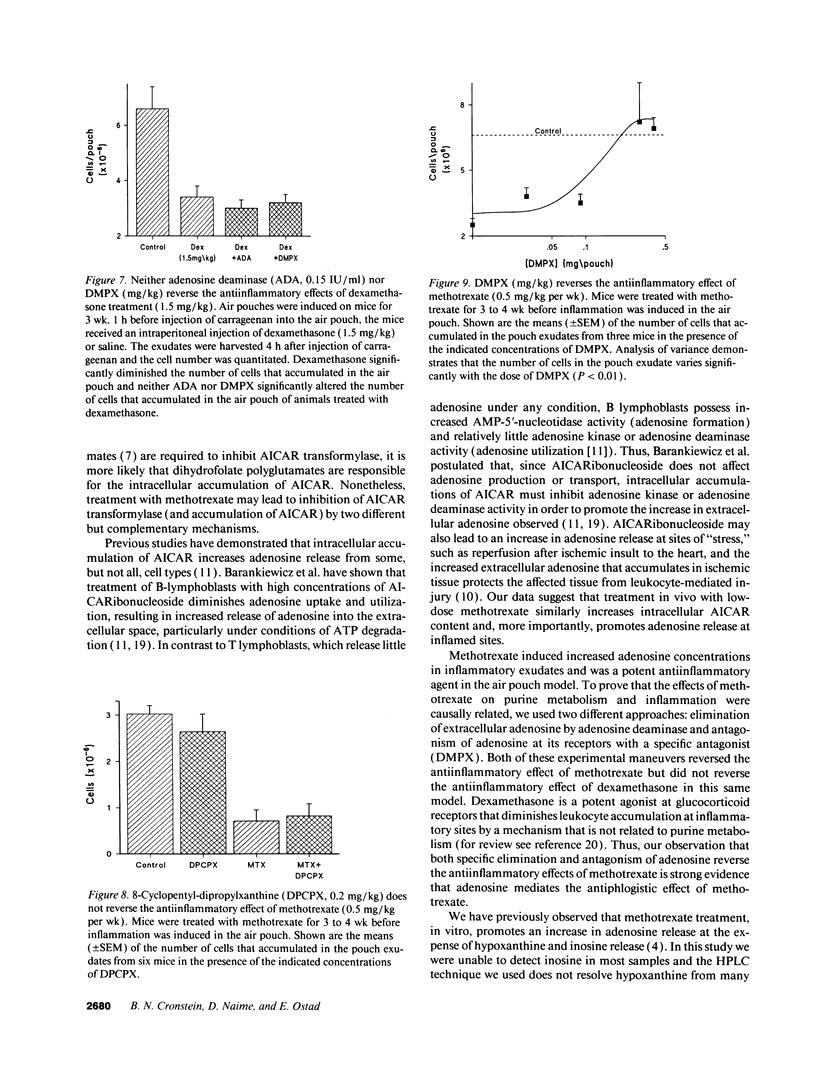


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allegra C. J., Drake J. C., Jolivet J., Chabner B. A. Inhibition of phosphoribosylaminoimidazolecarboxamide transformylase by methotrexate and dihydrofolic acid polyglutamates. Proc Natl Acad Sci U S A. 1985 Aug;82(15):4881–4885. doi: 10.1073/pnas.82.15.4881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allegra C. J., Hoang K., Yeh G. C., Drake J. C., Baram J. Evidence for direct inhibition of de novo purine synthesis in human MCF-7 breast cells as a principal mode of metabolic inhibition by methotrexate. J Biol Chem. 1987 Oct 5;262(28):13520–13526. [PubMed] [Google Scholar]
- Arend L. J., Sonnenburg W. K., Smith W. L., Spielman W. S. A1 and A2 adenosine receptors in rabbit cortical collecting tubule cells. Modulation of hormone-stimulated cAMP. J Clin Invest. 1987 Mar;79(3):710–714. doi: 10.1172/JCI112875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asako H., Wolf R. E., Granger D. N. Leukocyte adherence in rat mesenteric venules: effects of adenosine and methotrexate. Gastroenterology. 1993 Jan;104(1):31–37. doi: 10.1016/0016-5085(93)90832-w. [DOI] [PubMed] [Google Scholar]
- Baggott J. E., Vaughn W. H., Hudson B. B. Inhibition of 5-aminoimidazole-4-carboxamide ribotide transformylase, adenosine deaminase and 5'-adenylate deaminase by polyglutamates of methotrexate and oxidized folates and by 5-aminoimidazole-4-carboxamide riboside and ribotide. Biochem J. 1986 May 15;236(1):193–200. doi: 10.1042/bj2360193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barankiewicz J., Jimenez R., Ronlov G., Magill M., Gruber H. E. Alteration of purine metabolism by AICA-riboside in human B lymphoblasts. Arch Biochem Biophys. 1990 Nov 1;282(2):377–385. doi: 10.1016/0003-9861(90)90132-i. [DOI] [PubMed] [Google Scholar]
- Barankiewicz J., Ronlov G., Jimenez R., Gruber H. E. Selective adenosine release from human B but not T lymphoid cell line. J Biol Chem. 1990 Sep 15;265(26):15738–15743. [PubMed] [Google Scholar]
- Chabner B. A., Allegra C. J., Curt G. A., Clendeninn N. J., Baram J., Koizumi S., Drake J. C., Jolivet J. Polyglutamation of methotrexate. Is methotrexate a prodrug? J Clin Invest. 1985 Sep;76(3):907–912. doi: 10.1172/JCI112088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen S. C., Brown P. R., Rosie D. M. Extraction procedures for use prior to HPLC nucleotide analysis using microparticle chemically bonded packings. J Chromatogr Sci. 1977 Jun;15(6):218–221. doi: 10.1093/chromsci/15.6.218. [DOI] [PubMed] [Google Scholar]
- Cronstein B. N., Daguma L., Nichols D., Hutchison A. J., Williams M. The adenosine/neutrophil paradox resolved: human neutrophils possess both A1 and A2 receptors that promote chemotaxis and inhibit O2 generation, respectively. J Clin Invest. 1990 Apr;85(4):1150–1157. doi: 10.1172/JCI114547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cronstein B. N., Eberle M. A., Gruber H. E., Levin R. I. Methotrexate inhibits neutrophil function by stimulating adenosine release from connective tissue cells. Proc Natl Acad Sci U S A. 1991 Mar 15;88(6):2441–2445. doi: 10.1073/pnas.88.6.2441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cronstein B. N., Kimmel S. C., Levin R. I., Martiniuk F., Weissmann G. A mechanism for the antiinflammatory effects of corticosteroids: the glucocorticoid receptor regulates leukocyte adhesion to endothelial cells and expression of endothelial-leukocyte adhesion molecule 1 and intercellular adhesion molecule 1. Proc Natl Acad Sci U S A. 1992 Nov 1;89(21):9991–9995. doi: 10.1073/pnas.89.21.9991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cronstein B. N., Kramer S. B., Weissmann G., Hirschhorn R. Adenosine: a physiological modulator of superoxide anion generation by human neutrophils. J Exp Med. 1983 Oct 1;158(4):1160–1177. doi: 10.1084/jem.158.4.1160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cronstein B. N., Levin R. I., Belanoff J., Weissmann G., Hirschhorn R. Adenosine: an endogenous inhibitor of neutrophil-mediated injury to endothelial cells. J Clin Invest. 1986 Sep;78(3):760–770. doi: 10.1172/JCI112638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cronstein B. N., Rosenstein E. D., Kramer S. B., Weissmann G., Hirschhorn R. Adenosine; a physiologic modulator of superoxide anion generation by human neutrophils. Adenosine acts via an A2 receptor on human neutrophils. J Immunol. 1985 Aug;135(2):1366–1371. [PubMed] [Google Scholar]
- Dolphin A. C., Prestwich S. A. Pertussis toxin reverses adenosine inhibition of neuronal glutamate release. Nature. 1985 Jul 11;316(6024):148–150. doi: 10.1038/316148a0. [DOI] [PubMed] [Google Scholar]
- Eppell B. A., Newell A. M., Brown E. J. Adenosine receptors are expressed during differentiation of monocytes to macrophages in vitro. Implications for regulation of phagocytosis. J Immunol. 1989 Dec 15;143(12):4141–4145. [PubMed] [Google Scholar]
- Furst D. E., Kremer J. M. Methotrexate in rheumatoid arthritis. Arthritis Rheum. 1988 Mar;31(3):305–314. doi: 10.1002/art.1780310301. [DOI] [PubMed] [Google Scholar]
- García-Sáinz J. A., Torner M. L. Rat fat-cells have three types of adenosine receptors (Ra, Ri and P). Differential effects of pertussis toxin. Biochem J. 1985 Dec 1;232(2):439–443. doi: 10.1042/bj2320439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gruber H. E., Hoffer M. E., McAllister D. R., Laikind P. K., Lane T. A., Schmid-Schoenbein G. W., Engler R. L. Increased adenosine concentration in blood from ischemic myocardium by AICA riboside. Effects on flow, granulocytes, and injury. Circulation. 1989 Nov;80(5):1400–1411. doi: 10.1161/01.cir.80.5.1400. [DOI] [PubMed] [Google Scholar]
- Hirschhorn R., Roegner-Maniscalco V., Kuritsky L., Rosen F. S. Bone marrow transplantation only partially restores purine metabolites to normal in adenosine deaminase-deficient patients. J Clin Invest. 1981 Dec;68(6):1387–1393. doi: 10.1172/JCI110389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lappin D., Whaley K. Adenosine A2 receptors on human monocytes modulate C2 production. Clin Exp Immunol. 1984 Aug;57(2):454–460. [PMC free article] [PubMed] [Google Scholar]
- Londos C., Cooper D. M., Wolff J. Subclasses of external adenosine receptors. Proc Natl Acad Sci U S A. 1980 May;77(5):2551–2554. doi: 10.1073/pnas.77.5.2551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matherne G. P., Headrick J. P., Coleman S. D., Berne R. M. Interstitial transudate purines in normoxic and hypoxic immature and mature rabbit hearts. Pediatr Res. 1990 Oct;28(4):348–353. doi: 10.1203/00006450-199010000-00010. [DOI] [PubMed] [Google Scholar]
- Monaco L., DeManno D. A., Martin M. W., Conti M. Adenosine inhibition of the hormonal response in the Sertoli cell is reversed by pertussis toxin. Endocrinology. 1988 Jun;122(6):2692–2698. doi: 10.1210/endo-122-6-2692. [DOI] [PubMed] [Google Scholar]
- Nesher G., Moore T. L. The in vitro effects of methotrexate on peripheral blood mononuclear cells. Modulation by methyl donors and spermidine. Arthritis Rheum. 1990 Jul;33(7):954–959. doi: 10.1002/art.1780330706. [DOI] [PubMed] [Google Scholar]
- Parmely M. J., Zhou W. W., Edwards C. K., 3rd, Borcherding D. R., Silverstein R., Morrison D. C. Adenosine and a related carbocyclic nucleoside analogue selectively inhibit tumor necrosis factor-alpha production and protect mice against endotoxin challenge. J Immunol. 1993 Jul 1;151(1):389–396. [PubMed] [Google Scholar]
- Parsons W. J., Ramkumar V., Stiles G. L. Isobutylmethylxanthine stimulates adenylate cyclase by blocking the inhibitory regulatory protein, Gi. Mol Pharmacol. 1988 Jul;34(1):37–41. [PubMed] [Google Scholar]
- Parsons W. J., Stiles G. L. Heterologous desensitization of the inhibitory A1 adenosine receptor-adenylate cyclase system in rat adipocytes. Regulation of both Ns and Ni. J Biol Chem. 1987 Jan 15;262(2):841–847. [PubMed] [Google Scholar]
- Ramkumar V., Stiles G. L. A novel site of action of a high affinity A1 adenosine receptor antagonist. Biochem Biophys Res Commun. 1988 Jun 30;153(3):939–944. doi: 10.1016/s0006-291x(88)81318-4. [DOI] [PubMed] [Google Scholar]
- Ramkumar V., Stiles G. L. Reciprocal modulation of agonist and antagonist binding to A1 adenosine receptors by guanine nucleotides is mediated via a pertussis toxin-sensitive G protein. J Pharmacol Exp Ther. 1988 Sep;246(3):1194–1200. [PubMed] [Google Scholar]
- Rose F. R., Hirschhorn R., Weissmann G., Cronstein B. N. Adenosine promotes neutrophil chemotaxis. J Exp Med. 1988 Mar 1;167(3):1186–1194. doi: 10.1084/jem.167.3.1186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossi N. F., Churchill P. C., Churchill M. C. Pertussis toxin reverses adenosine receptor-mediated inhibition of renin secretion in rat renal cortical slices. Life Sci. 1987 Feb 2;40(5):481–487. doi: 10.1016/0024-3205(87)90114-7. [DOI] [PubMed] [Google Scholar]
- Salmon J. E., Brogle N., Brownlie C., Edberg J. C., Kimberly R. P., Chen B. X., Erlanger B. F. Human mononuclear phagocytes express adenosine A1 receptors. A novel mechanism for differential regulation of Fc gamma receptor function. J Immunol. 1993 Sep 1;151(5):2775–2785. [PubMed] [Google Scholar]
- Salmon J. E., Cronstein B. N. Fc gamma receptor-mediated functions in neutrophils are modulated by adenosine receptor occupancy. A1 receptors are stimulatory and A2 receptors are inhibitory. J Immunol. 1990 Oct 1;145(7):2235–2240. [PubMed] [Google Scholar]
- Schrier D. J., Lesch M. E., Wright C. D., Gilbertsen R. B. The antiinflammatory effects of adenosine receptor agonists on the carrageenan-induced pleural inflammatory response in rats. J Immunol. 1990 Sep 15;145(6):1874–1879. [PubMed] [Google Scholar]
- Sperling R. I., Coblyn J. S., Larkin J. K., Benincaso A. I., Austen K. F., Weinblatt M. E. Inhibition of leukotriene B4 synthesis in neutrophils from patients with rheumatoid arthritis by a single oral dose of methotrexate. Arthritis Rheum. 1990 Aug;33(8):1149–1155. doi: 10.1002/art.1780330815. [DOI] [PubMed] [Google Scholar]
- Trussell L. O., Jackson M. B. Dependence of an adenosine-activated potassium current on a GTP-binding protein in mammalian central neurons. J Neurosci. 1987 Oct;7(10):3306–3316. doi: 10.1523/JNEUROSCI.07-10-03306.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmerman C. L., Franz T. J., Slattery J. T. Pharmacokinetics of the poly-gamma-glutamyl metabolites of methotrexate in skin and other tissues of rats and hairless mice. J Pharmacol Exp Ther. 1984 Nov;231(2):242–247. [PubMed] [Google Scholar]
- Zurier R. B., Hoffstein S., Weissmann G. Suppression of acute and chronic inflammation in adrenalectomized rats by pharmacologic amounts of prostaglandins. Arthritis Rheum. 1973 Sep-Oct;16(5):606–618. doi: 10.1002/art.1780160505. [DOI] [PubMed] [Google Scholar]
- van Calker D., Müller M., Hamprecht B. Adenosine regulates via two different types of receptors, the accumulation of cyclic AMP in cultured brain cells. J Neurochem. 1979 Nov;33(5):999–1005. doi: 10.1111/j.1471-4159.1979.tb05236.x. [DOI] [PubMed] [Google Scholar]